Abstract
Medicinal herbs have been increasingly used worldwide for diseases prevention and treatment. Rheum turkestanicum Janisch. is a perennial shrub of the Polygonaceae family. Genus Rheum includes more than 60 species growing around the world which are used in foods and traditional medicines. R. turkestanicum is believed to be able to improve different kinds of disorders including diabetes, hypertension, jaundice and cancer. In recent years, this medicinal plant has been a subject of many experimental studies to document its health-beneficial properties. These studies have revealed antidiabetic, anticancer, nephroprotective, cardioprotective, and hepatoprotective properties of R. turkestanicum. The presence of flavonoids (e.g. epicatechin and quercetin) and anthraquinones (e.g. chrysophanol, physcion, and emodin) in R. turkestanicum justifies its health-beneficial effects. Nevertheless, possible therapeutic applications and safety of this plant still need to be elucidated in further clinical studies.
Keywords: Plant biology, Nutrition, Diabetes, Rhubarb, Anticancer, Toxicity, Rheum turkestanicum
1. Introduction
The use of medicinal herbs in the diseases prevention and treatment has been increased worldwide due to their privileged properties. A recent study showed that about one-third of adults in the United States are taking at least a dose of herbal supplement [1]. Numerous studies have provided evidence of the medicinal herbs effectiveness on various diseases such as diabetes, hypertension, cancer, and fatty liver [2, 3, 4, 5]. As a perennial shrub of the Polygonaceae family which mainly grows in central Asia, Rheum turkestanicum Janisch [6, 7]. includes more than 60 species around the world. These plants are widely used in foods and traditional medicines [7, 8, 9]. In recent years, experimental and clinical studies have revealed several biological effects for Rheum genus plants. Some of these effects include hypolipidemic, antibacterial, anti-inflammatory, and antioxidant activities [9, 10, 11, 12]. To the best of our knowledge, there is no comprehensive review of the literatures about pharmacological properties and ethnomedicinal uses of R. turkestanicum. In this paper we reviewed current knowledge about the phytochemistry, ethnomedicinal uses, and pharmacological activities of R. turkestanicum. The information reviewed in this paper may be useful for designing clinical studies to evaluate beneficial effects of R. turkestanicum in patients with diabetes, nephropathy, heart diseases, and liver damage.
1.1. Method of literature search
An online search of the literature was carried out at Pubmed/Medline, Scopus, and Google scholar covering all years until February 2019. The following key terms were used, usually in combinations: Rheum turkestanicum, Rheum, Rhubarb, Compounds, and Ethnomedicinal. All the authors independently performed the literature search. The bibliographies of all articles were checked for cross-references that were not found in the search of databases. Articles were selected if they reported any biological effects, ethnomedicinal uses, phytochemical compounds, and botanical descriptions for Rheum turkestanicum.
1.2. Botanical description
Rheum L. (family Polygonaceae) is a highly diversified genus, composed of about 60 species distributed in the mountainous and desert regions in Asia and Europe, with the highest diversity of species occurring in China [13]. Among them, R. turkestanicum (commonly known as Eshghan) is a highly reputable medicinal plant that grows widely in central Asia particularly in north-east of Iran [6, 7]. It is a perennial herb with long and stout roots. The leaves are cordate-reniformis, Inflorescence is spherical and high branches. Flowers are hermaphrodite with pedicles up to 7 mm long and perianth segments 2.5–3 mm [6, 14]. Fruits are trigonous nuts and their wings are cream or milk-white [6] (see Fig. 1). The only acceptable name according to “The Plant List” database for this plant is R. turkestanicum Janisch. and other synonyms including R. megalophyllum Sumner, R. renifolium Sumner, R. rupestre Litv. ex Losinsk., and R. turanicum Litv. are invalid [15].
Fig. 1.
Arial parts of Rheum turkestanicum Janisch in spring (A) and summer (B).
1.3. Ethnomedicinal uses
Several plants in Rheum genus are known as rhubarb and widely used in traditional medicines for the treatment of a wide range of human ailments in the world, particularly in Asia. Three main types of rhubarb are gaining significant attention globally and being the main subject of many investigations: the Chinese rhubarb, the Indian rhubarb, and the Rhapontic rhubarb [16]. In Traditional Chinese Medicine, R. officinale Baill., R. palmatum L., and R. tanguticum Maxim. ex Balf. are the Chinese rhubarbs with a long history of use as medicinal plants [17]. The Indian rhubarbs R. emodi Wall. ex Meisn. and R. webbianum Royle are used in Indian traditional medicine to treat a variety of diseases such as abdominal disorders, boils, and wounds [18]. Likewise, in Iranian Traditional Medicine, a number of Rheum taxa, particularly R. ribes L. and R. turkestanicum are popularly used in folk medicine [7]. The roots of R. turkestanicum are believed to improve diabetes, hypertension, jaundice, and cancer [7, 19, 20]. Due to the increasing demand, the short supply, and similar morphological traits, some Rheum taxa have been used as commercial substitutes in some regions of the world (e.g., R. turkestanicum may be mixed with R. ribes in markets, which can reduce the quality and efficacy of R. turkestanicum) [21]. Therefore, authentication of commercial frauds of Rheum taxa is necessary because without correct identification, their efficacy and safety cannot be guaranteed.
1.4. Bioactive compounds of Rheum turkestanicum
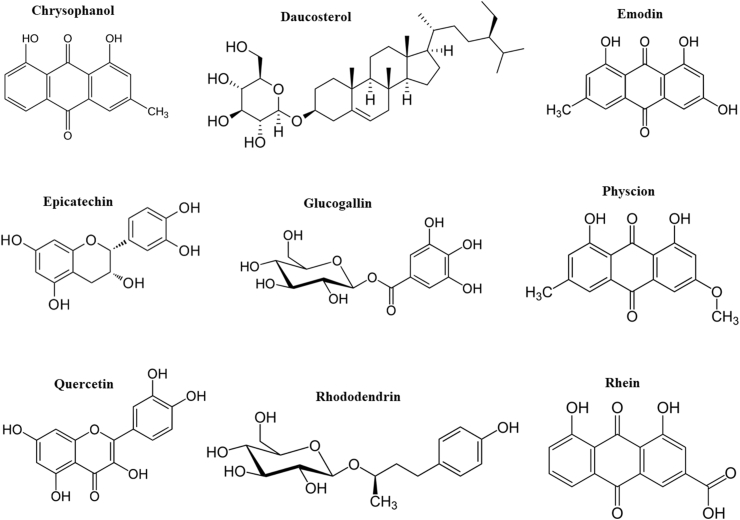
A wide range of bioactive constituents including anthraquinones, anthrones, acylglucosides, flavonoids, stilbenes, anthocyanins, organic acids, and vitamins have been characterized in Rheum species [16, 22]. However, the number of studies determined bioactive compounds of R. turkestanicum are limited. In our previous study, HPLC-MS/MS analysis on the hydroalcoholic extract of R. turkestanicum root suggested the presence of alkanes (e.g., eicosane and heneicosane), anthraquinones (e.g., aloe-emodin, emodin glycosides, physione, and rhein), fatty acids (e.g., linoleic acid and 9-octadecenoic acid), and flavonoids (e.g., epicatechin and quercetin). The extract also contained a high level of glucogallin, a phenolic compound that is formed from β-D-glucose and gallic acid [23]. Recently, Dehghan et al. analyzed ethyl acetate extract of R. turkestanicum root by 1H-,13C-, 2D-NMR, electron impact ionization mass spectra and single-crystal X-ray diffraction methods and isolated chrysophanol, daucosterol, emodin, physcion, and rhododendrin from the extract [24]. Daucosterol, a glucoside of β-sitosterol, is one of the major sterols found in higher plants [25]. Rhododendrin is a glycoside of arylbutanoid (a type of natural phenol) found in the inner bark of many plant species. Rhododendrin is hydrolyzed to rhododendrol when the plant is under drought stress [26]. Biological effects and chemical structures of some phytochemicals of R. turkestanicum are shown in Table 1 and Fig. 2, respectively. The yield of a macerated hydroalcoholic extract of R. turkestanicum root is about 30%. The level of phenolic compounds in each gram of the root extract is approximately 250 mg gallic acid equivalent [27].
Table 1.
Biological activities of some phytochemicals of Rheum turkestanicum.BPH: benign prostatic hyperplasia; *: This effect was reported for an unglycosylated form of the phytochemical compound. #: This effect was reported for a glycosylated form of the compound.
| Phytochemical | Classification | Biological activities (in vivo) | References |
|---|---|---|---|
| Chrysophanol | Anthraquinone | Anti-inflammatory | [96, 97] |
| Neuroprotective | [98, 99, 100] | ||
| Retinoprotective | [101] | ||
| Cardioprotective | [97] | ||
| Hepatoprotective | [102] | ||
| Daucosterol | Phytosterol | Neuroprotective | [103, 104] |
| Immunomodulatory | [105, 106] | ||
| Antihyperglycemic* | [107] | ||
| Improving BPH* | [108] | ||
| Emodin | Anthraquinone | Hepatoprotective | [109, 110] |
| Anti-inflammatory | [111] | ||
| Antihyperlipidemic | [35, 112, 113, 114] | ||
| Antihyperglycemic | [35, 113, 114] | ||
| Cardioprotective | [113] | ||
| Antifibrotic | [115, 116, 117] | ||
| Epicatechin | Flavonoid | Antihypertensive | [118, 119] |
| Angiogenic | [120] | ||
| Neuroprotective | [121] | ||
| Cardioprotective | [122] | ||
| Antiaging | [123] | ||
| Glucogallin | Phenolic glycosides | Hepatoprotective | [124] |
| Anti-inflammatory | [125] | ||
| Antihyperlipidemic* | [126] | ||
| Nephroprotective* | [127] | ||
| Cardioprotective* | [128] | ||
| Physcion (Parietin) | Anthraquinone | Neuroprotective | [129, 130] |
| Vasodilator | [131] | ||
| Anticancer# | [132] | ||
| Antisepsis# | [133] | ||
| Quercetin | Flavonoid | Anti-inflammatory | [134] |
| Neuroprotective | [135] | ||
| Wound healing | [136] | ||
| Hepatoprotective | [137] | ||
| Antiobesity | [138] | ||
| Rhein | Anthraquinone | Hepatoprotective | [139] |
| Nephroprotective | [139, 140] | ||
| Antihyperglycemic | [40, 141, 142] | ||
| Antihyperlipidemic | [142] | ||
| Antifibrotic | [143] | ||
| Anti-aging | [144] | ||
| Rhododendrin | Phenolic glycosides | Anti-inflammatory | [145, 146, 147] |
| Analgesic | [148] | ||
| Antipsoriasis | [146] |
Fig. 2.
Chemical structures of some phytochemicals of Rheum turkestanicum.
1.5. Pharmacological activities
1.5.1. Antidiabetic effects
Diabetes is identified as a metabolic disease, which leads to an increase in blood glucose due to insufficient insulin release or resistance to insulin actions. Lack of control of hyperglycemia, in long-term, causes microvascular (retinopathy and nephropathy) and macrovascular (ischemia or cardiac attack) complications [28, 29]. Todays, although insulin and oral antidiabetic drugs are available for management of diabetes, these complications are still being developed in most of the patients. Therefore, the researchers are attempting to find new antidiabetic drugs. Experimental and clinical studies have reported that some of the medicinal plants have beneficial effects on the reduction of blood glucose. In traditional medicine of Iran, R. turkestanicum has been recommended for management of diabetes [7]. Recent experimental studies also support the beneficial effects of this plant on diabetes. In streptozotocin (STZ)-induced diabetic rats, we found that administration of macerated hydroalcoholic extract of R. turkestanicum root (at 200 and 300 mg/kg) for 4 weeks improved diabetic dyslipidemia by reducing the levels of triglyceride, total cholesterol, and low density lipoprotein (LDL) [27]. The hypolipidemic effect of R. turkestanicum was also observed by Hadjzadeh et al. using decoction extract after 3 weeks (at doses of 200, 400 and 600 mg/kg) [30]. There are inconsistent findings about the effect of R. turkestanicum on the blood glucose. In our study, the macerated extract significantly reduced the levels of fasting blood glucose and HbA1c, while Hadjzadeh et al. showed that the decoction extract did not change serum glucose over a 3-week period [27, 30]. This discrepancy can be explained by the differences between the type of extract and duration of study. Increase of triglyceride, cholesterol, and ratio of LDL/HDL are risk factors for atherosclerosis and cardiovascular diseases. The reduction of these parameters by R. turkestanicum favors it as a complementary therapeutic along with current hypolipidemic drugs for the management of diabetic dyslipidemia. Elevation of the activity of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) is an indicator of liver disorders, which are observed more frequently in patients with diabetes than healthy individuals [31, 32, 33]. The hydroalcoholic extract of R. turkestanicum reduces the elevated levels of serum AST, ALT and ALP and improved histological changes of the liver in STZ-induced diabetic rats. In addition, the extract improved the serum biochemical indexes related to the kidney function (creatinine) and myocardial damage (lactate dehydrogenase and creatine phosphokinase) [27].
Antidiabetic effects observed for R. turkestanicum are in agreement with previous reports about beneficial effects of other plants in the genus Rheum in diabetes. For example, it has been shown that administration of R. ribes (350 mg, thrice daily, for 12 weeks) to patients with type 2 diabetes can improve the control of blood glucose [34]. Antidiabetic effects of Rheum genus may be related to the presence of high amounts of anthraquinones and flavonoids (e.g., quercetin) in these plants. Emodin was reported to activate peroxisomal proliferator-activated receptor-gamma and regulate other genes involved in the control of metabolism [35]. Also, it has been shown that chrysophanol and chrysophanol-8-O-b-D-glucopyranoside enhance the effect of insulin on glucose uptake through increasing Glut4 expression and insulin receptor phosphorylation [36]. The beneficial effects of flavonoids on diabetes are attributed to their antihyperglycemic, antioxidant, anti-inflammatory, nephroprotective, neuroprotective, and hepatoprotective effects. These compounds induce their antihyperglycemic effects through decreasing glucose absorption from the small intestine, enhancing glucose uptake in tissues, reducing gluconeogenesis, stimulating insulin release from beta cells, and preserving beta cell mass [37]. For example, quercetin was shown to reduce blood glucose in STZ diabetic rats via preserving pancreatic islets and increase of insulin secretion [38, 39]. Similarly, it has been shown that rhein as an anthraquinone which is found in high amount in Rheum genus can reduce blood glucose via enhancing insulin secretion and inhibiting apoptosis of beta cells [40]. In addition, rhein is able to protect the kidney against the development of diabetic nephropathy by antioxidant and anti-inflammatory properties [41].
1.5.2. Nephroprotective effects
Several biological agents (e.g., molds and fungi), minerals (e.g., mercury, arsenic, and lead), diseases (e.g., diabetes), and drugs (e.g., cisplatin and gentamicin) can influence renal function and may cause nephrotoxicity. Nephrotoxicity is associated with decreased glomerular filtration rate (GFR), which leads to a decrease in urine output and an increase in serum creatinine and urea [42]. Cisplatin is known as one of the nephrotoxic agent that can cause renal problems in 28–36% of patients after an initial dose [43]. This drug is an anticancer agent, which is widely used in the treatment of cancers of neck, head, and ovary [44]. Cisplatin leads to excessive generation of reactive oxygen species (ROS) and depletion of antioxidant enzymes in kidney. These changes along with inflammatory mechanisms result in apoptosis and necrosis of the kidney cells [45, 46]. Studies have shown that the combination of cisplatin with antioxidant agents may reduce the risk of its nephrotoxicity [47]. R. turkestanicum is a rich source of phenolic compounds with antioxidant properties [27]. The protective effect of R. turkestanicum against cisplatin-induced nephrotoxicity has been studied in vivo. In our recent study, the hydroalcoholic extract of this plant was prepared using 70% ethanol by soxhlet apparatus and administrated (100 and 200 mg/kg) to rats for 3 days (1 h before cisplatin injection). Both doses of the extract could inhibit the cisplatin-induced increase of serum urea, serum creatinine, urinary glucose and urinary protein. These renoprotective effects were confirmed by histopathological test [48]. In another study, protective effect of soxhlet extract of R. turkestanicum was evaluated in HgCl2 toxicity model. Administration of doses of 100 and 200 mg/kg of the extract attenuated HgCl2-induced atrophy and necrosis of the kidney via reduction of lipid-peroxidation and increase of thiol content [49]. This antioxidant activity was also observed in animal models of gentamicin- and hexachlorobutadiene-induced nephrotoxicity. Gentamicin is an aminoglycoside antibiotic that causes acute kidney injury through induction of oxidative stress, inflammation, endoplasmic reticulum stress, and apoptosis in tubular and glomerular cells [50, 51]. Hexachlorobutadiene, which is used in different industries particularly in the manufacture of rubber compounds has toxic effects on the kidney and respiratory system. Boroushaki et al., administrated soxhlet extract of R. turkestanicum (100 and 200 mg/kg) to rats 1 h before intraperitoneally injection of gentamicin (80 mg/kg for 6 days) or hexachlorobutadiene. The extract reduced proteinuria, decreased the serum levels of urea and creatinine, and improved the histopathological changes induced by these compounds. These effects were associated with a decrease of lipid peroxidation and an increase of thiol content in the kidney [52, 53, 54]. The exact compounds responsible for the nephroprotective property of R. turkestanicum remained to be identified. However, according to the previous studies, emodin and quercetin may be the nephroprotective phytochemicals of this plant [55, 56]. It has been reported that emodin can reduce the toxicity of cisplatin in human kidney cells (HEK 293) via scavenging of free radicals and elevation of antioxidant enzymes such as catalase, glutathione peroxidase, glutathione S-transferase, glutathione reductase and superoxide dismutase [55]. Also, quercetin was shown to protect the kidney against mercury toxicity by scavenging of free radicals and inhibition of apoptotic cells [56].
1.5.3. Cardio-protective effects
It is well known that some chemotherapeutic drugs such as doxorubicin, cyclophosphamide, and 5-fluourouracil may change blood pressure, induce arrhythmia and lead to cardiomyopathy [57]. The clinical studies have shown that consumption of doxorubicin may lead to cardiovascular problems such as dilated cardiomyopathy and congestive heart failure [58]. Doxorubicin enhances production of free radicals in cardiac cells, which leads to accumulation of Ca2+, an increase in permeability of mitochondria, release of cytochrome c, lipid-peroxidation, and DNA damage [59, 60]. Recent studies have suggested that medicinal herbs may attenuate cardiotoxicity of doxorubicin due to having anti-oxidant ingredients [61]. Our previous work showed that exposure of H9C2 cells to Soxhlet extract of R. turkestanicum at doses of 25–200 μg/mL reduced doxorubicin cardiotoxicity through reducing free radicals, lipid peroxidation and apoptosis [62]. This effect was comparable to N-acetylcysteine, as a control antioxidant. In addition, macerated hydroalcoholic extract of R. turkestanicum can reduce the level of lactate dehydrogenase and creatine phosphokinase (the indicators of myocardial damage) in the serum of STZ model of diabetic rats. Histological examination confirmed the cardioprotective activity of this plant in diabetic animals [27]. This effect of R. turkestanicum may be related to the presence of emodin, stilbene, quercetin and resveratrol in the active genus of Rheum [22]. Emodin was shown to reduce cardiac injury after ischemia-reperfusion via increasing the antioxidant enzymes [63]. Also it improved cardiac injury following viral myocarditis via decreasing the expression of Toll-like receptor 4 and P38 mitogen activating protein kinase [64]. Quercetin attenuated ischemia-reperfusion injury in cardiomyocytes via decrease of inflammatory response by reducing the activity of signal transducer and activator of transcription 3 (STAT3) [65]. Also, it has been shown that quercetin attenuated cardiotoxicity of doxorubicin in H9C2 cells and mice by enhancement of Bmi-1 expression and reduction of oxidative stress-induced lipid peroxidation [66]. Similarly, resveratrol (a phytoalexin) reduced toxicity of doxorubicin in H9C2 cells [67] and in animal studies via elevation of antioxidant capacity and decreasing the pro-apoptotic proteins such as p53, Bax, and caspase 3 [68, 69, 70].
1.5.4. Hepatoprotective effects
The plants of Rheum genus have the potential to protect the liver against environmental toxins and hepatotoxic drugs [49, 71, 72]. Administration of Soxhlet extract of R. turkestanicum (100 and 200 mg/kg, for 3 days) to rats can prevent mercury-induced liver toxicity as observed by reduction of liver enzymes AST and ALT. This hepatoprotective effect was induced via inhibition of oxidative stress [49]. Similarly, it has been shown that Rheum emodi reduced the levels of serum AST and ALT in rats exposed to paracetamol [73] and carbon tetrachloride [71]. Hepatoprotective effect of plants of Rheum genus can be mediated by different components found in these plants such as quercetin, rhein, emodin, anthraquinones and resveratrol. For example, quercetin is able to protect the liver against different toxins including ethanol, heavy metals (cadmium, lead acetate, copper and nickel), pesticides, drugs and chemical compounds [72]. Quercetin protects the liver through decreasing oxidative stress, inhibition of apoptosis, and prevention of inflammatory cytokines action through the IKK/NF-κB and MAPK signaling pathways [74, 75]. Rhein can attenuate the expression of pro-apoptotic proteins in human hepatocytes treated by methotrexate and reduce methotrexate-induced liver injury (reduction of ALT and AST) in rats [76]. An animal study in rats showed that emodin dose-dependently reduced acetaminophen-induced liver toxicity [77]. In rat model of α-naphthylisothiocyanate-induced cholestatic liver damage, free anthraquinones particularly aloe-emodin could improve function and morphological alterations of hepatocytes [78].
1.5.5. Anticancer effects
Clinical studies have suggested that some phytochemicals compounds show beneficial effects on various types of cancers [3]. One of the malignant cancers is acute promyelocytic leukemia (APL) [79]. Currently, arsenic trioxide (As2O3) is used for management of APL, because of its potential to induce apoptosis and differentiation of APL cells. However, at high concentrations, it leads to different side effects such as skin reaction, an increasing in white blood cell, vomiting, diarrhea and nausea, attenuation of appetite, hemorrhage of teeth and nose [80]. Effects of R. turkestanicum on acute promyelocytic leukemia (APL) cell line have been tested by Hosseini and co-workers. The Soxhlet extract of R. turkestanicum (60–500 μg/mL) increased cell death in HL60 and NB4 leukemia cells similar to As2O3 [81]. Also, Shiezadeh and co-workers have shown that ethyl acetate and n-hexane extracts of R. turkestanicum increased cell death (30–500 μg/mL) in cervical cancer (Hela) and breast cancer (MCF-7) cells similar to taxol (as positive control) via elevation of PARP expression [82] The antitumor activity of R. turkestanicum may be related to the presence of quercetin, emodin, and aloe-emodin in its extract [83, 84].
1.5.6. Neuroprotective effects
Elevation of glutamate can lead to neurodegenerative diseases via decreasing antioxidant enzymes and increasing ROS production [85]. It has been reported that exposure of neuronal cells (PC12 and N2a) to Soxhlet extract of R. turkestanicum (25–200 μg/mL) reduced glutamate toxicity via reducing the level of ROS, inhibition of lipid-peroxidation, and prevention of apoptosis [86]. Neuroprotective effect against glutamate toxicity was also shown for R. emodi in IMR32 cells (by up-regulating Nrf2/HO-1 expression) [87]. In the case of R. turkestanicum phytochemicals, it has been reported that quercetin at 100 μM has a neuroprotective effects against different neurotoxic agents including 1-methyl-4-phenylpyridinium (MPP +), ammonium chloride, and nocodazole by increasing α-synuclein expression, decreasing inflammation and inhibition of apoptosis [88, 89]. Also, aloe-emodin was shown to induce neuroprotective effect via inhibition of oxidative stress and may induce beneficial effects on Alzheimer's disease by preventing the activity of acetylcholinesterase [90, 91]. The neuroprotective effect of aloe-emodin may be induced via activation of the Akt serine/threonine kinase and elevation of the level of anti-apoptotic protein Bcl-2 [91].
1.5.7. Toxicity
Several studies have shown that not all herbs and phytochemicals are risk-free, as per common belief, and some of them can be toxic [92, 93]. Although R. turkestanicum has a long history of use in herbal medicine, evidence concerning its safety is limited. Presence of anthraquinones derivatives in this plants suggests that it should be used with caution, as they may cause adverse effects including genotoxicity, nephrotoxicity, liver damage, and gastrointestinal disorders [94]. Such toxic effects have also been reported for the other plants of Rheum species [94, 95]. However, in a recent study, we found no acute and sub-acute toxicity in animals treated with a hydroalcoholic extract of R. turkestanicum root. In acute toxicity experiment, male and female mice were orally treated with the extract at single doses of 300, 2000 and 3000 mg/kg. Up to 14 days, no deaths or signs of toxicity were observed in the mice that received the extract at a dose of 300 mg/kg (the no-observed-adverse-effect level, NOAEL). The extract at a dose of 3000 mg/kg led to the death of one male and one female mouse (LD50 > 3000 mg/kg). In the sub-acute experiment, the extract was administered at doses of 100 and 400 mg/kg to male rats for 28 consecutive days. These doses produced no mortality or significant changes in hematological parameters, serum biochemical indicators of kidney and liver function, and histopathology of the liver, kidney, heart, and brain [23]. Further studies will be necessary to elucidate whether R. turkestanicum has any unwanted effects in humans.
2. Conclusion
Nowadays, the interest in herbal medicine has increased all over the world. R. turkestanicum has a long history of use in traditional medicine of Central Asia to treat different diseases including diabetes, hypertension, jaundice, and cancer. Experimental studies performed in recent years have revealed a number of health-beneficial effects for R. turkestanicum. These effects include antidiabetic, anticancer, nephroprotective, cardioprotective, and hepatoprotective activities. It seems that this plant has the potential to protect the body tissues, particularly liver and kidney, against environmental toxins and side effects of drugs. The presence of flavonoids and anthraquinones in R. turkestanicum justifies its health-beneficial effects. However, the number of studies determined the bioactive constituents of this plant is small, and much work should be done. Also, future works are needed to reveal the exact molecular mechanisms responsible for health-beneficial effects of R. turkestanicum. In addition, more studies are needed to confirm the safety of extract of this plant. A main limitation of the current literature on the health-beneficial effects of R. turkestanicum is that these studies have been performed only on animal models. The information reviewed in this paper may be useful for designing clinical studies to evaluate beneficial effects of R. turkestanicum in patients with diabetes, nephropathy, heart diseases, and liver damage.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Rashrash M., Schommer J.C., Brown L.M. Prevalence and predictors of herbal medicine use among adults in the United States. J. Patient Exp. 2017;4(3):108–113. doi: 10.1177/2374373517706612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hosseini A., Shafiee-Nick R., Ghorbani A. Pancreatic beta cell protection/regeneration with phytotherapy. Braz. J Pharmaceut. Sci. 2015;51(1):1–16. [Google Scholar]
- 3.Hosseini A., Ghorbani A. Cancer therapy with phytochemicals: evidence from clinical studies. Avicenna J. Phytomed. 2015;5(2):84–97. [PMC free article] [PubMed] [Google Scholar]
- 4.Chrysant S.G., Chrysant G.S. Herbs used for the treatment of hypertension and their mechanism of action. Curr. Hypertens. Rep. 2017;19(9):77. doi: 10.1007/s11906-017-0775-5. [DOI] [PubMed] [Google Scholar]
- 5.Baghernya M., Nobili V., Blesso C.N., Sahebkar A. Medicinal plants and bioactive natural compounds in the treatment of non-alcoholic fatty liver disease: a clinical review. Pharmacol. Res. 2018;130:213–240. doi: 10.1016/j.phrs.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 6.Taheri G. A synopsis of the genus Rheum (Polygonaceae) in Iran with description of three new species. Rostaniha. 2013;14(1):85–93. [Google Scholar]
- 7.Amiri M.S., Joharchi M.R. Ethnobotanical investigation of traditional medicinal plants commercialized in the markets of Mashhad, Iran. Avicenna J. Phytomed. 2013;3(3):254–271. [PMC free article] [PubMed] [Google Scholar]
- 8.Schrader W.L. UCANR Publications; 2000. Rhubarb Production in California. [Google Scholar]
- 9.Lai F. A systematic review of rhubarb (a Traditional Chinese Medicine) used for the treatment of experimental sepsis. Evid. Based Complement Altern. Med. 2015;2015:131283. doi: 10.1155/2015/131283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fallah Huseini H. The efficacy of rheum ribes L. stalk extract on lipid profile in hypercholesterolemic type II diabetic patients: a randomized, double-blind, placebo-controlled, clinical trial. J. Med. Plants. 2008;3(27):92–97. [Google Scholar]
- 11.Li Y., Sun Y., Ma H., Chen X., Liu J. Rheum rhabarbarum extract promotes healing of the incision through relieving inflammation and stimulating angiogenesis. Pak. J. Pharm. Sci. 2016;29(4):1437–1441. [PubMed] [Google Scholar]
- 12.Fei Y. Phenolic constituents from Rheum nobile and their antioxidant activity. Nat. Prod. Res. 2017;31(24):2842–2849. doi: 10.1080/14786419.2017.1303691. [DOI] [PubMed] [Google Scholar]
- 13.Ruirui L., Wang A., Tian X., Wang D., Liu J. Uniformity of karyotypes in Rheum (Polygonaceae), a species-rich genus in the Qinghai-Tibetan Plateau and adjacent regions. Caryologia. 2010;63(1):82–90. [Google Scholar]
- 14.Rechinger K.H., Schiman-Czeika H. Akademische Druck-und Verlagsanstalt; 1968. Polygonaceae. [Google Scholar]
- 15.The Plant List. 2013. http://www.theplantlist.org version1.1. [Google Scholar]
- 16.Agarwal S.K., Singh S.S., Lakshmi V., Verma S., Kumar S. Chemistry and pharmacology of rhubarb (Rheum species)—a review. J. Sci. Ind. Res. 2001;60:1–9. [Google Scholar]
- 17.Xu G. Authentication of official Da-huang by sequencing and multiplex allele-specific PCR of a short maturase K gene. Genome. 2013;56(2):109–113. doi: 10.1139/gen-2012-0182. [DOI] [PubMed] [Google Scholar]
- 18.Singh A., Lal M., Samant S. Diversity, indigenous uses and conservation prioritization of medicinal plants in Lahaul valley, proposed Cold Desert Biosphere Reserve, India. Int. J. Biodivers. Sci. Manag. 2009;5(3):132–154. [Google Scholar]
- 19.Amiri M.S., Joharchi M.R., TaghavizadehYazdi M.E. Ethno-medicinal plants used to cure jaundice by traditional healers of Mashhad, Iran. Iran. J. Pharm. Res. (IJPR) 2014;13(1):157–162. [PMC free article] [PubMed] [Google Scholar]
- 20.Moradi M.-T., Asadi-Samani M., Bahmani M. Hypotensive medicinal plants according to Ethnobotanical evidence of Iran: a Systematic Review. Int. J. PharmTech Res. 2016;9(5):416–426. [Google Scholar]
- 21.Joharchi M.R., Amiri M.S. Taxonomic evaluation of misidentification of crude herbal drugs marketed in Iran. Avicenna J. Phytomed. 2012;2(2):105–112. [PMC free article] [PubMed] [Google Scholar]
- 22.Singh P., Rawat M. Phytochemistry and biological activity perspectives of Rheum species. Nat. Prod. J. 2016;6(2):84–93. [Google Scholar]
- 23.Jahani Yazdi A. Acute and sub-acute toxicity evaluation of the root extract of Rheum turkestanicum Janisch. Drug Chem. Toxilogy. 2019 doi: 10.1080/01480545.2018.1561713. in press. [DOI] [PubMed] [Google Scholar]
- 24.Dehghan H., Salehi P., Amiri M.S. Bioassay-guided purification of α-amylase, α-glucosidase inhibitors and DPPH radical scavengers from roots of Rheum turkestanicum. Ind. Crops Prod. 2018;117:303–309. [Google Scholar]
- 25.Pegel K.H. The importance of sitosterol and sitosterolin in human and animal nutrition. South Afr. J. Sci. 1997;93(6):263–268. [Google Scholar]
- 26.Santamour F.S., Jr., Lundgren L.N. Rhododendrin in Betula: a reappraisal. Biochem. Syst. Ecol. 1997;25(4):335–341. [Google Scholar]
- 27.Hosseini A., Mollazadeh H., Amiri M.S., Sadeghnia H.R., Ghorbani A. Effects of a standardized extract of Rheum turkestanicum Janischew root on diabetic changes in the kidney, liver and heart of streptozotocin-induced diabetic rats. Biomed. Pharmacother. 2017;86:605–611. doi: 10.1016/j.biopha.2016.12.059. [DOI] [PubMed] [Google Scholar]
- 28.Deshpande A.D., Harris-Hayes M., Schootman M. Epidemiology of diabetes and diabetes-related complications. Phys. Ther. 2008;88(11):1254–1264. doi: 10.2522/ptj.20080020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.A Adeshara K., Diwan A.G., Tupe R.S. Diabetes and complications: cellular signaling pathways, current understanding and targeted therapies. Curr. Drug Targets. 2016;17(11):1309–1328. doi: 10.2174/1389450117666151209124007. [DOI] [PubMed] [Google Scholar]
- 30.Mousa-Al-Reza Hadjzadeh Z.R., Khodaei E., Malek M., Ghanbari H. Rheum turkestanicum rhizomes possess anti-hypertriglyceridemic, but not hypoglycemic or hepatoprotective effect in experimental diabetes. Avicenna J. Phytomed. 2017;7(1):1. [PMC free article] [PubMed] [Google Scholar]
- 31.Forlani G. Prevalence of elevated liver enzymes in Type 2 diabetes mellitus and its association with the metabolic syndrome. J. Endocrinol. Investig. 2008;31(2):146–152. doi: 10.1007/BF03345581. [DOI] [PubMed] [Google Scholar]
- 32.Malenica M. Use of databases for early recognition of risk of diabetic complication by analysis of liver enzymes in type 2 diabetes mellitus. Acta Inf. Med. 2016;24(2):90. doi: 10.5455/aim.2016.24.90-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schindhelm R.K. Alanine aminotransferase as a marker of non-alcoholic fatty liver disease in relation to type 2 diabetes mellitus and cardiovascular disease. Diabetes/Metabolism Res. Rev. 2006;22(6):437–443. doi: 10.1002/dmrr.666. [DOI] [PubMed] [Google Scholar]
- 34.Adham A., Naqishbandi A. HPLC analysis and antidiabetic effect of Rheum ribes root in type 2 diabetic patients. Zanco J. Med. Sci. 2015;19(2) [Google Scholar]
- 35.Xue J., Ding W., Liu Y. Anti-diabetic effects of emodin involved in the activation of PPARγ on high-fat diet-fed and low dose of streptozotocin-induced diabetic mice. Fitoterapia. 2010;81(3):173–177. doi: 10.1016/j.fitote.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 36.Lee M.S., Sohn C.B. Anti-diabetic properties of chrysophanol and its glucoside from rhubarb rhizome. Biol. Pharm. Bull. 2008;31(11):2154–2157. doi: 10.1248/bpb.31.2154. [DOI] [PubMed] [Google Scholar]
- 37.Ghorbani A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017;96:305–312. doi: 10.1016/j.biopha.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 38.Vessal M., Hemmati M., Vasei M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2003;135(3):357–364. doi: 10.1016/s1532-0456(03)00140-6. [DOI] [PubMed] [Google Scholar]
- 39.Yang D.K., Kang H.-S. Anti-diabetic effect of cotreatment with quercetin and resveratrol in streptozotocin-induced diabetic rats. Biomol. Ther. 2018;26(2):130. doi: 10.4062/biomolther.2017.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Du H. Improvement of glucose tolerance by rhein with restored early-phase insulin secretion in db/db mice. J. Endocrinol. Investig. 2012;35(6):607–612. doi: 10.1007/BF03345796. [DOI] [PubMed] [Google Scholar]
- 41.Zeng C.-C. The molecular mechanism of rhein in diabetic nephropathy. Evid. Based Complement Altern. Med. 2014:2014. doi: 10.1155/2014/487097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barnett L.M., Cummings B.S. Nephrotoxicity and renal pathophysiology: a contemporary perspective. Toxicol. Sci. 2018;164(2):379–390. doi: 10.1093/toxsci/kfy159. [DOI] [PubMed] [Google Scholar]
- 43.Jelinek M.J. Predicting acute renal injury in cancer patients receiving cisplatin using urinary neutrophil gelatinase-associated lipocalin and cystatin C. Clin. Transl. Sci. 2018;11(4):420–427. doi: 10.1111/cts.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rabik C.A., Dolan M.E. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev. 2007;33(1):9–23. doi: 10.1016/j.ctrv.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arany I., Safirstein R.L. Seminars in Nephrology. Elsevier; 2003. Cisplatin nephrotoxicity. [Google Scholar]
- 46.Kart A., Cigremis Y., Karaman M., Ozen H. Caffeic acid phenethyl ester (CAPE) ameliorates cisplatin-induced hepatotoxicity in rabbit. Exp. Toxicol. Pathol. 2010;62(1):45–52. doi: 10.1016/j.etp.2009.02.066. [DOI] [PubMed] [Google Scholar]
- 47.Barabas K., Milner R., Lurie D., Adin C. Cisplatin: a review of toxicities and therapeutic applications. Vet. Comp. Oncol. 2008;6(1):1–18. doi: 10.1111/j.1476-5829.2007.00142.x. [DOI] [PubMed] [Google Scholar]
- 48.Hosseini A., Fanoudi S., Mollazadeh H., Aghaei A., Boroushaki M.T. Protective effect of Rheum turkestanicum against cisplatin by reducing oxidative stress in kidney tissue. J. Pharm. BioAllied Sci. 2018;10(2):66. doi: 10.4103/JPBS.JPBS_9_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hosseini A., Rajabian A., Fanoudi S., Farzadnia M., Boroushaki M.T. Protective effect of Rheum turkestanicum root against mercuric chloride-induced hepatorenal toxicity in rats. Avicenna J. Phytomed. 2018:1–11. [PMC free article] [PubMed] [Google Scholar]
- 50.Martinez-Salgado C., Lopez-Hernandez F.J., Lopez-Novoa J.M. Glomerular nephrotoxicity of aminoglycosides. Toxicol. Appl. Pharmacol. 2007;223(1):86–98. doi: 10.1016/j.taap.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 51.Lopez-Novoa J.M., Quiros Y., Vicente L., Morales A.I., Lopez-Hernandez F.J. New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney Int. 2011;79(1):33–45. doi: 10.1038/ki.2010.337. [DOI] [PubMed] [Google Scholar]
- 52.Green T., Lee R., Farrar D., Hill J. Assessing the health risks following environmental exposure to hexachlorobutadiene. Toxicol. Lett. 2003;138(1-2):63–73. doi: 10.1016/s0378-4274(02)00372-7. [DOI] [PubMed] [Google Scholar]
- 53.Boroushaki M.T., Fanoudi S., Mollazadeh H., Boroumand-Noughabi S., Hosseini A. Reno-protective effect of Rheum turkestanicum against gentamicin-induced nephrotoxicity. Iran. J. Basic Med. Sci. 2019;22(3):328–333. doi: 10.22038/ijbms.2019.31552.7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boroushaki M.T. Evaluation of rheum turkestanicum in hexachlorobutadien-induced renal toxicity. Drug Res. 2019 doi: 10.1055/a-0821-5653. [DOI] [PubMed] [Google Scholar]
- 55.Waly M.I., Ali B.H., Al-Lawati I., Nemmar A. Protective effects of emodin against cisplatin-induced oxidative stress in cultured human kidney (HEK 293) cells. J. Appl. Toxicol. 2013;33(7):626–630. doi: 10.1002/jat.1788. [DOI] [PubMed] [Google Scholar]
- 56.Shin Y.J. Protective effects of quercetin against HgCl2-induced nephrotoxicity in Sprague-Dawley rats. J. Med. Food. 2015;18(5):524–534. doi: 10.1089/jmf.2014.3242. [DOI] [PubMed] [Google Scholar]
- 57.Lamberti M. Animal models in studies of cardiotoxicity side effects from antiblastic drugs in patients and occupational exposed workers. BioMed Res. Int. 2014:2014. doi: 10.1155/2014/240642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Turakhia S. Doxorubicin-induced cardiotoxicity: direct correlation of cardiac fibroblast and H9c2 cell survival and aconitase activity with heat shock protein 27. Am. J. Physiol. Heart Circ. Physiol. 2007;293(5):H3111–H3121. doi: 10.1152/ajpheart.00328.2007. [DOI] [PubMed] [Google Scholar]
- 59.Bryant J. Use of cardiac markers to assess the toxic effects of anthracyclines given to children with cancer: a systematic review. Eur. J. Cancer. 2007;43(13):1959–1966. doi: 10.1016/j.ejca.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 60.Pacher P., Csordás G., Hajnóczky G. Mitochondrial Ca2+ signaling and cardiac apoptosis. Neurosignals. 2001;10(3-4):200–223. doi: 10.1159/000046888. [DOI] [PubMed] [Google Scholar]
- 61.Hosseini A., Sahebkar A. Reversal of doxorubicin-induced cardiotoxicity by using phytotherapy: a review. J. Pharmacopuncture. 2017;20(4):243. doi: 10.3831/KPI.2017.20.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hosseini A., Rajabian A. Protective effect of Rheum turkestanikum root against doxorubicin-induced toxicity in H9c2 cells. Revista Brasileira de Farmacognosia. 2016;26(3):347–351. [Google Scholar]
- 63.Du Y., Ko K.M. Effects of pharmacological preconditioning by emodin/oleanolic acid treatment and/or ischemic preconditioning on mitochondrial antioxidant components as well as the susceptibility to ischemia-reperfusion injury in rat hearts. Mol. Cell. Biochem. 2006;288(1-2):135–142. doi: 10.1007/s11010-006-9129-3. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Y. Effect of emodin on the expression of TLR4 and P38MAPK in mouse cardiac tissues with viral myocarditis. Int. J. Clin. Exp. Pathol. 2016;9(10):10839–10845. [Google Scholar]
- 65.Chen Y.-W. 2013. Cardioprotective Effects of Quercetin in Cardiomyocyte under Ischemia/reperfusion Injury; p. 2013. Evidence-Based Complementary and Alternative Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dong Q. Quercetin attenuates doxorubicin cardiotoxicity by modulating Bmi-1 expression. Br. J. Pharmacol. 2014;171(19):4440–4454. doi: 10.1111/bph.12795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu M.H., Shan J., Li J., Zhang Y., Lin X.L. Resveratrol inhibits doxorubicin-induced cardiotoxicity via sirtuin 1 activation in H9c2 cardiomyocytes. Exp. Ther. Med. 2016;12(2):1113–1118. doi: 10.3892/etm.2016.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sin T.K. Resveratrol protects against doxorubicin-induced cardiotoxicity in aged hearts through the SIRT1-USP7 axis. J. Physiol. 2015;593(8):1887–1899. doi: 10.1113/jphysiol.2014.270101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tatlidede E. Resveratrol treatment protects against doxorubicin-induced cardiotoxicity by alleviating oxidative damage. Free Radic. Res. 2009;43(3):195–205. doi: 10.1080/10715760802673008. [DOI] [PubMed] [Google Scholar]
- 70.Al-Harthi S.E. Amelioration of doxorubicin-induced cardiotoxicity by resveratrol. Mol. Med. Rep. 2014;10(3):1455–1460. doi: 10.3892/mmr.2014.2384. [DOI] [PubMed] [Google Scholar]
- 71.Hassan M., Malik S. Hepatoprotective potential of ‘rheum emodi wall’on carbon tetrachloride-induced hepatic damage. Ann. Pak. Inst. Med. Sci. 2008;4(3):152–155. [Google Scholar]
- 72.Miltonprabu S. Hepatoprotective effect of quercetin: from chemistry to medicine. Food Chem. Toxicol. 2017;108:365–374. doi: 10.1016/j.fct.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 73.Akhtar M., Amin M., Ahmad M., Alamgeer A. Hepatoprotective effect of Rheum emodi roots (Revand chini) and Akseer-e-Jigar against paracetamol-induced hepatotoxicity in rats. Ethnobotanical Leafl. 2009;2009(2):3. [Google Scholar]
- 74.Abo-Salem O.M., Abd-Ellah M.F., Ghonaim M.M. Hepatoprotective activity of quercetin against acrylonitrile-induced hepatotoxicity in rats. J. Biochem. Mol. Toxicol. 2011;25(6):386–392. doi: 10.1002/jbt.20406. [DOI] [PubMed] [Google Scholar]
- 75.Peng Z. Hepatoprotective effect of quercetin against LPS/d-GalN induced acute liver injury in mice by inhibiting the IKK/NF-κB and MAPK signal pathways. Int. Immunopharmacol. 2017;52:281–289. doi: 10.1016/j.intimp.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 76.Bu T. Hepatoprotective effect of rhein against methotrexate-induced liver toxicity. Eur. J. Pharmacol. 2018;834:266–273. doi: 10.1016/j.ejphar.2018.07.031. [DOI] [PubMed] [Google Scholar]
- 77.Bhadauria M. Dose-dependent hepatoprotective effect of emodin against acetaminophen-induced acute damage in rats. Exp. Toxicol. Pathol. 2010;62(6):627–635. doi: 10.1016/j.etp.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 78.Zhao Y.L., Wang J.B., Zhou G.D., Shan L.M., Xiao X.H. Investigations of free anthraquinones from rhubarb against α-Naphthylisothiocyanate-induced cholestatic liver injury in rats. Basic Clin. Pharmacol. Toxicol. 2009;104(6):463–469. doi: 10.1111/j.1742-7843.2009.00389.x. [DOI] [PubMed] [Google Scholar]
- 79.Wang Z.-Y., Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood. 2008;111(5):2505–2515. doi: 10.1182/blood-2007-07-102798. [DOI] [PubMed] [Google Scholar]
- 80.Evens A.M., Tallman M.S., Gartenhaus R.B. The potential of arsenic trioxide in the treatment of malignant disease: past, present, and future. Leuk. Res. 2004;28(9):891–900. doi: 10.1016/j.leukres.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 81.M. M., A. A., Sadeghnia H.R., Hosseini A. Rheum turkestanicum induced apoptosis through ROS without differential effect on human leukemic cells. Jundishapur J. Nat. Pharm. Prod. 2018 [Google Scholar]
- 82.Shiezadeh F. Cytotoxic and apoptotic potential of Rheum turkestanicum Janisch root extract on human cancer and normal cells. Iran. J. Pharm. Res.: IJPR. 2013;12(4):811. [PMC free article] [PubMed] [Google Scholar]
- 83.Rauf A. Anticancer potential of quercetin: a comprehensive review. Phytother Res. 2018 doi: 10.1002/ptr.6155. [DOI] [PubMed] [Google Scholar]
- 84.Guo J.-m. Anticancer effect of aloe-emodin on cervical cancer cells involves G 2/M arrest and induction of differentiation. Acta Pharmacol. Sin. 2007;28(12):1991. doi: 10.1111/j.1745-7254.2007.00707.x. [DOI] [PubMed] [Google Scholar]
- 85.Michaels R., Rothman S.M. Glutamate neurotoxicity in vitro: antagonist pharmacology and intracellular calcium concentrations. J. Neurosci. 1990;10(1):283–292. doi: 10.1523/JNEUROSCI.10-01-00283.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.H. A., HR S., A. R., M. M. Rheum turkestanicum reduces glutamate toxicity in PC12 and N2a cell lines. Folia Neuropathol. 2018 doi: 10.5114/fn.2018.80869. [DOI] [PubMed] [Google Scholar]
- 87.Hamid Z., Waza A., Haq E. Rheum emodi ameliorates glutamate toxicity in neuronal cells by up-regulating Nrf2/HO-1 expression. Int. J. Adv. Res. Eng. 2018;7(4):788–795. [Google Scholar]
- 88.Ahn T.-B., Jeon B.S. The role of quercetin on the survival of neuron-like PC12 cells and the expression of α-synuclein. Neural Regen. Res. 2015;10(7):1113. doi: 10.4103/1673-5374.160106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bao D., Wang J., Pang X., Liu H. Protective effect of quercetin against oxidative stress-induced cytotoxicity in rat pheochromocytoma (PC-12) cells. Molecules. 2017;22(7):1122. doi: 10.3390/molecules22071122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu T., Jin H., Sun Q.-R., Xu J.-H., Hu H.-T. Neuroprotective effects of emodin in rat cortical neurons against β-amyloid-induced neurotoxicity. Brain Res. 2010;1347:149–160. doi: 10.1016/j.brainres.2010.05.079. [DOI] [PubMed] [Google Scholar]
- 91.Tao L. Protective effects of aloe-emodin on scopolamine-induced memory impairment in mice and H 2 O 2-induced cytotoxicity in PC12 cells. Bioorg. Med. Chem. Lett. 2014;24(23):5385–5389. doi: 10.1016/j.bmcl.2014.10.049. [DOI] [PubMed] [Google Scholar]
- 92.Valdivia-Correa B., Gómez-Gutiérrez C., Uribe M., Méndez-Sánchez N. Herbal medicine in Mexico: a cause of hepatotoxicity. A critical review. Int. J. Mol. Sci. 2016;17(2):235. doi: 10.3390/ijms17020235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mounanga M.B., Mewono L., Angone S.A. Toxicity studies of medicinal plants used in sub-Saharan Africa. J. Ethnopharmacol. 2015;174:618–627. doi: 10.1016/j.jep.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 94.Shukla V. Advances in Molecular Toxicology. Elsevier; 2017. Toxicity of naturally occurring anthraquinones; pp. 1–50. [Google Scholar]
- 95.Deng N. Mechanism of nephrotoxicity of rhubarb in rats. China J. Chin. Mater. Med. 2018;43(13):2777–2783. doi: 10.19540/j.cnki.cjcmm.20180314.001. [DOI] [PubMed] [Google Scholar]
- 96.Kim S.-J. Anti-Inflammatory activity of chrysophanol through the suppression of NF-kB/caspase-1 activation in vitro and in vivo. Molecules. 2010;15(9):6436–6451. doi: 10.3390/molecules15096436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lian Y., Xia X., Zhao H., Zhu Y. The potential of chrysophanol in protecting against high fat-induced cardiac injury through Nrf2-regulated anti-inflammation, anti-oxidant and anti-fibrosis in Nrf2 knockout mice. Biomed. Pharmacother. 2017;93:1175–1189. doi: 10.1016/j.biopha.2017.05.148. [DOI] [PubMed] [Google Scholar]
- 98.Zhang N. Chrysophanol inhibits NALP3 inflammasome activation and ameliorates cerebral ischemia/reperfusion in mice. Mediat. Inflamm. 2014;2014:370530. doi: 10.1155/2014/370530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhao Y. Neuroprotective effects of Chrysophanol against inflammation in middle cerebral artery occlusion mice. Neurosci. Lett. 2016;630:16–22. doi: 10.1016/j.neulet.2016.07.036. [DOI] [PubMed] [Google Scholar]
- 100.Chu X. Chrysophanol relieves cognition deficits and neuronal loss through inhibition of inflammation in diabetic mice. Neurochem. Res. 2018;43(4):972–983. doi: 10.1007/s11064-018-2503-1. [DOI] [PubMed] [Google Scholar]
- 101.Lin F.-L. The natural retinoprotectant chrysophanol attenuated photoreceptor cell apoptosis in an N-methyl-N-nitrosourea-induced mouse model of retinal degenaration. Sci. Rep. 2017;7:41086. doi: 10.1038/srep41086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jiang W. Protective effect of chrysophanol on LPS/d-GalN-induced hepatic injury through the RIP140/NF-κB pathway. RSC Adv. 2016;6(44):38192–38200. [Google Scholar]
- 103.Ji Z.-H., Xu Z.-Q., Zhao H., Yu X.-Y. Neuroprotective effect and mechanism of daucosterol palmitate in ameliorating learning and memory impairment in a rat model of Alzheimer’s disease. Steroids. 2017;119:31–35. doi: 10.1016/j.steroids.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 104.Jiang L.-h. Daucosterol protects neurons against oxygen–glucose deprivation/reperfusion-mediated injury by activating IGF1 signaling pathway. J. Steroid Biochem. Mol. Biol. 2015;152:45–52. doi: 10.1016/j.jsbmb.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 105.Lee J.-H. Immunoregulatory activity by daucosterol, a β-sitosterol glycoside, induces protective Th1 immune response against disseminated Candidiasis in mice. Vaccine. 2007;25(19):3834–3840. doi: 10.1016/j.vaccine.2007.01.108. [DOI] [PubMed] [Google Scholar]
- 106.Desai F. Comparison of the immunomodulatory effects of the plant sterol β-sitosterol to simvastatin in peripheral blood cells from multiple sclerosis patients. Int. Immunopharmacol. 2009;9(1):153–157. doi: 10.1016/j.intimp.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 107.Gupta R., Sharma A.K., Dobhal M., Sharma M., Gupta R. Antidiabetic and antioxidant potential of β-sitosterol in streptozotocin-induced experimental hyperglycemia. J. Diabetes. 2011;3(1):29–37. doi: 10.1111/j.1753-0407.2010.00107.x. [DOI] [PubMed] [Google Scholar]
- 108.Berges R., Windeler J., Trampisch H., Senge T. Randomised, placebo-controlled, double-blind clinical trial of beta-sitosterol in patients with benign prostatic hyperplasia. Beta-sitosterol Study Group. Lancet (London, England) 1995;345(8964):1529–1532. doi: 10.1016/s0140-6736(95)91085-9. [DOI] [PubMed] [Google Scholar]
- 109.Alisi A. Emodin prevents intrahepatic fat accumulation, inflammation and redox status imbalance during diet-induced hepatosteatosis in rats. Int. J. Mol. Sci. 2012;13(2):2276–2289. doi: 10.3390/ijms13022276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dong M.-X. Emodin protects rat liver from CCl4-induced fibrogenesis via inhibition of hepatic stellate cells activation. World J. Gastroenterol. 2009;15(38):4753–4762. doi: 10.3748/wjg.15.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hwang J.-K. Emodin suppresses inflammatory responses and joint destruction in collagen-induced arthritic mice. Rheumatology. 2013;52(9):1583–1591. doi: 10.1093/rheumatology/ket178. [DOI] [PubMed] [Google Scholar]
- 112.Tzeng T.-F., Lu H.-J., Liou S.-S., Chang C.J., Liu I.-M. Emodin, a naturally occurring anthraquinone derivative, ameliorates dyslipidemia by activating AMP-activated protein kinase in high-fat-diet-fed rats. Evid. Based Complement Altern. Med. 2012;2012:781812. doi: 10.1155/2012/781812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wu Z., Chen Q., Ke D., Li G., Deng W. Emodin protects against diabetic cardiomyopathy by regulating the AKT/GSK-3β signaling pathway in the rat model. Molecules. 2014;19(9):14782–14793. doi: 10.3390/molecules190914782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Feng Y. Emodin, a natural product, selectively inhibits 11β-hydroxysteroid dehydrogenase type 1 and ameliorates metabolic disorder in diet-induced obese mice. Br. J. Pharmacol. 2010;161(1):113–126. doi: 10.1111/j.1476-5381.2010.00826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen X.H. Inhibitory effect of emodin on bleomycin-induced pulmonary fibrosis in mice. Clin. Exp. Pharmacol. Physiol. 2009;36(2):146–153. doi: 10.1111/j.1440-1681.2008.05048.x. [DOI] [PubMed] [Google Scholar]
- 116.Wang C.-H. Effect of emodin on pancreatic fibrosis in rats. World J. Gastroenterol. 2007;13(3):378–382. doi: 10.3748/wjg.v13.i3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Guan R. Emodin ameliorates bleomycin-induced pulmonary fibrosis in rats by suppressing epithelial-mesenchymal transition and fibroblast activation. Sci. Rep. 2016;6:35696. doi: 10.1038/srep35696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schroeter H. (–)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc. Natl. Acad. Sci. Unit. States Am. 2006;103(4):1024–1029. doi: 10.1073/pnas.0510168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gómez-Guzmán M. Epicatechin lowers blood pressure, restores endothelial function, and decreases oxidative stress and endothelin-1 and NADPH oxidase activity in DOCA-salt hypertension. Free Radic. Biol. Med. 2012;52(1):70–79. doi: 10.1016/j.freeradbiomed.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 120.Van Praag H. Plant-derived flavanol (−) epicatechin enhances angiogenesis and retention of spatial memory in mice. J. Neurosci. 2007;27(22):5869–5878. doi: 10.1523/JNEUROSCI.0914-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shah Z.A. The flavanol (−)-epicatechin prevents stroke damage through the Nrf2/HO1 pathway. J. Cereb. Blood Flow Metab. 2010;30(12):1951–1961. doi: 10.1038/jcbfm.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yamazaki K.G. Effects of (−)-epicatechin on myocardial infarct size and left ventricular remodeling after permanent coronary occlusion. J. Am. Coll. Cardiol. 2010;55(25):2869–2876. doi: 10.1016/j.jacc.2010.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Si H. Dietary epicatechin improves survival and delays skeletal muscle degeneration in aged mice. FASEB J. 2019;33(1):965–977. doi: 10.1096/fj.201800554RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Majeed M., Majeed S., Pande A., Karri S.K. Amelioration of carbon tetrachloride induced hepatotoxicity by Β-glucogallin, a gallic acid derivative of emblica officinalis gaertn.(euphorbiaceae) Science. 2015;4(7):696–701. [Google Scholar]
- 125.Chang K.-C. Beta-glucogallin reduces the expression of lipopolysaccharide-induced inflammatory markers by inhibition of aldose reductase in murine macrophages and ocular tissues. Chem. Biol. Interact. 2013;202:283–287. doi: 10.1016/j.cbi.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chao J. Gallic acid ameliorated impaired glucose and lipid homeostasis in high fat diet-induced NAFLD mice. PLoS One. 2014;9(6) doi: 10.1371/journal.pone.0096969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ahad A., Ahsan H., Mujeeb M., Siddiqui W.A. Gallic acid ameliorates renal functions by inhibiting the activation of p38 MAPK in experimentally induced type 2 diabetic rats and cultured rat proximal tubular epithelial cells. Chem. Biol. Interact. 2015;240:292–303. doi: 10.1016/j.cbi.2015.08.026. [DOI] [PubMed] [Google Scholar]
- 128.Ryu Y. Gallic acid prevents isoproterenol-induced cardiac hypertrophy and fibrosis through regulation of JNK2 signaling and Smad3 binding activity. Sci. Rep. 2016;6:34790. doi: 10.1038/srep34790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang P. Protective effects of physcion against cerebral injury induced by ischemiareperfusion in rats. Chin. J. Pathophysiol. 2005;21:1829–1833. [Google Scholar]
- 130.Xu N.-G., Xiao Z.-J., Zou T., Huang Z.-L. Ameliorative effects of physcion 8-O-β-glucopyranoside isolated from Polygonum cuspidatum on learning and memory in dementia rats induced by A β 1–40. Pharmaceut. Biol. 2015;53(11):1632–1638. doi: 10.3109/13880209.2014.997251. [DOI] [PubMed] [Google Scholar]
- 131.Zhou Z.Q. Physcion induces vasorelaxation in rat aorta through endothelium-dependent and endothelium-independent mechanisms. FASEB J. 2016;30 lb576-lb576. [Google Scholar]
- 132.Chen X., Guo H., Li F., Fan D. Physcion 8-O-β-glucopyranoside suppresses the metastasis of breast cancer in vitro and in vivo by modulating DNMT1. Pharmacol. Rep. 2017;69(1):36–44. doi: 10.1016/j.pharep.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 133.Wei-Jun F. In vivo and in vitro anti-sepsis effects of physcion 8-O-β-glucopyranoside extracted from Rumex japonicus. Chin. J. Nat. Med. 2017;15(7):534–539. doi: 10.1016/S1875-5364(17)30079-1. [DOI] [PubMed] [Google Scholar]
- 134.Lesjak M. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Food. 2018;40:68–75. [Google Scholar]
- 135.Godoy J.A. Quercetin exerts differential neuroprotective effects against H 2 O 2 and Aβ aggregates in hippocampal neurons: the role of mitochondria. Mol. Neurobiol. 2017;54(9):7116–7128. doi: 10.1007/s12035-016-0203-x. [DOI] [PubMed] [Google Scholar]
- 136.Polera N., Badolato M., Perri F., Carullo G., Aiello F. Quercetin and its natural sources in wound healing management. Curr. Med. Chem. 2018 doi: 10.2174/0929867325666180713150626. in press. [DOI] [PubMed] [Google Scholar]
- 137.Porras D. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free Radic. Biol. Med. 2017;102:188–202. doi: 10.1016/j.freeradbiomed.2016.11.037. [DOI] [PubMed] [Google Scholar]
- 138.Chen S., Jiang H., Wu X., Fang J. Therapeutic effects of quercetin on inflammation, obesity, and type 2 diabetes. Mediat. Inflamm. 2016;2016:9340637. doi: 10.1155/2016/9340637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhao Y.-L. Rhein protects against acetaminophen-induced hepatic and renal toxicity. Food Chem. Toxicol. 2011;49(8):1705–1710. doi: 10.1016/j.fct.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 140.Sheng-Nan P., Hui-Hong Z., Ai-Xiang F., Xiao-Wen C., Qing-Xian Z. Protection of rhein on IgA nephropathy mediated by inhibition of fibronectin expression in rats. Indian J. Pharmacol. 2013;45(2):174–179. doi: 10.4103/0253-7613.108309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu J. Rhein protects pancreatic β-cells from dynamin-related protein-1-mediated mitochondrial fission and cell apoptosis under hyperglycemia. Diabetes. 2013;62(11):3927–3935. doi: 10.2337/db13-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang Y. Rhein reduces fat weight in db/db mouse and prevents diet-induced obesity in C57Bl/6 mouse through the inhibition of PPARγ signaling. PPAR Res. 2012;2012:374936. doi: 10.1155/2012/374936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tsang S.W. Rhein, a natural anthraquinone derivative, attenuates the activation of pancreatic stellate cells and ameliorates pancreatic fibrosis in mice with experimental chronic pancreatitis. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0082201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhen Y.Z. Effects of rhein lysinate on D-galactose-induced aging mice. Exp. Ther. Med. 2016;11(1):303–308. doi: 10.3892/etm.2015.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jeon Y.-J. Rhododendrin ameliorates skin inflammation through inhibition of NF-κB, MAPK, and PI3K/Akt signaling. Eur. J. Pharmacol. 2013;714(1-3):7–14. doi: 10.1016/j.ejphar.2013.05.041. [DOI] [PubMed] [Google Scholar]
- 146.Jeon Y.-J. Rhododendrin inhibits toll-like receptor-7-mediated psoriasis-like skin inflammation in mice. Exp. Mol. Med. 2017;49(6):e349. doi: 10.1038/emm.2017.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fushiya S., Kabe Y., Ikegaya Y., Takano F. +)-rhododendrol and epi-rhododendrin suppress the NO production by activated macrophages in vivo. Planta Med. 1998;64(7):598–602. doi: 10.1055/s-2006-957529. [DOI] [PubMed] [Google Scholar]
- 148.Kim M.-H., Nugroho A., Choi J., Park J.H., Park H.-J. Rhododendrin, an analgesic/anti-inflammatory arylbutanoid glycoside, from the leaves of Rhododendron aureum. Arch Pharm. Res. (Seoul) 2011;34(6):971–978. doi: 10.1007/s12272-011-0614-1. [DOI] [PubMed] [Google Scholar]