Abstract
Sprouting and seed elicitor treatments stimulate the biosynthesis of health relevant phenolic bioactives in plants partly by upregulating proline-associated pentose phosphate pathway (PAPPP). This study investigated the upregulation of PAPPP-linked and antioxidant enzyme associated metabolic responses in elicitor-treated barley (Hordeum vulgare L.) sprouts previously established with stimulation of health relevant phenolic bioactives. Barley seeds were treated with bioprocessed elicitors marine protein hydrolysates (GroPro®, GP) and soluble chitosan oligosaccharide and germinated under dark conditions. Upregulation of PAPPP and subsequent stimulation of phenolic biosynthesis and antioxidant enzyme responses were monitored at day 2, 4, and 6 of sprouting. High PAPPP-linked antioxidant enzyme responses were observed at early stages of germination with selected doses of GP treatments, especially in cv. Pinnacle. Total soluble phenolic content remained at higher level, while guaiacol peroxidase activity increased over the course of sprouting indicating increased phenolic polymerization to support structural needs of sprouts.
Keywords: Antioxidant enzymes, Elicitors, Pentose phosphate pathway, Phenolics, Proline
Introduction
Phenolic compounds are an important class of plant secondary metabolites with diverse protective functions towards plant and human health (Naczk and Shahidi, 2004). Due to their significant antioxidant property, the role of phenolic compounds in human health relevant therapeutic applications has been widely investigated in recent decades (Crozier et al., 2008). Phenolic biosynthesis in plants is associated with the regulation of the protective pathways such as pentose phosphate (PPP), shikimate, and phenylpropanoid pathways (Shetty and Wahlqvist, 2004). The first rate limiting step of PPP involves the glucose-6-phosphate dehydrogenase (G6PDH)-catalyzed conversion of glucose-6-phosphate (G6P) to ribulose-5-phosphate, while generating reducing equivalents for other anabolic cellular processes (Shetty and Wahlqvist, 2004). Based on this rationale, a model was proposed for biosynthesis of phenolic metabolites in plants through up-regulation of protective PPP coupled with active metabolic role of proline under stress (Shetty, 1997). In proline-associated pentose phosphate pathway (PAPPP) model, the demand for NADPH2 during proline synthesis from glutamate in the cytosol can potentially increase the NADP+/NADPH2 ratio, which favors the activation of G6PDH (Shetty, 2004; Shetty and Wahlqvist, 2004). Simultaneously, during its oxidation in the mitochondria, proline can potentially act as a reducing equivalent instead of NADH to facilitate ATP synthesis via oxidative phosphorylation (Hare and Cress, 1997). Therefore, stimulation of the PAPPP can drive carbon flux in the form of erythrose-4-phosphate towards the shikimate and phenylpropanoid pathways, potentially elevating the rate of phenolic biosynthesis (Shetty and Wahlqvist, 2004). Further, this mechanism may also stimulate various antioxidant enzyme responses to counter stress-induced oxidative damage of cellular structures and their functions (Shetty, 1997; Shetty and McCue, 2003).
Various novel strategies have been previously investigated in plant models to stimulate protective phenolic-linked antioxidant enzyme responses through upregulation of PAPPP-linked metabolic responses (Randhir et al., 2002; Randhir et al., 2004; Shetty, 2004; Shetty and Wahlqvist, 2004). Among these strategies, bioprocessed elicitors from natural sources, such as marine protein hydrolysates and soluble chitosan oligosaccharide (COS) have been found to be particularly effective (Orwat, 2016; Sarkar et al., 2011). Further, PAPPP-linked metabolic responses were especially improved with dark germination, as biosynthetic processes associated with respiration could potentially be favored over photosynthesis, under such conditions (McCue and Shetty, 2002; Orwat, 2016; Randhir et al., 2009). In the absence of light, a greater amount of carbon flux derived from the amylolytic degradation of starch, may be channeled towards the PPP (McCue and Shetty, 2002). Thus, elicitor-primed dark germination of seeds represents a novel approach that may effectively partition carbon flux between primary and secondary metabolic processes and could potentially improve human health relevant phenolic bioactive profile in targeted food crops (Sarkar and Shetty, 2014; Shetty, 2004). Based on this rationale, dark germination of primed seeds can be employed as an effective and viable strategy to produce grain sprouts with enhanced phenolic bioactive profiles with human health benefits.
Among cereal grains, barley is widely consumed, containing a diverse profile of phenolic metabolites such as hydroxybenzoic, vanillic, syringic, ferulic, coumaric, and sinapic acids that are of relevance to human health, especially due to their high antioxidant potential (Baik and Ullrich, 2008; Idehen et al., 2017). Therefore, barley is an ideal choice for studying the overexpression of phenolic bioactives through PAPPP mediated metabolic regulation during germination. In a previous study, improvement of phenolic-linked antioxidant and anti-hyperglycemic properties were observed in dark germinated barley sprouts with seed elicitor treatments [(marine protein hydrolysate (GroPro®) and COS] (Ramakrishna et al., 2017). The major soluble phenolic compounds found in this previous study were catechin, gallic acid, protocatechuic acid, and dihydroxybenzoic acid (Ramakrishna et al., 2017). Therefore, the aim of this study was to investigate the efficacy of these bioprocessed elicitors (GroPro and COS) in stimulation of human health relevant phenolic biosynthesis and associated antioxidant enzyme responses in dark germinated barley sprouts through upregulation of critical control points of the anabolic PAPPP.
Materials and methods
Chemical reagents
All reagents and enzymes used in this study were purchased from Sigma Aldrich Chemical Co. (St Louis, MO, USA), unless otherwise mentioned.
Bioprocessed elicitors
Two different types of bioprocessed elicitors were targeted in the current study—marine protein hydrolysate derived from seaweed and marine fish extracts (GroPro®/GP; Icelandic Bio-Enhancers, Westchester, NY, USA) and soluble chitosan oligosaccharide derived from shells of crustaceans, with ascorbic acid side chains (Kong Poong Bio, Jeju, South Korea). Both elicitors were dissolved in distilled water to obtain solutions of the following concentrations—1 mL/L or 1 g/L (GP1/COS1), 2 mL/L or 2 g/L (GP2/COS2), 5 mL/L or 5 g/L (GP5/COS5) and 10 mL/L or 10 g/L (GP10/COS10). In all, two different elicitors with four different doses were used along with control (distilled water seed treatment).
Seed treatment and germination
Seeds of two malting barley cultivars (Pinnacle—2 row; Celebration—6 row) were obtained from the North Dakota Malting Barley Improvement Program at North Dakota State University (Fargo, ND, USA). The seeds were disinfected in 0.5% sodium hypochlorite solution for 5 min, rinsed 5 times with distilled water and extra water was blotted out using dry paper towels. The disinfected seeds were then transferred to conical flasks containing seed treatment solution (100 mL) and incubated on a rotary shaker at 150 rpm and at room temperature for 8 h. The seed treatment solutions were drained, the seeds were rinsed with distilled water and excess treatment solution was then blotted out with paper towels. Treated seeds were placed in sterile Petri-dishes lined with moist paper towels (10 seeds per plate; 5 plates per treatment) and transferred to an incubator for germination. Internal temperature and relative humidity were maintained throughout the experiment at 20 °C and > 95% respectively. Germination was monitored daily, and the sprouts were moistened as required. The developing dark germinated sprouts were used for biochemical analysis at 2, 4 and 6 days post elicitor treatment.
Sample extraction for total soluble phenolic content and antioxidant activity assays
Barley sprouts (100 mg fresh weight F.W.) were transferred to a glass vial containing 95% ethanol (5 mL) and stored at − 10 °C in the freezer (Sarkar et al., 2009). After 48 h, samples were homogenized using tissue tearor and centrifuged at 13,000 rpm for 5 min. The supernatant was analyzed to determine the total soluble phenolic content and antioxidant activity of the sprout samples in vitro.
Total soluble phenolic content
Total soluble phenolics content of the barley sprout extracts was measured using the Folin–Ciocalteu (FC) method, modified from Shetty et al. (1995). Sprout extracts (1 mL) were combined with 95% ethanol (1 mL), distilled water (5 mL), FC reagent (50% v/v; 0.5 mL), and sodium carbonate solution (5% v/v; 1 mL) in a test tube, mixed thoroughly and incubated in the dark for 60 min. Absorbance of the reaction mixtures were measured at 725 nm using a spectrophotometer (Genesys 10S UV–Vis, ThermoFisher Scientific; Waltham, MA, USA). A gallic acid calibration curve was prepared, using standard solutions (10–300 μg/mL) prepared in 95% ethanol, based on which sample absorbance values were converted to total soluble phenolic content and expressed as milligram equivalents of gallic acid per gram fresh weight of tissue sample.
2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) free radical scavenging assay
Antioxidant activity was measured using the ABTS [2, 2-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)] radical cation decolorization assay (Re et al., 1999). A stock solution of 7 mM ABTS containing 140 mM of potassium persulphate was prepared and allowed to mature in the dark at 4 °C for 12–16 h before use. Prior to performing the assay, a working solution of ABTS was prepared by diluting the stock solution with 95% ethanol, to adjust its absorbance to 0.70 ± 0.02 units at 734 nm. To determine the antioxidant activity, 1 mL of ABTS working solution was mixed with 50 μL of sprout extract, incubated at room temperature for 2.5 min and absorbance was measured at 734 nm. The antioxidant activity of the sprout extracts was expressed as percentage (%) inhibition of ABTS radical formation and was calculated as per the following formula:
where AControl and AExtract represent the absorbance of the control (95% ethanol) and extract (sample) at 734 nm.
Enzyme extraction
Barley sprouts (200 mg fresh weight; F.W.) were macerated thoroughly using a cold pestle and mortar, placed in an ice bath, with 2 mL of cold enzyme extraction buffer (0.5% polyvinylpyrrolidone (PVP), 3 mM EDTA, and 0.1 M potassium phosphate buffer; pH 7.5) (Sarkar et al., 2009). The sample extract was then centrifuged at 13,500 rpm for 10 min and immediately stored on ice. The supernatant was used for further biochemical analysis.
Total protein assay
Protein content was measured using the method described by Bradford (1976). One part of dye reagent (Bio-Rad protein assay kit II, Bio-Rad Laboratory; Hercules, CA, USA) was diluted with four parts of distilled water. A volume of 5 mL of diluted dye reagent was added to 50 µL of the barley extract; the mixture was then vortexed and incubated in the dark for 5 min at room temperature. The absorbance of the reaction mixture was measured at 595 nm against a blank (5 mL reagent and 50 µL buffer solution) using a Genesys 10S UV–Vis Spectrophotometer (Thermo-Fisher Scientific; Waltham, MA, USA). A calibration curve was prepared with standard solutions of bovine serum albumin dissolved in distilled water (1, 2.5, 5, 7.5, 10 µg/mL), and used to calculate the total protein content of the extracts.
Glucose-6-phosphate dehydrogenase (G6PDH) assay
A modified method described by Deutsch (1983) was used. The enzyme reaction mixture containing 5.88 µmol ß-NADP, 88.5 µmol MgCl2, 53.7 µmol glucose-6-phosphate, and 0.77 mmol maleimide was prepared. This mixture was used to set the baseline (zero) of the spectrophotometer (Evolution 200 UV–Vis, Thermo-Fisher Scientific; Waltham, MA, USA) reading at 340 nm. To 1 mL of this mixture, 100 µL of the extracted sample was added. The rate of change in absorbance per min was used to quantify the enzyme in the mixture with the help of the extinction coefficient of NADPH (6.22/mM/cm). The activity of G6PDH was expressed in nmol/g protein.
Guaiacol peroxidase (GPX) assay
A modified method described by Laloue et al. (1997) was used to measure the activity of guaiacol peroxidase. The enzyme reaction mixture containing 0.1 M potassium phosphate buffer (pH 6.8), 56 mM guaiacol solution, and 50 mM hydrogen peroxide was used. To 990 µL of this reaction mixture, 10 µL of enzyme sample was added. The absorbance was recorded immediately after the addition of reaction mixture and again after 3 min. The rate of change in absorbance per min was measured using an Evolution 200 UV–Vis spectrophotometer (Thermo-Fisher Scientific; Waltham, MA, USA) and used to quantify GPX activity in the sample based on the extinction coefficient of the oxidized product, i.e. tetraguaiacol (26.6/mM/cm). The activity of GPX was expressed in nmol/g protein.
Superoxide dismutase (SOD) assay
A competitive inhibition assay was performed that used xanthine oxidase generated superoxide to reduce nitrobluetetrazolium (NBT) to blue formazan. Spectrophotometric assay of SOD activity was carried out by monitoring the reduction of NBT at 560 nm (Oberley and Spitz, 1984). The reaction mixture contained 13.8 mL of 50 mM potassium phosphate buffer (pH 7.8) containing 1.33 mM diethylenetetraaminepentaacetic acid (DETEPAC); 0.5 mL of 2.45 mM NBT; 1.7 mL of 1.8 mm xanthine and 40 IU/mL catalase. Then 100 µL of phosphate buffer and 100 µL of xanthine oxidase were added to 0.8 mL of reagent mixture. The change in absorbance at 560 nm was measured immediately after adding the reagents and again after 1 min using an Evolution 200 UV–Vis spectrophotometer (Thermo-Fisher Scientific; Waltham, MA, USA). The concentration of xanthine oxidase was adjusted to obtain a linear curve with a slope of 0.024–0.026 absorbance per min. The phosphate buffer was replaced with the sample extract and the change in absorbance was monitored immediately after the addition of reaction mixture and after 1 min. One unit of SOD was defined as the amount of protein that inhibits NBT reduction to 50% of the maximum. The activity of SOD was expressed in units/mg protein.
Catalase (CAT) assay
A method originally described by Beers and Sizer (1952) was used to measure the activity of catalase. To 1.9 mL of distilled water, 1 mL of 0.059 M hydrogen peroxide (Fisher Scientific; Waltham, MA) in 0.05 M potassium phosphate (pH 7.0) was added. This mixture was incubated in a spectrophotometer (Evolution 200 UV–Vis, Thermo-Fisher Scientific; Waltham, MA, USA) for 4–5 min to achieve temperature equilibration and to establish blank rate. Then 0.1 mL of diluted enzyme was added, and the disappearance of peroxide was followed spectrophotometrically by recording the decrease in absorbance at 240 nm for 1 min. The rate of change in absorbance (ΔA240/min) from the initial linear portion of the curve was calculated. One unit of catalase activity was defined as amount that decomposes one micromole of H2O2. The activity of CAT was expressed in units/mg protein.
Proline dehydrogenase (PDH) assay
A method described by Costilow and Cooper (1978) was used to measure the activity of proline dehydrogenase. An enzyme reaction mixture containing 100 mM sodium carbonate buffer (pH 10.3), 20 mM l-proline solution and 10 mM NAD was used. To 1 mL of this reaction mixture, 200 µL of enzyme extract was added. The increase in absorbance was measured at 340 nm for 3 min, at 32 °C using an Evolution 200 UV–Vis spectrophotometer (Thermo-Fisher Scientific; Waltham, MA, USA). The absorbance was recorded at zero time and then after 3 min. In this spectrophotometric assay, one unit of enzyme activity is equal to the amount causing an increase in absorbance of 0.01 per min at 340 nm (1.0 cm light path). The activity of PDH was expressed in units/mg protein.
Succinate dehydrogenase (SDH) assay
To measure the activity of succinate dehydrogenase a modified method described by Bregman (1987) was used. The reaction mixture containing 1.0 mL of 0.4 M potassium phosphate buffer (pH 7.2), 40 µL of 0.15 M sodium succinate (pH 7.0), 40 µL of 0.2 M sodium azide, and 10 µL of 6.0 mg/mL 2,6-dichlorophenolindophenol (DCPIP) was prepared. This mixture was used to set the baseline (zero) of the spectrophotometer (Evolution 200 UV–Vis, Thermo-Fisher Scientific; Waltham, MA, USA) reading at 600 nm. To 1.0 mL of this mixture, 200 µL of the enzyme sample was added. The rate of change of absorbance per min was used to quantify the enzyme in the mixture based on the extinction coefficient of DCPIP (19.1/mM/cm). The activity of SDH was expressed in nmol/g protein.
Determination of free proline content using HPLC
High performance liquid chromatography (HPLC) analysis was performed using an Agilent 1260 series liquid chromatograph (Agilent Technologies, Santa Clara, CA, USA) equipped with a diode array detector (DAD 1100). A C-18 analytical column (Agilent Zorbax Eclipse Plus; 4.5 mm × 250 mm) with a packing material particle size of 5 μm was used as the stationary phase, and a 20 mM ortho-phosphoric acid solution was used as the mobile phase. The column temperature was maintained at 25 °C. Each sample extract was eluted isocratically for 10 min, at a flow rate of 1.0 mL/min and free proline was detected at 210 nm. Standard solutions of l-proline dissolved in 20 mM ortho-phosphoric acid were used to prepare the calibration curve. Four replicates were analyzed per sample, and the mean free proline content was expressed in mg/g fresh weight.
Statistical analysis
A completely randomized design (CRD) was used for this study. The sprouts were sampled in triplicate for each run of the assays, and the entire experiment was repeated thrice. Analysis of variance (ANOVA) for the data was performed using the Statistical Analysis Software (SAS; version 9.4; SAS Institute, Cary, NC, USA). Statistically significant differences between elicitor treatments, cultivars, and cultivar × elicitor treatment interactions were determined using the Tukey’s least mean square test at a confidence level of 95%.
Results and discussion
Total soluble phenolic content and antioxidant activity
Phenolic compounds are mostly found in the hull and aleurone layers of cereal grains bound to cell wall polysaccharides such as arabinoxylans providing structural support (Baik and Ullrich, 2008). Previous studies have reported that seed treatment with bioprocessed elicitors prior to sprouting further enhanced the total soluble phenolic (TSP) content of various cereal grains and legumes (Randhir et al., 2002; Randhir et al., 2004), which may have relevance in improving the health oriented functionality of grain sprouts. In our previous study with barley sprouts to investigate hyperglycemia related health benefits, improvement of TSP and individual phenolic acids (catechin, gallic acid, protocatechuic acid, and dihydroxybenzoic acid) content were observed with seed elicitor treatments (Ramakrishna et al., 2017). However, the TSP content and individual phenolic acid content of barley sprouts did not always change proportionately (Ramakrishna et al., 2017). Therefore, in this study the metabolic control associated with TSP content of dark-germinated barley sprouts was investigated. In the current study, the TSP content of sprouts of both Celebration and Pinnacle slightly reduced from day 2 to day 4, followed by a subsequent increase at day 6 post seed elicitor treatments (Table 1). The TSP content of sprouts of cultivar Celebration was found to be statistically higher than that of Pinnacle at day 4 (p < 0.05). However, at day 2 and 6, TSP content was found to be statistically at par between the two cultivars. Previously, Ramakrishna et al. (2017) found high TSP content at day 2 in aqueous extracts of Pinnacle and Celebration sprouts following GP and COS seed elicitor treatments. Similarly, fish protein hydrolysates, lactoferrin, and oregano extracts also elicited TSP content in 1-day old mung bean sprouts, followed by a gradual decline in TSP content as sprouting progressed (Randhir et al., 2004). This trend may be attributed to an increase in TSP content immediately after the sprouting induced activation of the seed, due to cleaving and solubilization of cell-wall bound phenolics, as well as the biosynthesis of new phenolic compounds to meet the anabolic needs of the developing plants (Singh and Sharma, 2017). The decline of TSP content over further seedling emergence may be linked to the conversion of simple soluble phenolics to more complex insoluble compounds of greater molecular weight, associated with developmental processes such as lignification (Randhir et al., 2004). However, in the current study, such a pattern of decline in TSP content was not observed over the course of sprouting and TSP content remained comparatively high. In our previous study (Ramakrishna et al., 2017), gallic acid was reported as the most dominant simple phenolic acid in all barley sprout samples, across cultivars and treatment types. Furthermore, the gallic acid content of the sprout samples was found to increase during dark germination over 6 days. Additionally, the content of catechin was found to be improved in barley sprouts in response to specific elicitor treatments (GP5, GP10 and COS5). Ramakrishna et al. (2017) also observed cultivar specific differences in the contents of individual phenolic acids. Sprouts of the cultivar Pinnacle demonstrated a higher content of dihydroxybenzoic acid in comparison with those of Celebration. Protocatechuic acid was predominantly found in Celebration sprouts at all time points, whereas it was detected at lower concentrations and only at day 6 in Pinnacle sprouts. The response observed in this particular study indicated the influence of genotype × environment interactions on the phenolic acid composition and their relative individual content, in addition to the effects of seed elicitor treatments.
Table 1.
Total soluble phenolics (TSP) (A) (mg/g GAE F.W.) and ABTS free radical inhibition capacity (B) (%) of dark germinated barley sprouts following seed elicitor treatments
| Day 2 | Day 4 | Day 6 | ||||
|---|---|---|---|---|---|---|
| Pinnacle | Celebration | Pinnacle | Celebration | Pinnacle | Celebration | |
| (A) | ||||||
| Control | 0.886 | 0.797 | 0.882 | 0.891 | 0.754cdef | 0.820abcd |
| GP1 | 0.784 | 0.618 | 0.839 | 0.739 | 0.927a | 0.784bcde |
| GP2 | 0.742 | 0.699 | 0.659 | 0.813 | 0.748cdef | 0.571g |
| GP5 | 0.722 | 0.767 | 0.647 | 0.720 | 0.701defg | 0.860abc |
| GP10 | 0.734 | 0.864 | 0.669 | 0.720 | 0.782bcde | 0.708defg |
| COS1 | 0.760 | 0.706 | 0.667 | 0.829 | 0.759cdef | 0.909ab |
| COS2 | 0.794 | 0.781 | 0.777 | 0.746 | 0.733cdef | 0.658efg |
| COS5 | 0.706 | 0.759 | 0.580 | 0.707 | 0.739cdef | 0.721cdef |
| COS10 | 0.759 | 0.768 | 0.687 | 0.748 | 0.638fg | 0.833abcd |
| (B) | ||||||
| Control | 24.88a | 16.25bcd | 21.19a | 13.33cdef | 12.33abc | 6.74gh |
| GP1 | 20.55ab | 10.91defg | 10.50defgh | 18.17ab | 14.34a | 7.30efgh |
| GP2 | 8.77fg | 12.75cdefg | 6.08ij | 13.86cde | 9.59bcdefg | 7.71defgh |
| GP5 | 8.36g | 10.50efg | 8.33ghij | 16.07bc | 12.75ab | 8.93cdefg |
| GP10 | 9.65efg | 9.98efg | 7.13hij | 16.28bc | 10.40bc | 11.01bcde |
| COS1 | 10.41efg | 9.40efg | 10.28efgh | 12.65cdef | 10.68bcde | 4.41h |
| COS2 | 17.15bc | 13.03cdefg | 12.65cdef | 9.72fghi | 8.32defg | 9.90bcdefg |
| COS5 | 13.96cdefg | 15.03bcde | 11.06defg | 5.30j | 10.24bcdef | 8.30defg |
| COS10 | 14.14cdef | 10.99defg | 13.95cd | 7.55ghij | 9.47bcdefg | 6.89fgh |
Different lowercase letters indicate significant differences in TSP content and antioxidant capacity activities due to cultivar × treatment interactions, during the corresponding sampling time point, at 95% confidence level
The high baseline value of TSP content in the current study over the period of germination and sprouting might have relevance for targeting barley sprout from different stages for designing phenolic bioactive enriched functional foods. However, no significant differences in TSP content were observed due to cultivar × elicitor treatment interaction at day 2 and 4. However at day 6, sprouts of cv. Pinnacle treated with GP1 (GroPro 1 mL/L) had significantly higher TSP content when compared to the control and other seed elicitor treatments (p < 0.05). For Celebration barley sprouts COS1 seed treatment had significantly higher TSP content, however it was at par with respective control. Previously, seed treatment with fish protein hydrolysates (5 mL/L) elicited the phenolic biosynthetic during the later stages of sprouting in primed sprouts of corn (Randhir and Shetty, 2005), while in mung bean sprouts 1 mL/L treatment corresponded with maximum phenolic stimulation on day 1 post priming (Randhir et al., 2004). Such variability could potentially arise from differences in response between monocots and dicots and anabolic demands of the developing plants during sprouting.
The ability of phenolic compounds to function as effective antioxidants and free radical scavengers is an important aspect of their biological activity, and it is vital for countering any deleterious deviations from optimal cellular redox homeostasis in plant tissues (Rice-Evans et al., 1997). Previous studies observed that elicitor-mediated stimulation of TSP content has corresponded with a simultaneous increase in the free radical scavenging capacities of sprout extracts of grains and legumes such as corn, pea, and mung beans (Andarwulan and Shetty, 1999; Randhir et al., 2004; Randhir and Shetty, 2005). In the current study antioxidant activity of the dark germinated sprouts was found to be highest at day 2, and steadily decreased over the course of sprouting (Table 1). A similar trend was observed in corn sprouts (Randhir and Shetty, 2005), where antioxidant activity (based on DPPH assay) was found to be highest at early stages of germination. Respiration is the dominant physiological process in the seed tissues immediately after water imbibition, which can lead to elevated levels of free radical generation and therefore necessitating a greater demand for antioxidants during this time (Randhir and Shetty, 2005). In earlier studies, declining antioxidant activity of barley and mung bean sprouts has corresponded with a decrease in TSP content (Ramakrishna et al., 2017; Randhir et al., 2004). The antioxidant activities of the two cultivars used in this study were significantly different at all three timepoints (p < 0.05). Pinnacle had significantly higher antioxidant potential at days 2 and 6, whereas Celebration was higher at day 4. At all time points, significant differences were observed in antioxidant activity due to cultivar × treatment interactions. At day 2 and 4, higher antioxidant activity was observed in control. However, at day 6, higher total antioxidant activity in Pinnacle sprouts was observed with GP1 seed elicitor treatment, while in cv. Celebration, GP10 had significantly higher antioxidant activity when compared to the respective control. For cv. Pinnacle, same elicitor treatment (GP1) resulted in higher TSP content at day 6 which has significant relevance for improving phenolic-linked antioxidant activity in dark germinated barley sprouts. Therefore, GroPro in lower doses (1 mL/L) can be targeted further to induce phenolic-linked antioxidant activity in barley sprout, especially at later sprouting stages. Such improvements in antioxidant activity may be linked to the increase in the content of specific soluble phenolic acids (Ramakrishna et al., 2017), rather than the total phenolic content of the sprouts.
Glucose 6 phosphate dehydrogenase (G6PDH) and succinate dehydrogenase (SDH) activity
The pentose phosphate pathway (PPP) is a critical protective pathway through which carbon flux are channeled into various anabolic pathways such as shikimic acid and phenylpropanoid pathways and leads to phenolic biosynthesis. The first rate limiting step of the PPP is the oxidation of glucose-6-phosphate to 6-phosopho-glucose-δ-lactone, catalyzed by G6PDH. Consequently, G6PDH activity in a specific tissue can be indicative of the amount of carbon flux from primary metabolism that is being potentially directed towards secondary metabolic pathways, including phenolic biosynthesis (Shetty and Wahlqvist, 2004).
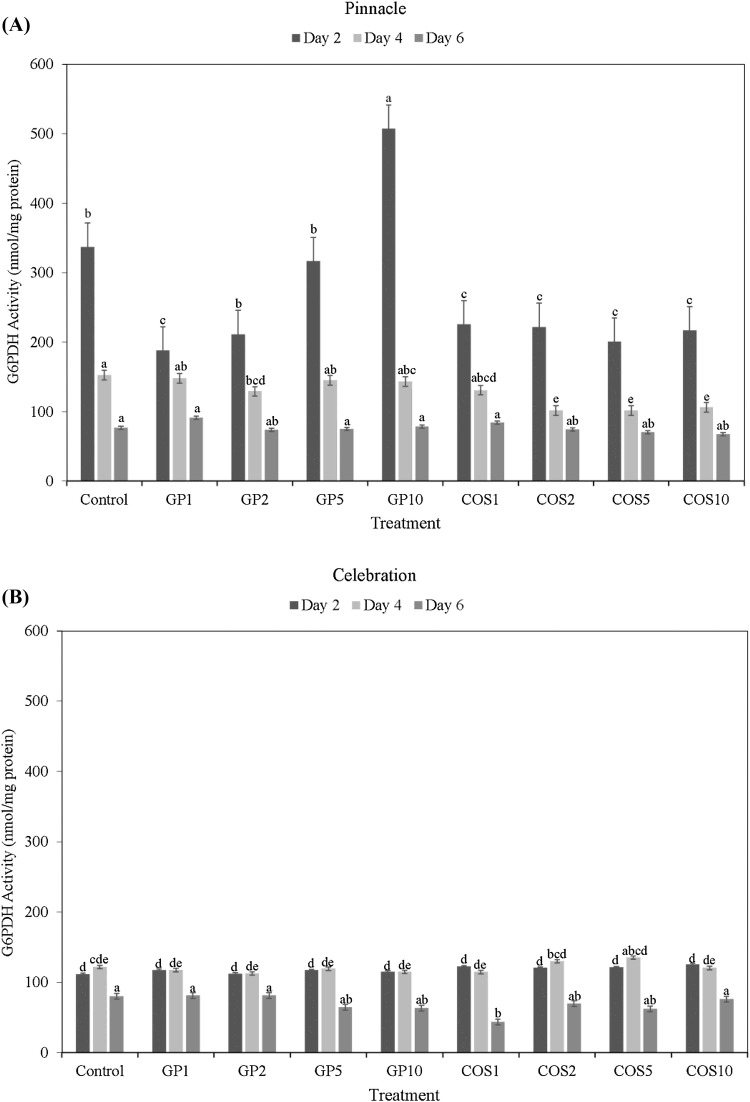
In barley sprouts of cv. Celebration and Pinnacle, G6PDH activity was at the highest levels at day 2 post seed elicitor treatments following which it steadily declined over the course of sprouting (Fig. 1A, B). Overall, Pinnacle sprouts had higher G6PDH enzyme activity than Celebration sprouts at all time points (at day 2, 4, and 6). At day 2, GP 10 treated Pinnacle sprouts had significantly higher G6PDH activity compared to its corresponding control as well as other elicitor treatments (p < 0.05). At day 4, G6PDH activity of most GP treated Pinnacle sprouts were significantly higher than that of Celebration sprouts with the exception of the GP2 seed elicitor treatments where it was statistically at par. No significant differences in G6PDH activities between Celebration and Pinnacle sprouts were observed at day 6.
Fig. 1.
Glucose-6-phosphate dehydrogenase (G6PDH) activity (nmol/mg protein) of dark germinated barley sprouts of cultivars Pinnacle (A) and Celebration (B) at day 2, day 4, and day 6 following seed elicitor treatments (GroPro and COS). Different lowercase letters indicate significant differences in G6PDH activity between cultivar × treatment interactions, during the corresponding sampling time point, at 95% confidence level
Previously, Randhir and Shetty (2005) found that corn seeds primed with fish protein hydrolysate (5 mL/L) had similar results whereby G6PDH activity was highest at the early stages of germination (day 3 post treatment), before declining in the intermediate stages. This may be attributed to elevated levels of carbohydrate solubilization and mobilization to meet the anabolic and energy demands of the developing seedling (Singh and Sharma, 2017). Furthermore, Randhir et al. (2004) also reported a positive correlation between G6PDH activity and TSP content in the intermediate stages of sprouting in elicitor treated mung bean sprouts, indicating the potential role of PPP upregulation in promoting phenolic biosynthesis. However, in the current study, TSP content maintained at higher level from day 2 to day 6, while G6PDH activity declined concurrently during the course of sprouting.
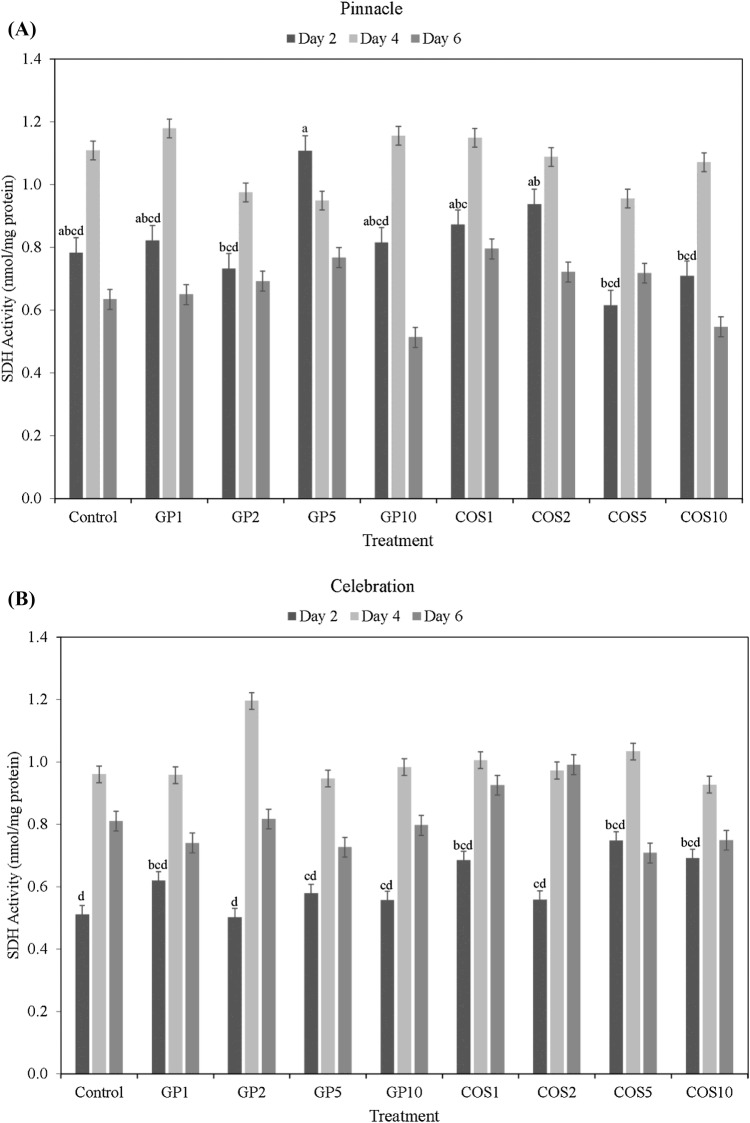
Succinate dehydrogenase (SDH) is a critical redox enzyme that couples the oxidation of succinic acid to fumaric acid in Kreb’s cycle with the reduction of ubiquinone to ubiquinol in the mitochondrial electron transport chain (ETC), accompanied by the formation of reducing equivalents (FADH2) and ATP (Huang and Millar, 2013). The activity of SDH activity was measured to evaluate whether the COS and GP elicitor treatments could upregulate the activity of Kreb’s cycle and overall respiration rate (Sarkar et al., 2011). For both barley cultivars, SDH activity was found to increase from day 2 to the highest level at day 4, before subsequently decreasing to the lowest level at day 6 (Fig. 2A, B). Among cultivars, sprouts of Pinnacle had higher SDH activity than those of Celebration at day 2 and 4, but statistically significant differences in SDH activity were observed only at day 2. However, at day 6 Celebration sprouts had higher SDH activity than those of Pinnacle for most seed elicitor treatments.
Fig. 2.
Succinate dehydrogenase (SDH) activity (nmol/mg protein) of dark germinated barley sprouts of cultivars Pinnacle (A) and Celebration (B) at day 2, day 4, and day 6 following seed elicitor treatments (GroPro and COS). Different lowercase letters indicate significant differences in SDH activity between cultivar × treatment interactions, during the corresponding sampling time point, at 95% confidence level. Bars without lowercase letters do not have statistical significant differences between cultivar × treatment interactions at 95% confidence level
Statistically significant difference in SDH activity due to cultivar × treatment interaction was observed only at day 2. At day 2, GP5 treated Pinnacle sprouts had the highest SDH activity (1.11 nmol/mg protein). However, this value was statistically at par with the SDH activities of other Pinnacle sprouts treated with other doses of GP, as well as those treated with COS1 and COS2. The improvement of both G6PDH and SDH activity at day 2 in Pinnacle with GroPro seed elicitor treatments (GP10 and GP5) might have relevance for mobilization of carbon flux through PPP and Kreb’s cycle to meet higher energy demands during germination and seedling emergence of barley. Therefore, GroPro can be targeted in selective doses to upregulate PPP to support anabolic needs during germination and seedling emergence of barley and other grain sprouts.
Proline content and proline dehydrogenase (PDH) activity
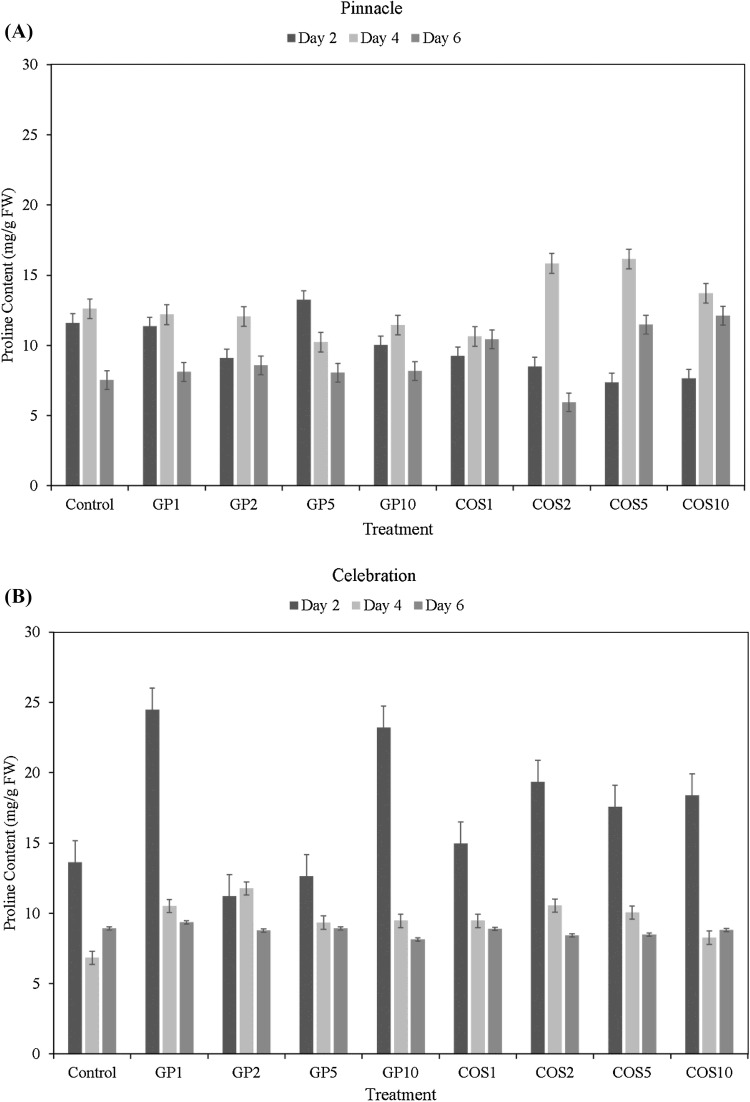
The free proline content of dark germinated barley extracts was measured to evaluate the modulation of proline metabolism and its coupling with PPP, in response to seed elicitor treatments. The free proline content of dark germinated sprouts of Celebration and Pinnacle was found to decline over the course of germination, with the highest levels being observed at day 2 (Fig. 3A, B). Extracts of Celebration sprouts treated with all doses of COS, as well as GP1 and GP10 treatments were found to have higher proline content than Pinnacle sprouts with same doses of elicitor treatments. In this study, Celebration sprouts treated with GP1 and GP10 had the highest free proline content at day 2 and 4. Overall, significantly higher proline content was observed in Pinnacle at day 2, while it was higher in Celebration at day 6. However, significant differences in proline content due to cultivar × treatment interactions were not observed at any stage of the study.
Fig. 3.
Total proline content (mg/g FW) of dark germinated barley sprouts of dark germinated barley sprouts of cultivars Pinnacle (A) and Celebration (B) at day 2, day 4, and day 6 following seed elicitor treatments (GroPro and COS)
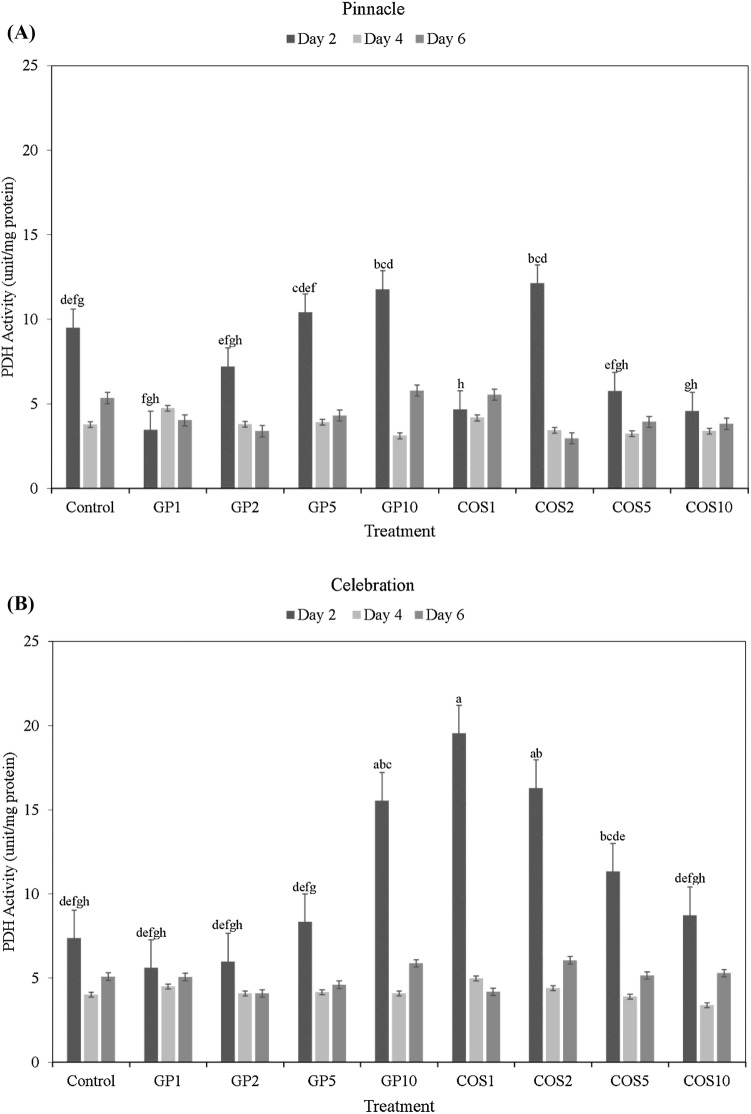
The enzyme PDH catalyzes the oxidation of l-proline to pyrroline-5-carboxylate in the mitochondria. Activity of the PDH in the barley sprouts was measured to assess the degree of cellular proline oxidation, and thereby to evaluate the role of elicitor treatments in upregulating proline mediated mitochondrial ATP synthesis. The highest PDH activity of dark germinated barley sprouts were observed at day 2. Activity of PDH decreased rapidly from day 2 to day 4, before reaching an intermediate level at day 6 (Fig. 4A, B). At day 2, Celebration sprouts treated with COS1, COS2, and GP10 elicitor treatments were found to have significantly higher PDH activities than that of the corresponding control and other treatments. However, differences in PDH activity due to cultivar × treatment interactions were found to be statistically significant only in the early stages of sprouting (day 2), and not in the intermediate or later stages.
Fig. 4.
Proline dehydrogenase (PDH) activity (unit/mg protein) of dark germinated barley sprouts of cultivars Pinnacle (A) and Celebration (B) at day 2, day 4, and day 6 following seed elicitor treatments (GroPro and COS). Different lowercase letters indicate significant differences in PDH activity between cultivar × treatment interactions, during the corresponding sampling time point, at 95% confidence level. Bars without lowercase letters do not have statistical significant differences between cultivar × treatment interactions at 95% confidence level
Overall, the trend observed for PDH activity during sprouting is very similar to that of free proline content. Therefore, the observed trends indicate that the seed treatment with seed elicitors such as COS and GP may play a potential role in stimulating proline oxidation by PDH which has relevance for improving abiotic stress modulation of food crops. Such active metabolic role of proline may support proline-linked ATP synthesis in the early stages of sprouting to meet the energy demand in the developing barley and other grain seedlings.
Guaiacol peroxidase (GPX), catalase (CAT) and superoxide dismutase (SOD) activity
The effect of COS and GP seed elicitor treatments on stimulating the protective antioxidant enzyme responses in barley sprouts at various growth stages was investigated by measuring the activities of GPX, SOD, and CAT enzyme activities. Peroxidases such as GPX are responsible for the polymerization of phenolic compounds by catalyzing the formation of oxidation-mediated cross linkages between phenolic precursors, during the biosynthesis of lignin in plant cell walls (Morales and Barceló, 1997).
The activity of GPX increased steadily over the course of sprouting, with the highest levels being observed at day 6 post seed elicitor treatments (Table 2A). The concurrent increase in GPX activity with the development of the barley seedlings is consistent with the trends observed in various other elicitor treated sprouts of corn, fava bean, black bean, velvet bean, and mung bean (Orwat, 2016; Randhir et al., 2004; Randhir et al., 2009; Randhir and Shetty, 2003). This can be attributed to the increasing necessity for lignification whereby free phenolics are utilized for the formation of robust polymeric complexes to lend structural and mechanical support and defense against environmental stresses to the growing plant. The early stages of germination are associated with the mobilization and production of phenolic precursors for the anabolic needs of the emerging seedling, because of which GPX levels are expected to have relatively low levels of activity (Randhir and Shetty, 2003). Dark germinated Pinnacle sprouts had higher GPX activity than those of Celebration. GroPro treated sprouts showed higher GPX activity at day 2 and 4 post seed treatment, while COS treated sprouts exhibited higher GPX activity in the later stages. Differences in GPX activity due to cultivar × treatment interactions were statistically significant (p < 0.05) at day 4 and 6.
Table 2.
Guaiacol peroxidase (GPX) (A) (nmol/mg protein), catalase (CAT) (B) (unit/mg protein), and superoxide dismutase (SOD) (C) (unit/mg protein) enzyme activity of dark germinated barley sprouts following seed elicitor treatments
| Day 2 | Day 4 | Day 6 | ||||
|---|---|---|---|---|---|---|
| Pinnacle | Celebration | Pinnacle | Celebration | Pinnacle | Celebration | |
| (A) | ||||||
| Control | 523.8 | 249.2 | 863.1ab | 637.2c | 1459.1abcd | 1117.6ef |
| GP1 | 525.9 | 251.2 | 795.5abc | 699.5bc | 1414.3bcde | 1172.1def |
| GP2 | 560.9 | 275.3 | 723.7bc | 742.4bc | 1341.5bcdef | 1115.9ef |
| GP5 | 582.1 | 281.7 | 772.2abc | 730.7bc | 1307.5cdef | 1061.1f |
| GP10 | 525.3 | 295.7 | 933.9a | 824.2ab | 1430.1abcd | 1165.3def |
| COS1 | 547.7 | 277.0 | 789.5abc | 766.0abc | 1337.0bcdef | 1247.0cdef |
| COS2 | 441.6 | 272.2 | 851.1ab | 819.5ab | 1616.5ab | 1231.3cdef |
| COS5 | 445.7 | 251.1 | 836.0ab | 870.7ab | 1489.9abc | 1179.2def |
| COS10 | 554.7 | 282.7 | 751.8abc | 783.0abc | 1720.5abcd | 1226.0def |
| (B) | ||||||
| Control | 210.7a | 219.3ab | 47.4d | 119.0abc | 42.7 | 24.3 |
| GP1 | 230.2ab | 227.2ab | 50.9d | 111.9bc | 43.9 | 25.5 |
| GP2 | 238.8a | 204.8ab | 51.8d | 99.9bc | 44.8 | 26.2 |
| GP5 | 255.7a | 237.4ab | 51.0d | 97.0c | 41.4 | 27.1 |
| GP10 | 230.9ab | 217.3ab | 58.3d | 111.8abc | 33.7 | 23.0 |
| COS1 | 242.5a | 229.3ab | 64.5d | 108.6abc | 47.5 | 27.3 |
| COS2 | 208.6ab | 223.1ab | 55.3d | 135.4a | 40.9 | 26.6 |
| COS5 | 184.3b | 256.8a | 56.1d | 126.8ab | 39.6 | 24.2 |
| COS10 | 220.0ab | 235.4ab | 51.7d | 135.5a | 40.5 | 27.5 |
| (C) | ||||||
| Control | 0.01 | 0.01 | 0.02 | 0.01 | 0.07 | 0.01 |
| GP1 | 0.03 | 0.01 | 0.02 | 0.01 | 0.05 | 0.01 |
| GP2 | 0.02 | 0.01 | 0.02 | 0.02 | 0.02 | 0.01 |
| GP5 | 0.02 | 0.01 | 0.02 | 0.01 | 0.03 | 0.01 |
| GP10 | 0.03 | 0.01 | 0.00 | 0.01 | 0.05 | 0.03 |
| COS1 | 0.02 | 0.01 | 0.02 | 0.02 | 0.02 | 0.01 |
| COS2 | 0.02 | 0.02 | 0.03 | 0.01 | 0.03 | 0.02 |
| COS5 | 0.03 | 0.01 | 0.03 | 0.02 | 0.04 | 0.01 |
| COS10 | 0.01 | 0.01 | 0.02 | 0.02 | 0.04 | 0.02 |
Different lowercase letters indicate significant differences in enzyme activities due to cultivar × treatment interactions, during the corresponding sampling time point, at 95% confidence level
Superoxide dismutase (SOD) regulates the elimination of highly deleterious superoxide free radicals by catalyzing its conversion to molecular oxygen and hydrogen peroxide and is critical to maintaining cellular redox homeostasis. It is a key component of the enzymatic antioxidant response mechanism that aids plants in overcoming ROS mediated cellular damage induced by biotic and abiotic stresses (Sarkar et al., 2011). During sprouting, the highest level of SOD activity was observed at day 6 for all combinations of cultivars × elicitor treatments (Table 2B). Among the cultivars, Pinnacle sprouts demonstrated significantly higher SOD activity when compared to Celebration (p < 0.05). High SOD activity of Pinnacle also positively correlated with G6PDH activity, TSP content, and total antioxidant activity.
Catalase is another key antioxidant enzyme which catalyzes the decomposition of hydrogen peroxide to water and oxygen, thereby aiding in protecting sub-cellular structures from the toxic effects of excessive hydrogen peroxide accumulation (Sarkar et al., 2011). In this study, the observed levels of CAT activity varied widely among cultivars across all time points (Table 2C). Significant differences in CAT activity due to cultivar × treatment interactions were observed at day 2 and 4 (p < 0.05). However, at day 4, CAT activity of Celebration sprouts was significantly higher than those of Pinnacle. Whereas the CAT activity of Celebration sprouts treated with COS2 and COS5 treatments were found to be statistically at par with the corresponding control treatment. However, it was significantly higher than that most GP treatments. From the trends observed in this study, it was evident that while seed treatment with elicitors did influence the activity of key antioxidant enzymes to varying extents, their response was cultivar specific (genetic make-up). Overall, the results of the current study indicate that GPX antioxidant enzyme has more relevance during germination and sprouting stages of barley and seed elicitor treatments in selective doses can be targeted to improve the antioxidant enzyme responses of some barley cultivars.
Sprouting has become a good strategy for functional food enrichments as many nutrients are mobilized or have higher bioavailability for assimilation during germination and seedling emergence of cereal grains and legumes. Therefore, sprouting in combination with seed elicitation strategy offers a cost-effective approach for improving phenolic biosynthesis and antioxidant enzyme responses through up-regulation of critical protective pathways such as PAPPP-linked metabolic regulation. In previous study, improvement of phenolic-linked antioxidant and anti-hyperglycemic functions was observed in dark germinated barley sprouts with seed elicitor treatments (GP and COS) (Ramakrishna et al., 2017). Based on this previous finding, the current study investigated the critical control points of PAPPP-linked metabolic responses for stimulating phenolic biosynthesis and antioxidant enzyme responses in dark germinated barley sprouts following seed elicitor treatments. Overall, PAPPP-linked improvement of phenolic biosynthesis and antioxidant enzyme response was observed in dark germinated barley sprouts with selected doses of GP as seed elicitor treatment. However, the response was more prominent in cv. Pinnacle when compared to the cv. Celebration barley sprouts which indicates that seed elicitation response might be cultivar specific (genetic make-up). Further studies are required with other barley cultivars or with other grains for further advancing this concept. However, bioprocessed elicitors such as GP can be targeted in selected doses to improve phenolic biosynthesis and antioxidant enzyme responses in barley and other grain sprouts which have dual function relevance in improving stress resilience in food crops and to enhance human health relevant bioactive profiles in plant-based foods.
Acknowledgements
We thank Dr. Richard Horsley, the coordinator of the North Dakota Malting Barley Improvement Program at North Dakota State University, Fargo, ND, USA, for providing malting barley seeds for this experiment.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- Andarwulan N, Shetty K. Improvement of pea (Pisum sativum) seed vigour response by fish protein hydrolysates in combination with acetyl salicylic acid. Process Biochem. 1999;35:159–165. doi: 10.1016/S0032-9592(99)00047-3. [DOI] [Google Scholar]
- Baik BK, Ullrich SE. Barley for food: characteristics, improvement, and renewed interest. J. Cereal Sci. 2008;48:233–242. doi: 10.1016/j.jcs.2008.02.002. [DOI] [Google Scholar]
- Beers RF, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952;195:133–140. [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bregman AA. Laboratory investigations in cell biology. 2. Hoboken: Wiley; 1987. [Google Scholar]
- Costilow RN, Cooper D. Identity of proline dehydrogenase and delta 1-pyrroline-5-carboxylic acid reductase in Clostridium sporogenes. J. Bacteriol. 1978;134:139–146. doi: 10.1128/jb.134.1.139-146.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozier A, Clifford MN, Ashihara H. Plant secondary metabolites: occurrence, structure and role in the human diet. Hoboken, NJ, USA: Wiley; 2008. [Google Scholar]
- Deutsch J. Methods of enzymatic analysis. 3. Deerfield Beach: Verlag Chemie Academic Press; 1983. [Google Scholar]
- Hare PD, Cress WA. Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul. 1997;21:79–102. doi: 10.1023/A:1005703923347. [DOI] [Google Scholar]
- Huang S, Millar HA. Succinate dehydrogenase: the complex roles of a simple enzyme. Curr. Opin. Plant Biol. 2013;16:344–349. doi: 10.1016/j.pbi.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Idehen E, Tang Y, Sang S. Bioactive phytochemicals in barley. J. Food Drug Anal. 2017;25:148–161. doi: 10.1016/j.jfda.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laloue H, Weber-Lofti F, Lucau-Danila A, Guillemaut P. Identification of ascorbate and guaiacol peroxidase in needle chloroplasts of spruce trees. Plant Physiol. Biochem. 1997;35:341–346. [Google Scholar]
- McCue P, Shetty K. Clonal herbal extracts as elicitors of phenolic synthesis in dark-germinated mung beans for improving nutritional value with implications for food safety. J. Food Biochem. 2002;26:209–232. doi: 10.1111/j.1745-4514.2002.tb00853.x. [DOI] [Google Scholar]
- Morales M, Barceló AR. A basic peroxidase isoenzyme from vacuoles and cell walls of Vitis vinifera. Phytochemistry. 1997;45:229–232. doi: 10.1016/S0031-9422(96)00825-4. [DOI] [Google Scholar]
- Naczk M, Shahidi F. Extraction and analysis of phenolics in food. J Chromatogr. A. 2004;1054:95–111. doi: 10.1016/S0021-9673(04)01409-8. [DOI] [PubMed] [Google Scholar]
- Oberley LW, Spitz DR. Assay of SOD activity in tumor tissue. Method Enzymol. 1984;105:457–461. doi: 10.1016/S0076-6879(84)05064-3. [DOI] [PubMed] [Google Scholar]
- Orwat J (2016) Phenolic antioxidant-linked bioactive enrichment in black beans (Phaseolus vulgaris L.) to screen for health benefits and enhancement of salinity resilience. M.S. thesis, North Dakota State University, Fargo, USA (2016)
- Ramakrishna R, Sarkar D, Manduri A, Iyer SG, Shetty K. Improving phenolic bioactive-linked anti-hyperglycemic functions of dark germinated barley sprouts (Hordeum vulgare L) using seed elicitation strategy. J. Food Sci. Technol. Mysore. 2017;54:1–13. doi: 10.1007/s13197-017-2828-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randhir R, Shetty K. Light-mediated fava bean (Vicia faba) response to phytochemical and protein elicitors and consequences on nutraceutical enhancement and seed vigour. Process Biochem. 2003;38:945–952. doi: 10.1016/S0032-9592(02)00219-4. [DOI] [Google Scholar]
- Randhir R, Shetty K. Developmental stimulation of total phenolics and related antioxidant activity in light- and dark-germinated corn by natural elicitors. Process Biochem. 2005;40:1721–1732. doi: 10.1016/j.procbio.2004.06.064. [DOI] [Google Scholar]
- Randhir R, Shetty P, Shetty K. L-DOPA and total phenolic stimulation in dark germinated fava bean in response to peptide and phytochemical elicitors. Process Biochem. 2002;37:1247–1256. doi: 10.1016/S0032-9592(02)00006-7. [DOI] [Google Scholar]
- Randhir R, Lin YT, Shetty K. Stimulation of phenolics, antioxidant and antimicrobial activities in dark germinated mung bean sprouts in response to peptide and phytochemical elicitors. Process Biochem. 2004;39:637–646. doi: 10.1016/S0032-9592(03)00197-3. [DOI] [PubMed] [Google Scholar]
- Randhir R, Kwon YI, Shetty K. Improved health-relevant functionality in dark germinated Mucuna pruriens sprouts by elicitation with peptide and phytochemical elicitors. Bioresource Technol. 2009;100:4507–4514. doi: 10.1016/j.biortech.2009.01.078. [DOI] [PubMed] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Bio. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Rice-Evans C, Miller N, Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–159. doi: 10.1016/S1360-1385(97)01018-2. [DOI] [Google Scholar]
- Sarkar D, Shetty K. Metabolic stimulation of plant phenolics for food preservation and health. Annual Rev. Food Sci. T. 2014;5:395–413. doi: 10.1146/annurev-food-030713-092418. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Bhowmik PC, In-Kwon Y, Shetty K. Cold acclimation responses of three cool-season turfgrasses and the role of proline-associated pentose phosphate pathway. J. Am. Soc. Hortic. Sci. 2009;134:210–220. doi: 10.21273/JASHS.134.2.210. [DOI] [Google Scholar]
- Sarkar D, Bhowmik PC, In-Kwon Y, Shetty K. The role of proline-associated pentose phosphate pathway in cool-season turfgrasses after UV-B exposure. Environ. Exp. Bot. 2011;70:251–258. doi: 10.1016/j.envexpbot.2010.09.018. [DOI] [Google Scholar]
- Shetty K. Biotechnology to harness the benefits of dietary phenolics: focus on Lamiaceae. Asia Pac. J. Clin. Nutr. 1997;6:162–171. [PubMed] [Google Scholar]
- Shetty K. Role of proline-linked pentose phosphate pathway in biosynthesis of plant phenolics for functional food and environmental applications: a review. Process Biochem. 2004;39:789–804. doi: 10.1016/S0032-9592(03)00088-8. [DOI] [Google Scholar]
- Shetty K, McCue P. Phenolic antioxidant biosynthesis in plants for functional food application: integration of systems biology and biotechnological approaches. Food Biotechnol. 2003;17:67–97. doi: 10.1081/FBT-120023073. [DOI] [Google Scholar]
- Shetty K, Wahlqvist M. A model for the role of the proline-linked pentose-phosphate pathway in phenolic phytochemical bio-synthesis and mechanism of action for human health and environmental applications. Asia Pac. J. Clin. Nutr. 2004;13:1–24. [PubMed] [Google Scholar]
- Shetty K, Curtis OF, Levin RE, Witkowsky R, Ang W. Prevention of vitrification associated with in vitro shoot culture of oregano (Origanum vulgare) by Pseudomonas spp. J. Plant Physiol. 1995;147:447–451. doi: 10.1016/S0176-1617(11)82181-4. [DOI] [Google Scholar]
- Singh A, Sharma S. Bioactive components and functional properties of biologically activated cereal grains: a bibliographic review. Crit. Rev. Food Sci. 2017;57:3051–3071. doi: 10.1080/10408398.2015.1085828. [DOI] [PubMed] [Google Scholar]