Abstract
Leptospirosis, an infectious zoonosis, is common to tropical areas. The clinical presentation varies from flu-like symptoms to a serious presentation called Weil’s syndrome. Fever and conjunctival suffusion are present in the majority of patients. This case report describes a resident of New York City who presented initially with gastroenteritis symptoms without fever or conjunctival suffusion to develop septic shock before being diagnosed with leptospirosis.
Keywords: Urban leptospirosis, Unusual presentation, Weil’s syndrome, Sepsis
Introduction
Leptospirosis is an infectious zoonosis caused by the pathogenic spirochetes of the genus Leptospira (L. interrogans). Although it has a higher incidence in tropical countries, the disease had several outbreaks globally [1]. Transmission to humans can happen directly through inoculation from tissues, body fluids, and urine of infected animals, or indirectly through entry via mucosal surfaces or breached skin [2]. The symptoms are abrupt in onset, and typically occur after an incubation period of five days to two weeks [3]. The clinical spectrum of the disease can range from an influenza-like fever to a serious presentation called Weil's syndrome, which involves an end-organ failure of the liver, kidneys, brain, and bone marrow [4].
Herein, we present the case of a 59-year-old female who presented for gastroenteritis symptoms with no fever or conjunctival suffusion then suffered from septic shock as a result of urban leptospirosis.
Case presentation
A 59-year-old Hispanic female resident of New York City (NYC) with past medical history of schizophrenia, hepatitis C, syphilis, and intravenous (IV) drug abuse on methadone program presented to the emergency department for a 10-day history of nausea, vomiting, and loose stool. The patient had multiple episodes of non-bloody and non-bilious vomiting with decreased oral intake. The diarrhea occurred several times per day being described as loose with no signs of blood or melena. The patient also reported having diffuse epigastric pain. She denied any fever, chills, or dysuria.
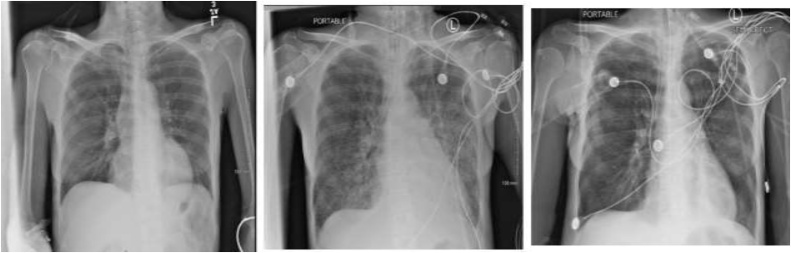
Upon admission, the patient's vital signs were unremarkable. On physical exam, she had a mild epigastric tenderness with no signs of rigidity. A dry oral mucous membrane was evident. Eyes were anicteric with no signs of jaundice or conjunctival suffusion. Clear breath sounds with regular heart rate and rhythm were present. Kernig's and Brudzinski's signs were absent. Laboratory studies demonstrated a white blood count of 12,600 cells/ mm3 with neutrophil predominance (90.5%), thrombocytopenia (48,000 cells/mm3), hyponatremia (131 mEq/L), and acute kidney injury (creatinine levels 1.35 mg/dL). Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were elevated (60 mEq/L and 46 mEq/L, respectively). There was mild hyperbilirubinemia (total 3.96 mEq/L, direct 3.3 mEq/L, hemolyzed sample). Color of urine was dark yellow. Urinalysis was negative for infection or bilirubin. Chest x-ray (CXR) upon presentation was completely normal (Fig. 1A).
Fig. 1.
A, 1B, and 1C (Left to right). Chest X-rays on the day of presentation (1A), 4 days post intubation (1B), and 10 days after starting the treatment (1C).
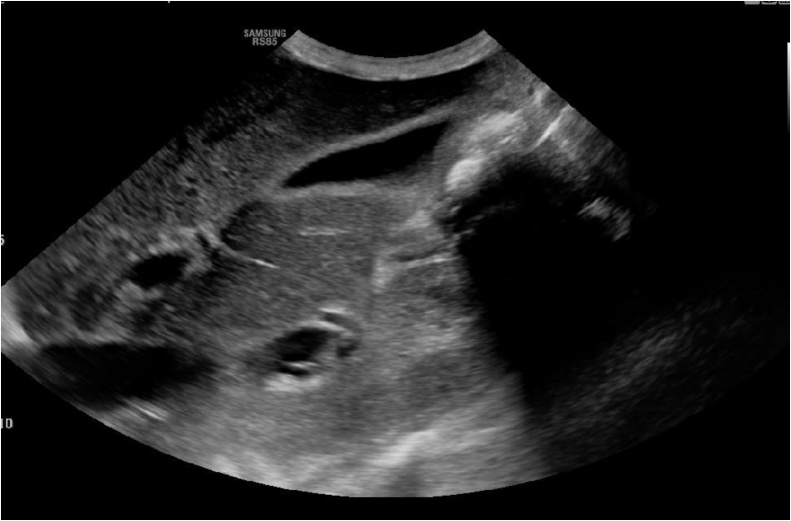
The patient was admitted to the medical floor due to inability to tolerate oral intake with a most likely diagnosis of gastroenteritis. On the second day after admission, the patient remained nauseated with continued inability to tolerate oral intake and worsening fatigue. Altered mental status with significant lethargy, tachycardia, tachypnea, and scleral icterus were noted on physical exam. Arterial blood gas testing on room air (ABG) showed hypoxemia with a PaO2 of 60 mmHg and no elevated lactate. Repeat laboratory studies showed worsening total and direct bilirubin (7.48 mEq/L, 4.6 mEq/L respectively) with worsening kidney function (Creatinine 3.04 mg/dL despite continuous IV hydration). Hepatobiliary ultrasound showed sludge in the gallbladder with normal common bile duct and no evidence of a focal obstructive biliary lesion (Fig. 2). Peripheral smear reading was not significant for any schistocytes or platelet clumps with well appearing red blood cells. The decision was taken to intubate the patient for impeding respiratory failure.
Fig. 2.
Hepatobiliary ultrasound showing mild thickening of the gallbladder wall with absent gall stones and no common bile dust dilatation.
CXR post intubation revealed bilateral non-cariodgenic pulmonary edema that was not present at the day of presentation (Fig. 1B). Bedside cardiac ultrasound revealed heart failure with an ejection fraction of 35–45%, with the apex and surrounding walls appearing severely hypokinetic to aneurysmal. The patient developed multiple episodes of ventricular tachycardia that was chemically cardioverted with amiodarone. Vasopressors including norepinephrine, phenylephrine, vasopressin, and dobutamine and empiric antibiotics including cefepime and doxycycline were started.
The differential diagnosis widened to include Leptospirosis, Salmonellosis, Ehrlichiosis, scrub typhus infection, and Hantavirus infection. Enzyme Linked Immunosorbent Assay (ELISA) showed positive IgM titers for Leptospira (88 IU/mL). Widal test was negative. IgM and IgG for Ehrlichia were negative. Weil–Felix test was negative. IgM and IgG for Hantavirus were negative. Upon questioning, the patient’s husband reported that the she used to eat food waste from the garbage, and that there were a lot of rats in her backyard and basement.
A 14-day course treatment with IV doxycycline for leptospirosis was started in addition to a 7-day course of IV ceftriaxone which was added as double coverage. The patient spent 3 weeks at the hospital. Her mental status improved. Platelet count normalized. Kidney function improved with a serum creatinine of 0.48 mg/dL 2 weeks after starting leptospirosis treatment while direct bilirubin dropped to a level of 1.4 mg/dL also 2 weeks after starting leptospirosis treatment. CXR showed significant improvement 10 days after starting the treatment (Fig. 1C).
Discussion
Leptospirosis has a common prevalence in tropical and subtropical regions. In the Unites States, most cases are reported in Hawaii. However, sporadic outbreaks in triathlons of Chicago and adventure race participants in Florida from exposure to common sources have been documented [5,6]. The incidence rate in urban populations is low. NYC reports only one to three cases every year. However, sporadic cases have occurred in people exposed to rat urine [7]. Three cases with exposure to a rodent infested environment were identified during 2017 confined to one block in the Bronx, New York area [8]. Our patient comes from urban NYC, and had a rat infestation of her house, which likely explains the disease transmission.
Leptospirosis can be mild or severe. The mild form is more common, and can be asymptomatic or present as flu-like illness. The illness generally presents with abrupt onset of fever, rigors, myalgia, and headache in 75%–100% of patients [9]. Marked conjunctival suffusion can present in about 50% of cases, and can be hallmark of the disease [10]. Antibodies usually appear 1 week after the onset of symptoms. The severe form has a similar onset to the mild form, but rapidly progresses. The classic presentation of the severe form is called Weil’s syndrome which is characterized by hemorrhage, hepatic, and renal impairment. Presence of jaundice coincides with poor prognosis [11]. The presence of icteric sclera, jaundice, tenderness over abdomen and costovertebral angles, acute kidney injury, and hepatic dysfunction in a febrile patient should raise suspicion for Weil’s syndrome. Our patient initially presented with no fever or conjunctival suffusion which delayed the diagnosis, and permitted disease progression.
In patients with leptospirosis, in case of presence of Systemic Inflammatory Response Syndrome (SIRS), clinical and laboratory findings can be mistaken for sepsis caused by other infectious factors [12]. Yilmaz et al. examined ICU patients and found that the clinical and laboratory findings of leptospirosis are similar to those of sepsis. They recommended to think about leptospirosis while examining a patient with SIRS/sepsis etiology in an area endemic for leptospirosis [13]. Our patient showed signs of sepsis within few days after presentation characterized by heart rate >90 beats/min, respiratory rate >20 breaths/min, white blood cell count >12,000 cells/mm3, and a valid source of infection.
In conclusion, it is important to focus on the social history in patients presenting for vague flu-like symptoms as these symptoms might further progress into a more complicated syndrome like Weil’s disease. Although leptospirosis presents typically with fever and conjunctival suffusion, unusual presentation might delay the diagnosis leading to complications such as septic shock.
CRediT authorship contribution statement
George El Hasbani: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing - original draft, Writing - review & editing. Sarmad Riaz Farooqui: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Validation, Visualization, Writing - original draft, Writing - review & editing. Ahmad Kofahi: Conceptualization, Funding acquisition, Resources, Software, Supervision, Validation, Visualization. Yasir Saeed: Funding acquisition, Investigation, Resources, Software, Supervision, Writing - review & editing. Omar Tayeh: Visualization, Writing - original draft, Writing - review & editing. Mohammad Abu-Hishmeh: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation. Herbeth Moran: Writing - review & editing. Marcelo Troya-Maldonado: Writing - review & editing. Rajan Khanna: Writing - review & editing. Jean-Pierre Assaker: Conceptualization, Data curation, Writing - original draft, Writing - review & editing. Richard Assaker: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing.
References
- 1.Chakurkar G., Vaideeswar P., Pandit S.P., Divate S.A. Cardiovascular lesions in leptospirosis: an autopsy study. J Infect. 2008;56(March (3)):197–203. doi: 10.1016/j.jinf.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Vijayachari P., Sugunan A.P., Shriram A.N. Leptospirosis: an emerging global public health problem. J Biosci. 2008;33(November (4)):557–569. doi: 10.1007/s12038-008-0074-z. [DOI] [PubMed] [Google Scholar]
- 3.Forbes A.E., Zochowski W.J., Dubrey S.W., Sivaprakasam V. Leptospirosis and Weil’s disease in the UK. QJM Mon J Assoc Physicians. 2012;105(December (12)):1151–1162. doi: 10.1093/qjmed/hcs145. [DOI] [PubMed] [Google Scholar]
- 4.Patil V.C., Patil H.V., Sakaria A., Tryambake S. An unusual case of Weil’s syndrome with paraparesis. Indian J Crit Care Med Peer-Rev Off Publ Indian Soc Crit Care Med. 2011;15(2):130–133. doi: 10.4103/0972-5229.83014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan J., Bornstein S.L., Karpati A.M., Bruce M., Bolin C.A., Austin C.C. Outbreak of leptospirosis among triathlon participants and community residents in Springfield, Illinois, 1998. Clin Infect Dis Off Publ Infect Dis Soc Am. 2002;34(June (12)):1593–1599. doi: 10.1086/340615. [DOI] [PubMed] [Google Scholar]
- 6.Stern E.J., Galloway R., Shadomy S.V., Wannemuehler K., Atrubin D., Blackmore C. Outbreak of leptospirosis among Adventure Race participants in Florida, 2005. Clin Infect Dis Off Publ Infect Dis Soc Am. 2010;50(March (6)):843–849. doi: 10.1086/650578. [DOI] [PubMed] [Google Scholar]
- 7.Vinetz J.M., Glass G.E., Flexner C.E., Mueller P., Kaslow D.C. Sporadic urban leptospirosis. Ann Intern Med. 1996;125(November (10)):794–798. doi: 10.7326/0003-4819-125-10-199611150-00002. [DOI] [PubMed] [Google Scholar]
- 8.Kupferman T., Coffee M.P., Eckhardt B.J. Case report: a cluster of three leptospirosis cases in a New York City Abattoir and an unusual complication in the index case. Am J Trop Med Hyg. 2017;97(December (6)):1679–1681. doi: 10.4269/ajtmh.17-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Ranke F.M., Zanetti G., Hochhegger B., Marchiori E. Infectious diseases causing diffuse alveolar hemorrhage in immunocompetent patients: a state-of-the-art review. Lung. 2013;191(February (1)):9–18. doi: 10.1007/s00408-012-9431-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vanasco N.B., Schmeling M.F., Lottersberger J., Costa F., Ko A.I., Tarabla H.D. Clinical characteristics and risk factors of human leptospirosis in Argentina (1999-2005) Acta Trop. 2008;107(September (3)):255–258. doi: 10.1016/j.actatropica.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Pothuri P., Ahuja K., Kumar V., Lal S., Tumarinson T., Mahmood K. Leptospirosis presenting with rapidly progressing acute renal failure and conjugated hyperbilirubinemia: a case report. Am J Case Rep. 2016;10(August (17)):567–569. doi: 10.12659/AJCR.897741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rhodes A., Evans L.E., Alhazzani W., Levy M.M., Antonelli M., Ferrer R. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(March (3)):304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 13.Yilmaz H., Turhan V., Yasar K.K., Hatipoglu M., Sunbul M., Leblebicioglu H. Characteristics of leptospirosis with systemic inflammatory response syndrome: a multicenter study. Ann Clin Microbiol Antimicrob [Internet] 2015;(December (21)) doi: 10.1186/s12941-015-0117-x. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4687284/ [cited 2019 May 17];14. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]