Abstract
The aim of this study was to develop and characterize the properties of khorasan wheat starch (KWS) films containing moringa leaf extract (MLE) as an antioxidative packaging material. KWS was isolated from khorasan wheat and used as a film base material. Different amounts (0, 0.4, 0.7, and 1.0%, w/v) of MLE were added to the KWS film-forming solution and the film properties were examined. Tensile strength of the KWS films decreased and elongation at break increased with increasing MLE content. In addition, the KWS films containing MLE possessed good antioxidative activities and ultraviolet light blocking ability. In particular, 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) and 2,2-diphenyl-1-picrylhydrazyl radical scavenging abilities of the KWS films with 1.0% MLE were 59.45% and 37.89%, respectively. Moreover, KWS films containing 1.0% MLE were biodegradable within 30 days. These findings indicate that the developed KWS films containing MLE can be applied as a biodegradable packaging material with antioxidative activity.
Keywords: Antioxidative activity, Biodegradable film, Khorasan wheat starch, Moringa leaf extract
Introduction
Lipid oxidation in foods negatively impacts visual and olfactory aspects of foods as well as quality (Barden and Decker, 2016). Thus, either foods are packaged properly to prevent lipid oxidation or antioxidants are added (Barbosa-Pereira et al., 2014). Synthetic plastics, which are mainly used as food packaging materials, have the advantages of favorable physical properties and low cost (Raheem, 2013). However, they can cause significant environmental pollution. Therefore, the interest in producing packaging materials with antioxidants or antimicrobials have increased (Ifuku et al., 2013), and several studies on the production of environmentally friendly film packaging materials using biopolymers such as starch have been conducted.
Khorasan wheat is an ancient crop that is resistant to pests and harsh conditions, while being similar to rye in appearance and nutrition (Saa et al., 2014). Khorasan wheat are abundant in starch, a carbohydrate suitable as a base material for biodegradable films (Sofi et al., 2013). However, films prepared from biopolymers often have poor mechanical properties compared to plastic packaging materials (Niranjana-Prabhu and Prashantha, 2018). Thus, there have been many studies attempting to improve the mechanical properties of the biopolymer films (Al-Mulla et al., 2011).
Synthetic antioxidants, which are mainly added to foods as additives, are typically avoided due to health concerns. Therefore, incorporation of natural antioxidants into the films is needed in order to produce antioxidative films (Moyo et al., 2012). Among natural antioxidants, essential oils or plant extracts are typically used due to their antioxidative properties by way of their polyphenols or short chain fatty acids content (Aleksic and Knezevic, 2014).
Moringa belongs to the family of Moringaceae and have the advantage that all parts including root, stem, flower, seed, and leaves may be used to create bioactive extracts (Choi and Jung, 2016; Saini et al., 2016). In particular, Moringa oleifera leaves contain various polyphenols, glycosylates, and isothiocyanates (Reddy et al., 2012) of which quercetin and kaempferol are the most abundant and possess excellent antioxidative and anti-inflammatory activities (Rodríguez-Pérez et al., 2015).
In this study, moringa leaf extract (MLE) was used to confer antioxidative activity to khorasan wheat starch (KWS) films. Thus, the objective of this study was to develop KWS films containing MLE and to examine the applicability as a novel antioxidant packaging material.
Materials and methods
Materials
Khorasan wheat (Kamut) and moringa (Moringa oleifera Lam.) leaf powder were purchased from Haneulsarang Co. (Incheon, Korea) and Haena Superfood Co. (Seoul, Korea), respectively. Fructose, glycerol, and sorbitol were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Isolation of KWS
KWS was isolated according to the method previously reported by Lim et al. (1992). Khorasan wheat (KW) was ground with a blender and sieved on a 600 μm sieve. KW powder in a 0.25% NaOH solution (1:6, w/v) was stirred at 4 °C for 24 h. The mixture was filtered through a 75 μm sieve to remove granules, and the filtrate was centrifuged (3000×g) at 4 °C for 10 min. Afterward, the supernatant was discarded and the precipitate was resuspended in distilled water (DW) and centrifuged again under the same condition. The above procedure was repeated until the yellow layer no longer appeared. The white starch precipitate was then dispersed in DW, neutralized to pH 6.5–7.0 with 1 M HCl, and centrifuged again as above. The supernatant was discarded while the pure starch was dissolved in DW and dried at 45 °C for 24 h. The isolated KWS was ground, filtered with a 200 μm sieve, and stored at 4 °C. Amylose content of the extracted KWS was 20.11%, and, besides starch, there were 0.01% crude fat, 0.01% crude protein, 1% ash content, and 10.16% moisture content.
Preparation of MLE
MLE was extracted following the method previously reported by Lee et al. (2016). Dried moringa leaf powder (100 g) and 70% ethanol (600 mL) were mixed and stirred at 25 °C for 24 h. After filtration with two layers of Whatman filter paper No. 2, the remaining solvent was removed using a rotary evaporator at 50 °C. The filtrate was filtered once again with two layers of filter paper to further remove remaining solid and finally lyophilized. After freeze-drying, the obtained MLE was ground with a dry mill, sieved with a 600 μm sieve, and stored at 4 °C.
Preparation of KWS films containing MLE
The KWS films containing MLE were prepared by the solvent casting method. Khorasan wheat starch (3.5%, w/v) and MLE (0, 0.4, 0.7, and 1.0%, w/v) were added to DW. Sorbitol (40% the weight of starch) was chosen as an appropriate plasticizer, based on preliminary experiments using various plasticizers (glycerol, sorbitol, and fructose). The starch film solution was gelatinized at 85 °C for 30 min with stirring. The gelatinized starch film solution was then filtered with 10 layers of cheesecloth. After cooling, the solution was sonicated for 7 min. The final film solution was cast on polypropylene coated plates (11.5 cm × 14 cm) and dried at 25 °C, 50% relative humidity (RH) for 16 h.
Mechanical properties
The thickness of the KWS films was measured with a micrometer (Model 2046-08, Mitutoyo, Tokyo, Japan). Tensile strength (TS) and elongation at break (E) of the films were measured with a Testometric machine (M250-2.5CT, Testometric Co., Lancashire, UK). All films were cut into 2.54 cm × 10 cm prior to measurements. The grip distance was 50 mm and the stretching speed of the films was 500 mm/min for the measurement. The experiment was repeated three times.
Water barrier properties
Polymethyl acrylate cups were used to measure the water vapor permeability (WVP) (Lee et al., 2015). Moisture content (MC) and water solubility (WS) of the KWS films containing MLE were evaluated following the methods previously reported by Lee et al. (2016).
Optical properties
To evaluate the color of KWS films incorporated with MLE, a colorimeter (CR-400, Minolta, Tokyo, Japan) was used. The L*, a*, and b* values were examined on the standard plate (L*: 96.79, a*: − 0.15, and b*: 2.00) according to the CIELAB color scale. The opacity and light transmittance (200–800 nm) of the KWS films with MLE were obtained based on the method reported by Baek and Song (2018b) using a spectrophotometer (UV-2450, Shimadzu Co., Kyoto, Japan).
Atomic force microscopy (AFM)
To investigate the morphology of the film surface, AFM analysis was carried out with an AFM-Raman spectrometer (INNOVA-LABRAM HR800, Horiba Jobin–Yvon Inc., New Jersey, NJ, USA). Three-dimensional images of the film surface (20 µm × 20 µm) were obtained and the roughness of the surface was calculated. With respect to the surface roughness, Ra was the average of height deviations from a mean surface (Ghasemlou et al., 2013).
Total polyphenolic content
In order to prepare film extract solutions for measurements of total phenolic content, the film samples (100 mg) were dissolved in 5 mL DW, vortexed for 3 min, and placed in a shaking incubator at 37 °C. After 30 min, the solution was centrifuged (3000×g) at 20 °C for 2 min, the supernatant was collected and subsequently used for the experiments. Total polyphenolic content was evaluated using Folin–Ciocalteu method (Siripatrawan and Vitchayakitti, 2016). Standard curves were obtained with various concentrations of gallic acid solution, and total polyphenolic content was expressed as gallic acid equivalents (GAE) (mg) per film (g).
Antioxidant activities
The antioxidative activities of the KWS films containing various amounts of MLE were examined using ABTS and DPPH radical scavenging assays (Kim et al., 2017; Lee et al., 2016). Film extract solutions were prepared by dissolving each film (0.1 g) in DW (10 mL). After 30 min shaking incubation, the film extract solutions were centrifuged (3000×g) at 4 °C for 5 min and the supernatants were collected and used for the determination of ABTS and DPPH radical scavenging activities.
Biodegradability
The biodegradability of KWS films containing 1.0% MLE, which had the highest antioxidant activity, was evaluated. Vegetable compost was purchased from Biocom Co. (Miryang, Korea). The compost was poured into a plastic tray (12 cm × 18 cm × 5 cm). The films were cut into 2 cm × 2 cm, placed on a plastic mesh, and buried in the soil. The plastic tray was stored at 25 °C and 70% RH. At various time intervals (1, 8, 16, and 30 d), samples were taken out and the biodegradability of the film was examined.
Statistical analysis
Statistical analysis was carried out using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) based on Duncan’s multiple range tests with significance at p < 0.05. The results are presented as mean ± standard deviation, and all experiments were repeated at least three times.
Results and discussion
Preparation of KWS films
To determine the proper plasticizer for the KWS film preparation, equal amounts (40%) of fructose, glycerol, and sorbitol were incorporated into the KWS film-forming solution. Regarding the mechanical characteristics, the addition of fructose formed a very weak film (TS: 5.45 MPa, E: 3.31%). When glycerol was incorporated into the KWS film, the TS and E were 23.71 MPa and 7.03%, respectively, whereas the KWS film containing sorbitol had 28.05 MPa of TS and 26.41% of E. Sorbitol was chosen as the plasticizer for all future experiments. After choosing sorbitol as the optimal plasticizer for the KWS film, various concentrations (30, 40, and 50%) of sorbitol were added to the film-forming solution. The KWS film containing 30% sorbitol had a TS-value similar to the film with 40% sorbitol, but a low E value (4.48%) was observed. Furthermore, the KWS film with 50% sorbitol had a lower TS (25.20 MPa) than that of the film with 40% sorbitol (28.05 MPa). Therefore, based on these results, 40% sorbitol was found to be an appropriate plasticizer for the KWS films in this study.
Mechanical and water barrier properties of KWS films with MLE
The mechanical and water barrier properties of KWS films with various concentrations of MLE were determined (Table 1). With the incorporation of MLE, the thickness of the KWS films increased slightly, and the TS value decreased, while the E value increased. This may be explained by MLE hindering intermolecular interactions between starch molecules and the reduced cohesive forces in the starch film network, resulting in a reduced TS value. This tendency was reported in several papers where low molecular weight materials, such as plant extracts or essential oils were incorporated into the polymer films (Medina-Jaramillo et al., 2015; Song et al., 2014). In addition, the increase in E value could be attributed to intermolecular interactions between starch molecules and phenolic compounds present in MLE, which may increase flexibility of starch chains (Homayouni et al., 2017). This change could be explained by the MLE molecules, which caused the interactions between starch molecules weak and had the films more elastic, resulting in the increase in E.
Table 1.
Mechanical and water barrier properties of KWS films containing moringa leaf extract
| Moringa leaf extract (%) | Thickness (mm) | Tensile strength (MPa) | Elongation at break (%) | Water vapor permeability (× 10−9 g/m s Pa) | Moisture content (%) | Water solubility (%) |
|---|---|---|---|---|---|---|
| 0 | 0.064 ± 0.004c | 28.05 ± 0.89a | 26.41 ± 2.30c | 2.82 ± 0.08c | 6.09 ± 0.17b | 42.09 ± 1.82b |
| 0.4 | 0.071 ± 0.002b | 21.74 ± 1.59b | 44.09 ± 2.39b | 3.09 ± 0.02b | 6.27 ± 0.41b | 43.69 ± 1.52b |
| 0.7 | 0.075 ± 0.003b | 17.44 ± 1.38c | 47.90 ± 3.34b | 3.17 ± 0.08b | 6.31 ± 0.27b | 46.93 ± 0.51a |
| 1.0 | 0.083 ± 0.002a | 13.10 ± 0.44d | 59.53 ± 1.76a | 3.53 ± 0.19a | 7.57 ± 0.46a | 49.16 ± 1.69a |
Mean ± S.D., n = 3
a–dAny means in the same column followed by different letters are significantly (p < 0.05) different by Duncan’s multiple range test
The WVP value of the KWS film was 2.82 × 10−9 g/m s Pa, and the WVP values increased with the addition of MLE (Table 1). MC and WS of the KWS films with MLE showed similar trends. These results could be explained by the characteristics of MLE and starch molecules, such as favorable interactions between starch and water molecules caused by the addition of MLE. In particular, the phenolic compounds of MLE in the films affected the interactions between starch and water molecules and caused the increases in WVP, WS, and MC with increase of MLE content (Pineros-Hernandez et al., 2017). Similarly, WVP, MC, and WS increased with increasing content of betel leaf extract added to sago starch films as previously observed (Nouri and Nafchi, 2014).
Optical properties
The KWS films containing MLE had yellowish green color, which turned dark green with increasing MLE content. As shown in Table 2, the KWS films containing MLE had lower L* values than those of the KWS film without MLE, indicating that lightness decreased. In contrast, the addition of MLE resulted in an increase in the yellowness (b*) values from 1.65 to 43.43. The opacity of the KWS films increased slightly with increasing MLE content. In general, the addition of plant extracts to the starch films decreases L* values and increases opacity. Similar results were reported with other starch films (Luchese et al., 2018; Xu et al., 2018).
Table 2.
Optical properties of KWS films containing moringa leaf extract
| Moringa leaf extract (%) | L* | a* | b* | ΔE | Opacity (A/mm) |
|---|---|---|---|---|---|
| 0 | 97.15 ± 0.05a | 0.04 ± 0.01a | 1.65 ± 0.03d | – | 0.43 ± 0.22c |
| 0.4 | 90.82 ± 0.05b | − 6.03 ± 0.05b | 25.56 ± 0.24c | 25.47 ± 0.24c | 0.92 ± 0.11b |
| 0.7 | 87.78 ± 0.13c | − 6.78 ± 0.01c | 34.64 ± 0.33b | 34.97 ± 0.35b | 0.95 ± 0.05b |
| 1.0 | 84.48 ± 0.23d | − 6.81 ± 0.03c | 43.43 ± 0.45a | 44.20 ± 0.48a | 1.41 ± 0.18a |
Mean ± S.D., n = 3
a–dAny means in the same column followed by different letters are significantly (p < 0.05) different by Duncan’s multiple range test
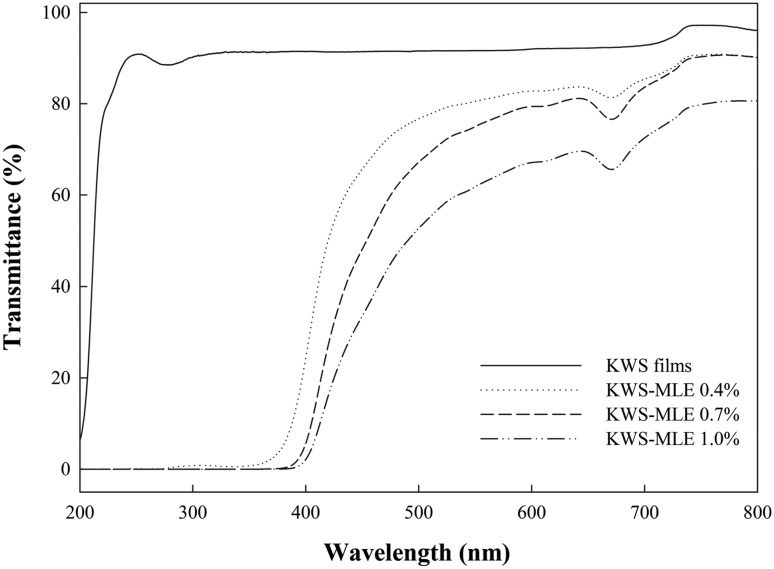
The results of UV–visible light transmission spectra of KWS films are presented in Fig. 1. The transmittance showed a steady decrease in the KWS films with increasing MLE content. In particular, UV light was completely blocked by the KWS films with MLE. Souza et al. (2017) also reported that chitosan films containing plant extracts had the color of the extract and decreased UV light transmittance. Baek and Song (2018a) reported that the incorporation of curcumin to proso millet starch film could improve the shelf life of foods due to enhanced blocking of UV light. Thus, the KWS films with MLE could also prevent lipid oxidation in foods during storage by blocking UV light.
Fig. 1.
UV-visible light transmission spectra of KWS films containing moringa leaf extract
Film morphology
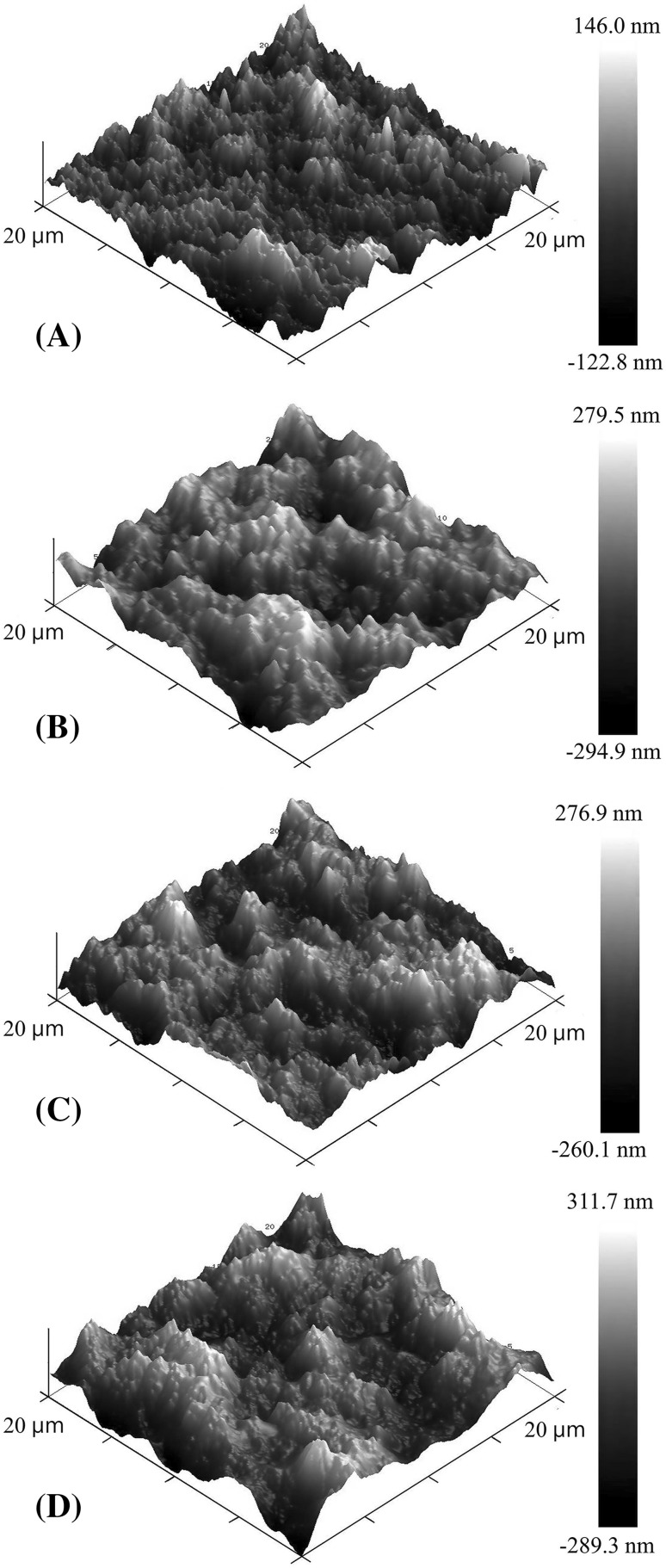
AFM was performed to compare the morphology of the film surface. The results were obtained as three-dimensional images (Fig. 2). AFM analysis showed that the Ra value of the KWS film was 22.9 nm, whereas the Ra value of the KWS films with MLE was 53.6–73.2 nm (Data not shown). These findings indicate that the incorporation of MLE increased the roughness of the film surface. However, there was not a significant difference between the KWS film with 0.4% MLE and the KWS film with 0.7% MLE, but there was a conspicuous difference between the KWS film and the KWS film with 1.0% MLE. The KWS film without MLE showed uniformly small peaks, whereas the films containing MLE showed relatively high and aggregated peaks. In addition, the peaks of the KWS films with MLE became higher with increasing MLE concentration. This tendency might be attributed to the solid insoluble particles generated during film preparation when MLE are added. Medina-Jaramillo et al. (2015) explained that the roughness of the film surface was mainly due to the hydrophobicity of the added material. It was shown that cassava starch film containing basil extract contained insoluble particles and the hydrophobic particles moved to the film surface during drying, causing the increase in roughness of the film surface. Therefore, roughness of KWS films with MLE could be due to the hydrophobic particles moving to the film surface during drying.
Fig. 2.
The morphology of KWS films containing moringa leaf extract. (A) KWS film; (B) KWS-MLE 0.4%; (C) KWS-MLE 0.7%; (D) KWS-MLE 1.0%
Total phenolic content and antioxidant activities
Total phenolic content and antioxidative activities of KWS films containing MLE are presented in Table 3. The TPC of MLE has been reported to be 118–132 mg/g in the literature, depending on the extraction and analysis methods (Pari et al., 2007; Vongsak et al., 2013). As expected, the total phenolic content increased with increasing MLE concentration in the KWS films. It should be noted that the control film without MLE did not have TPC. In addition, all KWS films containing MLE indicated scavenging activities for ABTS and DPPH radicals, suggesting that KWS films incorporated with MLE had antioxidative properties. This was especially apparent for the ABTS assays, where the scavenging activity increased from 34.36 to 59.45% as the concentration of MLE increased from 0.4 to 1.0%. The most important chemicals with antioxidative activity in the plant extracts are polyphenols (Kim et al., 2017). Therefore, the antioxidative activity of the KWS films with MLE is related to the number of polyphenols in the films. MLE contains quercetin and kaempferol (Lee et al., 2016), as well as isoquercetin and crypto-chlorogenic acid (Vongsak et al., 2013). Similar to our findings, cassava starch films containing rosemary extract showed increasing antioxidative activity with increasing phenolic content (Pineros-Hernandez et al., 2017). Furthermore, fish gelatin films also showed increasing ABTS radical scavenging activity with the incorporation of MLE (Lee et al., 2016). Taken together, these results suggest that KWS films containing MLE could be suitable as an antioxidative packaging material for foods.
Table 3.
Total phenolic contents and antioxidant activities of KWS films containing moringa leaf extract
| Moringa leaf extract (%) | Total phenolic content (mg GAE/g film) | ABTS radical scavenging activity (%) | DPPH radical scavenging activity (%) |
|---|---|---|---|
| 0 | – | – | – |
| 0.4 | 4.91 ± 0.29c | 34.36 ± 1.63c | 15.25 ± 3.52c |
| 0.7 | 8.28 ± 0.15b | 52.31 ± 1.33b | 30.55 ± 0.64b |
| 1.0 | 10.96 ± 0.27a | 59.45 ± 2.57a | 37.89 ± 2.47a |
Mean ± S.D., n = 5
a–cAny means in the same column followed by different letters are significantly (p <0.05) different
Biodegradability of KWS films
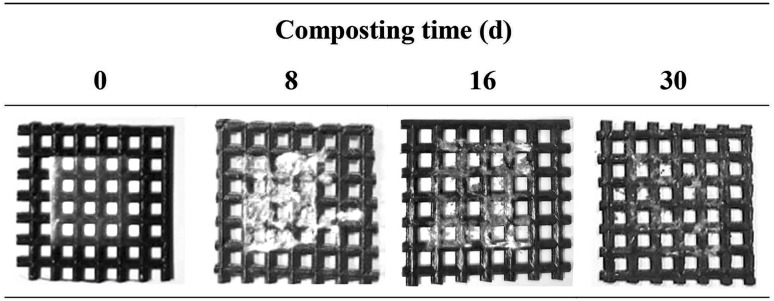
Biodegradation experiments were performed and morphological changes in the films were observed for 30 d (Fig. 3). In this study, the KWS film containing 1.0% MLE, which had the highest antioxidant activity, was selected for biodegradability test. On the eighth day of storage, partial decomposition of the films was observed, while the films were almost decomposed on the 30th day and complete degradation time could be more than 30 days. Overall, the developed KWS films containing MLE could be disintegrated, suggesting that it is suitable as a biodegradable packaging. Similar trends have been reported for starch films containing plant extracts (Assis et al., 2018; Jaramillo et al., 2016; Pineros-Hernandez et al., 2017).
Fig. 3.
Biodegradability of the KWS film containing 1.0% moringa leaf extract
In this study, MLE, a natural plant extract, was successfully incorporated into KWS films to provide antioxidative activity. The KWS films with MLE possessed excellent free radical scavenging activities, which increased further with increasing MLE content. Moreover, the KWS films containing MLE had outstanding UV light blocking ability. The biodegradability of the KWS films with MLE was also confirmed and could be more than 30 days. In conclusion, KWS films containing MLE are applicable as a novel antioxidative biodegradable packaging material to prevent lipid oxidation of foods during storage.
Acknowledgements
This study was supported by a grant from the National Research Foundation of Korea (Grant No. 2018R1D1A1B07047096).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ahreum Ju, Email: supernova3a@gmail.com.
Su-Kyoung Baek, Email: sukyoungb@naver.com.
Sujin Kim, Email: pppink32@gmail.com.
Kyung Bin Song, Phone: +82 42 821 6723, Email: kbsong@cnu.ac.kr.
References
- Al-Mulla EAJ, Suhail AH, Aowda SA. New biopolymer nanocomposites based on epoxidized soybean oil plasticized poly (lactic acid)/fatty nitrogen compounds modified clay: Preparation and characterization. Ind. Crops Prod. 2011;33:23–29. doi: 10.1016/j.indcrop.2010.07.022. [DOI] [Google Scholar]
- Aleksic V, Knezevic P. Antimicrobial and antioxidative activity of extracts and essential oils of Myrtus communis L. Microbiol. Res. 2014;169:240–254. doi: 10.1016/j.micres.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Assis RQ, Pagno CH, Costa TMH, Flôres SH, Rios ADO. Synthesis of biodegradable films based on cassava starch containing free and nanoencapsulated β-carotene. Packag. Technol. Sci. 2018;31:157–166. doi: 10.1002/pts.2364. [DOI] [Google Scholar]
- Baek SK, Song KB. Characterization of active biodegradable films based on proso millet starch and curcumin. Starch/Stärke. 2018;9:99. [Google Scholar]
- Baek SK, Song KB. Development of Gracilaria vermiculophylla extract films containing zinc oxide nanoparticles and their application in smoked salmon packaging. LWT-Food Sci. Technol. 2018;89:269–275. doi: 10.1016/j.lwt.2017.10.064. [DOI] [Google Scholar]
- Barbosa-Pereira L, Aurrekoetxea GP, Angulo I, Paseiro-Losada P, Cruz JM. Development of new active packaging films coated with natural phenolic compounds to improve the oxidative stability of beef. Meat Sci. 2014;97:249–254. doi: 10.1016/j.meatsci.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Barden L, Decker EA. Lipid oxidation in low-moisture food: a review. Crit. Rev. Food Sci. Nutr. 2016;56:2467–2482. doi: 10.1080/10408398.2013.848833. [DOI] [PubMed] [Google Scholar]
- Choi YJ, Jung KI. Anti-diabetic, alcohol-metabolizing, and hepatoprotective activities of moringa (Moringa oleifera Lam.) leaf extracts. J. Korean Soc. Food Sci. Nutr. 2016;45:819–827. doi: 10.3746/jkfn.2016.45.6.819. [DOI] [Google Scholar]
- Ghasemlou M, Aliheidari N, Fahmi R, Shojaee-Aliabadi S, Keshavarz B, Cran MJ, Khaksar R. Physical, mechanical and barrier properties of corn starch films incorporated with plant essential oils. Carbohydr. Polym. 2013;98:1117–1126. doi: 10.1016/j.carbpol.2013.07.026. [DOI] [PubMed] [Google Scholar]
- Homayouni H, Kavoosi G, Nassiri SM. Physicochemical, antioxidant and antibacterial properties of dispersion made from tapioca and gelatinized tapioca starch incorporated with carvacrol. LWT - Food Sci. Technol. 2017;77:503–509. doi: 10.1016/j.lwt.2016.12.007. [DOI] [Google Scholar]
- Ifuku S, Ikuta A, Egusa M, Kaminaka H, Izawa H, Morimoto M, Saimoto H. Preparation of high-strength transparent chitosan film reinforced with surface-deacetylated chitin nanofibers. Carbohydr. Polym. 2013;98:1198–1202. doi: 10.1016/j.carbpol.2013.07.033. [DOI] [PubMed] [Google Scholar]
- Jaramillo CM, Gutiérrez TJ, Goyanes S, Bernal C, Famá L. Biodegradability and plasticizing effect of yerba mate extract on cassava starch edible films. Carbohydr. Polym. 2016;151:150–159. doi: 10.1016/j.carbpol.2016.05.025. [DOI] [PubMed] [Google Scholar]
- Kim H, Yang HJ, Lee KY, Beak SE, Song KB. Characterization of red ginseng residue protein films incorporated with hibiscus extract. Food Sci. Biotechnol. 2017;26:369–374. doi: 10.1007/s10068-017-0050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Lee J, Song KB. Development of a chicken feet protein film containing essential oils. Food Hydrocoll. 2015;46:208–215. doi: 10.1016/j.foodhyd.2014.12.020. [DOI] [Google Scholar]
- Lee KY, Yang HJ, Song KB. Application of a puffer fish skin gelatin film containing Moringa oleifera Lam. leaf extract to the packaging of Gouda cheese. J. Food Sci. Technol. 2016;53:3876–3883. doi: 10.1007/s13197-016-2367-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim W, Liang Y, Seib P, Rao C. Isolation of oat starch from oat flour. Cereal Chem. 1992;69:233–236. [Google Scholar]
- Luchese CL, Garrido T, Spada JC, Tessaro IC, de la Caba K. Development and characterization of cassava starch films incorporated with blueberry pomace. Int. J. Biol. Macromol. 2018;106:834–839. doi: 10.1016/j.ijbiomac.2017.08.083. [DOI] [PubMed] [Google Scholar]
- Medina-Jaramillo C, Gonzalez-Seligra P, Goyanes S, Bernal C, Famá L. Biofilms based on cassava starch containing extract of yerba mate as antioxidant and plasticizer. Starch/Stärke. 2015;67:780–789. doi: 10.1002/star.201500033. [DOI] [Google Scholar]
- Moyo B, Oyedemi S, Masika P, Muchenje V. Polyphenolic content and antioxidant properties of Moringa oleifera leaf extracts and enzymatic activity of liver from goats supplemented with Moringa oleifera leaves/sunflower seed cake. Meat Sci. 2012;91:441–447. doi: 10.1016/j.meatsci.2012.02.029. [DOI] [PubMed] [Google Scholar]
- Niranjana-Prabhu T, Prashantha K. A review on present status and future challenges of starch based polymer films and their composites in food packaging applications. Polym. Composites. 2018;39:2499–2522. doi: 10.1002/pc.24236. [DOI] [Google Scholar]
- Nouri L, Nafchi AM. Antibacterial, mechanical, and barrier properties of sago starch film incorporated with betel leaves extract. Int. J. Biol. Macromol. 2014;66:254–259. doi: 10.1016/j.ijbiomac.2014.02.044. [DOI] [PubMed] [Google Scholar]
- Pari L, Karamac M, Kosinska A, Rybarczyk A, Amarowicz R. Antioxidant activity of the crude extracts of drumstick tree (Moringa oleifera Lam.) and sweet broomweed (Scoparia dulcis L.) leaves. Polish J. Food Nut. Sci. 2007;57:203–208. [Google Scholar]
- Pineros-Hernandez D, Medina-Jaramillo C, López-Córdoba A, Goyanes S. Edible cassava starch films carrying rosemary antioxidant extracts for potential use as active food packaging. Food Hydrocoll. 2017;63:488–495. doi: 10.1016/j.foodhyd.2016.09.034. [DOI] [Google Scholar]
- Raheem D. Application of plastics and paper as food packaging materials-An overview. Emir. J. Food Agric. 2013;25:177–188. doi: 10.9755/ejfa.v25i3.11509. [DOI] [Google Scholar]
- Reddy DHK, Seshaiah K, Reddy A, Lee S. Optimization of Cd (II), Cu (II) and Ni (II) biosorption by chemically modified Moringa oleifera leaves powder. Carbohydr. Polym. 2012;88:1077–1086. doi: 10.1016/j.carbpol.2012.01.073. [DOI] [Google Scholar]
- Rodríguez-Pérez C, Quirantes-Piné R, Fernández-Gutiérrez A, Segura-Carretero A. Optimization of extraction method to obtain a phenolic compounds-rich extract from Moringa oleifera Lam leaves. Ind. Crops Prod. 2015;66:246–254. doi: 10.1016/j.indcrop.2015.01.002. [DOI] [Google Scholar]
- Saa DT, Turroni S, Serrazanetti DI, Rampelli S, Maccaferri S, Candela M, Gianotti A. Impact of Kamut® Khorasan on gut microbiota and metabolome in healthy volunteers. Food Res. Int. 2014;63:227–232. doi: 10.1016/j.foodres.2014.04.005. [DOI] [Google Scholar]
- Saini RK, Sivanesan I, Keum YS. Phytochemicals of Moringa oleifera: a review of their nutritional, therapeutic and industrial significance. 3 Biotech. 2016;6:203. doi: 10.1007/s13205-016-0526-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siripatrawan U, Vitchayakitti W. Improving functional properties of chitosan films as active food packaging by incorporating with propolis. Food Hydrocoll. 2016;61:695–702. doi: 10.1016/j.foodhyd.2016.06.001. [DOI] [Google Scholar]
- Sofi F, Whittaker A, Cesari F, Gori A, Fiorillo C, Becatti M, Abbate R. Characterization of Khorasan wheat (Kamut) and impact of a replacement diet on cardiovascular risk factors: cross-over dietary intervention study. Eur. J. Clin. Nutr. 2013;67:190. doi: 10.1038/ejcn.2012.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song NB, Lee JH, Al Mijan M, Song KB. Development of a chicken feather protein film containing clove oil and its application in smoked salmon packaging. LWT-Food Sci. Technol. 2014;57:453–460. doi: 10.1016/j.lwt.2014.02.009. [DOI] [Google Scholar]
- Souza VGL, Fernando AL, Pires JRA, Rodrigues PF, Lopes AA, Fernandes FMB. Physical properties of chitosan films incorporated with natural antioxidants. Ind. Crops Prod. 2017;107:565–572. doi: 10.1016/j.indcrop.2017.04.056. [DOI] [Google Scholar]
- Vongsak B, Sithisarn P, Mangmool S, Thongpraditchote S, Wongkrajang Y, Gritsanapan W. Maximizing total phenolics, total flavonoids contents and antioxidant activity of Moringa oleifera leaf extract by the appropriate extraction method. Ind. Crops Prod. 2013;44:566–571. doi: 10.1016/j.indcrop.2012.09.021. [DOI] [Google Scholar]
- Xu Y, Rehmani N, Alsubaie L, Kim C, Sismour E, Scales A. Tapioca starch active nanocomposite films and their antimicrobial effectiveness on ready-to-eat chicken meat. Food Packag. Shelf Life. 2018;16:86–91. doi: 10.1016/j.fpsl.2018.02.006. [DOI] [Google Scholar]