Abstract
This work presents a new bioprocess process for the extraction of bioactive components from soy pulp by-product (okara) using an enzymatic technology that was compared to a conventional water extraction. Okara is rich in fiber, fat, protein, and bioactive compounds such as isoflavones but its low solubility hampers the use in food and fertilizer industry. After the enzymatic attack with endoproteases half of the original insoluble proteins were converted into soluble peptides. Linked to this process occured the solubilization of isoflavones trapped in the insoluble protein matrix. We were able to extract up to 62.5% of the total isoflavones content, specially aglycones, the more bioactive isoflavone forms, whose values rose 9.12 times. This was probably due to the increased solubilization and interconversion from the original isoflavones. In conclusion, our process resulted in the formulation of a new functional product rich in aglycones and bioactive peptides with higher antioxidant potency than the original source. Therefore, we propose that the enzymatic extraction of okara bioactive compounds is an advantageous tool to replace conventional extraction.
Keywords: Food science, Food chemistry, Enzymatic extraction, Subtilisin, Okara, Isoflavone, Antioxidant, Bioactive peptides
1. Introduction
The soy pulp, also called okara, is the insoluble by-product obtained during the production of soymilk and tofu. Despite its high content in proteins, fats, carbohydrates (including fiber), phytochemicals and minerals (Redondo-Cuenca et al., 2008; Villanueva-Suarez et al., 2013), okara is generally floccinaucinihilipilificated. Two main obstacles hamper its wider use in food industry. The first reason is that raw okara has to be processed quickly due to its high moisture content (about 80%), which leads to a fast fermentation process. Unfortunately, current methods to preserve okara, such as heat drying, lyophilization or pressing, still cannot guarantee a profitable practice at an industrial scale (Mateos-Aparicio, 2012). The second problem is that most of the nutrients in okara remain in the insoluble form, decreasing its nutritional value. With these arguments, okara is hardly used for human consumption in Western countries, and basically just applied as animal food, plant biostimulant, soil fertilizer (Rinaldi et al., 2000), or more recently, in the obtention of bioethanol.
Therefore, its introduction in the productive sector requires new strategies to valorize it and make it attractive. Our aim in this study was to develop a simple process for the obtention of such a product with new functional properties.
It is largely known that soy and its derivatives have several polyphenol compounds, such as isoflavones (Galanakis, 2012) that can be beneficial for promoting human health (Cederroth and Nef, 2009; Messina et al., 1994). In this sense, okara contains all the remnants that do not pass to soymilk and up to 40% of the soy isoflavones, despite this percentage may vary depending on the type of soy and elaboration method (Jackson et al., 2002; Rinaldi et al., 2000; Wang and Murphy, 1996). Isoflavones are classified as phytoestrogens and have shown potential clinical implications preventing diabetes, cancer, osteoporosis, neurodegeneration or cardiovascular diseases (Kalaiselvan et al., 2010). Up to date, twelve different types of isoflavones have been isolated from soybeans and they are grouped in four main categories: aglycones (daizein, glicitein, genistein), β-glucosides (daidzin, glicitin, genistin) and their malonyl- and acetyl-derivatives (Wang and Murphy, 1994). Since isoflavones have hydrophobic interactions with the proteins where they are embedded, a direct correlation between the solubilization of proteins and isoflavones has been observed in okara (Jankowiak et al., 2014a), suggesting the idoneity of proteolytic treatments for isoflavone recovery.
Soy is recommended for human and animal consumption due to the high biological value of its proteins. Besides, it is considered to be the cheapest source of proteins available worldwide, which may help solve numerous feeding and agronomical problems. On top of that, soybean-based products such as okara are a potential source of bioactive peptides that can be obtained using proteolytic enzymes. These peptides are of particular interest due to their antihypertensive, antioxidant, anticancer, antimicrobial or antidiabetic properties (Agyei, 2015; Puchalska et al., 2014a, 2014b, 2017). Several studies have analyzed the antioxidant properties (Yokomizo et al., 2002) and health-promoting effects of bioactive peptides (Jiménez-Escrig et al., 2010; Nishibori et al., 2017) and fiber (Jiménez-Escrig et al., 2008) derived from okara with promising results both in vitro and in animal models.
The extraction of isoflavones using alcohol has been proposed by other authors and is maximal in theory (Jankowiak el al., 2014b) but expensive and not practical. Therefore, using water as extractant is preferred (Jankowiak et al., 2014a) despite the lower yield. In this article we hypothesized that the direct enzymatic hydrolysis of crude okara in aqueous solutions would enhance the extraction of isoflavones while also favoring the generation of bioactive peptides, thus contributing to the affordable formulation of a new product with higher antioxidant potency and increased value for the food and fertilizer industry.
2. Materials and methods
2.1. Okara and enzymes
Okara from the same production batch was used for all experiments and it was supplied by Soria Natural S.L. (Garray, Soria, Spain). Briefly, soybeans were washed to eliminate impurities, soaked in cold water for 30 h at 33.3% (w/v) and heated up at 95 °C for 5 min to inactivate any tripsin inhibitors and lipoxygenase present. Next, the grains were grinded in the presence of hot water at a 1:1 proportion (w/v) and soymilk was obtained by pressure and filtration, with the remaining by-product being the soy pulp (okara). Okara hydrolysates were made using a liquid formulation of the serine-endoprotease subtilisin (EC 3.4.21.62) from Biocon (Les Franqueses del Valles, Barcelona, Spain) with an enzyme activity of 100,000 AU/g. The enzyme was stored at 4 °C.
2.2. Analysis of the chemical composition of okara
The chemical composition of the okara used was analyzed by the Spanish Association for Standardisation and Certification (AENOR) laboratory (Madrid, Spain).
The ash content was analysed according to standard AOAC methods (2016). The protein load was determined using the Kjeldahl procedure (AOAC, 2016) and multiplying the total nitrogen content in a protein by the conversion factor 5.80, which is applicable to most plant proteins. Crude fat was determined gravimetrically after extraction with hexane for 12 h in a Soxhlet extractor (Clemente et al., 1997). Total soluble carbohydrates were determined after extraction with a mixture of ethanol/water (2/3) for 2 h. After centrifugation at 4,000 g (MiniSpin, Eppendorf), the supernatant was filtered through a no. 1 Whatman paper and total soluble sugars were estimated colorimetrically at 490 nm (GeneQuant 1300, General Electric) by the phenol-sulphuric acid method, using a standard curve of glucose (Dubois et al., 1956). Organic matter was determined by the dry combustion method (MAPA, 1986).
2.3. Preparation of okara extracts
Crude okara was diluted in water to a final concentration of 10% (dry w/v). Enzymatic hydrolysis using water as extractant was performed at pH 4.0, 6.0, 8.0 and 10.0 according to the pH-stat method (Brocklehurst, 2002). Conditions relative to the amount of enzymes, enzyme type, times of incubation and temperatures used were optimized in preliminary experiments not shown here. Briefly, incubation with 0.3% of liquid subtilisin (v/v) for 2 h at 55 °C without stirring was chosen. This temperature is optimal for the subtilisin activity and kills most of the microorganisms present in the sample, thus deferring spoilage while contributing to the solubilization process. It has been described that after one hour at that temperature subtilisin retains 90% of its activity (Sigma Aldrich, 1996) and enzyme variants with increased stability have been developed too (Takagi et al., 1990). The samples were later centrifuged for 40 min at 4 °C and 10,000 g (Avanti J-26XP, Beckman-Coulter). The soluble part (hereafter also referred as hydrolyzed okara) was heat dried and analyzed, while the pellet was weighted and discarded. As negative controls, we performed the same extractions without any enzyme, using water only (hereafter also referred as soluble okara).
2.4. Protein quantification
Protein determinations were performed according to Bradford (1976), using bovine serum albumin as standard.
2.5. Analysis of the molecular weight of soluble proteins
The protein content of the soluble fraction of okara was studied by size-exclusion chromatography using an ÄKTA-purifier FPLC system (GE Healthcare), filtration chromatography and a Superdex Peptide 10/300GL column, with an exclusion range between 700 and 10,000 Da that discriminates free peptides and amino acids. Samples were centrifuged at 12,000 g for 30 min at room temperature (MiniSpin, Epperdorf) to remove insoluble molecules, and the supernatant was passed through a 0.2 μm filter and loaded into a 0.1 mL loop connected to an ÄKTA-purifier system. The column was equilibrated, and eluted with 0.25 M Tris-HCl buffer (pH 7.0) in isocratic mode, at a flow-rate of 0.5 mL/min, and proteins/peptides were detected at 280 and 215 nm with a GE Healthcare UV900 module coupled to the column elution.
2.6. Analysis of the amino acid composition
The amino acid composition was determined by reversed-phase HPLC analysis of 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC) derivatives using γ-aminobutyric acid as the internal standard. The samples were hydrolyzed using 6N HCl/1% (w/v) phenol vapor at 110 °C for 24 h in a vacuum (Bidlingmeyer et al., 1984). The amino acids were treated with AQC to form AQC derivatives using the commercial method described by Waters AccQ-Tag, which were then analyzed using a Waters HPLC system (Millipore Ltd) fitted with a reversed-APAse C18 column. For cysteine estimation, aliquots were first oxidized with performic acid and analyzed as above.
2.7. Analysis of isoflavones by UHPLC-MS/MS
Isoflavones were analyzed using a Thermo Scientific liquid chromatography system consisting of a quaternary UHPLC Dionex Ultimate 3000 SD, connected to a quadrupole-Orbitrap Qexactive hybrid mass spectrometer with HESI ionization probe. Xcalibur software was used for instrument control and data acquisition. Separation was performed on a C18 column Xbridge BEH (2.1 × 100 mm, 2.5 μm) (Waters). The injection volume was 5 μL and the flow rate was 0.4 mL/min. Two different solvents were used as mobile phase: solvent A (water with 0.1% formic acid) and solvent B (acetonitrile with 0.1% formic acid). The following gradient was used: 0–1 min 5% of solvent B, 1–10 min from 5% to 100% of solvent B, 10–12 min 100% of solvent B, then 5% of solvent B up to 15 min. The Parallel Reaction Monitoring (PRM) was acquired in positive mode and a resolution of 17500 at m/z 200 FWHM, with an isolation window of 1 m/z.
The HESI source parameters were as follows: spray voltage 3.5 kV, S lens level 50, capillary temperature of 320 °C and probe heater temperature of 400 °C, sheath flow, auxiliary flow and sweep gas flow (N2) of 50, 13 and 2 arbitrary units, respectively.
Quantitation was performed selecting the following fragments: m/z 255.06519 for daidzin, acetyl daidzin and malonyl daidzin, m/z 285.07575 for glycitin, acetyl glycitin and malonyl glycitin, m/z 271.06010 for genistin, acetyl genistin and malonyl genistin, m/z 137.02320 for daidzein, m/z 242.05736 for glycitein and m/z 153.01824 for genistein. External calibration was used for the majority of the isoflavones, except for the malonyl and acetyl derivatives, where the calibration curves were estimated as daidzin, glycitin, and genistin, respectively. TraceFinder 3.3 software was used for treatment of data.
2.8. Antioxidant capacity assays
The ability of okara samples to reduce Fe+3 to Fe+2 was determined by the FRAP (ferric reducing antioxidant power) assay (Benzie and Strain, 1996) at pH 3.6. Absorbance was measured at 734 nm and Trolox (Merck Millipore) was used for the standard curve.
2.9. Statistical analysis
Three independent experiments were performed and measured by triplicate using the same okara batch. Data was analyzed by a one-way analysis of variance (ANOVA) using Statgraphics Centurion XVIIII followed by Tukey's post-hoc test for multiple comparisons in order to confirm differences among the groups. A p-value <0.001 was considered significant.
3. Results and discussion
3.1. Chemical composition of okara
The chemical composition of crude okara can vary depending on the soybean variety and method of obtention during soymilk production. Therefore, a prior characterization of the dried product was performed as shown in Table 1. In our case, crude okara contained 76.5% of humidity, which is in agreement with the literature and predisposes okara to fast spoilage. On the other hand, proteins represented around 40% of the total dry weight. However, around 90% of them were insoluble, reflecting their poor bioavailability. When soluble proteins were analyzed by exclusion chromatography (Fig. 1, Table 2), about 90% of them corresponded to proteins of more than 10 kDa, while almost 7% were small peptides and free amino acids.
Table 1.
Chemical composition of okara (dry matter) used.
| OKARA | Dried okara | Water extraction | Subtilisin extraction |
|---|---|---|---|
| Moisture (%) | 0.0 | 0.0 | 0.0 |
| Dry matter (%) | 100.0 | 100.0 | 100.0 |
| C | 53.7 | 41.8 | 44.8 |
| N | 6.6 | 4.2 | 10.6 |
| Proteins (%N x 6.25) | 41.2 | 25.9 | 66.5 |
| Insoluble proteins | 36.9 | 0.0 | 0.0 |
| Soluble proteins | 4.4 | 100.0 | 100.0 |
| Carbohydrates (%) | 29.5 | 50.2 | 21.4 |
| Insoluble fiber | 26.0 | 4.9 | 5.1 |
| Soluble fiber | 2.8 | 6.0 | 4.9 |
| Starch | 0.5 | 0.5 | 0.5 |
| Fats (%) | 23.6 | 4.6 | 2.2 |
| Ashes (%) | 4.1 | 14.1 | 6.6 |
| Macroelements (g/100 g) | |||
| K | 0.9 | 4.5 | 1.7 |
| P | 0.7 | 2.1 | 1.1 |
| Na | 0.4 | 1.0 | 0.6 |
| Mg | 0.2 | 0.7 | 0.4 |
| Ca | 0.2 | 0.3 | 0.3 |
| Microelements (mg/100 g) | |||
| Fe | 1.3 | 0.7 | 10.2 |
| Mn | 1.1 | 0.2 | 7.2 |
| Zn | 0.6 | 0.6 | 4.7 |
| Cu | 0.3 | 0.3 | 0.4 |
Fig. 1.
Chromatography profile of the soluble protein content of okara according to its molecular weight using a Superdex Peptide 10/300GL column.
Table 2.
Distribution of the soluble protein content of okara according to its molecular weight using a Superdex Peptide 10/300GL column.
| Molecular weight (Da) | Water extraction (%) | Subtilisin extraction (%) |
|---|---|---|
| >10,000 | 91.14 | 7.85 |
| 10,000–5,000 | 1.09 | 15.87 |
| 5,000–1,000 | 0.80 | 0 |
| <1,000 | 6.98 | 75.74 |
The whole amino acid composition of dried okara (which includes both soluble and insoluble protein content) was further analyzed (Table 3). Demonstrating the high potential value of its protein, we observed that nearly 30% of the amino acids found were essential for the human diet. Among these, branched-chain amino acids (BCAA) represented 15% of the total, and their presence in diets may have beneficial effects on the ageing process, muscle synthesis, body weight and glucose homeostasis (Bifari and Nisoli, 2017). Aromatic amino acids summed a 6% and most remarkably, hydrophobic amino acids counted for around one third of all amino acids detected.
Table 3.
Whole amino acid composition from dry matter of okara used in the experiments. Essential amino acids are in bold. N.D.: not determined.
| (% ng) | |
|---|---|
| Glu + Gln | 19.27 |
| Asp + Asn | 12.20 |
| Leu | 7.84 |
| Lys | 6.88 |
| Arg | 6.62 |
| Ser | 6.35 |
| Pro | 5.88 |
| Phe | 5.44 |
| Ala | 5.04 |
| Gly | 4.79 |
| Thr | 4.19 |
| Val | 4.12 |
| Ile | 3.79 |
| His | 2.87 |
| Tyr | 2.83 |
| Met | 1.40 |
| Cys | 0.49 |
| Trp | N.D. |
Most soluble proteins are removed during soymilk production. These proteins contain less than 25% of hydrophobic amino acids (Puchalska et al., 2017). Therefore, it is expected that proteins and peptides with a higher content of hydrophobic amino acids are retained in okara. Interestingly, the prevalence of hydrophobic amino acids in bioactive peptides has been related to their antioxidant properties (Kitts and Weiler, 2003; Puchalska et al., 2017; Samardi and Ismail, 2010; Zhang et al., 2012). Altogether, these values reflect the high potential for generating bioactive peptides from okara.
Carbohydrates comprised nearly 30% of the total dry weight and for the most part were insoluble fiber. On the other hand, fats accounted nearly one fourth of the total weight (23.6%) and the most abundant oligoelements were K, P, S, Mg and Ca. Despite okara is rich in organic molecules, most of its proteins, carbohydrates and biomolecules embedded inside are insoluble, which hampers its use as a source of functional molecules. For this reason, we reasoned that the enzymatic hydrolysis would enable the extraction of compounds with functional activity, such as isoflavones, bioactive peptides and amino acids, hence contributing to the formulation of a new product with added value.
3.2. Chemical composition of the water extract and subtilisin extract of okara
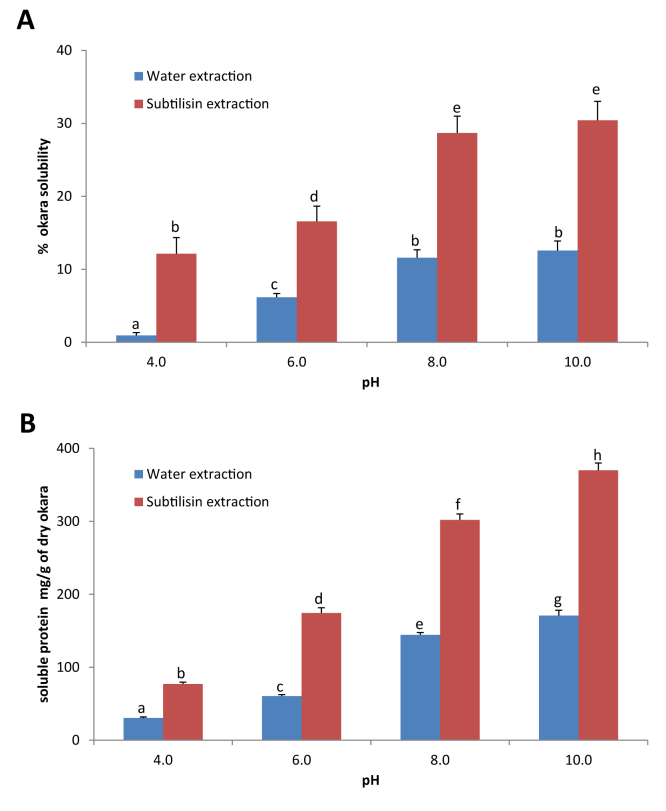
The chemical composition of the soluble extracts (treated with subtilisin or only with water) was analyzed after heat drying the samples (Table 1). Taking into account that the extraction efficiency at pH 10.0 was 30.4% for the subtilisin treatment and 12.6% for the water control (Fig. 2A), we were able to extract 49.1% of the total protein with the subtilisin treatment, compared to only 7.9% with water alone. As expected, no significant differences were observed in the extraction of carbohydrates (22.9% with subtilisin vs. 21.4% with water) and fats (2.9% with subtilisin vs 2.5% with water).
Fig. 2.
A. Solubility of okara at different pH values after subtilisin treatment or using water as negative control. B. Soluble protein (mg/g of dry okara) extracted after subtilisin treatment at different pH values or using water as negative control. Bars with different letters are significantly different (p < 0.001).
3.3. Effect of pH and subtilisin treatment on the solubilization of okara
Unless stated otherwise, previous experiments were carried out at the starting pH value of 6.7, which corresponds to the initial pH of okara, without pH control. However, we wanted to check the effect of different pH values on the solubilization of okara. As shown in Fig. 2A, the solubility was minimal at pH 4.0 and increased at higher pH values for both the aqueous controls and the 0.3% subtilisin hydrolyzed extracts. This data suggests that alkaline pH favors solubilization. After the treatment, the enzymatic extracts reached a solubility of 27.3% at pH 8.0 and 30.4% at pH 10.0, respectively, despite that difference was not statistically significant. This represented an additional 17.1% and 17.8% solubilization at pH 8.0 and 10.0, compared to their respective controls, and is consistent with the optimal pH range activity for subtilisin (pH 7.5–10.0). At acidic pH values subtilisin samples yielded an extra 11.2–10.4% than the controls. Overall, the solubilization achieved with the subtilisin treatments at pH 8.0 and 10.0 was significantly much higher than any of the controls at any pH value tested, hence justifying the use of subtilisin in the alkaline pH range.
3.3.1. Effect of pH and subtilisin treatment on the solubilization of proteins
After testing the enzymatic treatment at different pH values we observed that the solubilization of proteins increased at higher pH values, being maximal at pH 10.0 (Fig. 2B). The addition of subtilisin doubled the amount of protein extracted compared to the respective controls at all pH values tested. In particular, the increase was 2.5 fold at pH 4.0, 2.9 fold at pH 6.0, 2.1 fold at pH 8.0, and 2.2 fold at pH 10.0, which represents almost 200 mg extra of solubilized protein per g of dry okara.
When the soluble proteins of the enzymatic extract at pH 10.0 were analyzed by molecular exclusion chromatography and compared to the negative control extracted with water only (Fig. 1, Table 2) we observed that proteins with a molecular weight greater than 10 kDa decreased from 91.14% to only 7.85%, while the small protein/peptide (1–10 kDa) and small peptide/amino acid (<1 kDa) fractions increased from 1.09% to 15.87% and 6.98%–75.74%, respectively.
It is worthy to mention that bioactive peptides, which usually consist of 2–20 amino acids, may be generated by proteolytic digestion and therefore be contained in these fractions. The biological and antioxidant properties of these peptides are related to their content in hydrophobic and aromatic residues (Kitts and Weiler, 2003; Puchalska et al., 2017; Samardi and Ismail, 2010; Zhang et al., 2012), whilst the abundance of hydrophobic amino acids also contributes to the bitter taste of soy (FitzGerald and O'Cuinn, 2006). Strikingly, subtilisin hydrolysates of soy protein have been found to exhibit the highest bitter taste compared to hydrolysates with other proteases (Seo et al., 2008), thus reflecting their idoneity for the potential generation of bioactive peptides. Taken together, these results underscore the adequacy of the subtilisin treatment to solubilize the protein content of okara and its potential to generate bioactive peptides.
3.3.2. Effect of pH and subtilisin treatment on the solubilization of isoflavones
The solubilization of isoflavones was measured by mass spectrometry both in the presence and absence of subtilisin. Table 4 shows the total amount of isoflavones, aglycones, β-glucosides, and acetyl and malonyl derivatives extracted at different pH values. In general, we observed that alkaline pH values exerted the strongest effect on the solubilization of isoflavones. Thus, when the pH increased from 8.0 to 10.0 the total amount of solubilized isoflavones raised an extra 28% (763.40 vs 597.54 μg/g of dry okara) in the presence of subtilisin and 38% (469.89 vs 338.97 μg/g of dry okara) in the control samples. Compared to their water-soluble counterparts, the addition of subtilisin at pH 8.0 augmented the extraction of total isoflavones by 76% (597.54 vs 338.97 μg/g of dry okara) and 62% at pH 10.0 (763.40 vs 469.89 μg/g of dry okara). Remarkably, at pH 10.0 the subtilisin treatment increased 9.1 times the total amount of aglycones extracted (174.83 vs 19.18 μg/g of dry okara). This can be due to an increased efficiency of the extraction at pH 10.0, but also to a higher interconversion of the thermally unstable conjugated forms (malonyl and acetyl glucosides) to their respective heat-stable non-conjugated β-glucosides and aglycones (Mathias et al., 2006). Importantly, the higher recovery of aglycones is desired, since they are considered to be the most bioactive forms of isoflavone, due to their moderate lipophilicity that allows them to be better absorbed in the gut (Murota et al., 2002).
Table 4.
Effect of the subtilisin treatment at different pH values on the extraction of isoflavones from okara. Values with different letters are significantly different (p < 0.001).
| Isoflavones (μg/g dry okara) | Water extraction |
Subtilisin extraction |
||||||
|---|---|---|---|---|---|---|---|---|
| pH4 | pH6 | pH8 | pH10 | pH4 | pH6 | pH8 | pH10 | |
| Aglycones | 0.00 ± 0.0 | 0.01 ± 0.0 | 1.70 ± 0.1 | 19.18 ± 1.2 | 0.02 ± 0.0 | 0.06 ± 0.0 | 1.05 ± 0.2 | 174.83 ± 13.7 |
| Daidzein | 0.00 ± 0.0 | 0.01 ± 0.0 | 0.71 ± 0.1 | 11.06 ± 0.7 | 0.01 ± 0.0 | 0.04 ± 0.0 | 0.57 ± 0.0 | 77.48 ± 5.4 |
| Genistein | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.31 ± 0.0 | 7.15 ± 0.4 | 0.01 ± 0.0 | 0.01 ± 0.0 | 0.19 ± 0.0 | 82.29 ± 6. 6 |
| Glycitein | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.68 ± 0.0 | 0.97 ± 0.0 | 0.00 ± 0.0 | 0.01 ± 0.0 | 0.29 ± 0.1 | 15.06 ± 1.7 |
| β-glucosides | 26.65 ± 2.3 | 50.93 ± 4.1 | 134.60 ± 24.6 | 445.81 ± 25.8 | 29.56 ± 2.9 | 129.64 ± 9.3 | 320.86 ± 31.8 | 481.70 ± 37.6 |
| Daidzin | 15.86 ± 1.6 | 24.98 ± 2.1 | 65.93 ± 10.2 | 193.41 ± 9.3 | 14.41 ± 1.7 | 14.90 ± 0.7 | 139.78 ± 9.4 | 176.66 ± 11. 7 |
| Genistin | 10.22 ± 0.6 | 21.31 ± 1.6 | 59.34 ± 12.4 | 225.81 ± 11.4 | 14.62 ± 1.2 | 18.70 ± 1.4 | 177.81 ± 11.2 | 300.03 ± 21.1 |
| Glycitin | 0.57 ± 0.1 | 4.64 ± 0.4 | 9.33 ± 2.0 | 26.59 ± 5.1 | 0.53 ± 0.0 | 96.04 ± 7.2 | 3.27 ± 1.2 | 5.01 ± 0.8 |
| Derivatives | 18.13 ± 1.4 | 80.22 ± 3.7 | 202.67 ± 14.3 | 4.9 ± 0.8 | 26.56 ± 2.1 | 67.08 ± 5.3 | 275.63 ± 16.4 | 106.87 ± 10.2 |
| Acetyl Daidzin | 0.12 ± 0.1 | 0.89 ± 0.0 | 1.00 ± 0.0 | 0.00 ± 0.0 | 0.11 ± 0.0 | 0.58 ± 0.1 | 0.00 ± 0.0 | 0.00 ± 0.0 |
| Acetyl Genistin | 0.05 ± 0.0 | 0.45 ± 0.0 | 0.90 ± 0.0 | 0.00 ± 0.0 | 0.11 ± 0.0 | 0.54 ± 0.0 | 0.24 ± 0.0 | 0.00 ± 0.0 |
| Acetyl Glycitin | 0.01 ± 0.0 | 0.10 ± 0.0 | 0.12 ± 0.0 | 0.00 ± 0.0 | 0.02 ± 0.0 | 0.08 ± 0.0 | 0.01 ± 0.0 | 0.00 ± 0.0 |
| Malonyl Daidzin | 10.50 ± 1.0 | 33.41 ± 2.3 | 80.09 ± 1.1 | 0.12 ± 0.0 | 11.69 ± 0.9 | 20.77 ± 1.6 | 88.66 ± 5.3 | 0.08 ± 0.0 |
| Malonyl Genistin | 7.15 ± 0.3 | 41.57 ± 1.3 | 112.25 ± 12.9 | 4.75 ± 0.8 | 14.21 ± 1.2 | 42.84 ± 3.3 | 184.77 ± 11.0 | 105.39 ± 9.8 |
| Malonyl Glycitin | 0.30 ± 0.0 | 3.80 ± 0.1 | 8.31 ± 0.3 | 0.03 ± 0.0 | 0.42 ± 0.0 | 2.27 ± 0.3 | 1.95 ± 0.2 | 1.40 ± 0.4 |
| Total Isoflavones | 44.78 ± 3.7a | 131.16 ± 7.8b | 338.97 ± 38.9c | 469.89 ± 27.7d | 56.14 ± 5.0a,b | 196.78 ± 14.6e | 597.54 ± 38.4f | 763.40 ± 57.5g |
For each of the aglycones measured (daidzein, genistein and glycitein), for the β-glucosides daidzin and genistin, and for total isoflavones, the highest solubilization values were achieved at pH 10.0. For the β-glucoside glycitin the maximum solubilization (96.04 μg/g of dry okara) was achieved at pH 6.0 in the presence of subtilisin. Malonyl and acetyl derivatives increased gradually with pH and reached the highest values at pH 8.0. However, at pH 10.0 they were almost inexistent, except for the malonyl genistin extracted with subtilisin, which remained high (105.39 μg/g of dry okara) despite some interconversion to its non-conjugated forms. No other major differences were observed for the malonyl and acetyl derivatives in the presence or absence of subtilisin, hence indicating a small effect of the enzyme on their extraction.
In the okara hydrolyzed with subtilisin at pH 10.0, malonyl-derivatives accounted for 14.0% of the total isoflavones extracted, β-glucosides for the 63.1% and aglycones for the 22.9%. Also, genistein and its β-glucosides and malonyl-derivatives represented 63.9% of all the isoflavones extracted. The daidzein family represented one third of the total, while the glycitein-based isoflavones only summed 2.8%. Similar percentages were found at pH 8.0, being 60.8% genistein-based isoflavones, the remaining 38.3% daidzein-derivatives, and only 0.9% glycitein and its family. However, at pH 6.0 glycitein-based isoflavones, mainly in the glycitin form, accounted for half of the total isoflavones extracted in the presence of subtilisin. In agreement with other authors (Mathias et al., 2006), this behavior may be related to the extra H3CO- group present in the glycitein molecule and reflects how the chemical structure of isoflavones dictates their stability under variable pH conditions, which may be used to modulate the solubility of different isoflavone molecules.
Malonyl genistin and malonyl daidzin are the most abundant forms in raw soybeans (Mathias et al., 2006) but their lower thermal stability favors their conversion into non-conjugated forms. Thus, the solubilization of isoflavones during our enzymatic treatment involved an incubation step at 55 °C for 2 h. At this temperature isoflavones are relatively stable (Xu et al., 2002). Besides, it may increase the solubility of aglycones (Wu et al., 2010) and also favor the conversion of daidzin and genistin into their bioactive aglycone forms (Muliterno et al., 2017), particularly at pH 10.0. However, Jankowiak et al. (2014a) did not observe any significant difference in the total amount of isoflavones extracted within the 20–95 °C range, despite a significant decrease of the malonyl-glucosides above 65 °C.
Similar to them (Jankowiak et al., 2014a), we also used water as extractant instead of ethanol. Whereas they tested water to dry okara ratios ranging from 20:1 till 100:1, we just used a 10:1 ratio. However, only when they assayed the 20:1 ratio at pH 10.0, they were able to increase the aglycone content up to 340 μg/g of dry okara approximately and recover 911 μg of total isoflavones per gram of dry okara. In our hands, water extraction alone did not recover high yields, and we only obtained 19.18 μg of aglycones and 469.89 μg of total isoflavones per gram of dry okara at pH 10.0. Since both methods are theoretically equivalent, the differences in the aglycone and isoflavone content at pH 10.0 without any enzymatic treatment may be essentially attributed to the type of soy used and the processing conditions during soymilk manufacture, as well as the distinct liquid-to-solid ratio used (10:1 in our case and 20:1 for Jankowiak et al.). It should be expected to increase our aglycone and total isoflavone content to some extent by raising the liquid-to-solid ratio from 10:1 to 20:1. However, the addition in our case of 0.3% subtilisin increased the total amount of isoflavones solubilized (763.4 μg/g of dry okara) by 62.5% and raised the aglycone content 9.1 times up to 174.8 μg/g of dry okara, whereas for Jankowiak et al. their highest extraction yield using 70% ethanol increased their isoflavone content only by 11.7% (1018 μg/g of dry okara) and the aglycone levels barely added a 5% (357 μg/g of dry okara) extra.
3.4. Protein/isoflavone relationship in okara extracts
Remarkably, we found a direct correlation between the amount of proteins solubilized and total isoflavones extracted at all pH values tested, either with the subtilisin treatment or the water extraction (Fig. 3). However, after the enzymatic treatment, the yield of isoflavones extracted was lower than expected for the quantity of proteins solubilized. This reinforces the notion that the solubilization of isoflavones was an indirect effect provoked by the solubilization of proteins and that some isoflavones may remain trapped in the insoluble fiber matrix too, in agreement with previous works (Jankowiak et al., 2014a).
Fig. 3.
Linear relationship between soluble proteins and isoflavones extracted from okara.
3.5. Reducing activity of okara extracts
When the specific ability of the samples to reduce Fe+3 to Fe+2 was measured using the FRAP technique (Fig. 4), we found that the reducing activity of the samples was strongly affected by the pH, despite the fact that samples hydrolyzed with subtilisin were higher than their respective controls. At acidic values, the effect of the enzymatic treatment was considerable (3.2-fold at pH 4.0 and 2.4-fold at pH 6.0 over their respective controls), but slight at basic pH values, where it only represented a 1.2-fold at pH 8.0 and 1.1-fold at pH 10.0. Overall, such antioxidant capacity increased at higher pH values in all samples tested, except for the subtilisin extract at pH 8.0 that was lower than its equivalent at pH 6.0. That anomaly might be due to the solubilization of other compounds at pH 6.0 not investigated here.
Fig. 4.
Reducing activity of okara extracts at different pH values, with or without subtilisin treatment using the FRAP method. Bars with different letters are significantly different (p < 0.001).
It has been described that genistein and genistin have the highest antioxidant activities of all soy isoflavones (Lee et al., 2005). Accordingly, their elevated occurrence at pH 10.0 correlated with the highest antioxidant capacity of okara extracts at that pH value. Also, low-molecular weight peptides have been shown to have anti-oxidant activity (Jiménez-Escrig et al., 2010; Nishibori et al., 2017) that may also contribute to the total reducing activity observed in the okara extracts due to the solubilization of the protein content with the subtilisin treatment.
4. Conclusions
We developed a method for the valorization of okara based on the application of 0.3% subtilisin at pH 10.0 to 10% (dry w/v) crude okara. This approach rendered high levels of soluble isoflavones and peptides, and a linear correlation between their extractions was observed reflecting potential interactions among them. Our process skips the drying of okara, which is energy intensive and may damage its components, decreasing its properties and economic value. However, it contains an initial step at 55 °C that reduces the risk of spoilage by microorganisms and extends the half-life of crude okara. This technology avoids the use of ethanol, which is not profitable at industrial scale, but achieves comparable rates for the solubilization of proteins and isoflavones. In particular, it is noteworthy the rich content of aglycones. The subtilisin activity also facilitates the generation of bioactive peptides with potential uses in health and agriculture, which all in all results in the formulation a new functional product with high antioxidant potency. Therefore, this protocol may represent a functional alternative for the valorization of okara potentially suitable for industrial scale-up.
Declarations
Author contribution statement
Angel Orts: Performed the experiments; Analyzed and interpreted the data.
Elisa Revilla, Albert García-Quintanilla, Angélica Castaño: Analyzed and interpreted the data; Wrote the paper.
Bruno Rodriguez-Morgado, Manuel Tejada: Conceived and designed the experiments.
Juan Parrado: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by Grant of Ministerio de Ciencia e Innovación, CTM2015-64354-C3-1-R, and Junta de Andalucía, Proyecto de Excelencia P11-RNM-7887.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Agyei D. Bioactive proteins and peptides from soybeans. Recent Pat. Food, Nutr. Agric. 2015;7(2):100–107. doi: 10.2174/2212798407666150629134141. [DOI] [PubMed] [Google Scholar]
- AOAC International . twentieth ed. Association of Official Analytical Chemists; Washington, DC: 2016. Official Methods of Analysis. [Google Scholar]
- Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay. Anal. Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bidlingmeyer B.A., Cohen S.A., Tarvin T.L. Rapid analysis of amino acids using pre-column derivatization. J. Chromatogr. 1984;336(1):93–104. doi: 10.1016/s0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- Bifari F., Nisoli E. Branched-chain amino acids differently modulate catabolic and anabolic states in mammals: a pharmacological point of view. Br. J. Pharmacol. 2017;174(11):1366–1377. doi: 10.1111/bph.13624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K. Electrochemical assays: the pH-stat. In: Eisenthal R., Danson M.J., editors. Enzyme Assays. A Practical Approach. second ed. Oxford University Press; Oxford: 2002. pp. 157–170. [Google Scholar]
- Cederroth C.R., Nef S. Soy, phytoestrogens and metabolism: a review. Mol. Cell. Endocrinol. 2009;304(1–2):30–42. doi: 10.1016/j.mce.2009.02.027. [DOI] [PubMed] [Google Scholar]
- Clemente A., Sánchez-Vioque R., Vioque J., Bautista J., Millan F. Chemical composition of extracted dried olive pomaces containing two and three phases. Food Biotechnol. 1997;11(3):273–291. [Google Scholar]
- Dubois M., Gilles K., Hamilton J., Rebers P., Smith F. Colorimetric method for determination of sugars and substances. Anal. Chem. 1956;28(3):350–353. [Google Scholar]
- FitzGerald R.J., O'Cuinn G. Enzymatic debittering of food protein hydrolysates. Biotechnol. Adv. 2006;24(2):234–237. doi: 10.1016/j.biotechadv.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Galanakis C.M. Recovery of high added-value components from food wastes: conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012;26(2):68–87. [Google Scholar]
- Jackson C.J.C., Dini J.P., Lavandier C., Rupasinghe H.P.V., Faulkner H., Poysa V., Buzzell D., DeGrandis S. Effects of processing on the content and composition of isoflavones during manufacturing of soy beverage and tofu. Process Biochem. 2002;37(10):1117–1123. [Google Scholar]
- Jankowiak L., Kantzas N., Boom R., Van der Goot A.J. Isoflavone extraction from okara using water as extractant. Food Chem. 2014;160:371–378. doi: 10.1016/j.foodchem.2014.03.082. [DOI] [PubMed] [Google Scholar]
- Jankowiak L., Trifunovic O., Boom R., van der Goot A.J. The potential of crude okara for isoflavone production. J. Food Eng. 2014;124:166–172. [Google Scholar]
- Jiménez-Escrig A., Alaiz M., Vioque J., Rupérez P. Health-promoting activities of ultra-filtered okara protein hydrolysates released by in vitro gastrointestinal digestion: identification of active peptide from soybean lipoxygenase. Eur. Food Res. Technol. 2010;230:655–663. [Google Scholar]
- Jiménez-Escrig A., Tenorio M.D., Espinosa-Martos I., Rupérez P. Health-promoting effects of a dietary fiber concentrate from the soybean byproduct okara in rats. J. Agric. Food Chem. 2008;56(16):7495–7501. doi: 10.1021/jf800792y. [DOI] [PubMed] [Google Scholar]
- Kalaiselvan V., Kalaivani M., Vijayakumar A., Sureshkumar K., Venkateskumar K. Current knowledge and future directions of research on soy isoflavones as therapeutic agents. Pharm. Rev. 2010;4(8):111–1117. doi: 10.4103/0973-7847.70900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitts D.D., Weiler K. Bioactive proteins and peptides from food sources. Applications of bioprocesses used in isolation and recovery. Curr. Pharmaceut. Des. 2003;9(16):1309–1323. doi: 10.2174/1381612033454883. [DOI] [PubMed] [Google Scholar]
- Lee C.H., Yang L., Xu J.Z., Yeung S.Y.V., Huang Y., Chen Z. Relative antioxidant activity of soybean isoflavones and their glycosides. Food Chem. 2005;90(4):735–741. [Google Scholar]
- MAPA . 1a ed. Secretaría General Técnica del Ministerio de Agricultura, Pesca y Alimentación; España: 1986. Métodos oficiales de análisis; pp. 221–285. [Google Scholar]
- Mateos-Aparicio I. Beans by-products, potential sources for functional ingredients. In: Popescu E., Golubev I., editors. Beans: Nutrition, Consumption and Health. Nova Publishers Inc.; New York: 2012. pp. 233–248. [Google Scholar]
- Mathias K., Ismail B., Corvalan C.M., Hayes K.D. Heat and pH effects on the conjugated forms of genistin and daidzin isoflavones. J. Agric. Food Chem. 2006;54(20):7495–7502. doi: 10.1021/jf061322a. [DOI] [PubMed] [Google Scholar]
- Messina M.J., Persky V., Setchell K.D.R., Barnes S. Soy intake and cancer risk – a review of the in vitro and in vivo data. Nutr. Canc. 1994;21(2):113–131. doi: 10.1080/01635589409514310. [DOI] [PubMed] [Google Scholar]
- Muliterno M.M., Rodrigues D., Sanches de Lima F., Ida E.L., Kurozawa L.E. Conversion/degradation of isoflavones and color alterations during the drying of okara. Food Sci. Technol. (N. Y.) 2017;75:512–519. [Google Scholar]
- Murota K., Shimizu S., Miyamoto S., Izumi T., Obata A., Kikuchi M., Terao J. Unique uptake and transport of isoflavone aglycones by human intestinal caco-2 cells: comparison of isoflavonoids and flavonoids. J. Nutr. 2002;132(7):1956–1961. doi: 10.1093/jn/132.7.1956. [DOI] [PubMed] [Google Scholar]
- Nishibori N., Kishibuchi R., Morita K. Soy pulp extract inhibits angiotensin I-converting enzyme (ACE) activity in vitro: evidence for its potential hypertension-improving action. J. Diet. Suppl. 2017;14(3):241–251. doi: 10.1080/19390211.2016.1207744. [DOI] [PubMed] [Google Scholar]
- Puchalska P.M., García M.C., Marina M.L. Advances in the determination of bioactive peptides in foods. In: Aguilar V., Otero C., editors. Frontiers in Bioactive Compounds. Bentham Science Publishers; Sharjah, UAE: 2017. pp. 24–53. [Google Scholar]
- Puchalska P.M., García M.C., Marina M.L. Identification of native angiotensin-I converting enzyme inhibitory peptides in commercial soybean based infant formulas using HPLC-Q-ToF-MS. Food Chem. 2014;157:62–69. doi: 10.1016/j.foodchem.2014.01.130. [DOI] [PubMed] [Google Scholar]
- Puchalska P.M., Marina M.L., García M.C. Isolation and identification of antioxidant peptides from commercial soybean-based infant formulas. Food Chem. 2014;148:147–154. doi: 10.1016/j.foodchem.2013.10.030. [DOI] [PubMed] [Google Scholar]
- Redondo-Cuenca A., Villanueva-Suárez M.J., Mateos-Aparicio I. Soybean seeds and its by-product okara as sources of dietary fibre. Measurement by AOAC and Englyst methods. Food Chem. 2008;108:1099–1105. doi: 10.1016/j.foodchem.2007.11.061. [DOI] [PubMed] [Google Scholar]
- Rinaldi V.E.A., Ng P.K.W., Bennink M.R. Effects of extrusion on dietary fiber and isoflavone contents of wheat extrudades enriched with wet okara. Cereal Chem. 2000;7(2):237–240. [Google Scholar]
- Sarmadi B.H., Ismail A. Antioxidative peptides from food proteins: a review. Peptides. 2010;3(10):1949–1956. doi: 10.1016/j.peptides.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Seo W.H., Lee H.G., Baek H.H. Evaluation of bitterness in enzymatic hydrolysates of soy protein isolate by taste dilution analysis. J. Food Sci. 2008;73(1):S41–46. doi: 10.1111/j.1750-3841.2007.00610.x. [DOI] [PubMed] [Google Scholar]
- Sigma-Aldrich . 1996. Subtilisin.https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma/Product_Information_Sheet/1/p5380pis.pdf [Google Scholar]
- Takagi H., Takahashi T., Momose H., Inouye M., Maeda Y., Matsuzawa H., Ohta T. Enhancement of the thermostability of subtilisin E by introduction of a disulfide bond engineered on the basis of structural comparison with a thermophilic serine protease. J. Biol. Chem. 1990;265(12):6874–6878. http://www.jbc.org/content/265/12/6874.long [PubMed] [Google Scholar]
- Villanueva-Suárez M.J., Pérez-Cózar M.L., Redondo-Cuenca A. Sequential extraction of polysaccharides from enzymatically hydrolyzed okara byproduct: physicochemical properties and in vitro fermentability. Food Chem. 2013;141(2):1114–1119. doi: 10.1016/j.foodchem.2013.03.066. [DOI] [PubMed] [Google Scholar]
- Wang H.J., Murphy P.A. Isoflavone content in commercial soybean foods. J. Agric. Food Chem. 1994;42(8):1666–1673. [Google Scholar]
- Wang H.J., Murphy P.A. Mass balance study of isoflavones during soybean processing. J. Agric. Food Chem. 1996;44(8):2377–2383. [Google Scholar]
- Wu J., Ge J., Zhang Y., Yu Y., Zhang X. Solubility of genistein in water, methanol, ethanol, propan-2-ol, 1-butanol, and ethyl acetate from (280 to 333) K. J. Chem. Eng. Data. 2010;55(11):5286–5288. [Google Scholar]
- Xu Z., Wu Q., Godber J.S. Stabilities of daidzin, glycitin, genistin, and generation of derivatives during heating. J. Agric. Food Chem. 2002;50(25):7402–7406. doi: 10.1021/jf025626i. [DOI] [PubMed] [Google Scholar]
- Yokomizo A., Takenaka Y., Takenaka T. Antioxidative activity of peptides prepared from okara protein. Food Sci. Technol. Res. 2002;8(4):357–359. [Google Scholar]
- Zhang H., Yokoyama W.H., Zhang H. Concentration-dependent displacement of cholesterol in micelles by hydrophobic rice bran protein hydrolysates. J. Sci. Food Agric. 2012;92(7):1395–1401. doi: 10.1002/jsfa.4713. [DOI] [PubMed] [Google Scholar]