Abstract
The aim of this study was to investigate the combined effects of Diospyros lotus leaves extracts (DLE) and Muscat Bailey A grape stalk extracts (MGSE) in obesity induced by high-fat diet (HFD) in mice. The mice were fed with HFD and orally administered daily with DLE, MGSE, a mixture of DLE and MGSE, and Garcinia cambogia extract over a period of 16 weeks. The results revealed that daily administration of DLE and MGSE mixtures markedly prevented HFD-induced weight gain, plasma lipid profile, hepatic steatosis, hepatic fibrosis, diabetic symptoms, and the risk of developing cardiovascular diseases. Also, DLE and MGSE mixtures administration greatly prevented oxidative stress and liver toxicity. The combined effects of DLE and MGSE mixtures were higher than effects of the single extracts and of G. cambogia, a plant known for its anti-obesity effects. In summary, these findings demonstrated that DLE and MGSE mixtures, exhibit anti-obesity activity in HFD-fed mice.
Keywords: Combined effect, Diospyros lotus leaf, Muscat Bailey A grape stalk, Obesity
Introduction
Obesity is one of the most leading preventable cause of death in the 21st century. The most recent data shows that worldwide obesity has nearly tripled over the past 40 years with 39% of adults aged 18 years and above being overweight and 13% being obese (WHO, 2018). Obesity is associated with the accumulation of fat in the body and secondary pathologic disorders such as metabolic syndrome, cardiovascular diseases, type 2 diabetes, hypertension, hyperlipidemia, liver disorders, and other pathologies (Clain and Lefkowitch, 1987; Manna and Kalita, 2016; Paccaud et al., 2000; Wang et al., 2018). Diet control and exercise have been used to prevent and control obesity. However, these methods have proven to be insufficient in preventing or controlling obesity and obesity-associated oxidative stress (Galili et al., 2007). This has led to the development of anti-obesity agents such as orlistat, lorcaserin, liraglutide, phentermine/topiramate and naltrexone/bupropion, which are currently in use for chronic weight management in obesity (Fujioka, 2015). These drugs come with considerable side effects such as stomach ache, paresthesia, vomiting, insomnia, constipation, headache, and nausea. Interestingly, most of the drugs are contraindicated in cardiovascular diseases patients and those with a high risk of cardiovascular diseases (Greco, 2015; Shin and Gadde, 2013; Wadden et al., 2013). For these reasons, there is a need to develop other anti-obesity agents with lesser side effects. Herbal medicines have been recently exploited and used for weight control in many countries. Garcinia cambogia and its main compound hydroxycitric acid have been shown to have no toxic effects and is widely becoming a popular natural product ingredient in weight loss supplements (Chuah et al., 2013).
Diospyros lotus is a plant of the Ebenaceae family, native to China and Asia. The fruit of this plant is eaten for its anti-diabetic and anti-hypertension properties (Rashed et al., 2012; Uddin et al., 2011). The roots of D. lotus have antinociceptive and anti-inflammatory activities (Uddin et al., 2014). We recently showed that D. lotus leaves ameliorated atopic dermatitis-like skin lesion in mice and had UV-protective properties (Cho et al., 2017a; 2017b).
Muscat Bailey A grape (Vitis labrusca × V. vinifera) is one of the major grape varieties grown in Korea. The grape stalk is an organic waste, produced in great amounts during the industrialization processes of grape. It is rich in bioactive phenolic compounds and displays antioxidant and UV-protective activities in mice studies (Cárcel et al., 2010; Cho et al., 2018). Due to the continuous search for better treatment of obesity and its associated disorders, there is a growing interest in the combined effects of phytochemicals in the treatment of diseases. In this light, the aim of the present study was to investigate the combined effects of D. lotus leaf and Muscat Bailey A grape stalk extracts in HFD-induced obesity and its associated disorders in mice.
Materials and methods
Plant extraction
Fresh D. lotus leaves were harvested from Bugwi-Myeon, Jinan-Gun, Jeonbuk, Korea. The plant was authenticated by Prof Kim Hong-Jun of the college of oriental medicine, Woosuk University, and a voucher specimen was kept in our laboratory in the Department of Health Management, Jeonju University. The leaves were washed in distilled water and dried at 60 °C for 16 h. One hundred grams of grind D. lotus leaves were placed in a container containing 2000 mL of 50% (V/V) ethanol for 72 h on a shaker to obtain the extract. The extracted sample was filtered with 0.45 μm filter paper (ADVANTEC, Togo, Japan), concentrated, lyophilized and subsequently stored at −20 °C until used.
Muscat Bailey A grape stalk came from Sintaein-eup, Jeongeup-si, Jeollabuk-do, Korea. The stalks were washed in distilled water and dried at 40 °C for 72 h. One hundred grams of grind Muscat Bailey A grape stalk was placed in a container containing 2000 mL of 80% (V/V) ethanol for 72 h on a shaker to obtain the extract. The extracted sample was then filtered with 0.45 μm filter paper (ADVANTEC), concentrated and lyophilized to obtain the dry extract which was subsequently stored at −20 °C for further use.
Animals and diet
Ethical approval for the present study was obtained from Jeonju University Institutional Animal Care and Use Committee (#JJU-IACUC-2017-011). Male 3-week-old C57BL/6N mice were purchased from Orient Bio Inc. (Iksan, Korea). The mice were maintained in standard environmental conditions of temperature 22 ± 2 °C, humidity 50–60%, and 12/12 h light–dark cycle, following Jeonju University Institutional Animal Care and Use Committee guidelines. Mice were fed with commercial standard laboratory diet (AIN-76A, Research Diet Inc., New Brunswick, NJ, USA) and water ad libitum. After 1 week of acclimatization, the mice were divided into 6 groups (n = 10) and fed for 16 weeks as follows: Group (1) Non-fat fed diet (Control). Group (2) High-fat fed diet (HFD, D12492, Rodent Diet with 60 kcal % fat, Research Diet Inc.). Group (3) High-fat fed diet and 200 mg/kg D. lotus leaf extracts (DLE). Group (4) High-fat fed diet and 200 mg/kg Muscat Bailey A grape stalk extracts (MGSE), Group (5) High-fat fed diet and 100 mg/kg DLE + 100 mg/kg MGSE. Group (6) High-fat fed diet and 200 mg/kg of G. cambogia extracts (GCE).
Weight, food intake and histochemical analysis
Before the start of the experiment, the weights and food intake of the mice in each group were recorded. At the end of the experimental period, the mice fasted for 16 h and the weights of the mice were again recorded before they were sacrificed. After blood collection, epididymal adipose and the liver tissues were immediately removed and weighed. For histological analysis, epididymal adipose and liver tissues were fixed in 10% neutral formalin for 42 h. The tissues were washed in three changes of phosphate buffered saline (30 min each); cleared in three changes of xylene (30 min each) before they were embedded in 3 changes of paraffin at 70 °C (1 h each). Tissue blocks were then prepared with the paraffin wax and the tissues were sectioned to a thickness of 5 μm and stained with either hematoxylin (Sigma-Aldrich, St. Louis, MO, USA) and eosin (Muto Pure Chemicals Co., Ltd., Tokyo, Japan) or trichrome stain (Abcam, Cambridge, UK).
Serum biochemical analysis
After the mice were sacrificed, blood was immediately obtained through the intraorbital vein. The blood samples were centrifuged at 2000×g for 15 min at 4 °C, and then stored at − 70 °C for subsequent experiments. Enzyme kits were employed to determine the serum concentrations of total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG) (All from Asan Pharmaceutical Co., Ltd., Gyeonggi, Korea), leptin (Enzo Life Sciences, Inc., Farmingdale, NY, USA), insulin (Alpco Diagnostics, Windham, NH, USA), and aspartate transaminase (AST) and alanine transaminase (ALT) (both from Sigma–Aldrich). Low-density lipoprotein cholesterol (LDL-C) was calculated by the following formula, LDL-C = TC − HDL-C − (TG/5). Atherogenic index and cardiac risk factor were calculated by the following formula: Atherogenic Index = TC − HDL-C)/HDL-C and Cardiac Risk Factor = TC/HDL-C. All experiments were carried out following the manufacturer’s instructions at an absorbance of 490 nm using a spectrophotometer (Tecan Group, Männedorf, Switzerland).
Liver tissue analysis
After the mice were sacrificed, liver tissues were obtained and 0.3 g were homogenized in 0.5 mL of phosphate-buffered saline and then centrifuged at 2000×g for 15 min at 4 °C to obtain the supernatant which was stored at − 70 °C. The concentrations of malondialdehyde (MDA, Cell Biolabs, Inc., San Diego, CA, USA), glutathione (GSH, Cell Biolabs, Inc.), glutathione peroxidase (GPx, Cayman Chemicals, Ann Arbor, MI, USA), and superoxide dismutase (SOD, Cayman Chemicals) were measured using commercially assay kits, following the manufacturer’s instructions at an absorbance at 490 nm using the Tecan spectrophotometer.
Statistical analysis
Differences between groups were appraised by analysis of variance (ANOVA) followed by Duncan’s multiple-range test or students T test. All data are given as the mean ± standard deviations (SD). A p value of < 0.05 was set to indicate statistically significant results.
Results and discussion
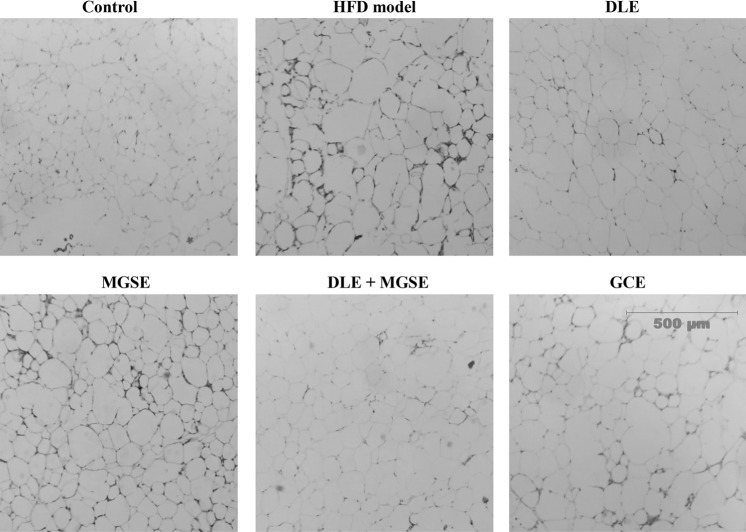
As available drugs/therapies designated for the prevention and/or treatment of obesity and its associated abnormalities are either insufficient or renders patients with severe consequences, many studies are focused on alternative methods from agricultural and forest products for the prevention and treatment of obesity. In the present study, we investigated the combined effects of DLE and MGSE, in HFD-induced obesity in mice. We found that the group of mice fed with HFD significantly gained weight with an increase in liver tissue weight, epididymal fat weight, and adipocyte size compared to the group that received the normal diet during the entire period of the study (Table 1). However, treatment of the HFD-fed mice with a combination of DLE and MGSE mixtures significantly prevented the body weight gain than the single extracts used alone or GCE, with no significant changes in food intake recorded in the various groups (Table 1). Administration of DLE and MGSE mixtures decreased liver and epididymal adipose weight than the single extracts administered alone, although the differences were not significant (Table 1). Administration of DLE and MGSE either alone or as a mixture significantly decreased liver and epididymal adipose weight compared to the HFD-treated group, although the differences were not significant among the treated groups (Table 1). Though not conclusive, it can be speculated that one of the anti-obesity mechanism of action of DLE, MGSE or its mixture in decreasing body weight gain with HFD consumption is by decreasing epididymal fat in the mice. Detailed studies of other visceral fat masses like mesenteric and perirenal, intramuscular or subcutaneous fats and lean mass weight which was not done in this study will be needed to further under the mechanism DLE and MGSE and its mixtures in weight gain in obesity. This result was also supported by comparative microscopic observations of the adipocytes of stained adipose tissues and liver tissue sections that showed that DLE and MGSE mixture administration appeared to be most effective in inhibiting adipocyte expansion in adipose tissues (Fig. 1). The inhibitory effects of DLE and MGSE mixtures on the body weight gain, epididymal fat, adipocyte expansion, liver weight, and fat accumulation in the liver tissues were similar to that of GCE, a well-known extract that has been shown to have anti-obesity effects.
Table 1.
Effect of DLE and MGSE mixture on body weight, weight gain, food intake, liver and epididymal fat weight in HFD-induced obese mice
| Group | Body weight (g) | Weight gain (g) | Food intake (g/day) | Liver weight (g) | Epididymal fat weight (g) | |
|---|---|---|---|---|---|---|
| Initial | Final | |||||
| Control | 21.58 ± 1.58 | 28.32 ± 2.78d | 6.74 ± 2.46d | 2.92 ± 0.46a | 0.97 ± 0.14c | 1.20 ± 0.20c |
| HFD | 20.65 ± 0.52 | 48.78 ± 1.36a | 28.13 ± 1.67a | 2.68 ± 0.30a | 2.12 ± 0.31a | 2.39 ± 0.34a |
| HFD + DLE | 21.76 ± 2.04 | 46.22 ± 1.69b | 24.46 ± 1.09b | 2.50 ± 0.24a | 1.56 ± 0.26b | 1.87 ± 0.17b |
| HFD + MGSE | 21.96 ± 1.49 | 45.94 ± 0.96b | 23.98 ± 0.92b | 2.66 ± 0.30a | 1.62 ± 0.23b | 1.89 ± 0.21b |
| HFD + DLE/MGSE mixture | 21.86 ± 0.65 | 44.16 ± 1.78c | 21.80 ± 1.18c | 2.48 ± 0.34a | 1.44 ± 0.23b | 1.85 ± 0.21b |
| HFD + GCE | 21.72 ± 1.14 | 45.87 ± 1.40b | 24.15 ± 1.58b | 2.58 ± 0.34a | 1.51 ± 0.27b | 1.99 ± 0.14b |
Values are mean ± SD (n = 10). Mean values with unlike letters are significantly different (p < 0.05)
Fig. 1.
Combined effects of DLE and MGSE mixtures on adipocyte size. Histological observations of hematoxylin and eosin stained epididymal adipose tissues. Representative photographs of hematoxylin and eosin stained adipose tissue. Magnification: × 100
With the effects of DLE and MGSE on body weight gain in obese mice, we proceeded to investigate the effects of these mixtures on lipid biomarkers to determine its potency to prevent cardiovascular diseases in obesity. HFD led to a significant elevation of plasma TG, TC, and LDL-cholesterol in serum compared to the normal group, demonstrating the development of hyperlipidemia, a cardiovascular disease risk factor (Fig. 2). On the other hand, administration of DLE and MGSE mixtures significantly decreased plasma concentrations of TC (Fig. 2A), TG (Fig. 2B), and LDL-cholesterol (Fig. 2D). The HDL-cholesterol was not significantly affected in all groups (Fig. 2C). In addition, DLE and MGSE mixtures significantly decreased atherogenic index (Fig. 2E) and cardiac risk factors (Fig. 2F). The efficacy of DLE and MGSE mixtures was higher than the single extracts alone or GCE. These results reveal that combined DLE and MGSE mixtures can be used to prevent and/or treat hyperlipidemia and cardiovascular diseases associated with obesity.
Fig. 2.
Combined effects of DLE and MGSE mixtures on plasma lipid profiles (A–D), atherogenic index (E), and cardiac risk factor (F) in HFD-induced obesity in mice. Data are presented as the mean ± SD (n = 10). *p < 0.05, **p < 0.01, ***p < 0.001 with respect to the DLE or MGSE alone
Possible roles of leptin production and insulin resistance in type 2 diabetes in obesity have been suggested. For instance, in type 2 diabetes, insulin sensitivity is reduced, while insulin secretion may be increased leading to hyperinsulinemia (Considine et al., 1996). Also, hyperinsulinemia has been reported to stimulates leptin expression and secretion in mice and humans (Kolaczynski et al., 1996; Saladin et al., 1995). Therefore, leptin and insulin can be used as an indicator of type 2 diabetes. Indeed, in this present study, leptin and insulin levels were increased in mice fed with HFD indicating type 2 diabetes in obesity (Fig. 3). However, the mice fed with HFD and treated with combined DLE and MGSE had a significantly lowered leptin concentration (Fig. 3A). Combined DLE and MGSE also lowered insulin levels more than the single extracts, but no significant differences were obtained between the groups (Fig. 3B). GCE demonstrated similar results and had no significant difference with the combined DLE and MGSE treatment groups. These results thus suggest that DLE and MGSE mixture can be beneficial in type 2 diabetes caused by HFD in obesity.
Fig. 3.

Combined effects of DLE and MGSE mixtures on leptin (A) and insulin (B) concentrations. Data are presented as the mean ± SD (n = 10). *p < 0.05 with respect to the DLE or MGSE alone. Representative photographs showing the combined effects of DLE and MGSE mixtures on fat accumulation (hepatic steatosis) (C) and collagen deposition (D). Tissue sections were stained with hematoxylin and eosin stain for hepatic steatosis and trichrome stain for collagen visualization. Magnification: × 200
HFD has previously been shown to induce liver damage, hepatic fibrosis, and hepatic steatosis in mice and in rats (Meli et al., 2013). This was true in this study as HFD also induced liver damage and hepatosteatosis (Fig. 3C). In histological observations, the liver of HFD control group contained macrovesicular lipid droplets as well as numerous micro lipid droplets, demonstrating a typical hepatic steatosis. Also, liver tissues showed collagen deposition in the liver of mice fed with HFD, which was prominent in the zone near the central veins, indicating liver fibrosis (Fig. 3D). Plasma concentration of AST and ALT was markedly elevated in the HFD group, an indication of liver damage (Fig. 4). DLE and MGSE groups showed less accumulation of lipid droplet in the liver (Fig. 3C); less deposition of collagen around the central vine in the liver (Fig. 3D); and also decrease the concentration of AST and ALT in blood plasma (Fig. 4A, B). Again, the effects of DLE and MGSE mixtures were higher than those of the single extracts alone and GCE. It can be deduced from the results that DLE and MGSE mixtures may help to prevent and/or treat hepatic steatosis, hepatic fibrosis and overall liver damage in HFD-induced obesity. In addition, the AST and ALT result further demonstrates that these extracts cause no detectable adverse toxic effects and can protect the liver to some extent.
Fig. 4.
Combined effects of DLE and MGSE mixtures on AST (A) and ALT (B) concentrations in plasma of mice, (C) lipid peroxidation, (D) glutathione (GSH) concentration, (E) superoxide dismutase activity and (F) glutathione peroxidase activity in liver tissues of mice. Data are presented as the mean ± SD (n = 10). *p < 0.05, **p < 0.01 with respect to the DLE or MGSE alone
In an attempt to investigate the biological pathways that have the potential to induce insulin resistance in mice fed with high-fat diet, a previous study demonstrated that the pathways for the production of reactive oxygen species (ROS) and oxidative stress were coordinately up-regulated in both the liver and adipose tissues of mice fed with HFD before the onset of insulin resistance (Matsuzawa-Nagata et al., 2008). Many other studies have also demonstrated that this oxidative stress process generates different responses that activates signaling pathways implicated in protecting cells against oxidative damage, such as heat shock proteins (HSP) and mitogen-activated protein kinase (MAPK), peroxidation of lipids and modification of proteins (Heinrichsen et al., 2014; Satapati et al., 2012; Sitnick et al., 2009). In our study, markers of oxidative stress were worsened in the HFD group as demonstrated by the significant increase in lipid peroxidation (indicated by TBARS levels), and a significant decrease in antioxidant concentration and activities (GSH concentration and SOD and GPX activities) (Fig. 4). However, when the HFD-fed mice were administered with either DLE, MGSE, DLE, and MGSE, or GCE. Combined DLE and MGSE mixtures, demonstrated higher effects than DLE, MGSE and even GCE alone, in preventing oxidative stress by significantly reducing lipid peroxidation (Fig. 4C), and up-regulating GSH concentration and SOD and GPx activities (Fig. 4D–F) in the liver. The results confirmed that HFD might promote oxidative stress in addition to weight gain, and dyslipidemia, while DLE and MGSE mixtures will prevent such HFD-induced changes.
In conclusion, we demonstrated for the first time, the combined effects of DLE and MGSE mixtures in HFD-induced. We showed that combined DLE and MGSE significantly lowered body weight gain; prevented adipocyte expansion; played a positive role in preventing type 2 diabetes and improved positively the plasma lipid profiles. We also showed that DLE and MGSE mixtures remarkably prevented liver oxidative stress, hepatic steatosis, and hepatic fibrosis without incurring any toxicity to the liver. Further studies are necessary to determine the exact molecular mechanism of action of combined DLE and MGSE in obesity.
Acknowledgements
This research was financially supported by the Ministry of SMEs and Startups (MSS), Korea, under the “Regional Specialized Industry Development Program (R&D, Project Number R0006168)” supervised by the Korea Institute for Advancement of Technology (KIAT).
Compliance with ethical standards
Conflict of interest
Theses authors declare that there are no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Denis Nchang Che, Email: chedenis88@gmail.com.
Hyun Ju Kang, Email: dkgk0608@naver.com.
Byoung Ok Cho, Email: enzyme21@naver.com.
Jae Young Shin, Email: sjy8976@naver.com.
Seon Il Jang, Phone: +82-63-220-3124, Email: sonjjang@jj.ac.kr.
References
- Cárcel JA, García-Pérez JV, Mulet A, Rodríguez L, Riera E. Ultrasonically assisted antioxidant extraction from grape stalks and olive leaves. Phys. Procedia. 2010;3:147–152. doi: 10.1016/j.phpro.2010.01.021. [DOI] [Google Scholar]
- Cho BO, Che DN, Shin JY, Kang HJ, Jang SI. Ameliorative effects of fruit stem extract from Muscat Bailey A against chronic UV-induced skin damage in BALB/c mice. Biomed. Pharmacother. 2018;97:1680–1688. doi: 10.1016/j.biopha.2017.12.003. [DOI] [PubMed] [Google Scholar]
- Cho BO, Che DN, Shin JY, Kang HJ, Kim JH, Kim HY, Cho WG, Jang SI. Ameliorative effects of Diospyros lotus leaf extract against UVB-induced skin damage in BALB/c mice. Biomed. Pharmacother. 2017;95:264–274. doi: 10.1016/j.biopha.2017.07.159. [DOI] [PubMed] [Google Scholar]
- Cho BO, Che DN, Yin HH, Shin JY, Jang SI. Diospyros lotus leaf and grapefruit stem extract synergistically ameliorate atopic dermatitis-like skin lesion in mice by suppressing infiltration of mast cells in skin lesions. Biomed. Pharmacother. 2017;89:819–826. doi: 10.1016/j.biopha.2017.01.145. [DOI] [PubMed] [Google Scholar]
- Chuah LO, Ho WY, Beh BK, Yeap SK. Updates on antiobesity effect of Garcinia origin (-)-HCA. Evid. Based Complement. Altern. Med. 2013 doi: 10.1155/2013/751658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clain DJ, Lefkowitch JH. Fatty liver disease in morbid obesity. Gastroenterol. Clin. North Am. 1987;16:239–252. [PubMed] [Google Scholar]
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, Caro JF. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- Fujioka K. Current and emerging medications for overweight or obesity in people with comorbidities. Diabetes Obes. Metab. 2015;17:1021–1032. doi: 10.1111/dom.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili O, Versari D, Sattler KJ, Olson ML, Mannheim D, McConnell JP, Chade AR, Lerman LO, Lerman A. Early experimental obesity is associated with coronary endothelial dysfunction and oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H904–H911. doi: 10.1152/ajpheart.00628.2006. [DOI] [PubMed] [Google Scholar]
- Greco D. Normal pregnancy outcome after first-trimester exposure to liraglutide in a woman with Type 2 diabetes. Diabet. Med. 2015;32:e29–e30. doi: 10.1111/dme.12726. [DOI] [PubMed] [Google Scholar]
- Heinrichsen ET, Zhang H, Robinson JE, Ngo J, Diop S, Bodmer R, Joiner WJ, Metallo CM, Haddad GG. Metabolic and transcriptional response to a high-fat diet in Drosophila melanogaster. Mol. Metab. 2014;3:42–54. doi: 10.1016/j.molmet.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaczynski JW, Nyce MR, Considine RV, Boden G, Nolan JJ, Henry R, Mudaliar SR, Olefsky J, Caro JF. Acute and chronic effects of insulin on leptin production in humans: studies in vivo and in vitro. Diabetes. 1996;45:699–701. doi: 10.2337/diab.45.5.699. [DOI] [PubMed] [Google Scholar]
- Manna P, Kalita J. Beneficial role of vitamin K supplementation on insulin sensitivity, glucose metabolism, and the reduced risk of type 2 diabetes: A review. Nutrition. 2016;32:732–739. doi: 10.1016/j.nut.2016.01.011. [DOI] [PubMed] [Google Scholar]
- Matsuzawa-Nagata N, Takamura T, Ando H, Nakamura S, Kurita S, Misu H, Ota T, Yokoyama M, Honda M, Miyamoto K, Kaneko S. Increased oxidative stress precedes the onset of high-fat diet-induced insulin resistance and obesity. Metabolism. 2008;57:1071–1077. doi: 10.1016/j.metabol.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Meli R, Mattace Raso G, Irace C, Simeoli R, Di Pascale A, Paciello O, Pagano TB, Calignano A, Colonna A, Santamaria R. High fat diet induces liver steatosis and early dysregulation of iron metabolism in rats. PLoS One. 2013;8:e66570. doi: 10.1371/journal.pone.0066570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paccaud F, Schluter-Fasmeyer V, Wietlisbach V, Bovet P. Dyslipidemia and abdominal obesity: an assessment in three general populations. J. Clin. Epidemiol. 2000;53:393–400. doi: 10.1016/S0895-4356(99)00184-5. [DOI] [PubMed] [Google Scholar]
- Rashed K, Zhang XJ, Luo MT, Zheng YT. Anti-HIV-1 activity of phenolic compounds isolated from Diospyros lotus fruits. Phytopharmacology. 2012;3:199–207. [Google Scholar]
- Saladin R, De Vos P, Guerre-Millo M, Leturque A, Girard J, Staels B, Auwerx J. Transient increase in obese gene expression after food intake or insulin administration. Nature. 1995;377:527–529. doi: 10.1038/377527a0. [DOI] [PubMed] [Google Scholar]
- Satapati S, Sunny NE, Kucejova B, Fu X, He TT, Mendez-Lucas A, Shelton JM, Perales JC, Browning JD, Burgess SC. Elevated TCA cycle function in the pathology of diet-induced hepatic insulin resistance and fatty liver. J. Lipid Res. 2012;53:1080–1092. doi: 10.1194/jlr.M023382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JH, Gadde KM. Clinical utility of phentermine/topiramate (Qsymia™) combination for the treatment of obesity. Diabetes Metab. Syndr. Obes. 2013;6:131–139. doi: 10.2147/DMSO.S43403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitnick M, Bodine SC, Rutledge JC. Chronic high fat feeding attenuates load-induced hypertrophy in mice. J. Physiol. 2009;587:5753–5765. doi: 10.1113/jphysiol.2009.180174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin G, Rauf A, Siddiqui BS, Muhammad N, Khan A, Shah SU. Anti-nociceptive, anti-inflammatory and sedative activities of the extracts and chemical constituents of Diospyros lotus L. Phytomedicine. 2014;21:954–959. doi: 10.1016/j.phymed.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Uddin G, Waliullah, Rauf A, Siddiqui BS, Ahmed A, Bibi C, Qaisar M, Azam S. Phytochemical Screening and Antimicrobial Activity of Cornus macrophylla Wall. ex Roxb. Middle-East J. Sci. Res. 2011;9:516–519. [Google Scholar]
- Wadden TA, Hollander P, Klein S, Niswender K, Woo V, Hale PM, Aronne L. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int. J. Obes. 2013;37:1443–1451. doi: 10.1038/ijo.2013.120. [DOI] [PubMed] [Google Scholar]
- Wang S, Wang C, Turdi S, Richmond KL, Zhang Y, Ren J. ALDH2 protects against high fat diet-induced obesity cardiomyopathy and defective autophagy: role of CaM kinase II, histone H3K9 methyltransferase SUV39H, Sirt1, and PGC-1alpha deacetylation. Int. J. Obes. 2018;42:1073–1087. doi: 10.1038/s41366-018-0030-4. [DOI] [PubMed] [Google Scholar]
- WHO. Obesity and overweight. Fact sheet, Reviewed February 2018. Available from http://www.who.int/mediacentre/factsheets/fs311/en/. Accessed April 1, 2018.