Abstract
Mounting evidence suggests that mild traumatic brain injuries (mTBI) have long-term effects that interact with the aging process to precipitate cognitive decline. This line of research predicts that early exposure to brain trauma is particularly detrimental to long-term brain integrity. However, a second line of research into the effects of age at trauma onset predict that older brains are more vulnerable to the effects of mTBI than younger brains. We sought to determine whether patients who sustain a mTBI earlier in life fare better than patients who sustain a mTBI at an older age. We conducted a multi-cohort, case-control study, with participants randomly sampled from a population of patients with a history of mTBI. We recruited two cohorts of aging participants (N = 74, mean [SD] = 61.16 [6.41]) matched in age and education levels that differed in only one respect: age at mTBI onset. One cohort sustained their concussion in their early twenties (24.60 [6.34] y/o), the other in their early sixties (61.05 [4.90] y/o). Each mTBI cohort had its own matched control group. Participants underwent high-resolution MRI at 3 Tesla for T1 and diffusion-weighted images (DWI) acquisition. Images were processed and analyzed using Deformation-Based Morphometry and DWI Tract-Based Spatial Statistics to identify group differences in a 2 × 2 ANOVA design. Results showed a significant interaction on DWI measures of white matter integrity indicating larger anomalies in participants who sustained a mTBI at a younger age (F1,70, P < .05, FDR corrected). These findings suggest that mTBI initiates a lifelong neurodegeneration process that outweighs the risks associated with sustaining a mTBI at an older age. Implications are important for young athletes' populations exposed to the risk of mTBI in the practice of their sports and for retired athletes aging with a history of concussions sustained at a younger age.
Keywords: Mild traumatic brain injury, mTBI, Concussion, Neuroimaging, Diffusion tensor imaging, Morphometry
Highlights
-
•
In aging adults, early-life mTBI leads to worst brain outcome than late-life mTBI.
-
•
Brain anomalies are mostly visible using DWI measures of white matter integrity.
-
•
Brain anomalies are visible even in neurologically normal individuals.
1. Introduction
Time is often seen as beneficial after an injury; with time comes recovery. Certain injuries, however, do not seem to heal with time. These often include injuries to the central nervous system (CNS) (Horner and Gage, 2000). A particular CNS injury has been making headlines over the last few years (Ken Belson, 2013). Mild traumatic brain injuries (mTBI), also called concussions in a sports context, have progressively been associated with degeneration of the brain in professional athletes (Crane et al., 2016; Henry et al., 2016; Martini and Broglio, 2017). These brain anomalies appear to affect primarily white matter tracts in the frontal and parietal cortices and correlate with a diffuse symptomatology spanning cognitive, somatic, and emotional domains (De Beaumont et al., 2009; Tremblay et al., 2014; Martini and Broglio, 2017; Alosco et al., 2018b). The long-term pathophysiological mechanism of chronic mTBI is still misunderstood and there currently exists no approved treatment to alleviate its effects.
The aging process is thought to interact with the physiological effects of mTBI and exacerbate difficulties. In this regard, two distinct risk factors have been identified: aging with a history of remote mTBI, and sustaining a mTBI at a later age (Peters, 2016; Griesbach et al., 2018). In the first case, the aging concussed brain is thought to no longer be able to compensate for brain damage sustained decades earlier, letting sub-clinical anomalies rise above the clinical threshold (Henry et al., 2016). In the second scenario, the aging brain is considered to be already weakened by the normal aging process and to therefore be more vulnerable to new injuries sustained later in life (Griesbach et al., 2018). These two risk factors have had their independent line of research in the mTBI literature, making it difficult to dissociate the effects of aging with a mTBI history from the effects of age at trauma onset. Moreover, those two risk factors make opposite predictions about brain health: 1) in the first case, mTBI sustained earlier in life are more detrimental because of their longer interaction with the aging process and/or the vulnerability of the developing brain, and in the second case, 2) mTBI sustained at an older age are more problematic because of the higher vulnerability of the aging brain, comorbid pathology, and/or its lack of capacity for compensation.
In the current study, we sought to disentangle these two effects and to answer the following question: Do aged patients who sustained a mTBI earlier in life fare better than aged patients who sustain a mTBI at an older age? To answer that question, we compared two cohorts of participants with mTBI who differed only in one respect: age at mTBI onset. One cohort, called the recent mTBI cohort, sustained their brain injury about two years prior to the study. The second cohort, called the remote mTBI cohort, sustained their brain injury more than three decades ago. Otherwise, the two aged cohorts have the same average age and level of education. Each cohort is matched with a respective control group. With this study design, we wish to compare deviations from age-typical brain health between the two cohorts and gain insights as to whether the effect of time after a mTBI is beneficial, neutral, or detrimental. Specifically, if the remote mTBI group shows more age-atypical degenerative changes compared to the recent mTBI group, it will be concluded that mTBI sets off a life-long neurodegenerative process that outweigh the protective effects of young age at trauma onset.
In the current study, we used sensitive MRI-based measures of brain structure integrity to compare our two cohorts. Deformation-based morphometry was used to assess white and grey matter atrophy, and diffusion-weighted imaging was used to assess the integrity of white matter tracts with more precision. Both techniques have been used in the past to investigate the effects of concussions in young and older adults (Gardner et al., 2012; Tremblay et al., 2013; Trotter et al., 2015; Bigler, 2017; Tremblay et al., 2017; Alosco et al., 2018b).
2. Methods
2.1. Participants
All 74 participants in the study provided written informed consent before testing in accordance with institutional review boards. In this retrospective cohort study, participants were grouped in two cohorts depending on the time since last mild traumatic brain injury (mTBI): a remote mTBI cohort and a recent mTBI cohort (Table 1). The recent mTBI cohort had a mean age of 62.23 (SD 5.92), mean level of education of 16.81 (SD 2.12), and mean time since injury of 22.06 months (SD 13.12) or 1.8 years. The remote mTBI cohort had a mean age of 59.50 (SD 6.41), mean level of education of 16.97 (SD 3.72), and mean time since injury of 443.20 months (SD 77.30) or 36.9 years. The two cohorts were not different in age or years of education (both P > .5, two-sample t-tests). To control for confounding effects of comorbidities at an advancing age, all mTBI subjects were asymptomatic at the time of testing, were fully functional (working or retired), and did not present with post-concussion syndrome. Each cohort included a matched control group that was equivalent in age, sex, and years of education, but who never sustained a mTBI or a TBI of higher severity over their lifetime. These matched control groups allowed to account for remaining differences between the two patient populations, such as sex (see Table 1; the recent cohort included both sexes). Sex is an important variable to control for given the differential susceptibility of each sex to the effects of mTBI (Broshek et al., 2005). By comparing each patient sample to its matched control sample, we could compare deviations from the control group across both cohorts (i.e. difference in deviations from the norm) and eliminate confounding variables that would contaminate a direct comparison between the mTBI cohorts.
Table 1.
Demographic data for study participants (Mean +/− SD).
| Recent cohort |
Remote cohort |
|||
|---|---|---|---|---|
| mTBI | controls | mTBI | controls | |
| N | 19 | 25 | 15 | 15 |
| Age | 62.89 (4.92) | 61.76 (6.60) | 60.87 (7.51) | 58.13 (5.28) |
| Sex (% male) | 39 | 44 | 100 | 100 |
| Education | 16.67 (1.97) | 16.92 (2.25) | 16.67 (4.06) | 17.27 (3.45) |
| % Caucasian | 100 | 100 | 100 | 100 |
| Time since mTBI (months) | 22.06 (13.12) | 443.20 (77.30) | ||
| Age at mTBI onset | 61.05 (4.90) | 24.60 (6.34) | ||
| Mini Mental State Examination | 29.00 (1.02) | 29.59 (0.62) | 29.20 (0.86) | 29.40 (1.12) |
| Clinical imaging (CT or MRI) | negative | negative | negative | negative |
Participants were excluded if they presented any of the following characteristics: a history of alcohol and/or substance abuse, a history of neurological or psychiatric condition (e.g. MCI, Alzheimer's disease, depression), a medical condition requiring daily medication or radiotherapy (malignant cancer, diabetes, hypertension, and/or other cardiovascular diseases), or a learning disability (e.g. dyslexia). Participants from the recent mTBI cohort were recruited from emergency room (ER) admissions at a Level 1 trauma hospital, the Sacred Heart Hospital in Montreal, Canada. The mechanisms of injury for this cohort were falls (55%), motor vehicle accidents (17%), or others (sports concussion, falling objects). Participants from this cohort were excluded if they were still symptomatic from their mTBI. Controls for this cohort were recruited from hospital registers and newspaper/web ads. Participants from the remote mTBI cohort were recruited with the help of university athletics organizations in Montreal. Both control and mTBI participants were randomly sampled from the same list of alumni. These mTBI participants were former university level athletes who suffered from concussions/mTBI in their early twenties, but who never experienced another mTBI later in life. About 70% of these participants were former ice hockey players, while 13% were former American football players. All suffered from sports concussions (mean number of concussions = 2.08, SD = 1.31) during the practice of their sports. Detailed information about this cohort and mTBI diagnosis methodology is included in previous publications (Tremblay et al., 2013, Tremblay et al., 2014). A standardized concussion history questionnaire (Collins et al., 2002; De Beaumont et al., 2009) was administered in an interview setting by an experienced sports physician to obtain detailed information about the number of previous mTBI, their approximate date, the description of the accident, and the nature and duration of on-field post-concussion severity markers (confusion and/or disorientation, retrograde and/or anterograde amnesia, and loss of consciousness [LOC]). A concussion was defined according to the definition provided by the 2009 Consensus Statement on Concussion in Sports (Mccrory et al., 2009) as “a complex pathophysiological process affecting the brain, induced by traumatic biomechanical forces, that results in the rapid onset of short-lived impairment of neurologic function that may or may not include loss of consciousness (LOC).”
2.2. Magnetic resonance imaging
All participants in this study underwent a MRI examination on the same scanner (Siemens 3T Magnetom TIM Trio, 32-channel head coil) housed at the Unité de Neuroimagerie Fonctionnelle (UNF) in Montreal, Canada. T1-weighted and diffusion-weighted imaging (DWI) acquisition sequence parameters differed slightly between the two cohorts. Acquisition parameters for the recent mTBI cohorts are the following: (T1-weighted: TR = 2300, TE = 2.98, number of slices = 176, resolution = 1 mm3); (DWI: TR = 9100, TE = 94, number of slices = 66, resolution = 2 mm3, 65 directions, b0 = 1000 s/mm2). Acquisition parameters for the remote mTBI cohorts are the following: (T1-weighted: TR = 2300, TE = 2.91, number of slices = 176, resolution = 1 mm3); (DWI: TR = 9200, TE = 84, number of slices = 75, resolution = 2 mm3, 32 directions, b0 = 1000 s/mm2). As explained above, to account for these small differences in parameters, comparisons between mTBI cohorts are made only on the basis of deviations from their matched control group (i.e. difference in differences). The matched control group was scanned with identical acquisition parameters as its associated patient group. No direct comparisons were made between the two patient populations to avoid contamination by acquisition parameters differences. A direct comparison between the two control groups was made to quantify the effect of acquisition parameters differences on our measures (see Results). All mTBI participants' MRI were reviewed by a board-certified radiologist for presence/absence of clinical anomalies. Participants presenting visible anomalies (e.g. hematomas, contusions, microbleeds) at conventional clinical exam (MRI or CT) were excluded from the study.
2.3. Deformation-based morphometry
Deformation-based morphometry (DBM) provides an estimate of volume changes across the entire T1-weigthed brain image by computing the Jacobian determinant for each vector in the deformation field recovered during nonlinear spatial normalization of images to a standardized template. T1 MRIs were first linearly registered to the ICBM152 nonlinear 6th-generation template with a 12-parameter linear transformation (Collins et al., 1994) and RF inhomogeneity corrected (Sled et al., 1998). An iterative, nonlinear fitting procedure was then used to create a study-specific template (Fonov et al., 2011) which subsequently served as the target for all nonlinear registrations yielding DBM measures. Displacement data were convolved with a 4 mm FWHM 3D Gaussian blurring kernel. Statistical thresholds were determined by application of the False Discovery Rate technique to correct for multiple comparisons (Genovese et al., 2002). Age and education were included as covariates of no interest in all DBM analyses.
2.4. Tract-based spatial statistics
Tract-based spatial statistics (TBSS) allow to compare various DWI metrics across groups to localise brain changes in diffusion at a voxel-wise level. Using the FMRIB's Diffusion Toolbox (FDT) from FSL (Smith et al., 2004), we corrected for eddy current distortions, extracted the brain from the full images, and fit the local diffusion tensors to obtain the following voxel-wise metrics for all subjects: Fractional Anisotropy (FA), Mean Diffusivity (MD), Radial Diffusivity (RD), and Axial Diffusivity (AD). Voxel-wise statistical analysis of the FA, MD, RD, and AD data was carried out using TBSS (Smith et al., 2006). All subjects' diffusion data were then aligned into a common space using the nonlinear registration tool FNIRT, which uses a b-spline representation of the registration warp field. Next, the mean diffusion image was created and thinned to create a mean diffusion skeleton which represents the centres of all tracts common to the group (skeleton thresholded at 0.3). Each subject's aligned diffusion data was then projected onto this skeleton and the resulting data fed into voxel-wise cross-subject statistics. Non-parametric permutation-based statistics were employed using the Randomize tool with threshold-free cluster enhancement and 5000 permutations. A threshold of P < .05 was then applied on the results, corrected for multiple comparisons. Age and education were included as covariates of no interest in all TBSS analyses. The tbss_fill script from FSL was run on the thresholded images for easier visualisation of final diffusion results.
2.5. Statistical analyses
No direct patient group comparisons were performed in this cohort study to account for slight variations in demographics and MRI acquisition sequence parameters. Each patient group is only compared to its matched control group, and relative group differences (mTBI - controls) are then compared across cohorts. To perform this analysis, we use a two-way, 2 × 2 ANOVA with factors Cohort (recent Vs remote) and Group (mTBI Vs controls). We focus on the interaction term in this regression model, namely the Group * Cohort interaction, which models the difference in differences. In brief, this interaction term asks the following question: are the deviations from the norm larger (or smaller) in one cohort compared to the other? When statistically significant, interaction effects will be decomposed using simple effects (contrasts) to identify group differences (mTBI Vs controls) within cohorts. Age and education were included as covariates of no interest in all analyses.
3. Results
3.1. Deformation-based morphometry
Total brain volume did not differ between mTBI and control participants in the recent (t-test(42), P > .05) and remote cohort (t-test(28), P > .05). Analyses of T1-weighted images using DBM revealed no significant Group * Cohort interaction effects following correction for multiple comparisons (F(1,70), P > .05). Main effects of Group and Cohort on DBM measures of white and grey matter integrity were also negative (F(1,70), P > .05).
3.2. Tract-based spatial statistics
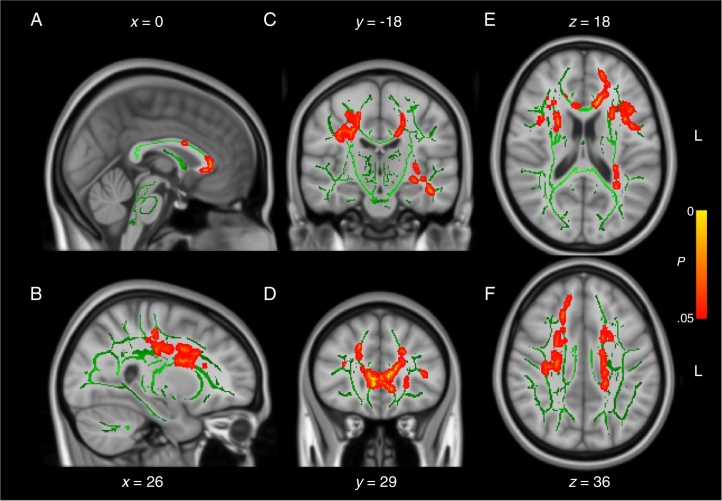
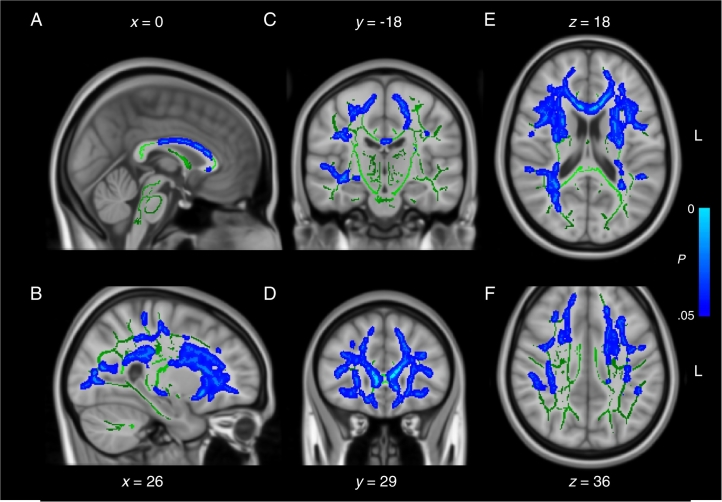
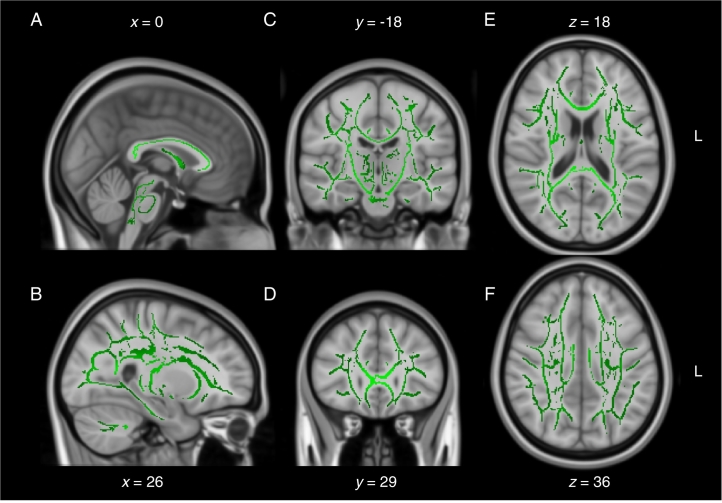
When compared directly, the two control groups were not significantly different from one another on all DWI measures tested (2 sample t-test, P > .05), suggesting the small differences in MRI acquisition sequence parameters between the two cohorts had negligible effects (as a reminder, all participants were scanned on the same scanner). Nonetheless, to be conservative, we only analyzed interaction effects between Group and Cohort using a two-way ANOVA rather than comparing both patient cohorts directly with t-tests. Analyses of mean diffusivity (MD) images revealed a significant Group * Cohort interaction effect following correction for multiple comparisons (F(1,70), P < .05) (Fig. 1). Regions affected included primarily the anterior aspect of the corpus callosum and frontal white matter. Decomposition of the interaction term into simple effects revealed large areas of increased MD in patients from the remote mTBI cohort relative to their matched control participants (t-test(28), P < .05) (Fig. 2). On the contrary, contrasts between the mTBI and the control groups of the recent mTBI cohort revealed no statistically significant group differences on MD (t-test(42), P > .05) (Fig. 3). Similar trends were observable for RD, AD and FA after correction for multiple comparisons (all P < .10), but none reached the statistical threshold. Overall, deviations from the control group were observable only in the remote mTBI cohort and not in the recent mTBI cohort.
Fig. 1.
Mean diffusivity interaction effect. Sagittal (A and B), coronal (C and D), and axial (E and F) slices of the interaction effect Cohort * Group on mean diffusivity (red traces). The significant voxels are overlaid on the mean fractional anisotropy skeleton (in green) and the standard MNI152 T1 1 mm brain template. The results are thresholded at P < .05, corrected for multiple comparisons. L = left.
Fig. 2.
Mean diffusivity in remote mTBI cohort. Sagittal (A and B), coronal (C and D), and axial (E and F) slices of the simple effect (mTBI vs controls) in the remote mTBI cohort on mean diffusivity (blue traces). The significant voxels are overlaid on the mean fractional anisotropy skeleton (in green) and the standard MNI152 T1 1 mm brain template. The results are thresholded at P < .05, corrected for multiple comparisons. L = left.
Fig. 3.
Mean diffusivity in recent mTBI cohort. Sagittal (A and B), coronal (C and D), and axial (E and F) slices of the simple effect (mTBI vs controls) in the recent mTBI cohort on mean diffusivity (no traces). The significant voxels are overlaid on the mean fractional anisotropy skeleton (in green) and the standard MNI152 T1 1 mm brain template. There are no voxels crossing the statistical threshold in this map. The results are thresholded at P < .05, corrected for multiple comparisons. L = left.
4. Discussion
In the current study, we sought to answer the following question: Do patients who sustain a mTBI earlier in life fare better or worse than patients who sustain a mTBI at an older age? To answer this question, we compared cohorts that differed vastly on the independent variable of interest (age at mTBI onset) to account for possible slow, decades-long effects of mTBI on brain integrity. The results reported herein support the thesis that patients who sustained a mTBI in young adulthood present with more severe structural brain anomalies than patients of a similar age who sustained their mTBI in late adulthood. In summary, results from the present study indicate that the deleterious interaction between the long-term effects of mTBI and the aging process appear to outweigh the negative effects of sustaining a mTBI at an older age.
The idea that mTBI affects white matter structural integrity is well documented (Gardner et al., 2012; Shenton et al., 2012). Although the evidence is still controversial in young adults (Koerte et al., 2012; Ilvesmäki et al., 2014), the evidence is strong for older adults (McKee et al., 2013; Tremblay et al., 2014; Stamm et al., 2015; Trotter et al., 2015), as demonstrated both by in vivo studies and by post-mortem histology. It is often concluded from these differing observations in young and older adults that aging is a critical factor in the process of mTBI-related neurodegeneration. It is proposed that the normal aging process interacts with the deleterious long-term effects of mTBI to precipitate cognitive decline in this population. This proposition is supported by cognitive examinations of both young and older adults that show neuropsychological tests alterations in older adults with a history of mTBI that are not so easily detectable in their younger counterparts (Mccrea et al., 2003; Guskiewicz et al., 2005; Bruce and Echemendia, 2009; De Beaumont et al., 2009). This is also supported by evidence that indicate accelerated brain aging (Cole et al., 2015) and earlier age-related cognitive decline in populations with a history of mTBI (Li et al., 2016).
In the current study, we add support to this notion. By comparing two cohorts that are similar with regards to age, we eliminate this factor and shine light on an important confounding variable: age at trauma onset. By controlling for age at time of examination and using the same neuroimaging pipeline for both cohorts, our analysis supports the idea that neurodegeneration is more likely to take place over extended delays following a mTBI, irrespective of brain health at mTBI onset. Moreover, this study suggests that long-term neurodegeneration is a greater threat to brain health than the vulnerability to insult characteristic of the aging brain. The regional pattern of white matter anomalies in our remote mTBI sample (i.e. primarily frontal cortex and corpus callosum) is similar to the one observed in other populations with a history of repetitive head trauma (Stamm et al., 2015), or even in normal aging (Gunning-Dixon et al., 2009; Fjell et al., 2013). These results have implications for pediatric and young athlete populations at risk of mTBI. It could be proposed that these younger populations are more vulnerable to mTBI-related neurodegeneration because of their young age at trauma onset which translates to a lifelong interaction between mTBI and aging. This proposition would agree with a growing body of evidence relating age of first exposure to contact sports with worse later-life neurologic outcomes (Stamm et al., 2015; Alosco et al., 2017, Alosco et al., 2018a).
The current study presents some caveats that are intrinsically linked to cohort studies. For one, the two patient cohorts were not recruited with the same approach. The recent cohort was recruited through a list of ER admissions for mTBI, whereas the remote cohort was recruited through alumni lists of university athletics organizations. The latter participants were not admitted to a hospital for their mTBI. This difference could predict a bias in favor of higher mTBI severity in the recent cohort who were admitted to a hospital for their injury. However, our results are contrary to this bias, showing that the remote cohort who did not seek medical examination at the time of injury exhibit larger brain structure anomalies respective to their control group. It is likely that if we compared a remote cohort who was admitted to a hospital for their mTBI more than three decades ago, the differences reported in this study would have been accentuated rather than diminished. Unfortunately, hospitals do not keep track of ER mTBI admissions over three decades, so this comparison is not feasible at the moment. Another limitation of this study is that the number of repetitive, sub-concussive head impact (RHI) was not directly modeled in our analyses. It is possible that RHI differed across groups and explains some of the variance observed in MRI-based brain integrity metrics. We did try to control for this factor by recruiting participants and controls matched on the level of athletic competition played, but a more direct statistical control of years of exposure to contact sport would have strengthened our findings. Finally, the study would have benefited from adding neuropsychological assessments to compare the cognitive functioning across groups and cohorts and relate MRI anomalies to neuropsychiatric function.
The negative findings from the DBM analysis were not completely unexpected. In the past, our group has failed to report grey matter anomalies in similar cohorts using techniques such as optimized voxel-based morphometry (VBM) and MRI-based cortical thickness measurements (Tremblay et al., 2013). It is noteworthy that other groups have been able to detect grey matter anomalies, namely in the hippocampal region (Singh et al., 2014; Sussman et al., 2017). We are inclined to think that our observed discrepancy between grey matter and white matter findings might be explained by a greater sensitivity of diffusion weighted imaging to capture mTBI-related anomalies in vivo compared to alternative T1-weighted based techniques such as DBM and VBM (Tremblay et al., 2017). Interestingly, the current investigation was able to detect a significant interaction effect only on the DWI measure of MD, with P-values close to significance for all other DWI metrics (FA, RD, AD, all P < .10)). Given the limited power of the current study (N = 74), interpretation as to why only MD crossed the statistical threshold warrants caution. Although more research is needed to adequately address such study results discrepancy across DWI metrics, it appears likely that statistical significance could have been achieved with other DWI metrics given a larger sample size.
In conclusion, this study probed the effect of age at mTBI onset on brain structure integrity. Our findings indicate that brain structure does not recover over time from a mTBI, but rather is inflicted by a long, insidious degeneration process. Moreover, this degeneration process extending over decades outweighs potential coping mechanisms that the young brain might have in comparison to the aged brain. We do not propose that this degenerative process is directly relevant to CTE, as CTE appears to be related to repetitive head trauma as opposed to single mTBI events (Baugh et al., 2012; Alosco et al., 2018c). Our findings have important implications for young athletes at risk of mTBI, and for aging retired athletes with a history of remote concussions.
Declaration of Competing Interests
The authors report no conflict of interest.
Acknowledgements
This work was supported by grants to L.D.B from the Canadian Institutes of Health Research (CIHR) and the Natural Sciences and Engineering Research Council of Canada (NSERC). The CIHR Postdoctoral Fellowship supported S.T.
References
- Alosco M.L. Age of first exposure to American football and long-term neuropsychiatric and cognitive outcomes. Transl. Psychiatry. 2017;7:e1236. doi: 10.1038/tp.2017.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alosco M.L. Age of first exposure to tackle football and chronic traumatic encephalopathy. Ann. Neurol. 2018;83:886–901. doi: 10.1002/ana.25245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alosco M.L., Koerte I.K., Tripodis Y., Mariani M., Chua A.S., Jarnagin J., Rahimpour Y., Puzo C., Healy R.C., Martin B., Chaisson C.E., Cantu R.C., Au R., McClean M., McKee A.C., Lin A.P., Shenton M.E., Killiany R.J., Stern R.A. White matter signal abnormalities in former National Football League players. Alzheimers Dement (Amst) 2018;10:56–65. doi: 10.1016/j.dadm.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alosco M.L., Tripodis Y., Fritts N.G., Heslegrave A., Baugh C.M., Conneely S., Mariani M., Martin B.M., Frank S., Mez J., Stein T.D., Cantu R.C., McKee A.C., Shaw L.M., Trojanowski J.Q., Blennow K., Zetterberg H., Stern R.A. Cerebrospinal fluid tau, Aβ, and sTREM2 in former National Football League Players: modeling the relationship between repetitive head impacts, microglial activation, and neurodegeneration. Alzheimers Dement. 2018;14:1159–1170. doi: 10.1016/j.jalz.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baugh C.M., Stamm J.M., Riley D.O., Gavett B.E., Shenton M.E., Lin A., Nowinski C.J., Cantu R.C., McKee A.C., Stern R.A. Chronic traumatic encephalopathy: neurodegeneration following repetitive concussive and subconcussive brain trauma. Brain Imaging and Behavior. 2012;6:244–254. doi: 10.1007/s11682-012-9164-5. [DOI] [PubMed] [Google Scholar]
- Belson Ken. N.F.L. agrees to settle concussion suit for $765 million. NY Times. 2013 [Google Scholar]
- Bigler E.D. Structural neuroimaging in sport-related concussion. Int. J. Psychophysiol. 2017;132:105–123. doi: 10.1016/j.ijpsycho.2017.09.006. [DOI] [PubMed] [Google Scholar]
- Broshek D.K., Kaushik T., Freeman J.R., Erlanger D., Webbe F., Barth J.T. Sex differences in outcome following sports-related concussion. J. Neurosurg. 2005;102:856–863. doi: 10.3171/jns.2005.102.5.0856. [DOI] [PubMed] [Google Scholar]
- Bruce J.M., Echemendia R.J. History of multiple self-reported concussions is not associated with reduced cognitive abilities. Neurosurgery. 2009;64:100–6–discussion106. doi: 10.1227/01.NEU.0000336310.47513.C8. [DOI] [PubMed] [Google Scholar]
- Cole J.H., Leech R., Sharp D.J. Alzheimer's disease neuroimaging initiative (2015) prediction of brain age suggests accelerated atrophy after traumatic brain injury. Ann. Neurol. 2015;77:571–581. doi: 10.1002/ana.24367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins D.L., Neelin P., Peters T.M., Evans A.C. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J. Comput. Assist. Tomogr. 1994;18:192–205. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=8126267&retmode=ref&cmd=prlinks Available at. [PubMed] [Google Scholar]
- Collins M.W., Lovell M.R., Iverson G.L., Cantu R.C., Maroon J.C., Field M. Cumulative effects of concussion in high school athletes. Neurosurgery. 2002;51:1175–1181. doi: 10.1097/00006123-200211000-00011. [DOI] [PubMed] [Google Scholar]
- Crane P.K., Gibbons L.E., Dams-O'Connor K., Trittschuh E., Leverenz J.B., Keene C.D., Sonnen J., Montine T.J., Bennett D.A., Leurgans S., Schneider J.A., Larson E.B. Association of Traumatic Brain Injury with Late-Life Neurodegenerative Conditions and Neuropathologic Findings. JAMA Neurol. 2016;73:1062. doi: 10.1001/jamaneurol.2016.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Beaumont L., Théoret H., Mongeon D., Messier J., Leclerc S., Tremblay S., Ellemberg D., Lassonde M. Brain function decline in healthy retired athletes who sustained their last sports concussion in early adulthood. Brain. 2009;132:695–708. doi: 10.1093/brain/awn347. [DOI] [PubMed] [Google Scholar]
- Fjell A.M., Westlye L.T., Grydeland H., Amlien I., Espeseth T., Reinvang I., Raz N., Holland D., Dale A.M., Walhovd K.B., Alzheimer Disease Neuroimaging Initiative Critical ages in the life course of the adult brain: nonlinear subcortical aging. Neurobiol. Aging. 2013;34:2239–2247. doi: 10.1016/j.neurobiolaging.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonov V., Evans A.C., Botteron K., Almli C.R., McKinstry R.C., Collins D.L., Brain Development Cooperative Group Unbiased average age-appropriate atlases for pediatric studies. Neuroimage. 2011;54:313–327. doi: 10.1016/j.neuroimage.2010.07.033. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=20656036&retmode=ref&cmd=prlinks Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner A., Kay-Lambkin F., Stanwell P., Donnelly J., Williams W.H., Hiles A., Schofield P., Levi C., Jones D.K. A systematic review of diffusion tensor imaging findings in sports-related concussion. J. Neurotrauma. 2012;29:2521–2538. doi: 10.1089/neu.2012.2628. [DOI] [PubMed] [Google Scholar]
- Genovese C.R., Lazar N.A., Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Griesbach G.S., Masel B.E., Helvie R.E., Ashley M.J. The impact of traumatic brain injury on later life: effects on Normal aging and neurodegenerative diseases. J. Neurotrauma. 2018;35:17–24. doi: 10.1089/neu.2017.5103. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon F.M., Brickman A.M., Cheng J.C., Alexopoulos G.S. Aging of cerebral white matter: a review of MRI findings. Int. J. Geriatr. Psychiatry. 2009;24:109–117. doi: 10.1002/gps.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guskiewicz K.M., Marshall S.W., Bailes J., Mccrea M., Cantu R.C., Randolph C., Jordan B.D. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery. 2005;57:719–726. doi: 10.1093/neurosurgery/57.4.719. [DOI] [PubMed] [Google Scholar]
- Henry L.C., Tremblay S., De Beaumont L. Long-term effects of sports concussions: bridging the neurocognitive repercussions of the injury with the newest neuroimaging data. Neuroscientist. 2016;23:567–578. doi: 10.1177/1073858416651034. [DOI] [PubMed] [Google Scholar]
- Horner P.J., Gage F.H. Regenerating the damaged central nervous system. Nature. 2000;407:963–970. doi: 10.1038/35039559. [DOI] [PubMed] [Google Scholar]
- Ilvesmäki T., Luoto T.M., Hakulinen U., Brander A., Ryymin P., Eskola H., Iverson G.L., Ohman J. Acute mild traumatic brain injury is not associated with white matter change on diffusion tensor imaging. Brain. 2014;137:1876–1882. doi: 10.1093/brain/awu095. [DOI] [PubMed] [Google Scholar]
- Koerte I.K., Ertl-Wagner B., Reiser M., Zafonte R., Shenton M.E. White matter integrity in the brains of professional soccer players without a symptomatic concussion. JAMA. 2012;308:1859–1861. doi: 10.1001/jama.2012.13735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Risacher S.L., McAllister T.W., Saykin A.J. Traumatic brain injury and age at onset of cognitive impairment in older adults. J. Neurol. 2016;263:1280–1285. doi: 10.1007/s00415-016-8093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini D.N., Broglio S.P. Long-term effects of sport concussion on cognitive and motor performance: a review. Int. J. Psychophysiol. 2017;132:25–30. doi: 10.1016/j.ijpsycho.2017.09.019. [DOI] [PubMed] [Google Scholar]
- Mccrea M., Guskiewicz K.M., Marshall S.W., Barr W., Randolph C., Cantu R.C., Onate J.A., Yang J., Kelly J.P. Acute effects and recovery time following concussion in collegiate football players: the NCAA concussion study. JAMA. 2003;290:2556–2563. doi: 10.1001/jama.290.19.2556. [DOI] [PubMed] [Google Scholar]
- Mccrory P., Meeuwisse W., Johnston K., Dvorak J., Aubry M., Cantu R. Consensus statement on concussion in sport: the 3rd international conference on concussion in sport held in Zurich, November 2008. Br. J. Sports Med. 2009;43:i76–i84. doi: 10.1136/bjsm.2009.058248. [DOI] [PubMed] [Google Scholar]
- McKee A.C. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136:43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters M.E. Traumatic brain injury (TBI) in older adults: aging with a TBI versus incident TBI in the aged. Int. Psychogeriatr. 2016;28:1931–1934. doi: 10.1017/S1041610216001666. [DOI] [PubMed] [Google Scholar]
- Shenton M.E., Hamoda H.M., Schneiderman J.S., Bouix S., Pasternak O., Rathi Y., Vu M.A., Purohit M.P., Helmer K., Koerte I., Lin A.P., Westin C.F., Kikinis R., Kubicki M., Stern R.A., Zafonte R. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behavior. 2012;6:137–192. doi: 10.1007/s11682-012-9156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R., Meier T.B., Kuplicki R., Savitz J., Mukai I., Cavanagh L., Allen T., Teague T.K., Nerio C., Polanski D., Bellgowan P.S.F. Relationship of collegiate football experience and concussion with hippocampal volume and cognitive outcomes. JAMA. 2014;311:1883–1888. doi: 10.1001/jama.2014.3313. [DOI] [PubMed] [Google Scholar]
- Sled J.G., Zijdenbos A.P., Evans A.C. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans. Med. Imaging. 1998;17:87–97. doi: 10.1109/42.668698. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=9617910&retmode=ref&cmd=prlinks Available at. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E.J., Johansen-Berg H., Bannister P.R., De Luca M., Drobnjak I., Flitney D.E., Niazy R.K., Saunders J., Vickers J., Zhang Y., De Stefano N., Brady J.M., Matthews P.M. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., Watkins K.E., Ciccarelli O., Cader M.Z., Matthews P.M., Behrens T.E.J. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Stamm J.M., Koerte I.K., Muehlmann M., Pasternak O., Bourlas A.P., Baugh C.M., Giwerc M.Y., Zhu A., Coleman M.J., Fritts N.G., Martin B., Chaisson C., McClean M.D., Lin A.P., Cantu R.C., Tripodis Y., Stern R., Shenton M.E. Age at first exposure to football is associated with altered Corpus callosum white matter microstructure in former professional football players. J. Neurotrauma. 2015;32:1768–1776. doi: 10.1089/neu.2014.3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman D., da Costa L., Chakravarty M.M., Pang E.W., Taylor M.J., Dunkley B.T. Concussion induces focal and widespread neuromorphological changes. Neurosci. Lett. 2017;650:52–59. doi: 10.1016/j.neulet.2017.04.026. [DOI] [PubMed] [Google Scholar]
- Tremblay S., De Beaumont L., Henry L.C., Boulanger Y., Evans A.C., Bourgouin P., Poirier J., Théoret H., Lassonde M. Sports concussions and aging: a neuroimaging investigation. Cereb. Cortex. 2013;23:1159–1166. doi: 10.1093/cercor/bhs102. [DOI] [PubMed] [Google Scholar]
- Tremblay S., Henry L.C., Bedetti C., Larson-Dupuis C., Gagnon J.-F., Evans A.C., Théoret H., Lassonde M., De Beaumont L. Diffuse white matter tract abnormalities in clinically normal ageing retired athletes with a history of sports-related concussions. Brain. 2014;137:2997–3011. doi: 10.1093/brain/awu236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay S., Iturria-Medina Y., Mateos-Pérez J.M., Evans A.C., De Beaumont L. Defining a multimodal signature of remote sports concussions. Eur. J. Neurosci. 2017;46:1956–1967. doi: 10.1111/ejn.13583. [DOI] [PubMed] [Google Scholar]
- Trotter B.B., Robinson M.E., Milberg W.P., McGlinchey R.E., Salat D.H. Military blast exposure, ageing and white matter integrity. Brain. 2015;138:2278–2292. doi: 10.1093/brain/awv139. [DOI] [PMC free article] [PubMed] [Google Scholar]