Abstract
Objectives
Transforaminal percutaneous endoscopic discectomy (TPED) is one of the most commonly used minimally invasive spine surgeries around the world. However, conventional surgical planning and intraoperative procedures for TPED have relied on surgeons’ experience, which limits its standardization and popularization. Virtual reality (VR) is a novel technology for pre‐surgical planning in various fields of medicine, while isocentric navigation can guide intraoperative procedures for TPED. The present study aimed to explore the feasibility of applying VR combined with isocentric navigation in TPED on cadavers.
Methods
The surgical levels were L3/L4 and L4/L5 as well as L5/S1 of both sides of each cadaver specimen. First, the surgeon manually conducted the above procedures on the left side of every specimen without preoperative simulation and isocentric navigation (Group A). Then the same surgeon conducted the VR simulation for surgical planning of the right side (Group B). After VR simulation, the same surgeon made the percutaneous punctures and placed the working channel on the right side of the specimen at all levels.
Results
At the L3/L4 level, the puncture‐channel time was 11.36 ± 2.13 min in Group A and 11.29 ± 2.23 min in Group B (t = 0.097, P = 0.938). The exposure time was 17.21 ± 2.91 s in Group A and 14.64 ± 1.60 s in Group B (t = 2.534, P = 0.025). At the L4/L5 level, the puncture‐channel time was 13.86 ± 3.90 min in Group A and 11.93 ± 2.95 min in Group B (t = 2.291, P = 0.039). Exposure time was 20.64 ± 3.84 s in Group A and 16.43 ± 2.47 s in Group B (t = 6.118, P < 0.01). There were 7 patients undergoing foraminotomy in Group A and 3 patients undergoing foraminotomy in Group B (t = 2.280, P = 0.236). At the L5/S1 level, the puncture‐channel time was 18.21 ± 1.85 min in Group A and 15.71 ± 3.20 min in Group B (t = 2.476, P = 0.028). Exposure time was 26.07 ± 3.17 s in Group A and 22.50 ± 2.68 s in Group B (t = 2.980, P = 0.011). There were 14 patients receiving foraminotomy in Group A and 13 patients receiving foraminotomy in Group B (t = 1.000, P = 1.000).
Conclusions
Virtual reality combined with isocentric navigation is feasible in TPED. It enables precise surgical planning and improves intraoperative procedures, and has the potential for application in clinical practice.
Keywords: Isocentric navigation, Transforaminal percutaneous endoscopic discectomy, Virtual reality
Introduction
Low back pain and sciatic pain are commonly attributed to symptomatic lumbar disc herniation (LDH)1, 2. With the development of medical instruments and optic techniques, minimally invasive spine surgery (MISS) has been widely used in LDH all over the world3, 4, 5. MISS has several identified advantages compared to open spine surgery, including less tissue injury, reduced blood loss, minimal postoperative pain, lower postoperative expense, and shorter hospital stays6, 7, 8, 9. Transforaminal percutaneous endoscopic discectomy (TPED) is a frequently used MISS because of the abovementioned advantages10. TPED has been demonstrated to have non‐inferior efficacy to open microdiscectomy11. However, learning and mastering TPED is a great challenge, because the surgeon needs to blindly build an optimal surgical trajectory, including an ideal puncture followed by the placement of the working channel, which largely relies on surgeons’ experience12. TPED requires repeated and frequent fluoroscopy to localize and calibrate the medical instruments to the optimal trajectory, but the inconvenient truth is that many surgeons do not have the capacity to transform the two‐dimensional fluoroscopy images into three‐dimensional (3D) images. In addition, the repeated X‐ray fluoroscopy may result in radiation exposure for the surgeons and patients, potentially negatively impacting junior surgeons’ confidence as well as surgeons’ and patients’ health13, 14, 15. Preoperative virtual reality (VR) simulation of TPED may help improve surgical efficiency and surgeons’ confidence as well as decrease radiation exposure.
In surgery, a detailed and clear anatomy understanding of the surgical target and surrounding tissue plays a very important role in safety and surgical outcomes. Feasibility studies have attempted to simulate TPED for surgeons in software based on 3D views prior to surgery16, 17. As a novel technology, VR is receiving increasing attention and has bright prospects in many medical fields when surgical planning, pain management, and therapeutic treatment of mental illness are considered18, 19. In terms of surgical planning, the benefits of VR have been demonstrated in preoperative planning for various surgeries20, 21, 22. The successful application of VR in surgical planning can be explained by the overwhelming advantages over simple 3D simulation. VR enables more accurate, realistic, vivid, and intuitive surgical analysis. However, the application of VR in spinal surgery is very limited, and it has been suggested that the paucity of neurosurgical simulator devices in the spine discipline is the greatest issue within the neurological surgical subspecialty23. To the best of our knowledge, no reported study has adopted VR technology in simulating TPED prior to surgery.
In previous studies, we introduced an isocentric navigation system based on two‐dimensional image planning for TPED24, 25. The surgical planning for TPED based on two‐dimensional images is certainly limited and not very efficient. However, we must acknowledge that foraminotomy is the key to the success of TPED. The reliance on surgeon experience for preoperative location and repeat intraoperative fluoroscopy in the puncture‐channel process make TPED difficult to standardize, which also affects TPED's development. The aim of the study is to explore the feasibility of applying VR in TPED combined with isocentric navigation in cadavers.
The use of VR is becoming increasingly popular, and previous research has revealed that isocentric navigation is beneficial for lumbar surgery. The aim of the study was: (i) to verify whether it is feasible to apply VR in preoperative planning; (ii) to research the feasibility of VR combined with isocentric navigation in TPED; and (iii) to explore whether VR could improve intraoperative procedures in TPED.
Materials and Methods
General Information
Participates
The study was approved by the local institutional review board (2015‐RES‐127). From September 2015 to March 2018, a total of 12 cadaver specimens were included in the study, which were all from the Spinal Endoscopy Training Courses. There were eight male cadaver specimens and four female cadaver specimens. Ten cadaver specimens were intact; one had no upper limbs and one had no lower limbs. All the cadaver specimens had undergone no previous lumbar surgery. All the cadaver specimens had no obvious lumbar deformity or vertebrae fractures, as confirmed by X‐ray fluoroscopy. All the involved operating processes and procedures met the specifications of the cadaver management standards.
Interventions
All specimens were placed in the prone position for the operation. A total of four experienced surgeons were asked to perform all the percutaneous punctures and the placement of the working channel. The surgical levels were L3/L4 and L4/L5 as well as L5/S1 of both sides of each cadaver specimen.
Comparisons
First, the surgeon manually conducted the above procedures on the left side of every specimen without preoperative simulation and isocentric navigation (Group A). Then the same surgeon conducted the VR simulation for surgical planning of the right side (Group B). oAfter VR simulation, the same surgeon performed the percutaneous punctures and the placement of the working channel on the right side of the specimen at all levels.
Outcomes
We hypothesized that all surgical levels had central disc herniation, which was regarded as the surgical target of all trajectories. A successful puncture is defined as the tip of the needle docked in the superior facet joint of the inferior vertebrae, while the needle points to the surgical target. Placement success of the working channel is defined as the tip of the working channel arriving at the surgical target through the brim of the facet joint. The observational parameters included puncture frequency, puncture‐channel time, and radiation exposure time.
Virtual Reality for Surgical Planning
All the specimens underwent CT scans of the lumbar prior to operation in prone position and the thin‐layer CT images were saved as DICOM data, which were then imported into VR software (Fig. 1). The software automatically reconstructed the skeleton of the lumbar (Fig. 2). The surgeon was asked to wear the glasses and become immersed in the VR to have a spatial sense of the specific anatomy. Then, the surgeon selected the puncture target point and the software automatically generated a suboptimal puncture trajectory. After that, the surgeon could precisely calibrate the puncture trajectory in VR and present the working channel along with the puncture trajectory (Fig. 3). The software will reveal how much bone you may remove for foraminotomy and automatically calculate the angle θ (between the trajectory and the coronary plane) and angle ξ (between the lateral axis that is perpendicular to the cephalocaudal axis on the coronal plane and the posterior projection of the trajectory, Fig. 4A). These two angles can be used for isocentric navigation to guide the trajectory in practice.
Figure 1.
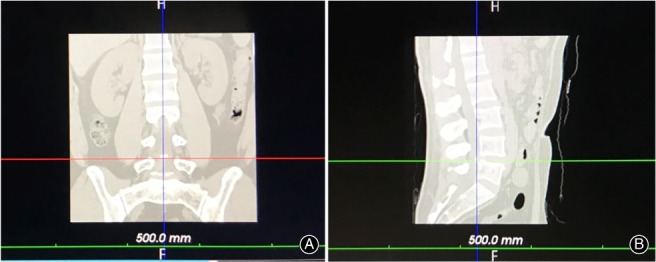
(A) The specimens underwent coronal CT scan of the lumbar prior to surgery and the thin‐layer CT were saved as DICOM data. (B) The specimens underwent sagittal CT scan of the lumbar prior to surgery and the thin‐layer CT were saved as DICOM data.
Figure 2.
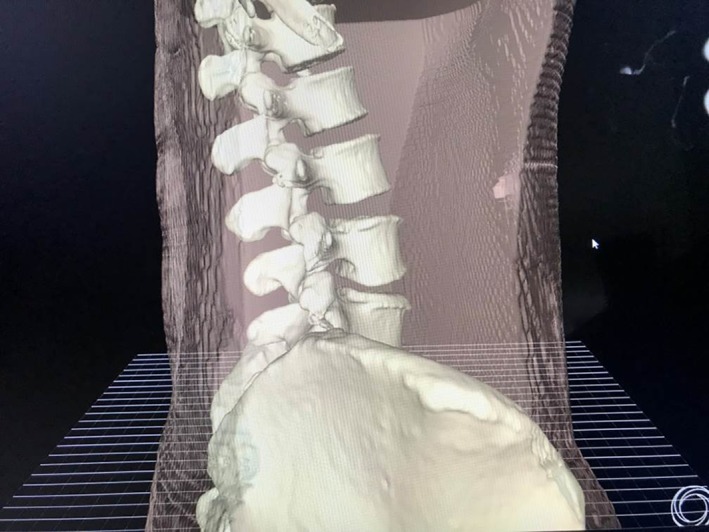
Three‐dimensional skeleton of the lumbar reconstructed by virtual reality software.
Figure 3.
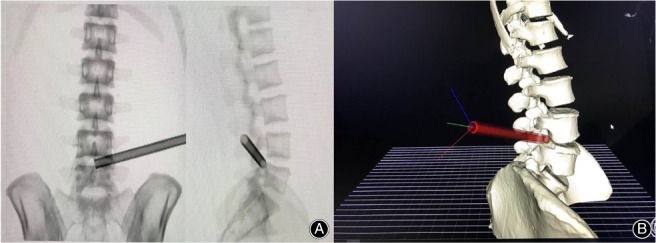
(A) The two‐dimensional working channel calibrated by the surgeon in virtual reality (VR). (B) The three‐dimensional working channel calibrated by the surgeon in VR.
Figure 4.
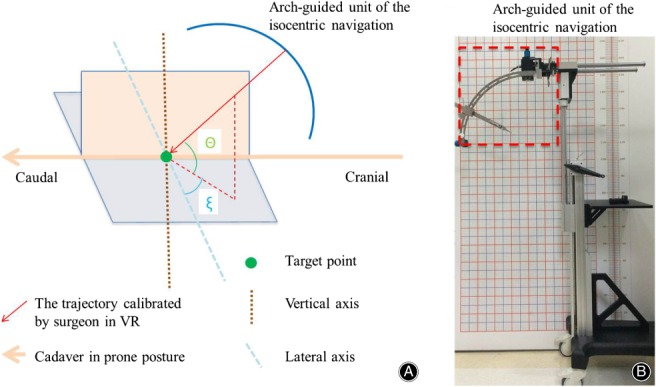
(A) Angle θ stands for the angle between the trajectory calibrated by the surgeon in virtual reality (VR) and its posterior projection on coronal plane. The lateral axis is the line that is perpendicular to the cephalocaudal axis on the coronal plane. Angle ξ represents the angle between the posterior projection of the trajectory and the lateral axis on the coronal plane. (B) The isocentric navigation device. The part circled by a red line represents the arch‐guided unit of the isocentric navigation.
Isocentric Navigation
The isocentric navigation for TPED has been well documented in previous literature24, 25 (Fig. 4B). Briefly, we attached two radiopaque grids to the back skin and lateral skin of the cadaver (Fig. 5A). Then we conducted the X‐ray fluoroscopy and identified the target point on anteroposterior fluoroscopy and lateral fluoroscopy. Given the relative relationship by two radiopaque grids, we identified the posterior projection point and the lateral projection point of the target on the back skin and the lateral skin, respectively. After that, we ensured that the posterior and lateral laser beams of the isocentric navigation were directed onto the two projection points (Fig. 5B). As a result, every trajectory of the isocentric navigation always points to the center of the target. Then, we selected the trajectory based on the angle ξ and angle θ. Finally, a hard needle was inserted along with the selected trajectory. Considering a certain length of the radius, we can control the insertion depth of the hard needle.
Figure 5.
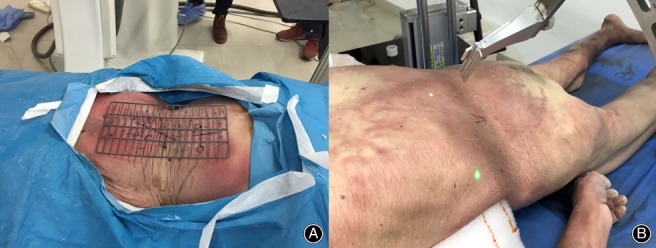
(A) The radiopaque grid was attached to the cadaveric specimen. (B) The posterior and lateral laser beams of the isocentric navigation directing onto the two projection points.
Statistical Analysis
All the data was analyzed using the software package SPSS 12.0 (SPSS, USA). The data were presented as mean ± SD. Continuous variables between two groups were compared by Student's t test. Categorical variables between Group A and Group B were analyzed by χ2‐test. P < 0.05 was regarded as statistically significant.
Results
Puncture Frequency
All procedures were completed successfully. At the L3/L4 level, the puncture frequency was 3.14 ± 1.35 in Group A and 1.14 ± 0.36 in Group B (t = 4.926, P < 0.01). At the L4/L5 level, the puncture frequency was 3.43 ± 1.65 in Group A and 1.36 ± 0.74 in Group B (t = 5.025, P < 0.01). It was 4.71 ± 1.38 in Group A and 1.50 ± 0.76 in Group B at the L5/S1 level (t = 7.623, P < 0.01). This showed that VR combined with isocentric navigation could decrease puncture frequency at L3/L4, L4/L5, and L5/S1 levels significantly (Table 1).
Table 1.
Surgical outcomes of puncture frequency (mean ± SD)
| Groups | L3/L4 level | L4/L5 level | L5/S1 level |
|---|---|---|---|
| Group A | 3.14 ± 1.35 | 3.43 ± 1.65 | 4.71 ± 1.38 |
| Group B | 1.14 ± 0.36 | 1.36 ± 0.74 | 1.50 ± 0.76 |
| t value | 4.926 | 5.025 | 7.623 |
| P value | <0.01 | <0.01 | <0.01 |
Puncture‐channel Time
At the L3/L4 level, the puncture‐channel time was 11.36 ± 2.13 min in Group A and 11.29 ± 2.23 min in Group B (t = 0.079, P = 0.938). There was no significant benefit in applying VR combined with isocentric navigation at the L3/L4 level. At the L4/L5 level, the puncture‐channel time was 13.86 ± 3.90 min in Group A and 11.93 ± 2.95 min in Group B (t = 2.291, P = 0.039). It was 18.21 ± 1.85 min in Group A and 15.71 ± 3.20 min in Group B at the L5/S1 level (t = 2.476, P = 0.028). This revealed that VR combined with isocentric navigation could make the puncture‐channel procedure easier at L4/L5 and L5/S1 levels (Table 2).
Table 2.
Surgical outcomes of puncture‐channel time (minutes, mean ± SD)
| Groups | L3/L4 level | L4/L5 level | L5/S1 level |
|---|---|---|---|
| Group A | 11.36 ± 2.13 | 13.86 ± 3.90 | 18.21 ± 1.85 |
| Group B | 11.29 ± 2.23 | 11.93 ± 2.95 | 15.71 ± 3.20 |
| t value | 0.079 | 2.291 | 2.476 |
| P value | 0.938 | 0.039 | 0.028 |
Exposure Time
Exposure time was 17.21 ± 2.91 s in Group A and 14.64 ± 1.60 s in Group B at the L3/L4 level (t = 2.534, P = 0.025). At the L4/L5 level, the exposure time was 20.64 ± 3.84 s in Group A and 16.43 ± 2.47 s in Group B (t = 6.118, P < 0.01). At the L5/S1 level, the exposure time was 26.07 ± 3.17 s in Group A and 22.50 ± 2.68 s in Group B (t = 2.980, P = 0.011). VR combined with isocentric navigation could protect surgeons and patients by reducing radiation exposure (Table 3).
Table 3.
Surgical outcomes of exposure time (s, mean ± SD)
| Groups | L3/L4 level | L4/L5 level | L5/S1 level |
|---|---|---|---|
| Group A | 17.21 ± 2.91 | 20.64 ± 3.84 | 26.07 ± 3.17 |
| Group B | 14.64 ± 1.60 | 16.43 ± 2.47 | 22.50 ± 2.68 |
| t value | 2.534 | 6.118 | 2.980 |
| P value | 0.025 | <0.01 | 0.011 |
Foraminotomy
For some cases, foraminotomy was needed to achieve satisfactory placement of the working channel. At the L3/L4 level, there was no patient receiving foraminotomy in either group. At the L4/L5 level, there were 7 patients receiving foraminotomy in Group A and 3 patients receiving foraminotomy in Group B (χ 2 = 2.280, P = 0.236). At the L5/S1 level, there were 14 patients receiving foraminotomy in Group A and 13 patients receiving foraminotomy in Group B (χ 2 = 1.000, P = 1.000). This showed that VR combined with isocentric navigation did not decrease the need for foraminotomy (Table 4).
Table 4.
Surgical outcomes of foraminotomy (cases)
| Groups | L4/L5 level | L5/S1 level |
|---|---|---|
| Group A | 7 | 14 |
| Group B | 3 | 13 |
| χ 2 value | 2.280 | 1.000 |
| P value | 0.236 | 1.000 |
Discussion
Difficulties of Mastering Transforaminal Percutaneous Endoscopic Discectomy
Puncture and working channel placement are the most challenging procedures for TPED, which rely on ideal trajectory planning and skillful manipulation of the surgeon. In this study, the combination of VR and isocentric navigation is found to be feasible, and it reduced the puncture frequency, the puncture‐channel time, and the radiation exposure time.
Precision is the key to the success of minimally invasive spine surgery, when you try to arrive at the lesion using a tiny incision. Considering the complicated anatomy of the lumbosacral structure, surgical planning plays an important role in achieving precision in MISS, especially in TPED. This is because the trajectory of TPED is a cranial–caudal, dorsal–ventral, and lateral–medial approach through skin, subcutaneous tissue, muscle, and foramina. It is very tricky because the ideal trajectories for every lesion are limited. If you select an inappropriate trajectory, you may injure the nerve root or visceral organs or important vessels. Even when you select a suboptimal trajectory without any injuries, you may increase the difficulty of foraminotomy or of removing the herniated disc. The inconvenient truth is that the patient is sober on the operation table. Larger foraminotomy will induce more pain for the patient. In this study, we observed that VR combined with isocentric navigation had great potential to reduce the necessity for foraminotomy in practice.
Present Strategies to Help Master Transforaminal Percutaneous Endoscopic Discectomy
During the development of TPED, experienced surgeons have proposed a practical marking method for surgical planning. It is recommended that you can mark the entry point with a rough distance from the midline; namely, 8–10 cm for L3‐4 level, 11–14 cm for L4‐5 level, and 12–16 cm for L5S1 level 26, 27. However, the exact distance for every individual patient is judged by surgeons’ experience based on the gender, body size, and anatomy of the patient14. Therefore, this process cannot be standardized. Marking the planned trajectory on the patient's skin is still associated with potential errors because the skin is flexible and surgeons tend to touch the skin a lot. Some studies have conducted surgical planning of TPED in numerical 3D models reconstructed by thin‐layer CT16, 17. However, surgeons still may not have a strong spatial sense of the planned trajectory, especially in practice without any reference for the planned trajectory.
Virtual Reality Combined with Isocentric Navigation in Transforaminal Percutaneous Endoscopic Discectomy
Virtual reality technology has existed for many years, but use was originally limited to, for instance, the military and space flight practice due to the large and expensive hardware required. As the hardware is evolving, VR is becoming a popular technology and has been applied in a wide range of fields, including surgical training28, rehabilitation29, psychological therapy30, and pain management31. The core of VR technology is simulation, which enables an immersive interactive environment based on computable information. In the virtual environment, users interact with objects in a natural manner with the aid of necessary devices, thereby generating feelings and experiences that are almost the same as in the real world environment. We believe that immersed feedback from VR allows the surgeon to develop a more accurate spatial concept of the ideal trajectory, and how much bone should be removed for foraminotomy. The exciting thing is that VR can be perfectly combined with isocentric navigation for intraoperative procedures. It has been indicated in this study that VR combined with isocentric navigation could significantly reduce puncture frequency, puncture‐channel time, and radiation exposure.
Limitations
Some issues should be clarified when interpreting the data of this study. First, this feasibility study did not investigate the potential merits of applying VR in training surgeons for TPE. This is because we have not developed a force‐feedback module for simulating foraminotomy. We do believe that VR combined with isocentric navigation also has potential to train junior surgeons for TPED. However, because the purpose of the study is to investigate the feasibility of combining VR and isocentric navigation for TPED, we still believe that this study has significance in improving intraoperative navigation with precise surgical planning. Second, we only hypothesized that all the specimens had central disc herniation at each level due to the limited number of specimens. Thus, VR combined with isocentric navigation for conditions with other types of disc herniation will be investigated in future clinical applications.
Conclusion
Virtual reality combined with isocentric navigation is feasible in TPED with advantages including precise surgical planning and improved intraoperative procedures, and has the potential to be applied in clinical practice.
Acknowledgments
Thanks to the Shanghai Tenth People's Hospital for providing spinal endoscopy training courses.
Grant Sources: The study was funded by the project of Shanghai Hospital Development Center (Grant No. 16CR3017A).
Disclosure: The authors declare no conflicts of interest.
Contributor Information
Guo‐xin Fan, Email: gfan@tongji.edu.cn.
Shi‐sheng He, Email: tjhss7418@tongji.edu.cn.
References
- 1. Mahesha K. Percutaneous endoscopic lumbar discectomy: results of first 100 cases. Ind J Orthop, 2017, 51: 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rasouli MR, Rahimi‐Movaghar V, Shokraneh F, Moradi‐Lakeh M, Chou R. Minimally invasive discectomy versus microdiscectomy/open discectomy for symptomatic lumbar disc herniation. Cochrane Database Syst Rev, 2014, 9, Sep 4: CD010328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hu W, Tang J, Wu X, Zhang L, Ke B. Minimally invasive versus open transforaminal lumbar fusion: a systematic review of complications. Int Orthop, 2016, 40: 1883–1890. [DOI] [PubMed] [Google Scholar]
- 4. Ahn Y. Transforaminal percutaneous endoscopic lumbar discectomy: technical tips to prevent complications. Expert Rev Med Devices, 2012, 9: 361–366. [DOI] [PubMed] [Google Scholar]
- 5. Zhu Y, Zhao Y, Fan G, et al Comparison of the effects of local anesthesia and epidural anesthesia for percutaneous transforaminal endoscopic discectomy in elderly patients over 65 years old. Int J Surg, 2017, 48: 260–263. [DOI] [PubMed] [Google Scholar]
- 6. Goldstein CL, Macwan K, Sundararajan K, Rampersaud YR. Perioperative outcomes and adverse events of minimally invasive versus open posterior lumbar fusion: meta‐analysis and systematic review. J Neurosurg Spine, 2016, 24: 416–427. [DOI] [PubMed] [Google Scholar]
- 7. McAnany SJ, Overley SC, Kim JS, Baird EO, Qureshi SA, Anderson PA. Open versus minimally invasive fixation techniques for thoracolumbar trauma: a meta‐analysis. Global Spine J, 2016, 6: 186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Phan K, Mobbs RJ. Minimally invasive versus open laminectomy for lumbar stenosis: a systematic review and meta‐analysis. Spine (Phila Pa 1976), 2016, 41: E91–E100. [DOI] [PubMed] [Google Scholar]
- 9. Ahn Y. Percutaneous endoscopic decompression for lumbar spinal stenosis. Expert Rev Med Devices, 2014, 11: 605–616. [DOI] [PubMed] [Google Scholar]
- 10. Gadjradj PS, Harhangi BS. Percutaneous transforaminal endoscopic discectomy for lumbar disk herniation. Clin Spine Surg, 2016, 29: 368–371. [DOI] [PubMed] [Google Scholar]
- 11. Ahn SS, Kim SH, Kim DW, Lee BH. Comparison of outcomes of percutaneous endoscopic lumbar discectomy and open lumbar microdiscectomy for young adults: a retrospective matched cohort study. World Neurosurg, 2016, 86: 250–258. [DOI] [PubMed] [Google Scholar]
- 12. Ruetten S, Komp M, Merk H, Godolias G. Use of newly developed instruments and endoscopes: full‐endoscopic resection of lumbar disc herniations via the interlaminar and lateral transforaminal approach. J Neurosurg Spine, 2007, 6: 521–530. [DOI] [PubMed] [Google Scholar]
- 13. Ahn Y, Kim CH, Lee JH, Lee SH, Kim JS. Radiation exposure to the surgeon during percutaneous endoscopic lumbar discectomy: a prospective study. Spine (Phila Pa 1976), 2013, 38: 617–625. [DOI] [PubMed] [Google Scholar]
- 14. Fan G, Gu X, Liu Y, et al Lower learning difficulty and fluoroscopy reduction of transforaminal percutaneous endoscopic lumbar discectomy with an accurate preoperative location method. Pain Physician, 2016, 19: E1123–E1134. [PubMed] [Google Scholar]
- 15. Fan G, Guan X, Zhang H, et al Significant improvement of puncture accuracy and fluoroscopy reduction in percutaneous transforaminal endoscopic discectomy with novel lumbar location system: preliminary report of prospective hello study. Medicine (Baltimore), 2015, 94: e2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hu Z, Li X, Cui J, et al Significance of preoperative planning software for puncture and channel establishment in percutaneous endoscopic lumbar DISCECTOMY: a study of 40 cases. Int J Surg, 2017, 41: 97–103. [DOI] [PubMed] [Google Scholar]
- 17. Chen X, Cheng J, Gu X, Sun Y, Politis C. Development of preoperative planning software for transforaminal endoscopic surgery and the guidance for clinical applications. Int J Comput Assist Radiol Surg, 2016, 11: 613–620. [DOI] [PubMed] [Google Scholar]
- 18. Mazur T, Mansour TR, Mugge L, Medhkour A. Virtual reality‐based simulators for cranial tumor surgery: a systematic review. World Neurosurg, 2018, 110: 414–422. [DOI] [PubMed] [Google Scholar]
- 19. Li L, Yu F, Shi D, et al Application of virtual reality technology in clinical medicine. Am J Transl Res, 2017, 9: 3867–3880. [PMC free article] [PubMed] [Google Scholar]
- 20. Kim Y, Kim H, Kim YO. Virtual reality and augmented reality in plastic surgery: a review. Arch Plast Surg, 2017, 44: 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pelargos PE, Nagasawa DT, Lagman C, et al Utilizing virtual and augmented reality for educational and clinical enhancements in neurosurgery. J Clin Neurosci, 2017, 35: 1–4. [DOI] [PubMed] [Google Scholar]
- 22. Bernhardt S, Nicolau SA, Soler L, Doignon C. The status of augmented reality in laparoscopic surgery as of 2016. Med Image Anal, 2017, 37: 66–90. [DOI] [PubMed] [Google Scholar]
- 23. Pfandler M, Lazarovici M, Stefan P, Wucherer P, Weigl M. Virtual reality‐based simulators for spine surgery: a systematic review. Spine J, 2017, 17: 1352–1363. [DOI] [PubMed] [Google Scholar]
- 24. Fan G, Wang T, Hu S, Guan X, Gu X, He S. Isocentric navigation of percutaneous endoscopic transforaminal discectomy at the L5/S1 level in difficult puncture cases: a technical note. Pain Physician, 2017, 20: E531–E540. [PubMed] [Google Scholar]
- 25. Fan G, Wang C, Gu X, Zhang H, He S. Trajectory planning and guided punctures with isocentric navigation in posterolateral endoscopic lumbar discectomy. World Neurosurg, 2017, 103: 899–905.e4. [DOI] [PubMed] [Google Scholar]
- 26. Hoogland T, Schubert M, Miklitz B, Ramirez A. Transforaminal posterolateral endoscopic discectomy with or without the combination of a low‐dose chymopapain: a prospective randomized study in 280 consecutive cases. Spine (Phila Pa 1976), 2006, 31: E890–E897. [DOI] [PubMed] [Google Scholar]
- 27. Guan X, Gu X, Zhang L, et al Morphometric analysis of the working zone for posterolateral endoscopic lumbar discectomy based on magnetic resonance neurography. J Spinal Disord Tech, 2015, 28: E78–E84. [DOI] [PubMed] [Google Scholar]
- 28. Mahmood F, Mahmood E, Dorfman RG, et al Augmented reality and ultrasound education: initial experience. J Cardiothorac Vasc Anesth, 2018, 32: 1363–1367. [DOI] [PubMed] [Google Scholar]
- 29. Rose T, Nam CS, Chen KB. Immersion of virtual reality for rehabilitation ‐ review. Appl Ergon, 2018, 69: 153–161. [DOI] [PubMed] [Google Scholar]
- 30. Williams B, Reddy P, Marshall S, Beovich B, McKarney L. Simulation and mental health outcomes: a scoping review. Adv Simul (Lond), 2017, 2: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Indovina P, Barone D, Gallo L, Chirico A, De Pietro G, Antonio G. Virtual reality as a distraction intervention to relieve pain and distress during medical procedures: a comprehensive literature review. Clin J Pain, 2018, 34: 858–877. [DOI] [PubMed] [Google Scholar]


