Abstract
The root bark of Lycium chinense (LC) has been reported to have potent antioxidant and antidiabetic properties. In the present study, we investigated the attenuative effect of LC against diabetes induced sexual dysfunction and testicular damages in animal models. Diabetic animals were treated with LC (100 and 400 mg/kg) once daily for 6 weeks. At the end of the treatment, mating behavior tests were performed and the animals were sacrificed for the determination of hormonal profile, oxidative stress indices and sperm analysis. LC administration significantly decreased blood glucose level, enhancement of the antioxidant enzyme activities, restored altered sperm characteristics and markedly improved levels of luteinizing hormone, follicle-stimulating hormone and testosterone as compared to the untreated diabetic animals. Furthermore, LC also improved sperm count, viability, motility, increased the reproductive organs weight. The results obtained indicated that L. chinense has beneficial effects in diabetes sexual dysfunction.
Keywords: Lycium chinensis, Oxidative stress, Spermatogenic dysfunction, Antioxidant, Diabetes mellitus
Introduction
Diabetes mellitus is a lifelong metabolic disease which bears a hallmark of hyperglycemia due to deficiency in the action of insulin, insulin secretion or both. Reproductive dysfunction is one of the several diabetic complications affecting about 90% of diabetic patients (Bener et al., 2009; Isidro, 2012). Some of the reproductive dysfunction experienced in diabetic situation includes testicular dysfunction, decreased fertility, reduced spermatogenesis, production of dysfunctional sperm and retrograde ejaculations (Mohamed et al., 2018). Oxidative stress, increased free radicals generation and impaired antioxidant defense mechanisms has been implicated in the pathogenesis of reproductive dysfunction in diabetes mellitus (Shi et al., 2017; Shrilatha and Muralidhara, 2007). Therefore, substances with antioxidant effect may be a possible remedy in mitigating the adverse effect of diabetes and its associated sexual dysfunction (Thakur et al., 2009).
Lycium chinense (family Solanaceae) popularly known as Chinese boxthorn is famous for its application as a functional food and medicine in many Asian countries especially China. The root bark of L. chinense (popularly referred to as Cortex Lycii) has a long history of treating several diseases such as diabetes, cough and inflammatory disorders (Yao et al., 2011). Several bioactive constituents are richly found in the root bark of L. chinense, flavonoids, phenolics, cyclopeptides, and phenolic glycosides (Chen et al., 2017). Previous findings have attributed actions such as neuroprotection, antioxidant, anti-inflammatory, antiulcer and anti-obesity activities to L. chinense (Chen et al., 2016; Olatunji et al., 2016). The antidiabetic action of L. chinense have been previously reported in pancreatic beta cells and obese-diabetic rats (Wang and Ye, 2016; Ye et al., 2008). On the basis of these previous findings, we envisaged that L. chinense might have the potentials of improving diabetic induced sexual dysfunction. Thus, this study investigated the protective effects of the root bark of L. chinense (LC) on male sexual dysfunction and testicular oxidative damage in streptozotocin-induced diabetic rats.
Materials and methods
Plant collection
Lycium chinense was collected at Dongtai, Jiangsu Province, China and was identified at the School of Pharmacy Jiangsu University, China where the reference specimen was deposited.
Preparation of plant material
The root bark of L. chinense was refluxed with 95% ethanol for 4 h and the extract was concentrated with a rotary evaporator. The ethanolic extract was preserved at 4 °C until further use.
Animals
Wistar rats (150–220 g) were housed in cages and kept under suitable controlled environment (temperature; 23 ± 2 °C; relative humidity; 40–55%). The rats were acclimatized for 7 days and were allowed access to food and water ad libitum. The protocols for the animal experiment were in line with the guidelines for the care and use of laboratory animals of the National Institutes of Health. Ethical approval was obtained from the Ethics Committee on the Use of Animals (UJS-LAER-2017112101).
Induction of diabetes
Overnight fasted rats were intraperitoneally administered with a single injection of freshly prepared streptozotocin (in sodium citrate buffer; pH 4.5, 65 mg/kg). Seventy hours after streptozotocin injection, fasting blood glucose level of the rats was measured using a glucometer. Rats having blood sugar level ≥ 250 mg/dL were adjudged as diabetic and were used for further studies.
Experimental design
The diabetic animals were divided into five groups of six rats per group; Group I is the normal control group treated with distilled water; Group II is the diabetic treated with distilled water, Groups III and IV were treated with LC (100 and 400 mg/kg, respectively); Group V was the positive control group and treated with Sildenafil citrate (5 mg/kg), an approved oral therapy for the treatment of erectile dysfunction. The extract and drug were administered by oral gavage for 6 weeks.
Copulatory behavior test
The mating behavior test behavioral test adopted the previous method of Suresh and Prakash (2012). Female rats were ovariectomised and made sexual receptivity by administering 10 µg/0.1 mL of 3-benzoyloxy-17β-estrol (subcutaneously) 48 h before testing and 500 µg progesterone in 0.1 mL sesame oil 4 h prior to the sexual behavioral test (Nusier et al., 2007). Normal and diabetic male rats that have been treated were kept in cages under dim red light and each male rat was allowed 10 min of adaptation period before pairing the sexually receptive female rats with the male rats in the cages on a one to one basis. The sexual training activity was terminated if any of the male or female rats failed to initiate sexual activity. After the placement of the female rats, the following male mating behaviors were evaluated: Intromission latency (IL): is the time of the introduction of the female until the first intromission by the male, Post- ejaculatory interval (PEI): is the time interval between first ejaculation until the next intromission; Mount latency (ML): is the time interval of the introduction of the female until the first mount by the male and Ejaculation latency (EL): is the of the first intromission until ejaculation. The test was performed on day 42 after administration of LC and the positive control drug.
Blood, semen and tissue sampling
After the treatment and experimentation period, the male rats were anaesthetized and sacrificed by cervical dislocation. The blood samples, testes and epididymis of the rats were collected for biochemical estimation. The serum obtained from the blood sample was used for the determination of levels of male reproductive hormones (follicle-stimulating hormone (FSH), luteinizing hormone (LH) and testosterone) with the aid of a commercially available ELISA kit (Wuhan Fine Biological Technology, Wuhan, Hubei, China and ERBA Fertikit, Germany). The testes of each rat were immediately excised and weighed and homogenized in ice cold saline, centrifuged at 2500g for 15 min which was used for the assessment of markers of oxidative stress namely reactive oxygen species (ROS), malondialdehyde (MDA), superoxide dismutase (SOD), glutathione (GSH) and catalase (CAT) using ELISA kit based on the instruction of the manufacturers manual (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Sperm analysis
Sperm analysis was determined based on previously described method (Suresh and Prakash, 2012). The percentage of sperm motility was evaluated according to the method of Sonmez et al. (2005), while the percentage of sperm viability was evaluated by using eosin-nigrosin stain based on previous method (Kose et al., 2012).
Statistical data analysis
Values were expressed as mean ± SD (n = 6). One way Analysis of Variance (ANOVA) followed by Tukey Post hoc multiple comparison test was used for statistical analysis. Statistical significant difference was considered at p < 0.05. Analyses was accomplished using SPSS statistical software (version 16.0, SPSS Inc. Chicago, Illinois, USA).
Results and discussion
L. chinense decreases fasting blood glucose levels in diabetic rats
The intraperitoneal administration of streptozotocin led to a state of hyperglycemia, which is a distinct feature of diabetes mellitus. Streptozotocin induces the generation of ROS resulting in insulin-dependent diabetes due to the selective destruction of beta cells (Lenzen, 2008). It was observed that after 6 weeks of administering L. chinense extract, the blood sugar levels was markedly decreased in comparison with the non-treated diabetic group (Table 1). The untreated diabetic rats suffered from consistent alteration in blood glucose level, which increased over time during the course of the study. The blood sugar level in the non-treated diabetic rats was approximately three fold higher than the normal control rats. The administration of L. chinense led to a significant reduction in the blood glucose levels of the treated diabetic rat group in a concentration dependent manner (Table 1).
Table 1.
Effect of LC on FBG, body weight and testis weight in diabetic rats
| Group (mg/kg) | Fasting blood glucose (mg/dL) | Final body weight (g) | Testis weight (g) |
|---|---|---|---|
| NC | 115.50 ± 2.65 | 305.76 ± 11.07 | 1.57 ± 0.21 |
| DC | 429.10 ± 10.09# | 158.46 ± 7.20# | 0.93 ± 0.08# |
| LC 100 | 308.25 ± 6.41* | 197.03 ± 8.44* | 1.17 ± 0.14* |
| LC 400 | 189.70 ± 5.11* | 258.60 ± 11.18* | 1.37 ± 0.26* |
| SC5 | 205.25 ± 6.90* | 233.94 ± 12.00* | 1.19 ± 0.19* |
Values are expressed as mean ± SD (n = 6)
NC normal control group, DC diabetic control, LC 100 Lycium chinense 100 mg/kg, LC 400 Lycium chinense 400 mg/kg, SC 5 Sildenafil citrate 5 mg/kg
#p < 0.05 as compared to normal control, *p < 0.05 when compared to diabetic control
L. chinense increases body and testes weights in treated diabetic rats
The decline in the body weight is one of the obvious signs of poor glycemic control experienced in diabetes mellitus. The non-treated diabetic animals had significantly observable mean body weight reduction as compared to the non-diabetic control rats (Table 1). The mean final body weight of the control rats was 305.76 ± 11.07 g. Diabetic rats had mean final body weight of 158.46 ± 7.20 g. In contrast, the administration of L. chinense significantly increased the body weight of the treated diabetic rats when compared to the non-treated diabetic control rats. Furthermore, the results displayed in Table 1 indicated that the diabetic rats had a significant reduction in the testes weight of as compared to the normal control. After the administration of L. chinense, the reduced weight of testes in the treated diabetic rats was markedly increased in comparison with the untreated diabetic rat group.
L. chinense upregulated the antioxidant defense system in testis of diabetic rats
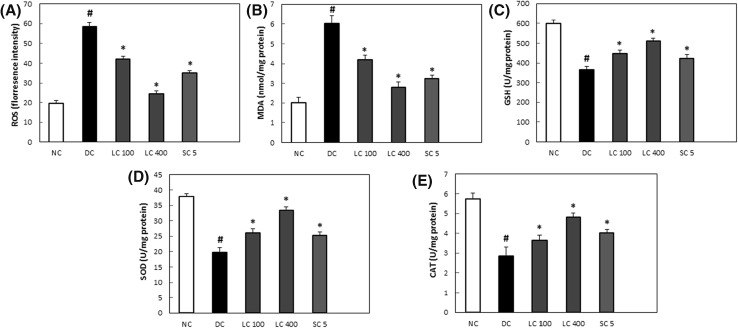
Accumulating evidences have continuously implicated oxidative stress as a critical parameter responsible for the pathophysiology of diabetic complication including spermatogenic dysfunction (Khaki et al., 2010; Kanter et al., 2013; Walczak-Jedrzejowska et al., 2013). The over production of ROS as well as the depletion of the antioxidants enzymes in the testicular tissues leads to a cascade of cytotoxic process which damages the proteins, unsaturated fatty acids and the nucleic acids of the spermatozoa plasma membrane (Khaki et al., 2010; Shi et al., 2017). Endogenous antioxidant enzymes are vital in maintaining proper antioxidant defense in the testicular tissues by protecting against ROS and oxidative stress (Fujii et al., 2003). Oxidative damage in the diabetic induced rats was evaluated by the determination of ROS, MDA and antioxidant enzymes SOD, GSH and CAT. MDA levels is increased in several tissues and endogenous antioxidant enzymes levels are diminished in diabetic conditions (Oladipo et al., 2018; Zhao et al., 2017). In addition, a number of prior studies confirmed the negative effect of oxidative stress in the testis of diabetic rats by inducing apoptotic cell death of the testicular tissue (Baynes and Thorpe, 1999; Cai et al., 2000). The levels of oxidative stress markers namely MDA, ROS and the antioxidant enzymes GSH, CAT and SOD activities in the testes of non-treated diabetic animals are depicted in Fig. 1. The antioxidant enzyme levels in the testis of non-treated diabetic animals was observed to be significantly downregulated in comparison to the normal non diabetic rats, while the MDA and ROS levels showed an opposite pattern as revealed by their significantly higher levels as compared with the non-diabetic control rats. L. chinense significantly reduced the elevated levels of MDA and ROS levels in the testis of treated diabetic rats. In addition, the reduced levels of CAT, GSH and SOD in the testis of diabetic rats were significantly upregulated in a concentration dependent fashion after the administration of L. chinense. This study indicated that administering L. chinense extract to the diabetic rats attenuated testicular tissue damage by preventing against ROS generation and upregulating the endogenous antioxidant diabetic treated rats.
Fig. 1.
Effect of L. chinense on oxidative stress parameters (A) ROS, (B) MDA, (C) GSH, (D) SOD, (E) CAT activities in streptozotocin induced diabetic rats. Values are expressed as mean ± SD (n = 6). *p < 0.05 compared with the diabetic control group; #p < 0.05 compared with the normal control group. NC normal control group, DC diabetic control, LC 100 Lycium chinense 100 mg/kg, LC 400 Lycium chinense 400 mg/kg, SC 5 Sildenafil citrate 5 mg/kg
L. chinense increases hormone levels in the serum of diabetic rats
In diabetes, the levels of testosterone is reduced due to the reduction in insulin secretion which impedes the production of FSH and LH. These hormones are responsible for the stimulation of the production of androgens such as testosterone. Testosterone regulates spermatogenesis, the activities of sertoli cell, testes weight and the male phenotype (Dkhil et al., 2016; Pitteloud et al., 2005). The non-treated diabetic rats had significantly reduced levels of serum hormonal profiles (testosterone, FSH and LH) as compared to the non-diabetic normal control rats. L. chinense at the treatment doses induced a marked elevation of serum testosterone, FSH and LH levels when juxtaposed with the non-treated diabetic rats. These increase was observed to correspond to the administered concentration (Table 2).
Table 2.
Effect of LC on hormonal profile in diabetic rats
| Group (mg/kg) | Testosterone (ng/mL) | FSH (ng/mL) | LH (ng/mL) |
|---|---|---|---|
| NC | 4.08 ± 0.35 | 6.08 ± 0.42 | 7.08 ± 0.89 |
| DC | 1.84 ± 0.15# | 3.49 ± 0.51# | 5.37 ± 1.05# |
| LC 100 | 2.74 ± 0.29* | 4.05 ± 0.47* | 5.93 ± 1.07* |
| LC 400 | 3.59 ± 0.42* | 4.88 ± 0.38* | 6.44 ± 1.17* |
| SC 5 | 2.19 ± 0.36 | 3.61 ± 0.22 | 5. 52 ± 0.38 |
Values are expressed as mean ± SD (n = 6)
LH luteinizing hormone, FSH follicle stimulating hormone, NC normal control group, DC diabetic control, LC 100 Lycium chinense 100 mg/kg, LC 400 Lycium chinense 400 mg/kg, SC 5 Sildenafil citrate 5 mg/kg
#p < 0.05 as compared to normal control, *p < 0.05 when compared to diabetic control
L. chinense increases sperm parameters in diabetic rats
Spermatogenesis is a vital function of the testes and previous studies have indicated that hyperglycemia impairs the quality, shape, form and structure of the sperm (Agarwal et al., 2014; Khaki et al., 2010). Furthermore, the thickness of the basal lamina in somniferous tubules is increased during diabetes leading to reduction in the production of sperm (Rohrbach and Martin, 1982). As shown in Table 3, sperm motility, sperm count and sperm viability were significantly reduced in non-treated diabetic rat group indicating impaired spermatogenesis. However, diabetic rat group administered with L. chinense exhibited significant upregulation and reversed the decline in the sperm parameters in the treated diabetic rats (Table 3).
Table 3.
Effect of LC on sperm parameters in diabetic rats
| Group (mg/kg) | Sperm count (millions/mL) | Sperm viability (%) | Sperm motility (%) |
|---|---|---|---|
| NC | 121.40 ± 7.01 | 67.05 ± 3.11 | 45.38 ± 3.09 |
| DC | 49.82 ± 5.27# | 34.90 ± 1.82# | 17.41 ± 2.33# |
| LC 100 | 60.48 ± 4.92* | 42.77 ± 2.02* | 24.17 ± 0.14* |
| LC 400 | 89.33 ± 6.08* | 54.28 ± 2.80* | 35.37 ± 4.42* |
| SC 5 | 51.00 ± 3.07 | 39.16 ± 1.99 | 20.10 ± 5.04 |
Values are expressed as mean ± SD (n = 6)
NC normal control group, DC diabetic control, LC 100 Lycium chinense 100 mg/kg, LC 400 Lycium chinense 400 mg/kg, SC 5 Sildenafil citrate 5 mg/kg
#p < 0.05 as compared to normal control, *p < 0.05 when compared to diabetic control
L. chinense improves male sexual behaviour in diabetic rats
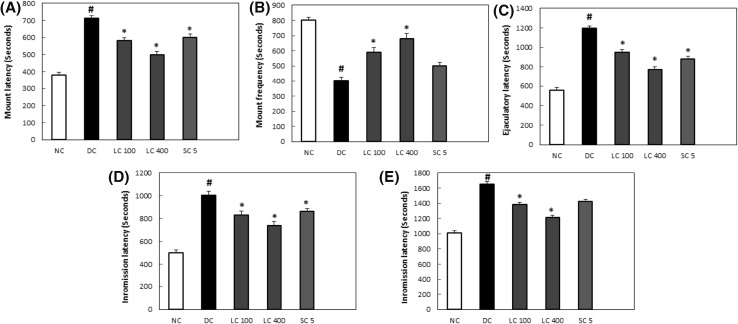
Alterations in male sexual parameters such as ML, EL, PEI, IL and MF in diabetic rats suggest an impairment in sexual performance (De et al., 2016). Sexual motivation is evaluated by a decrease in mount latency and intromission latency (Yakubu et al., 2005). The diabetic rat group that were treated with L. chinense had increased male sexual activities which was significant when compared to the non-treated diabetic rats. These was evident in the improvement in MF and a decline in EL, PEI, ML and IL in the groups of diabetic rats administered with L. chinense. As for the diabetic rats, decrease in sexual behavior was observed by noticeable decline in MF and increase in EL, PEI, IL and ML (Fig. 2).
Fig. 2.
Effect of L. chinense on male sexual behaviour in diabetic rats (A) mount latency, (B) mount frequency, (C) ejaculatory latency, (D) intromission latency, (E) post ejaculatory interval in streptozotocin induced diabetic rats. Values are expressed as mean ± SD (n = 6). *p < 0.05 compared with the diabetic control group; #p < 0.05 compared with the normal control group. NC normal control group, DC diabetic control, LC 100 Lycium chinense 100 mg/kg, LC 400 Lycium chinense 400 mg/kg, SC 5 Sildenafil citrate 5 mg/kg
Diabetes mellitus is rapidly becoming one of the foremost severe metabolic disorder. The increasing number of secondary complications such as neuropathy, nephropathy, retinopathy, cardiovascular diseases, limb amputation and sexual dysfunction that accompanies diabetes is mainly responsible for the impairment of numerous organs and functions of the organism leading to morbidity and mortality (El Rabey et al., 2017). Diabetes induced reproductive disorder is one of serious secondary complication of diabetes which adversely affects the quality of life of patients. Medicinal plant have been in existence for thousands of years as a mean of preventing and treating several chronic disorders including diabetes. The root back of L. chinense is a famous traditional Chinese medicine (TCM) with several health enhancing properties. It has a long history of been used in TCM as an antidiabetic agent.
In conclusion, this present study demonstrated that L. chinense attenuated impaired male sexual dysfunction in diabetic rats by significantly increasing testes weight, spermatogenic quality and quantity and upregulating the antioxidant enzyme status by way of decreasing oxidative stress. These findings suggest the possible use of L. chinense in the enhancement or protection against diabetic induced sexual dysfunction.
Acknowledgements
Thanks to the Jiangsu Provincial Platform for Conservation and Utilization of Agricultural Germplasm (No. BM 2014047) and the Public Science and Technology Research Funds Projects of Ocean (No. 201505023) for their grants.
Compliance with ethical standards
Conflict of interest
All authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yifeng Zhou, Email: njgzhou@163.com.
Opeyemi Joshua Olatunji, Email: Opeyemi.j@psu.ac.th.
Hongxia Chen, Email: chhx2001@ujs.edu.cn.
References
- Agarwal A, Virk G, Ong C, Du PS. Effect of oxidative stress on male reproduction. World J. Mens Health. 2014;32:1–17. doi: 10.5534/wjmh.2014.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- Bener A, Alansari AA, Zirie M, Alhamaq AO. Is male fertility associated with type 2 diabetes mellitus? Int. Urol. Nephrol. 2009;41:777–784. doi: 10.1007/s11255-009-9565-6. [DOI] [PubMed] [Google Scholar]
- Cai L, Chen S, Evans T, Deng DX, Mukherjee K, Chakrabarti S. Apoptotic germ-cell death and testicular damage in experimental diabetes: prevention by endothelin antagonism. Urol. Res. 2000;28:342–347. doi: 10.1007/s002400000134. [DOI] [PubMed] [Google Scholar]
- Chen H, Li YJ, Sun YJ, Gong JH, Du K, Zhang YL, Su CF, Han QQ, Zheng XK, Feng WS. Lignanamides with potent antihyperlipidemic activities from the root bark of Lycium chinense. Fitoterapia. 2017;122:119–125. doi: 10.1016/j.fitote.2017.09.004. [DOI] [PubMed] [Google Scholar]
- Chen H, Olatunji OJ, Zhou Y. Anti-oxidative, anti-secretory and anti-inflammatory activities of the extract from the root bark of Lycium chinense (Cortex Lycii) against gastric ulcer in mice. J. Nat. Med. 2016;70:610–619. doi: 10.1007/s11418-016-0984-2. [DOI] [PubMed] [Google Scholar]
- Dkhil MA, Zrieq R, Al-Quraishy S, Abdel Moneim AE. Selenium nanoparticles attenuate oxidative stress and testicular damage in streptozotocin-induced diabetic rats. Molecules. 2016;21:1517. doi: 10.3390/molecules21111517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De A, Singh MF, Singh V, Ram V, Bisht S. Treatment effect of l-norvaline on the sexual performance of male rats with streptozotocin induced diabetes. Eur J Pharmacol. 2016;771:247–254. doi: 10.1016/j.ejphar.2015.12.008. [DOI] [PubMed] [Google Scholar]
- El Rabey HA, Al-Seeni MN, Bakhashwain AS. The antidiabetic activity of Nigella sativa and propolis on streptozotocin-induced diabetes and diabetic nephropathy in male rats. Evid. Based Complement. Alternat. Med. 2017;2017:5439645. doi: 10.1155/2017/5439645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii J, Iuchi Y, Matsuki S, Ishii T. Cooperative function of antioxidant and redox systems against oxidative stress in male reproductive tissues. Asian J. Androl. 2003;5:231–242. [PubMed] [Google Scholar]
- Isidro ML. Sexual dysfunction in men with type 2 diabetes. Postgrad. Med. J. 2012;88:152–159. doi: 10.1136/postgradmedj-2011-130069. [DOI] [PubMed] [Google Scholar]
- Kanter M, Aktas C, Erboga M. Curcumin attenuates testicular damage, apoptotic germ cell death, and oxidative stress in streptozotocin-induced diabetic rats. Mol Nutr Food Res. 2013;57:1578–1585. doi: 10.1002/mnfr.201200170. [DOI] [PubMed] [Google Scholar]
- Khaki A, Fathiazad F, Nouri M, Khaki A, Maleki NA, Khamnei HJ, Ahmadi P. Beneficial effects of quercetin on sperm parameters in streptozotocin-induced diabetic male rats. Phytother Res. 2010;24:1285–1291. doi: 10.1002/ptr.3100. [DOI] [PubMed] [Google Scholar]
- Kose E, Sarsılmaz M, Tas U¸ Kavakl A, Turk G, Ozlem Dabak D, Sapmaz H, Ogeturk M. Rose oil inhalation protects against formaldehyde-induced testicular damage in rats. Andrologia. 44: 342–348 (2012) [DOI] [PubMed]
- Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51:216–226. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- Mohamed NA, Ahmed OM, Hozayen WG, Ahmed MA. Ameliorative effects of bee pollen and date palm pollen on the glycemic state and male sexual dysfunctions in streptozotocin-Induced diabetic wistar rats. Biomed. Pharmacother. 2018;97:9–18. doi: 10.1016/j.biopha.2017.10.117. [DOI] [PubMed] [Google Scholar]
- Nusier MK, Bataineh HN, Daradkah HM. Adverse effects of rosemary (Rosmarinus officinalis L.) on reproductive function in adult male rats, Exp. Biol. Med. 232: 809–813 (2007) [PubMed]
- Oladipo GO, Nlekerem CM, Ibukun EO, Kolawole AO. Quail (Coturnix japonica) egg yolk bioactive components attenuate streptozotocin-induced testicular damage and oxidative stress in diabetic rats. Eur. J. Nutr. 2018;57:2857–2867. doi: 10.1007/s00394-017-1554-4. [DOI] [PubMed] [Google Scholar]
- Olatunji OJ, Chen H, Zhou Y. Lycium chinensis Mill attenuates glutamate induced oxidative toxicity in PC12 cells by increasing antioxidant defense enzymes and down regulating ROS and Ca 2+ generation. Neurosci. Lett. 2016;616:111–118. doi: 10.1016/j.neulet.2015.10.070. [DOI] [PubMed] [Google Scholar]
- Pitteloud N, Hardin M, Dwyer AA, Valassi E, Yialamas M, Elahi D, Hayes FJ. Increasing insulin resistance is associated with a decrease in leydig cell testosterone secretion in men. J. Clin. Endocrinol. Metab. 2005;90:2636–2641. doi: 10.1210/jc.2004-2190. [DOI] [PubMed] [Google Scholar]
- Rohrbach DH, Martin GR. Structure of basement membrane in normal and diabetic tissue. Ann. N Y Acad. Sci. 1982;401:2203–2211. doi: 10.1111/j.1749-6632.1982.tb25719.x. [DOI] [PubMed] [Google Scholar]
- Shi GJ, Zheng J, Wu J, Qiao HQ, Chang Q, Niu Y, Sun T, Li YX, Yu JQ. Beneficial effects of Lycium barbarum polysaccharide on spermatogenesis by improving antioxidant activity and inhibiting apoptosis in streptozotocin-induced diabetic male mice. Food Funct. 2017;8:1215–1226. doi: 10.1039/C6FO01575A. [DOI] [PubMed] [Google Scholar]
- Shrilatha B, Muralidhara. Occurrence of oxidative impairments, response of antioxidant defenses and associated biochemical perturbations in male reproductive milieu in the Streptozotocin-diabetic rat. Int. J. Androl. 30: 508–518 (2007) [DOI] [PubMed]
- Sonmez M, Turk G, Yuce A. The effect of ascorbic acid supplementation on sperm quality: lipid peroxidation and testosterone levels in male Wistar rats. Theriogenology. 2005;63:2063–2072. doi: 10.1016/j.theriogenology.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Suresh S, Prakash S. Effect of Mucuna pruriens(Linn.) on sexual behavior and sperm parameters in streptozotocin‐induced diabetic male rat. J. Sex Med. 2012;9:3066–3078. doi: 10.1111/j.1743-6109.2010.01831.x. [DOI] [PubMed] [Google Scholar]
- Thakur M, Bhargava S, Dixit VK. Effect of Asparagus racemosus on sexual dysfunction in hyperglycemic male rats. Pharm. Biol. 2009;47:390–395. doi: 10.1080/13880200902755234. [DOI] [PubMed] [Google Scholar]
- Walczak-Jedrzejowska R, Wolski JK, Slowikowska-Hilczer J. The role of oxidative stress and antioxidants in male fertility. Cent European J Urol. 2013;66:60–67. doi: 10.5173/ceju.2013.01.art19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Ye Z. Cortex lycii radicis extracts protect pancreatic beta cells under high glucose conditions. Curr Mol Med. 2016;16:591–595. doi: 10.2174/1566524016666160523143757. [DOI] [PubMed] [Google Scholar]
- Yao X, Peng Y, Xu LJ, Li L, Wu QL, Xiao PG. Phytochemical and biological studies of Lycium medicinal plants. Chem. Biodivers. 2011;8:976–1010. doi: 10.1002/cbdv.201000018. [DOI] [PubMed] [Google Scholar]
- Yakubu MT, Akanji MA, Oladiji AT. Aphrodisiac potentials of the aqueous extract of Fadogia agrestis (Schweinf. Ex Hiern) stem in male albino rats. Asian J Androl. 7: 399–404 (2005) [DOI] [PubMed]
- Ye Z, Huang Q, Ni HX, Wang D. Cortex Lycii Radicis extracts improve insulin resistance and lipid metabolism in obese-diabetic rats. Phytother Res. 2008;22:1665–1670. doi: 10.1002/ptr.2552. [DOI] [PubMed] [Google Scholar]
- Zhao L, Gu Q, Xiang L, Dong X, Li H, Ni J, Wan L, Cai G, Chen G. Curcumin inhibits apoptosis by modulating Bax/Bcl-2 expression and alleviates oxidative stress in testes of streptozotocin-induced diabetic rats. Ther Clin Risk Manag. 2017;13:1099–1105. doi: 10.2147/TCRM.S141738. [DOI] [PMC free article] [PubMed] [Google Scholar]