Highlights
-
•
FT-IR is non-invasive technique used to detect salt induced changes in Sesuvium portulacastrum.
-
•
Changes in Lipids, carbohydrates, proteins and cell wall components were detected and confirmed by some biochemical assays.
-
•
Root and Shoot showed differential responses to salt stress.
-
•
Principle component analysis confirmed differential response of root and shoot.
Keywords: Fourier Transform Infrared Spectroscopy, Sesuvium portulacastrum, Salt stress, Metabolic changes
Abstract
In a halophyte, Sesuvium portulacastrum (L.) L., we have applied Fourier Transform InfraRed (FT-IR) spectroscopy to detect the corresponding changes associated with salt-induced physiological changes under long- term treatment with 0, 100 and 500 mM NaCl. FT-IR profiles showed changes in chemical composition and functional groups of proteins, lipids and carbohydrates due to salt treatments, evident as differential FT-IR profiles in both roots and leaves specific to these metabolites. Further, the Principle Component Analysis (PCA) was applied to identify the main sources of variation in FT-IR data due to differential treatment. In PCA, the PC1 showed 85.55% and PC2 showed 18.18% variability in data and confirmed differential response of root and leaves to salt treatment in Sesuvium. The results suggest that FT-IR spectrometry can be used to study stress-induced metabolic changes in plants in relation to their salt tolerance.
1. Introduction
Sesuvium portulacastrum (L.) L., a facultative halophyte belonging to family Aizoaceae, has a remarkable ability to thrive under high salinity, drought, metal and other abiotic stresses [1]. Apart from stress tolerance, it is a suitable species for phytoremediation, desalination, sand dune fixation and production of industrially important compounds (Reviewed in [1,2]). Physiological and biochemical studies under salt stress have shown the role of osmolytes accumulation, antioxidant defense and V-ATPase, H+ ATPase and F- ATPase activity [[2], [3], [4], [5]]. However, salt stress induced changes in chemical composition are less understood and relationship with physiological status remains to be known. The aim of this study was to analyze the effect of salt treatment on chemical composition of Sesuvium by using Fourier transform infrared (FT-IR) spectroscopy. In the recent past, this non-invasive method has shown demonstrative usefulness to determine the composition, structural characterization and detection of biomolecules [6,7].
FT-IR is highly sensitive method which offers several advantages over other analytical methods. This non destructive technique has ability to recognize functional groups and thus provides information about structural and chemical changes with respect to any biotic or abiotic stress. This technique has been extensively used to study protein secondary structures in the 1800 to 1500 cm−1 spectral region [8] Differences in the C O stretching vibrations of the peptide groups (the amide-I region between 1600–1700 cm−1) provide information on the type of secondary structure, such as α-helix, β-strands, and different kinds of turn structures. In maize, the technique was used to study overall protein secondary structure during dehydration in the embryo and pollen [9] and, to analyze cell wall components and their in muro organization to monitor plant cell wall modifications [10]. In Pea, the involvement of proteins and lipids of intact pollen for heat stress stress tolerance was confirmed by ATR-FT-IR spectroscopy [11]. In Calophyllum inophyllum, FT-IR spectroscopy was successfully used to delineate differences between control and salt treated plants [12].
In plants, this technique has been applied to discern chemical changes under salt stress however; there is paucity of information in case of halophyte plant species. In Mesembryanthemum leaves, Yang and Yen [8] used this technique to study early salt stress effects on the chemical changes. Halophytes are endowed with a higher adaptability to saline environment and hence offer as excellent candidates to investigate salt tolerance mechanism. The salt tolerance and productivity under saline habitat emanates from morphological adaptations to osmotic adjustment to efficient energy dissipation mechanisms based on electron fluxes adjustment inside the chloroplast [13]. It is hence interesting to study such physiological and biochemical modulations by using biophysical tools, and derive insight to improve salt stress tolerance in salt sensitive (glycophytes) crops.
The present study has revealed applicability of FT-IR based metabolic profiling for deducing salinity induced responses in roots and leaves of Sesuvium portulacastrum. The salinity-induced modulations in biochemical and metabolic changes were compared and the results indicated that root and shoot show differential response to salt exposure.
2. Materials and methods
2.1. Plant material and salt treatments
The axillary shoots of Sesuvium were collected from the coastal plains of Vashi, Navi Mumbai. The shoots were surface sterilized with 1% bavistin and few drops of tween twenty for five minutes and subsequently washed with distilled water to remove chemical traces. This was followed by 0.1% mercuric chloride treatment for 2 min and washing for five times with sterile distilled water. The surface sterilized, 5–6 cm long shoots with two nodes and two opposite leaves were grown in half strength Hoagland’s medium under hydroponic conditions. The four weeks old plants were subjected to 0 (control, No salt), 150 mM (Low salt, LS) and 500 mM (High salt, HS) treatment (based on previous work, [14]) in half strength Hoagland’s solution and losses due to evaporation was make up with the same strength salt solution. The plants were harvested after 28 days of treatment.
2.2. Estimation of Protein Content
Fresh plant material (500 mg) was homogenized in 100 mM chilled potassium phosphate buffer (pH 7.0) containing 0.1 mM EDTA and 1% polyvinyl pyrrolidone (w/v) at 4oC. Homogenate was squeezed through four layers of cheese cloth and extract thus obtained was centrifuged at 15,000×gfor 15 min at 4oC. The supernatant was used to measure protein according to [15].
2.3. IR spectroscopy
Pellets for FT-IR spectroscopy were prepared in an agate mortars, by mixing leaves or roots powder (2 mg) with KBr (1:100 p/p). The absorbance spectra were measured between 400 and 4000 cm−1 on Jasco (Model FT-IR-660 plus) FT-IR Spectrometer. At least three samples were collected for each treatment and at least three spectra were obtained from each sample. KnowItall software was used to find the functional groups for preliminarily analyzing IR spectra collected. Each peak was assigned its characteristic [16].
2.4. Statistical analysis
All experiments were carried out in completely randomized design with minimum three independent biological replicates. Biochemical data was expressed as mean ± SE. The principle component analysis was performed using XLSTAT software to study behavior of roots and leaves under range of salt treatments.
3. Results and discussion
The basic tenet of FT-IR relies on vibration of chemical bonds in the IR region. In the IR region, chemical bonds absorb radiation between 4000 and 400 cm−1. As per Griffiths and de Haseth [17], each functional group in a molecule has its own characteristic absorption frequency in the IR Spectrum. The sensitivity of IR spectroscopy has been successfully applied to in vitro and in vivo detection of biological systems.
In this study, FT-IR profiling of both leaves and roots was done so as to confirm physiological response of Sesuvium to varying salt treatment. All the samples were scanned from 400-4000 cm−1 wave number [8] and 3000-2000 cm−1 region was assigned for lipids, 1800-1500 cm−1 for proteins, 1500-1200 cm−1 for carbohydrates and 1000-600 cm−1 for cell wall components. The FT-IR spectra peaks and their probable functional groups are showed in control and salt treated root (Table 1) and leaves (Table 2) (Fig. 1).
Table 1.
FT-IR spectra showing observed peaks and probable functional groups in salt treated roots.
| Sr. no | Wave number (cm−1) |
Probable functional group | |||
|---|---|---|---|---|---|
| 0 mM NaCl | 150 mM NaCl | 500 mM NaCl | |||
| Lipids (3000-2000 cm−1) | |||||
| 1 | 3787.51 | 3783.65 | O—H stretch (Alcohols) | ||
| 2 | 3699.76 | O—H stretch(Alcohols), N—H stretch(Amines, Amides) | |||
| 3 | 3300.57 | N—H stretch (Amines) | |||
| 4 | 3295.75 | N—H stretch (Amides), O—H stretch(Alcohols), S, O—H stretch (Carboxylic acids) | |||
| 5 | 2934.16 | 2942.84 | 2940.91 | S, O—H stretch(Carboxylic acids), C—H stretch(Alkenes) | |
| 6 | 2894.63 | S, O—H stretch(Carboxylic acids), C—H stretch (Alkanes), (CO)-H (Aldehydes) | |||
| 7 | 2426.98 | ……………… | |||
| 8 | 2361.41 | 2361.41 | 2362.37 | C C stretch(Alkynes) | |
| 9 | 2335.37 | 2334.41 | C C (Alkynes) | ||
| 10 | 2086.6 | ………………… | |||
| Proteins (1800- 1500 cm−1) | |||||
| 11 | 1727.91 | 1729.83 | C O (Esters, Carboxylic acids, ketones, Aldehydes), C C (benzenes) | ||
| 12 | 1637.27 | 1658.48 | 1646.91 | C—O stretch (Amides), N—H bend(Nitro compounds, Amines), C O stretch (Carboxylic acids, ketones), C C (Alkenes, benzenes) (Proline) | |
| 13 | 1532.17 | 1529.27 | 1522.52 | N-H bend (Nitro compounds), C—O stretch (Amides), C C (benzenes), C O (ketones) | |
| Carbohydrates (1500-1200 cm−1) | |||||
| 14 | 1383.68 | 1380.78 | 1381.75 | N O stretch (Nitro compounds), C—O stretch (Esters), CO—H bend (Aldehydes), O—H bend (Alcohols) | |
| 15 | 1320.04 | 1324.86 | 1326.79 | S( O)2 stretch (Sulfones), N O stretch (Nitro compounds), O—H bend (Carboxylic acids, Alcohols) | |
| 16 | 1245.79 | 1245.79 | 1246.75 | C—N stretch (Amines), C—O stretch (Esters, Ethers, alcohols), O—H band (Carboxylic acids) | |
| Cell wall components (1000- 600 cm-1) | |||||
| 17 | 1059.69 | 1060.66 | 1060.66 | S O stretch (Sulfoxides), C—N stretch (Amines), C—O stretch (Esters, Ethers, Alcohols), C—H bend (Benzene, Alkenes) (Cellulose) | |
| 18 | 897.701 | 897.701 | C—N stretch (Amines), C—H bend (Benzene, Alkynes) (Xyloglucan) | ||
| 19 | 766.566 | 778.136 | 776.208 | C—N stretch (Amines), C—H bend (Benzene), C—C stretch | |
| 20 | 666.285 | C—N stretch (Amines), C—H bend (Benzene), C—C stretch (Chlorides) | |||
Table 2.
FT-IR spectra showing observed peaks and probable functional groups in salt treated leaves.
| Sr. No. | Wave number (cm−1) |
Probable functional group | ||
|---|---|---|---|---|
| 0 mM NaCl | 150 mM NaCl | 500 mM NaCl | ||
| Lipids (3000-2000 cm−1) | ||||
| 1 | 3772.08 | 3852.11, 3782.69 | 3884.9, 3852.11, 3782.69 | O—H stretch (Alcohols) |
| 2 | 3285.14 | 3284.18 | 3279.36 | N—H stretch (Amides, Amines), O—H stretch (Alcohols), S, O—H stretch (Carboxylic acids), C—H (Benzene, Alkynes, Alkenes) |
| 3 | 2928.38 | 2930.31 | 2930.31 | S, O—H stretch (Carboxylic acids), C—H stretch (Alkanes) |
| 4 | 2426.98 | P-H phosphine | ||
| 5 | 2397.08, 2370.09 | 2361.41 | P-H phosphine | |
| 6 | 2067.32 | 2066.35 | ------- | |
| Proteins (1800-1500 cm−1) | ||||
| 7 | 1885.08 | 1885.08 | 1883.15 | C O stretch (Ketone), C C (Benzene) |
| 8 | 1728.87 | C O (Saturated Aldehyde), C C stretch (Benzene) | ||
| 9 | 1642.09 | 1645.95 | 1636.3 | N—H bend (Nitro compounds, Amides), C—C stretch (Amides), C O stretch (Carboxylic acid, Ketone), C C (Benzene, Alkenes) |
| 10 | 1538.92 | 1537.95 | 1539.88 | N—H bend (Nitro compounds), C—O stretch (Amides, Ketone), C C (Benzene) |
| Carbohydrates (1500-1200 cm−1) | ||||
| 11 | 1383.68 | 1388.5 | 1385.6 | N O stretch (Nitro compounds), CO—H bend (Aldehydes), O—H bend (Alcohols) |
| 12 | 1321.96 | 1327.75 | 1327.75 | S( O)2 stretch (Sulfones), N O stretch (Nitro compounds), O—H bend (Carboxylic acids, Alcohols) |
| 13 | 1240 | 1242.9 | 1241.93 | C—N stretch (Amines), C—O stretch (Esters), C—O stretch (Ethers, Alcohols), O—H band (Carboxylic acids) |
| Cell wall components and chlorophyll (1000- 600 cm−1) | ||||
| 14 | 1026.91 | 1063.55 | 1069.33 | S O stretch (Sulfoxides), C—N stretch (Amines), C—O stretch (Esters, Ether, Alcohol), C—H bend (Alkenes) (Rhamnogalacturonan and Chlorophyll) |
| 15 | 953.627 | 958.448 | C—H bend (Alkenes) (Pectin) | |
| 16 | 830.205 | 828.277 | 830.205 | C—N stretch (Amines), C—H bend (Benzene, Alkenes), C—C (Chlorides) |
| 17 | 774.279 | 776.208 | 775.244 | C—N stretch (Amines), C—H bend (Benzene), C—C stretch (Chlorides) |
| 18 | 637.358 | 665.321 | C—N stretch (Amines), C—H bend (Benzene), C—C stretch (Chlorides) | |
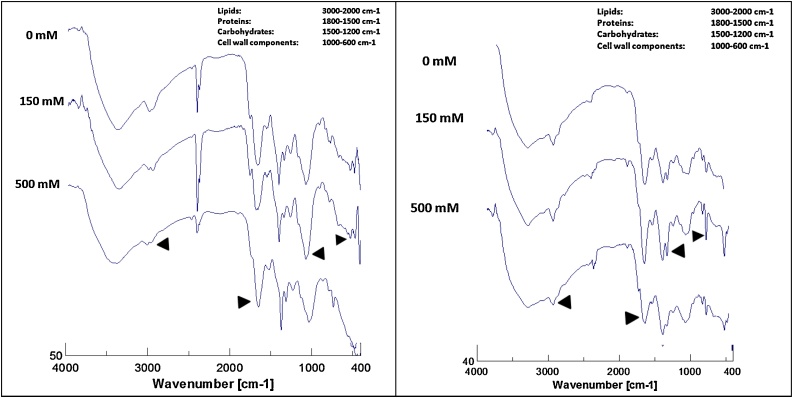
Fig. 1.
A) FT-IR spectra (range 4000- 400 cm−1) of leaves under NaCl (0, 150, 500 mM) treatment. B) FT-IR spectra (range 4000-400 cm−1) of roots under NaCl (0, 150, 500 mM) treatment.
In the lipid region, the peak at 2981 cm−1 of root was splitted into two peaks in both LS and HS (Fig. 1a). This peak is characteristic of S, O—H stretch (Carboxylic acids), C—H stretch (Alkanes), (CO)-H (Aldehydes) (Table 1). The leaves showed differential spectra in lipid region (Fig. 1b). The peak at 2981 cm-1 was not spitted in salt treatments; only peak area was increased in LS. To confirm this Malondialdehyde accumulation pattern in Sesuvium under salt stress was checked in our previous work [14]. MDA is an indicator of oxidative stress and product of lipid peroxidation. It was directly proportional to magnitude of stress. In our previous work, In root, it was 1.7 fold and 5.5 fold higher in low and high salt respectively as compared to control while in leaves, it was not significantly different in control and low salt but it was increased by 1.7 fold in high salt treatment [14]. In addition, Metabolic profiling of Sesuvium under salt stress revealed involvement of glycolipids in salt adaptation Nikalje et al. [14] In Salt treated Jojoba plant, the FT-IR data identified a peak at 2935 cm-1 belongs to -OCH2-group which represents peroxides and indicates peroxidation of lipids Afifi et al. [20].
In the FT-IR profile, protein region showed differential response of root and leaves to salt stress. In root, the peak at 1732 cm−1 showed increase peak area with increment in salt treatment. Also a small peak at 1733 cm−1 was disappeared in high salt treatment. These peaks are characteristic of C O (Esters, Carboxylic acids, Ketones, Aldehydes), C C (benzenes), C—N (amino group) these functional groups. To confirm these results protein content was estimated using Lowry’s method. In roots, under salt influence, protein content was significantly increased in both treatments in concentration dependant manner. It was 2 fold higher in low salt and 3 fold higher in high salt treatment. In leaf, FT-IR profile also peak 1733 cm-1 showed difference in peak area. It was higher in LS. Leaves protein content of both control and high salt treatment was not significant but in low salt treatment it was 1.5 fold more (Table 3). The isolated proteins of Soybean mutants was studied for relative content of irregular confirmations, α-helix and β-sheets in amide I region using FT-IR technique and salt tolerant mutants of Soybean were identified Akyuz et al. [19].
Table 3.
Effect of different salt treatments on protein content of roots and leaves of Sesuvium portulacastrum.
| NaCl | Protein (mg. g−1FW) |
|
|---|---|---|
| Root | Leaves | |
| 0 mM | 5.14 ± 0.88 | 15 ± 0.98 |
| 150 mM | 11.43 ± 0.62 | 22 ± 0.44 |
| 500 mM | 14.95 ± 0.55 | 17 ± 0.32 |
Carbohydrate region was assigned at 1500–1200 cm−1 wave number. In this region, in root and leaves showed similar FTIR profile. The area of peak at 1219 cm-1wave number was increased in LS of both plant parts. This peak indicates presence of C—N stretch (Amines), C—O stretch (Esters, Ethers, Alcohols) or O—H band (Carboxylic acids) functional groups. This was confirmed by assaying total soluble sugar (TSS) content in previous reports. In biochemical analysis, TSS content also showed differential response of root and leaves to salt stress. Previously we have shown that root TSS content of control and high salt treatments was not significant while under low salt treatment, sugar content was enhanced by 1.6 fold higher [14]. Leaf sugar accumulation pattern was not treatment dependant as low salt treatment enhanced sugar content by five- fold while high salt treatment enhanced sugar content by 2.4 fold as compared to control [14]. However, FT-IR analysis of Jojoba plant under salt stress reaveled no accumulation of carbohydarates, the band area of peaks in carbohydate region was decreased by 19% and conformation of carbohydrate was changed Afifi et al. [20].
The wave number 1000-600 cm−1 represents cell wall components. In root, the peak at 818 wave number [Characteristic of C—N stretch (Amines), C—H bend (Benzene, Alkynes) (Xyloglucan)] was disappeared completely which indicates cell wall components are highly susceptible to high salt stress treatment. In leaves, the area of peak at 754 wave number (characteristic of C—N stretch (Amines), C—H bend (Benzene), C—C stretch functional groups) was increased significantly in LS as compared to control and HS. In a halophyte, Atriplex prostrata salinity reduced lignifications of cell wall because extensin may replace lignin during salt adaptation [17]. The study by Yang and Yen [8] revealed that under salt stress Arabidopsis plants never accumulates precrsors of cell wall components while a halophyte, Mesembryanthemum tend to generate precurssors of cell wall components along with pectin. Considering impact of salt on both tissues in terms of cell wall components, it can be infered that roots of Sesuvium have susceptibility and leaf tissue have tolerent nature towards salinity stress.
3.1. PCA analysis
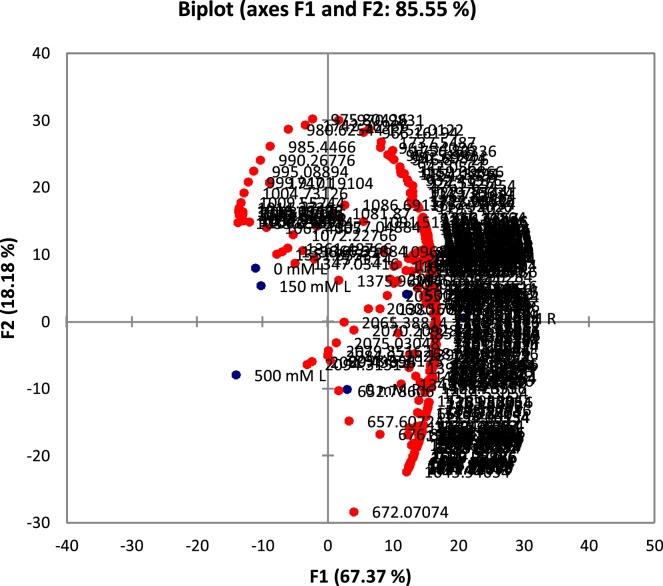
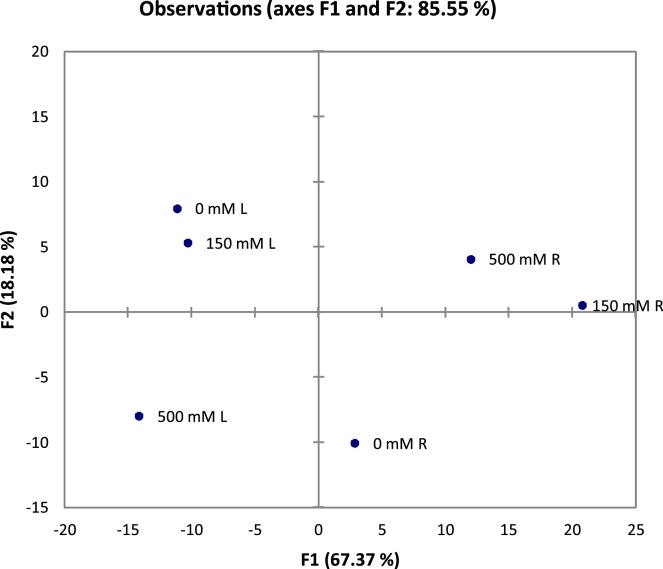
The principle component analysis (PCA) is a powerful statistical tool which can be used to reduce dimensionality of huge and multivariate data sets Adiani et al. [22]. In large data sets with multiple factors, interpretation of data is very difficult and complicated. PCA is useful to understand and interpret visually complicated data with relative ease. In spectroscopic data PCA helps to analyze large number of variables and makes data analysis easier. The PCA was applied to FT-IR spectra of 4000- 400 nm region. For multivariate analysis, Biplot and PCA plots were plotted to study variations occurring due to different salt treatments. In PC1 and PC2, the variability was 85.55% and 18.18% respectively (Fig. 2). In both biplot and PCA plot, roots and leaves showed differential response to salt treatment (Fig. 2). In PCA plot, roots and leaves were placed on opposite quadrates, which confirmed that root and leaves have contrasting strategies to cope up with salinity (Fig. 3). In roots, 150 mM and 500 mM salt treatments were in close vicinity and no salt treatment was on separate quadrate. In contrast, in leaves, 0 and 150 salt treatments were in close vicinity and 500 mM salt treatment was on different quadrate. This suggests that roots are more susceptible to salinity than areal parts. In wheat, finger millet and kodo millet, multivariate analysis enabled the identification of biochemical variables under salt stress Bhatt et al. [21]. Shelke et al. [18] have used PCA for multivariate analysis and screened different soybean varieties based on their salt tolerance ability. The PCA analysis FT-IR data of Jojoba plant showed descremination between salt tretaed and control plants.There was large intrisic variation and about 77% was accounted for PC1 Afifi et al. [20]. In Calophyllum inophyllum, the PCA score plot of FT-IR data showed clear grouping of salt treated and control plants Westworth et al. [12].
Fig. 2.
Biplot of FT-IR spectra of root and leaves of FT-IR spectra (range 4000- 400 cm−1) NaCl (0, 150, 500 mM) treatment.
Fig. 3.
Principal Component Analysis of FT-IR spectra (range 4000- 400 cm−1) of roots and leaves under NaCl (0, 150, 500 mM) treatment.
Elucidation of the mechanism of tolerance in halophytes at morphological, anatomical, physiological, biochemical, and molecular levels is crucial to understand the strategies that these plants to cope up with salinity.This information will help to improve the tolerance of the crop plants to abiotic stress conditions and to grow them in problematic soils. Among different analytical methods, FT-IR is becoming faster and sensitive method for evaluation of stress responses in stress biology. In present work, the FT-IR spectra revealed changes in carbohydrates, proteins, lipids and cell wall components in relatively less time and small sample amount as compared to physiological and biochemical assays. The organ specific response of Sesuvium to salt stress was confirmed by PCA analysis of FT-IR data. Hence, the results validate applicability of FT-IR technique in characterization of biomolecules and prove its further applications in unraveling salt stress adaptive mechanism in plants.
Acknowledgement
GCN is thankful to Bhabha Atomic Research Centre- Savitribai Phule Pune University Collaborative Ph.D. programme for Fellowship.
Contributor Information
G.C. Nikalje, Email: ganeshnikalje7@gmail.com.
P. Suprasanna, Email: prasanna@barc.gov.in.
References
- 1.Lokhande V.H., Gor B.K., Desai N.S., Nikam T.D., Suprasanna P. Sesuvium portulacastrum, a plant for drought, salt stress, and fixation, food and phytoremediation. A review. Agro. Sust. Devel. 2013;33:329–348. [Google Scholar]
- 2.Nikalje G.C., Srivastava A.K., Pandey G.K., Suprasanna P. Halophytes in Biosaline Agriculture: mechanism, utilization and value added products. Land Degrad. Dev. 2017;29(4):1081–1095. doi: 10.1002/ldr.2819. [DOI] [Google Scholar]
- 3.Ramani B., Reeck T., Debez A., Stelzerd R., Huchzermeyera B., Schmidt A., Papenbrock J. Aster tripolium L. and Sesuvium portulacastrum L.: two halophytes, two strategies to survive in saline habitats. Plant Physiol. Biochem. 2006;44:395–408. doi: 10.1016/j.plaphy.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Xiaoping Y., Yong S., Qian Y., Anping G., Lili C., Dan W., Zheng T., Xiang J., Limin W., Jianlan Y., Wenhai J., Yongming X., Xuchu W. Quantitative proteomics of Sesuvium portulacastrum leaves revealed that ion transportation by V-ATPase and sugar accumulation in chloroplast played crucial roles in halophyte salt tolerance. J. Proteomics. 2014;99:84–100. doi: 10.1016/j.jprot.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 5.Muchate N., Nikalje G.C., Rajurkar N., Suprasanna P., Nikam T.D. Flora - Morphology Distribution Functional Ecology of Plants. 2016. Physiological responses of the halophyte Sesuvium portulacastrum to salt stress and their relevance for saline soil bio-reclamation; pp. 96–105. [Google Scholar]
- 6.Dukor R.K. In: Handbook of Vibrational Spectroscopy. Chalmers J.M., Griffiths P.R., editors. John Wiley and Sons Ltd; New York: 2002. pp. 3335–3361. [Google Scholar]
- 7.Komal Kumar J., Devi Prasad A.G. Fourier transform infrared spectroscopy an advanced technique for identification of biomolecules. Drug Invent. Today. 2012;4:616–618. [Google Scholar]
- 8.Yang J., Yen H.C.E. Early salt stress effects on the changes in chemical composition in leaves of ice plant and Arabidopsis. A Fourier Transform Infrared Spectroscopy Study. Plant Physiol. 2002;130:1032–1042. doi: 10.1104/pp.004325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolkers W.F., Hoekstra F.A. Aging of dry desiccation-tolerant pollen does not affect protein secondary structure. Plant Physiol. 1995;109:907–915. doi: 10.1104/pp.109.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alonso-Simon A., Garcia-Angulo P., Melida H., Encina A., Alvarez J.M., Acebes J.L. Plant Signal. Behav. 2011;6:1104–1110. doi: 10.4161/psb.6.8.15793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lahlali R., Jiang Y., Kumar S., Karunakaran C., Liu X., Borondics F., Hallin E., Bueckert R. ATR–FTIR spectroscopy reveals involvement of lipids and proteins of intact pea pollen grains to heat stress tolerance. Front. Plant Sci. 2014;5:747. doi: 10.3389/fpls.2014.00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Westworth S., Ashwath N., Cozzolino D. Application of FTIR-ATR spectroscopy to detect salinity response in Beauty Leaf Tree (Calophyllum inophyllum L) Energy Procedia. 2019;160:761–768. [Google Scholar]
- 13.Duarte B., Sleimi N., Cacador I. Biophysical and biochemical constraints imposed by salt stress: learning from halophytes. Front. Plant Sci. 2014;5:746. doi: 10.3389/fpls.2014.00746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nikalje G.C., Variyar P.S., Joshi M.V., Nikam T.D., Suprasanna P. Temporal and spatial changes in ion homeostasis and accumulation of flavanoids and glycolipid in a halophyte Sesuvium portulacastrum (L.) L. PLoS One. 2018;13(4) doi: 10.1371/journal.pone.0193394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowry O.H., Roenbrough N.J., Farr A.L., Randal E.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 16.Griffiths P.R., de Haseth J.A. John Wiley and Sons; New York: 1986. Fourier Transform Infrared Spectrometry. [Google Scholar]
- 17.Wang L.W., Showalter A.M., Ungar I.A. Effect of salinity on growth, ion content, and cell wall chemistry in Atriplex prostrata (Chenopodiaceae) Am. J. Bot. 1997;84(9):1247–1255. [PubMed] [Google Scholar]
- 18.Shelke D.B., Padey M., Nikalje G.C., Suprasanna P., Zaware B.N., Nikam T.D. Salt responsive physiological, photosynthetic and biochemical attributes at early seedling stage for screening soybean genotypes. Plant Physiol. Biochem. 2017;118:519–528. doi: 10.1016/j.plaphy.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Akyuz S., Akyuz T., Celik O., Atak C. FTIR spectroscopy of protein isolates of salt-tolerant soybean mutants. J. Appl. Spectrosc. 2018;84(6):1019–1023. [Google Scholar]
- 20.Afifi A.A., Youssef R.A., Hussein M.M. Fourier transform infrared spectometry study on early stage of salt stress in jujube plant. Life Sci. J. 2013;10(4):1973–1981. [Google Scholar]
- 21.Bhatt R., Asopa P.P., Sihag S., Sharma R., Kachhwaha S., Kothari S.L. Comparative three way analysis of biochemical responses in cereal and millet crops under salinity stress. J. Appl. Biol. Biotech. 2015;3(6):22–28. [Google Scholar]
- 22.Adiani V., Gupta S., Ambolikar R., Variyar P.S. Development of rapid method to assess microbial quality of minimally processed pomegranate arils using FTIR. Sens. Actuators B: Chem. 2018;260:800–807. [Google Scholar]