Abstract
Astaxanthin is widely used in food, feed and nutraceutical industries. Xanthophyllomyces dendrorhous is one of the most promising natural sources of astaxanthin. However, the astaxanthin yield in the wild-type X. dendrorhous is considered low for industrial application. In the present study, X. dendrorhous ATCC 66272 was subjected to two-staged mutagenesis: (i) UV light and (ii) N-methyl-N′-nitro-N-nitroso-guanidine (NTG) toward attaining higher astaxanthin yield. The UV-irradiation mutant, X. dendrorhous SK974 showed 1.7-fold (1.07 mg/g) higher astaxanthin production as compared with the wild-type strain (0.65 mg/g). The UV mutant strain was then treated with NTG, designated as X. dendrorhous SK984, displayed further 1.4-fold (1.45 mg/g) higher astaxanthin production. Furthermore, the oak leaf extract (5%, v/v) and inorganic phosphate (KH2PO4, 3 mM) supplementation resulted about 1.4-fold (1.98 mg/g) higher astaxanthin production as compared with control (1.45 mg/g) in X. dendrorhous SK984. These findings serve as a platform suggesting that intersecting approaches might be aimed toward systematically enhanced astaxanthin production.
Keywords: Astaxanthin, Inorganic phosphate, Mutagenesis, Oak leaf extract, X. dendrorhous
Introduction
Astaxanthin (3,3′-dihydroxy-β,β-carotene-4,4′-dione), trivially known as “the king of carotenoids” has widespread biotechnological applications, ranging from animal nutrition to human health (Nguyen, 2013). Astaxanthin has been widely used as a pigment in aquaculture (salmon, trout, and shrimp) and poultry (Han et al., 2013), as a potent antioxidant in pharmaceuticals products (Baker and Guenther, 2004), in cosmetics for its anti-aging properties (Hussein et al., 2006) as well as for the fortification of foods and beverages (Mayne, 1996).
The global market of astaxanthin (~ USD 550 million, 2017) is dominated by synthetic pigment (more than 95%) which is characterized with a higher yield and lower production costs than its natural alternative. Another key point is that due to the cancer-causing petrochemical origin, synthetic astaxanthin is considered inadequate for human consumption, and hence galvanizing the research which explores the viable natural sources of astaxanthin including bacteria, yeasts, algae or shrimp by-products (Han et al., 2013). The yeast Xanthophyllomyces dendrorhous (teleomorphic state of Phaffia rhodozyma) is one of the most promising microbial sources for the commercial astaxanthin production coupled with the several advantages including high attainable biomass, rapid self-propagation, and an easy level of cultivation (Jiang et al., 2017). In addition, the astaxanthin obtained from the yeast is free from heavy metal contaminants and contains 100% 3R, 3′R configuration product (Johnson and Schroeder, 1995; Bhatt et al., 2013). However, the specific astaxanthin production from wild-type strains of X. dendrorhous is very low ~0.2–0.4 mg/g of yeast dry weight (Johnson and Schroeder, 1995; Barredo et al., 2017). Several approaches have been applied for improving the astaxanthin production from microorganisms namely random (physical or chemical) and targeted mutagenesis, genetic and metabolic engineering, and optimization of process parameters (Gassel et al., 2013). The development of low-cost media using by-products and residues of agro-industrial origin could also reduce the production costs of astaxanthin (Tinoi et al., 2006). Different plant sources including Jerusalem artichoke, cane sugar molasses, grape juice, carrot juice, coconut milk, mustard wastes, and pineapple juice have already been explored as potential substrates for enhanced fermentative astaxanthin production (Tinoi et al., 2006; Jiang et al., 2017; Kim et al., 2007; Stachowiak, 2012). The present study adopts a systematic methodology involving two-staged mutagenesis (i.e. UV-irradiation and treatment with NTG) of X. dendrorhous and its subsequent fermentative cultivation with different plant extracts as well as inorganic phosphate (Pi) for enhanced astaxanthin production.
Materials and methods
Yeast strain and culture conditions
The X. dendrorhous ATCC 66272 (designated wild-type, X. dendrorhous KCTC 7800) was grown in Yeast-Malt extract (YM) medium (3 g yeast extract, 3 g malt extract, 5 g bacto-peptone, and 10 g glucose per liter, pH 5.0) or Yeast extract bacto-peptone (YP) medium (10 g yeast extract, 3 g malt extract, 10 g bacto-peptone, and 50 g sucrose per liter, pH 5.0) (Difco, BD, USA) at 22 °C and 120 rpm for 5 days. KH2PO4 was used as a source of Pi.
Mutagenesis
X. dendrorhous ATCC 66272 (wild-type) was grown in 100 mL YM broth at 22 °C and 120 rpm for 12 h. Aliquot (2.5 mL) of this culture suspension (OD600 ~ 0.6) was mixed with sterile distilled water (2.5 mL) in a petri dish and irradiated with UV light (6 W, 254 nm; Sankyo Denki, Japan) at a distance of ca. 55 cm for 20 and 30 min. Following irradiation, the X. dendrorhous culture was kept in the dark for 24 h at 22 °C. After that, the culture was serially diluted and grown in selective YM agar plates containing 0.5 mM β-ionone at 22 °C for 5 days to screen the mutants (Lewis et al., 1990). The colonies with enhanced pigmentation were selected and later transferred to the fresh YM broth. The UV mutant strains of X. dendrorhous were further subjected to chemical mutagenesis, using NTG (10–40 μg/mL) according to the method of Kim et al. (2007). The selected two-staged mutant was sub-cultured and stored as glycerol stocks (15%, v/v) at − 80 °C.
Analysis of carotenoid content
The main carotenoids (90–95%) in X. dendrorhous represents astaxanthin (An et al., 1989) and carotenoids extracted in this study were expressed as astaxanthin. Briefly, the yeasts were harvested from YM broth (5 days) by centrifugation, washed twice in sterile distilled water, and re-suspended in 1 mL of dimethyl sulfoxide (DMSO) to disrupt cell membrane. The DMSO cell suspension was transferred to a 15 mL tube, followed by the addition of 1 mL acetone, 4 mL petroleum ether, and 1 mL NaCl (20%, w/v) serially. The resulting solution was then centrifuged at 3000 rpm for 15 min, and the absorbance of the supernatant layer (petroleum ether) at 478 nm was measured (model UV-1601, Shimadzu, Japan). Astaxanthin content was calculated using the following equation (An et al., 1989):
To measure dry cell weight (DCW), the harvested cells were resuspended in water and then subsequently filtered. The cell biomass collected on the filter was dried in oven at 70 °C for at least 16 h. The DCW was expressed as g/L of the X. dendrorhous culture.
Effects of plant extracts and inorganic phosphate on astaxanthin production
Different types of plants (9 edible vegetables, 10 fruits, and 2 tree leaves) were either purchased or collected from local markets and farms. In particular, the aqueous extracts were prepared from sweet potatoes, water parsley, broccoli, head lettuce, oak leaves (2:1 w/v), tangerine peels, garlic, red cabbage, orange peels, Hanrabong peels (1:1 w/v), maple leaves (1:6 w/v), and carrots (4:1 w/v). The other plants were homogenized using a juice extractor (Oscar, Korea) without water. All the plant extracts were filtered (0.2 µm filter, Sartorius, Germany), blended at a concentration of 1% (v/v) into the YM medium, and employed toward the fermentative cultivation of mutant. The effect of Pi (KH2PO4) on the astaxanthin production was also studied. Furthermore, the effect of YP medium on the astaxanthin production and the use of oak leaf extract as carbon source were examined. The culture conditions were identical to those described above.
Statistical analysis
Data were presented as mean values ± standard deviation (SD). The DCW, astaxanthin concentration, and astaxanthin content were compared by using the one-way factor analysis of variance (ANOVA). Significant differences (p < 0.05) among the groups were determined by using the Duncan’s multiple range test. All statistical analyses were carried out using SPSS 24.0 software (USA).
Results and discussion
Mutagenesis
Thirteen strongly pigmented colonies were screened after UV irradiation (data not shown) and the mutant with maximum astaxanthin content was designated as X. dendrorhous SK974 (SK974). Intriguingly, it was noted that the astaxanthin content of SK974 (1.06 mg/g) was approximately 1.7-fold higher as compared with that of the wild-type strain (0.65 mg/g) at day 5 (Table 1 and Fig. 1). Previously, De la Fuente et al. (2010) suggested that the yeast exposure to the UV radiations engendered the higher generation of reactive oxygen species potentially up-regulating the carotenogenesis to mitigate the oxidative stress. NTG was also reported as the potent mutagen for generating considerable variation in the pigmentation pattern among yeast strains by alkylating purines and pyrimidines (An et al., 1989; Kim et al., 2007). Hence, the UV mutant (SK974) was treated with NTG (10–40 μg/mL) and 40 µg/mL of NTG was found to be effective in generating the mutant with enhanced astaxanthin production, designated as X. dendrorhous SK984 (SK984) (Fig. 1). The astaxanthin content of SK984 (1.45 mg/g) was 1.4-fold higher when compared with that of the UV mutant SK974 (1.07 mg/g), and 2.2-fold higher than that of the wild-type strain X. dendrorhous ATCC 66272 (0.65 mg/g) at day 5 (Table 1). However, the cell growth of SK984 (DCW, 3.8 g/L) was reduced as compared with that of the wild-type (DCW, 5.1 g/L), suggesting the case of a potentially compromised growth at the expense of elevated pigment production.
Table 1.
Cell growth and astaxanthin production in X. dendrorhous strains
| Strains | Cell growth (DCW, g/L) | Astaxanthin concentration (mg/L) | Astaxanthin content (mg/g) |
|---|---|---|---|
| X. dendrorhous ATCC 66272 | 5.1 ± 0.15 | 3.3 ± 0.10 | 0.65 ± 0.01 |
| X. dendrorhous SK974 (UV mutant) | 5.0 ± 0.21 | 5.3 ± 0.20 | 1.07 ± 0.01 |
| X. dendrorhous SK984 (UV-NTG mutant) | 3.8 ± 0.06 | 5.5 ± 0.07 | 1.45 ± 0.01 |
Data are represented as mean of triplicate ± SD
Fig. 1.
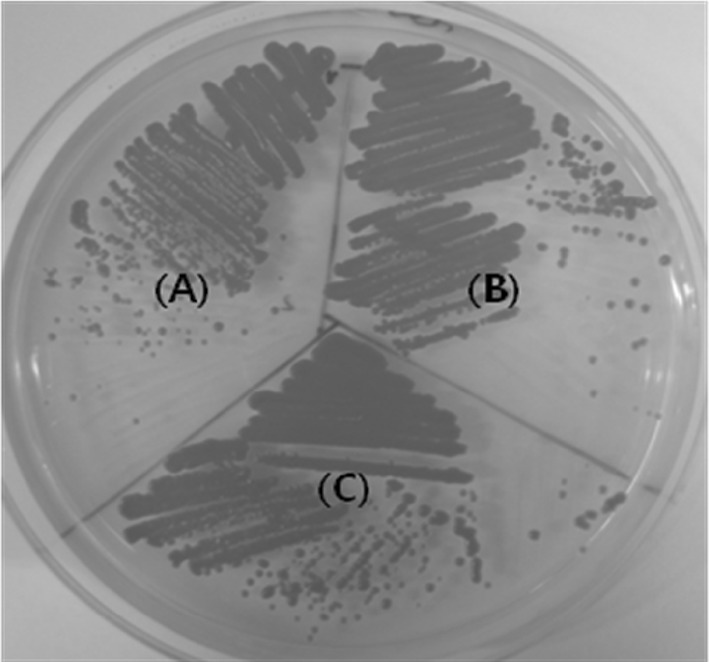
Comparison of astaxanthin production between wild-type and mutant strains of X. dendrorhous. (A) X. dendrorhous ATCC 66272 (wild-type), (B) X. dendrorhous SK974 (UV mutant), and (C) X. dendrorhous SK984 (UV-NTG mutant)
Effects of plant extracts and inorganic phosphate on astaxanthin production
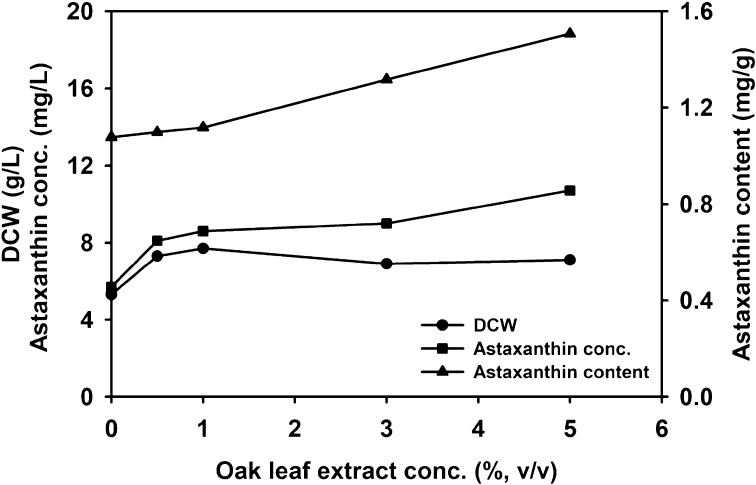
To increase astaxanthin production, various plant extracts of vegetables, fruits, and tree leaves were added at a final 1% (v/v) concentration in YM medium (Table 2). Most of the plant extracts increased cell growth but decreased astaxanthin production. Among the 24 plant extracts used, only head lettuce, maple leaf, and oak leaf extracts significantly enhanced the astaxanthin production. The head lettuce and maple leaf extract supplementation resulted lower cell growth as compared with that of the oak leaf extract supplementation. Therefore, the oak leaf extract was selected as the best media supplement which enhanced astaxanthin concentration (11.3 mg/L) and astaxanthin content (1.75 mg/g) coupled with the higher cell growth (DCW, 6.5 g/L) (Table 2). Whereas, the control without addition of any plant extracts showed astaxanthin concentration, astaxanthin content, and DCW of 5.8 mg/L, 1.42 mg/g, and 4.1 (g/L), respectively. The oak leaf contains chlorophyll a, chlorophyll b, lutein, β-carotene, neoxanthin, violaxanthin, antheraxanthin, and zeaxanthin, which may serve as the precursors for enhanced astaxanthin production in X. dendrorhous (Wolkerstorfer et al., 2011). To further check the optimal oak leaf supplementation, varying concentrations ranging from 0.5 to 5% (v/v) were examined, and it was noted that a 5% (v/v) oak leaf extract induced the maximum astaxanthin production with the concurrent increase in cell growth (Fig. 2).
Table 2.
Effect of various plant extracts on cell growth and astaxanthin production in X. dendrorhous SK984
| Plant extract supplement | Cell growth (DCW, g/L) | Astaxanthin concentration (mg/L) | Astaxanthin content (mg/g) | |
|---|---|---|---|---|
| Control | No plant extract | 4.1 ± 0.14a | 5.8 ± 0.01a,b,c | 1.42 ± 0.05a |
| Vegetables | Sweet potato (Ipomoea batatas) | 7.3 ± 0.07b | 5.7 ± 0.03d | 0.78 ± 0.00b,c |
| Carrot (Daucus carota) | 5.0 ± 0.14c | 6.7 ± 0.07e | 1.34 ± 0.05d | |
| Garlic (Allium sativum) | 5.4 ± 0.07d | 4.7 ± 0.08f | 0.87 ± 0.00e | |
| Water Parsley (Apium crispum) | 8.6 ± 0.07e,f | 4.8 ± 0.07f,g | 0.56 ± 0.01f | |
| Broccoli (Brassica oleracea) | 7.6 ± 0.07g | 5.9 ± 0.06b,c | 0.78 ± 0.01b,c | |
| Head lettuce (Lactuca sativa) | 2.9 ± 0.07h | 5.1 ± 0.11h,i | 1.76 ± 0.01g | |
| Ginger (Zingiber officinale) | 3.2 ± 0.07i | 4.3 ± 0.09j | 1.37 ± 0.06d,h | |
| Red cabbage (Brassica oleracea) | 8.7 ± 0.07f | 5.6 ± 0.07d | 0.64 ± 0.03i | |
| Red onion (Allium cepa) | 7.9 ± 0.07j | 5.9 ± 0.09b,c | 0.75 ± 0.05b | |
| Fruits | Apple (Malus domestica) | 6.2 ± 0.07k | 6.0 ± 0.04c,k | 0.97 ± 0.00j,k |
| Strawberry (Fragaria vesca) | 6.2 ± 0.07k | 5.8 ± 0.03a,b | 0.94 ± 0.01j | |
| Cherry tomato (Lycopersicon lycopersicum) | 8.5 ± 0.07e | 6.4 ± 0.02l | 0.75 ± 0.01b | |
| Tomato (Solanum lycopersicum) | 5.0 ± 0.07c | 7.1 ± 0.10m | 1.42 ± 0.00a,h | |
| Pear (Pyrus pyrifolia) | 6.3 ± 0.07k | 5.2 ± 0.04i | 0.83 ± 0.01e,l | |
| Orange (Citrus sinensis) | 6.2 ± 0.07k | 6.1 ± 0.04k | 0.99 ± 0.00k | |
| Orange peel (Citrus sinensis) | 9.1 ± 0.00l | 5.2 ± 0.01i | 0.57 ± 0.00f | |
| Kiwi (Apteryx mantelli) | 8.9 ± 0.07m | 6.1 ± 0.1k | 0.69 ± 0.01i | |
| Grape (Vitis vinifera) | 3.2 ± 0.07i | 4.4 ± 0.17j | 1.39 ± 0.02a,h | |
| Hanrabong (Citrus reticulata) | 6.5 ± 0.07n | 4.8 ± 0.06f | 0.74 ± 0.00b | |
| Hanrabong peel (Citrus reticulata) | 6.3 ± 0.07k | 5.0 ± 0.12h | 0.80 ± 0.01c | |
| Tangerine (Citrus reticulata) | 8.5 ± 0.07e | 5.8 ± 0.01a | 0.68 ± 0.01i | |
| Tangerine peel (Citrus reticulata) | 4.1 ± 0.07a | 5.0 ± 0.07g | 1.22 ± 0.00m | |
| Tree leaves | Maple leaf (Acer pseudoplatanus) | 4.4 ± 0.14o | 7.3 ± 0.07n | 1.65 ± 0.03n |
| Oak leaf (Quercus aliena) | 6.5 ± 0.07n | 11.3 ± 0.00o | 1.75 ± 0.02g |
Data are represented as mean of duplicate ± SD. Values with different superscripts in a column are significantly different (p < 0.05)
Fig. 2.
Effect of oak leaf extract concentration on astaxanthin production in X. dendrorhous SK984. Cells were grown in YM medium at 22 °C and 190 rpm for 5 days
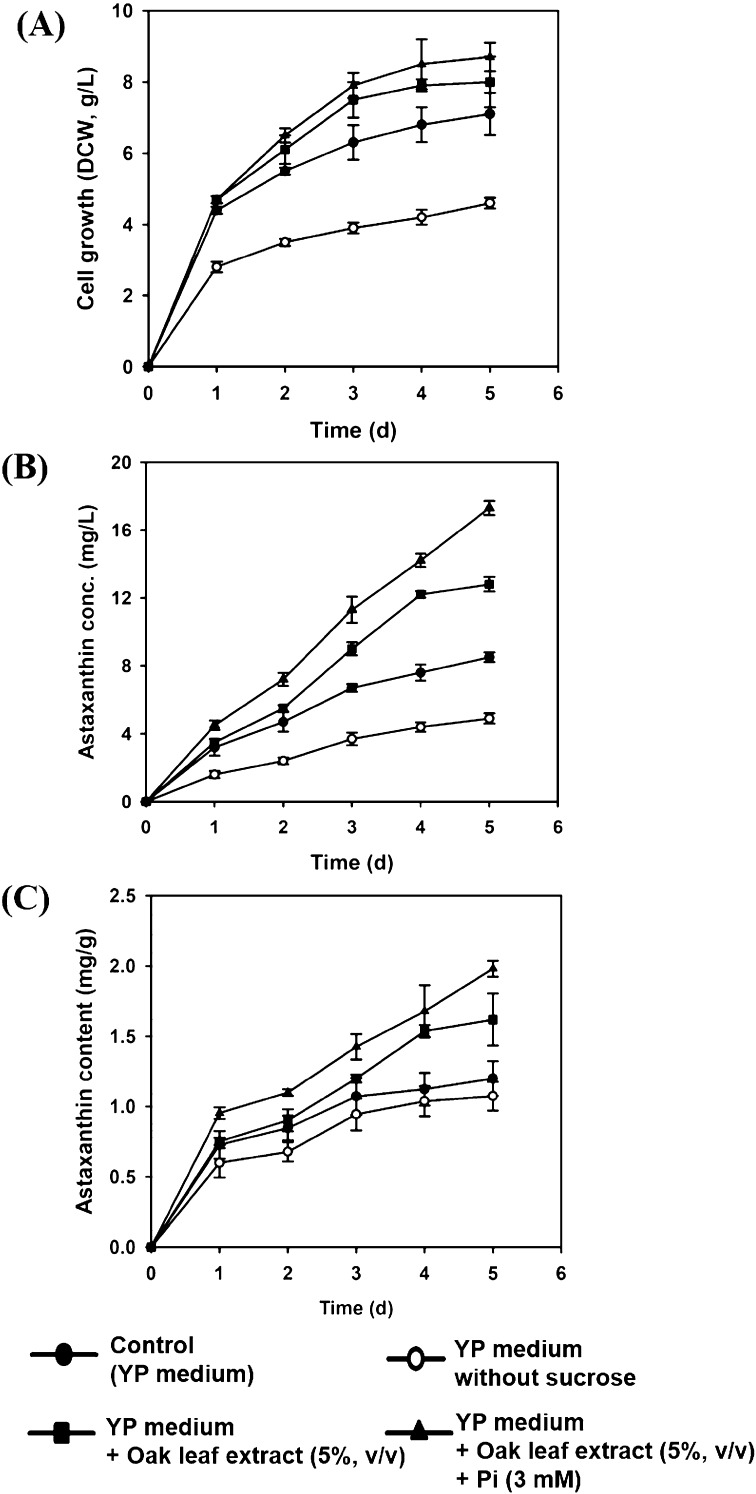
The low Pi levels are reported to increase the final astaxanthin concentration by limiting the protein biosynthesis (Flores-Cotera et al., 2001). However, the present study reported a considerable increase in astaxanthin production following the supplementation of 3 mM Pi (optimal concentration) (data not shown). This might be due to the difference in strain and culture media used. The effect of supplementation of oak leaf extract and Pi on astaxanthin production in YP medium was further investigated (Fig. 3). Notably, the pigment production (1.3–1.4 fold) was higher in YP medium than YM medium, which is consistent with the previous report by Kim et al. (2007). The addition of 5% oak leaf extract together with 3 mM Pi enhanced astaxanthin production as compared with YP medium only. The medium without sucrose displayed minimum values for DCW and astaxanthin production, suggesting the requirement of simple sugar as preferred carbon source for X. dendrorhous growth. Furthermore, the cell growth and astaxanthin production of mutant in the oak leaf extract and Pi supplemented YP medium at day 3, were observed similar or higher as compared with that of the commercial YP medium at day 5 (Fig. 3), indicating that these supplementations might have shortened the cultivation time of X. dendrorhous SK984 toward astaxanthin production.
Fig. 3.
Effect of oak leaf extract and Pi supplementation on astaxanthin production in X. dendrorhous SK984. Cells were grown in YP medium at 22 °C and 190 rpm for 5 days. (A) Cell growth (DCW, g/L), (B) astaxanthin concentration (mg/L), and (C) astaxanthin content (mg/g)
In conclusion, X. dendrorhous SK984 was isolated by UV and NTG mutagenesis with improved astaxanthin production. The addition of oak leaf extract and Pi in YP medium further improved cell growth and astaxanthin production. Future studies are needed to delineate the mechanisms that led to an improved pigment production in the mutant X. dendrorhous SK984.
Acknowledgements
This work was supported by the Konkuk University (Seoul, Republic of Korea) in 2018.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Damini Kothari, Email: damini.kth@gmail.com.
Jun-Hyeong Lee, Email: dairy25@hanmail.net.
Jung-Whan Chon, Email: alvarmar@naver.com.
Kun-Ho Seo, Email: bracstu3@konkuk.ac.kr.
Soo-Ki Kim, Phone: +82-2-450-3728, Email: sookikim@konkuk.ac.kr.
References
- An GH, Schuman, DB, Johnson EA. Isolation of X. dendrorhous mutants with increased astaxanthin content. Appl. Environ. Microbiol. 55: 116–124 (1989) [DOI] [PMC free article] [PubMed]
- Baker R, Guenther C. The role of carotenoids in consumer choice and the likely benefits from their inclusion into products for human consumption. Trends Food Sci. Technol. 2004;15:484–488. doi: 10.1016/j.tifs.2004.04.0094. [DOI] [Google Scholar]
- Barredo JL, García-Estrada C, Kosalkova K, Barreiro C. Biosynthesis of astaxanthin as a main carotenoid in the heterobasidiomycetous yeast Xanthophyllomyces dendrorhous. J. Fungi. 2017;3:44. doi: 10.3390/jof3030044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt PC, Ahmad M, Panda BP. Enhanced bioaccumulation of astaxanthin in Phaffia rhodozyma by utilising low-cost agro products as fermentation substrate. Biocat. Agric. Biotechnol. 2013;2:58–63. doi: 10.1016/j.bcab.2012.11.002. [DOI] [Google Scholar]
- de la Fuente JL, Rodríguez-Sáiz M, Schleissner C, Díez B, Peiro E, Barredo JL. High-titer production of astaxanthin by the semi-industrial fermentation of Xanthophyllomyces dendrorhous. J. Biotechnol. 2010;148:144–146. doi: 10.1016/j.jbiotec.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Flores-Cotera LB, Martin R, Sanchez S. Citrate, a possible precursor of astaxanthin in Phaffia rhodozyma: influence of varying level of ammonium, phosphate and citrate in a chemically defined medium. Appl. Microbiol. Biotechnol. 2001;55:341–347. doi: 10.1007/s002530000498. [DOI] [PubMed] [Google Scholar]
- Gassel S, Schewe H, Schmidt I, Schrader J, Sandmann G. Multiple improvement of astaxanthin biosynthesis in Xanthophyllomyces dendrorhous by a combination of conventional mutagenesis and metabolic pathway engineering. Biotechnol. Lett. 2013;35:565–569. doi: 10.1007/s10529-012-1103-4. [DOI] [PubMed] [Google Scholar]
- Han D, Li Y, Hu Q. Astaxanthin in microalgae: pathways, functions and biotechnological implications. Algae. 2013;28:131. doi: 10.4490/algae.2013.28.2.131. [DOI] [Google Scholar]
- Hussein G, Sankawa U, Goto H, Matsumoto K, Watanabe H. Astaxanthin, a carotenoid with potential in human health and nutrition. J. Nat. Prod. 2006;69:443–449. doi: 10.1021/np050354+. [DOI] [PubMed] [Google Scholar]
- Jiang GL, Zhou LY, Wang YT, Zhu MJ. Astaxanthin from Jerusalem artichoke: Production by fed-batch fermentation using Phaffia rhodozyma and application in cosmetics. Process Biochem. 2017;63:16–25. doi: 10.1016/j.procbio.2017.08.013. [DOI] [Google Scholar]
- Johnson EA, Schroeder WA. Singlet oxygen and peroxyl radicals regulate carotenoid biosynthesis in Phaffia rhodozyma. J. Biol. Chem. 1995;270:18374–18379. doi: 10.1074/jbc.270.31.18374. [DOI] [PubMed] [Google Scholar]
- Kim SK, Lee JH, Lee CH, Yoon YC. Increased carotenoid production in Xanthophyllomyces dendrorhous G276 using plant extracts. J. Microbiol. 2007;45:128–132. [PubMed] [Google Scholar]
- Lewis MJ, Ragot N, Berlant MC, Miranda M. Selection of astaxanthin-overproducing mutants of Phaffia rhodozyma with β-ionone. Appl. Environ. Microbiol. 1990;56:2944–2945. doi: 10.1128/aem.56.9.2944-2945.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayne ST. Beta–carotene, carotenoids, and disease prevention in humans. FASEB J. 1996;10:690–701. doi: 10.1096/fasebj.10.7.8635686. [DOI] [PubMed] [Google Scholar]
- Nguyen KD. Astaxanthin: A comparative case of synthetic vs. natural production. Chemical and Biomolecular Engineering Publications and Other Works. http://trace.tennessee.edu/utk_chembiopubs/94 (2013)
- Stachowiak B. Astaxanthin synthesis by yeast Xanthophyllomyces dendrorhous and its mutants on media based on plant extracts. Indian J. Microbiol. 2012;52:654–659. doi: 10.1007/s12088-012-0306-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoi J, Rakariyatham N, Deming RL. Utilization of mustard waste isolates for improved production of astaxanthin by Xanthophyllomyces dendrorhous. J. Ind. Microbiol. Biotechnol. 2006;33:309–314. doi: 10.1007/s10295-005-0054-3. [DOI] [PubMed] [Google Scholar]
- Wolkerstorfer SV, Wonisch A, Stankova T, Tsvetkova N, Tausz M. Seasonal variations of gas exchange, photosynthetic pigments, and antioxidants in Turkey oak (Quercus cerris L.) and Hungarian oak (Quercus frainetto Ten.) of different age. Trees 25: 1043–1052 (2011)