Abstract
Metastatic spine disease is a heterogeneous clinical condition commonly requiring surgical intervention. Despite this heterogeneity, all cases share the common theme of altered tumor metabolism, characterized by aerobic glycolysis and high lactate production. Here we review the existing literature on lactate metabolism as it pertains to tumor progression, metastasis, and the formation of painful bone lesions. We included articles from the English literature addressing the role of lactate metabolism in the following: (I) primary tumor aggressiveness, (II) local tissue invasion, (III) metastasis formation, and (IV) generation of oncologic pain. We also report current investigations into restoring normal lactate metabolism as a means of impeding tumor growth and the formation of bony metastases. Both in vivo and in vitro experiments suggest that high lactate levels may be necessary for tumor cell growth, as small molecules inhibitors of lactate dehydrogenase (LDH5/LDHA) decrease both the rate of tumor growth and formation of metastases. Additionally, in vitro evidence strongly implicates lactate in tumor cell migration by driving the amoeboid movements of these cells. Acidification of the local bony tissue by excess lactate production activates CGRP+ neurons in the bone marrow and periosteum to generate oncologic bone pain. High lactate may also increase expression of acid sensing receptors in these neurons to generate the neuropathic pain seen in some patients with metastatic disease. Lastly, investigation into lactate-directed therapeutics is still early in development. Initial preclinical trials looking at LDH5/LDHA inhibitors as well as inhibitors of lactate transporters (MCT1) have demonstrated promise, but clinical work has been restricted to a single phase I trial. Lactate appears to play a crucial role in the pathogenesis of metastatic spine disease. Efforts are ongoing to identify small molecules inhibitors of targets in the lactogenic pathway capable of preventing the formation of osseous metastatic disease.
Keywords: Lactic acid, spinal metastases, Warburg effect, neoplasms, tumor metabolism
Introduction
Each year more than 1.7 million Americans receive a new cancer diagnosis, most commonly of the prostate, lung, or breast (1). Though prognosis varies wildly, for the vast majority of patients, disease is progressive and forms metastases, most commonly in the lungs, liver, and bones (2). More importantly, between 40% and 70% of patients will have metastatic cancer to the spine at some point during the course of their disease (3,4). Though the symptoms of metastatic spine disease can have significant overlap with the symptoms produced by primary vertebral body pathologies e.g., osteosarcoma, this clinical entity represents a highly heterogeneous group with a varied array of dysregulated cell signaling pathways, genetic mutations, and clinical interventions. Despite this, some commonalities have been observed, notably shared metabolic disruptions (5). Here we review the role of these metabolic perturbations, specifically the proposed mechanisms involved in lactate metabolism, as they pertain to primary tumor growth, the formation of osseous metastasis, and the generation of oncologic bone pain.
Proposed mechanisms
From the Greek karkinos, or crab, cancer has been known to humans since pre-literary times. Despite this relatively benign nomenclature, advances in sanitation, childhood mortality, and preventative medicine, have led cancer to become a common clinical entity that is now the second leading cause of death in the US behind only heart disease (6). Much of this increase occurred during the 20th century, the same period during which cancer was demonstrated to be a disease of genetic mutation (7). Occurring concurrently with this research on cancer genetics were investigations into neoplastic metabolism with the goal of identifying how tumor cells fed and could thus be killed (8). This work culminated in the description of the Warburg effect, which posits that tumor cells are glucose-consuming and lactate-producing, despite normoxic conditions (9,10).
During this same time, research in muscle physiology and lactate metabolism was transitioning from an era where lactate was thought to be a “dead-end waste product” formed under hypoxic conditions, to our current understanding of it being a dynamic metabolite seen under normoxic conditions (5). Lactate is readily mobilized during times of stress and is the mechanism by which whole body metabolism is coordinated. In fact, lactate flux often exceeds glucose flux, and lactate accumulation is rarely if ever due to hypoxia in vivo (5). For a more detailed look at normal lactate metabolism, the reader is encouraged to see part 1 of this review.
The first work demonstrating a role for lactate in tumor metabolism was generated by the Cori’s and Warburg in the 1920s. The latter demonstrated increased venous lactate levels in tumor-bearing limbs of rats (11) as compared to control limb, as well as increased lactate production by tumor cells cultured in the presence of glucose (12). Incorporating contemporaneous research, lactate was suggested to be a metabolic waste product attributable to anaerobic cellular respiration within tumor cells, demonstrating a selective preference for anaerobic metabolism (5). Subsequent studies also reported increased glycolysis within cancer cells, which led to the formalized description of the Warburg effect as a key part of tumor metabolism in the 1970s by Racker (9,13).
Research by Sonveaux et al. intimated that the increased lactate production might occur in the hypoxic tumor regions and then get shuttled to normoxic regions for utilization as a metabolic fuel (14). This shuttling of lactate between tumor cells was in line with work in muscle physiology, where numerous cell-to-cell lactate shuttles had been described by Brooks and others (15). Work to determine which tumors cells produce lactate, and which tumor cells use lactate is ongoing and dependent on numerous dynamic factors (see part 1).
More recently, tumor cells have been demonstrated to preferentially express lactate dehydrogenase type A (LDHA or LDH-5) (13), an isoform thought to more efficiently convert pyruvate to lactate in the presence of high pyruvate levels as may be seen in Warburg-type cancer cells (5). Consistent with this, both LDHA knockdown (16,17) and administration of LDH inhibitors seem to hinder the growth of tumor cells in vitro (18-20) suggesting lactate metabolism to be an essential component of carcinogenesis (13). Specifically, lactate has been implicated in the role LDH plays in promoting tumor aggressiveness and metastatic disease. Tumor aggressiveness has been described as the ability to grow or spread quickly, forming metastatic foci throughout the body. Three main steps characterize this process of progressive tumor invasion: (I) angiogenesis, (II) immune system evasion, and (III) extracellular matrix (ECM) degradation and tumor cell migration. The role of lactate in each of these will be discussed in turn (Figure 1).
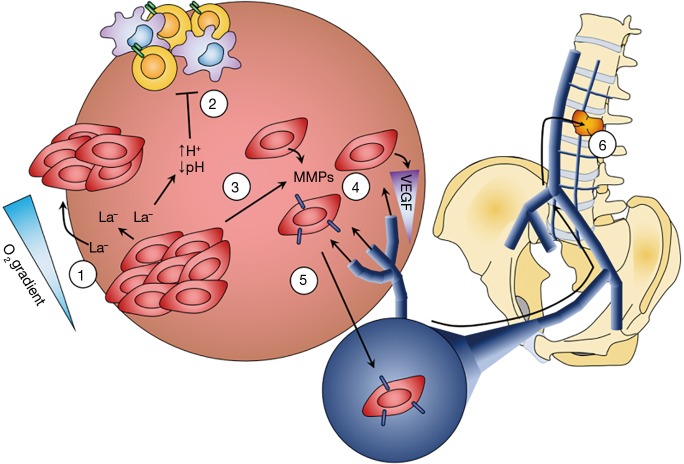
Figure 1.
Lactate contributes to local tumor invasiveness and the formation of metastatic disease. 1: Warburg-type cancer cells, commonly thought to be enriched in the hypoxic regions, upregulate proteins essential for the production (LDHA/LDH5) and export (MCT4) of lactate. By contrast, reverse Warburg cells—thought to be more enriched in normoxic tumor regions—upregulate enzymes and transporters (e.g., MCT1) important for using lactate as a metabolic fuel, setting up a lactate shuttle within the tumor based upon the tumor’s oxygen gradient. 2: Increased lactate production and export creates a strong ion gradient and concurrent drop in extracellular pH. This acidification leads to inhibition of immunosurveillance by impairing recognition of phenotypically abnormal cells by T-cells and NK cells, by impairing NK cell generation, and by decreasing monocyte motility. 3: Additionally, extracellular acidification disrupts the binding of cell surface integrins to the surrounding ECM, which in conjunction with downregulation of surface cadherins allows tumor cells to fragment from the central mass, leading to local invasion. This is further compounded by increases in the activity of tumor “invadopodia”, increasing tumor cell motility. 4: Further facilitating tumor cell invasiveness is upregulation of matrix metalloproteinases, which digest the local ECM, and CD44, enabling tumor cells to bind and migrate along hyaluronan-based proteoglycans. By inhibiting the citric acid cycle, lactate indirectly results in upregulation of vascular endothelial growth factor (VEGF), which drives angiogenesis and vascularization of the tumor mass. 5: A secondary function of VEGF is to promote the detachment of pericytes from local blood vessels, creating fenestrations in the vessel wall that facilitate tumor cell diapedesis and subsequent dissemination to distant sites, including the bones of the vertebral column through the veins of Batson’s plexus (6) (credit Z Pennington).
Angiogenesis
Angiogenesis is an essential component of bone tumor growth and the formation of metastases (21), especially metastases to the vertebral column, which are commonly attributed to retrograde migration through Batson’s plexus (22,23). In support of this, it has been demonstrated that increases in the density of tumor microvessel formation are directly correlated to the risk of metastasis (24).
The mechanisms by which lactate mitigates angiogenesis are largely derived from investigations into the mechanisms of wound healing (25). Local increases in extracellular lactate diffuse down their concentration gradient into the cell via monocarboxylate transporters (MCTs). Once intracellular, the law of mass action drives lactate to form pyruvate, catalyzed by LDH (26). Pyruvate accumulation inhibits the formation of α-ketoglutarate (2-oxoglutarate). In the canonical view of angiogenesis, oxygen-rich environments prevent accumulation of pyruvate and consequently α-ketoglutarate levels are high. Alpha-ketoglutarate then binds to prolyl hydroxylases, resulting in hydroxylation and subsequent degradation of hypoxia-inducible factor 1α (HIF1α) (27,28). As α-ketoglutarate is depleted though, prolyl hydroxylase activity drops, decreasing HIF1α degradation (5). HIF1α then migrates to the nucleus and upregulates pro-angiogenic genes, including vascular endothelial growth factor (VEGF). HIF1α upregulation can additionally feedback to shift tumor cell metabolism towards glycolysis by inactivating pyruvate dehydrogenase, resulting in additional lactate production (13). This is further compounded by HIF-1α-mediated upregulation of LDH type A (LDHA) (29) and MCT4 (30), leading to increased lactate efflux from tumor cells (31).
HIF1α upregulation increases VEGF expression by tumor cells. High VEGF levels promote vascular pericyte detachment, degradation of the surrounding basement membrane, and blood vessel dilatation (32). This fenestrates the vessel, allowing plasma proteins to extravasate and lay down ECM into which endothelial cells migrate, creating new vessels that feed the tumor. Inhibition of VEGF signaling has proven to offer selective survival benefits in patients with advanced disease (32), though it remains to be seen whether such benefits are associated with changes in lactate metabolism.
Immune system evasion
At baseline, the intrinsic immune system, chiefly natural killer cells, regularly monitors the body’s tissues for alterations in cell phenotype, such as those that characterize neoplastic cells (33). When said cells are encountered, they are targeted for death through either the expression of Fas/Fas ligand or release of granzymes/perforin, both of which activate the apoptotic machinery (34). Successful downregulation of these pathways through loss of heterozygosity or hypermethylation, along with upregulation of anti-apoptotic proteins, allows tumor cells to escape immune system-mediated death (35). Similarly, downregulation of the highly immunogenic antigens, the instigating entity in the extrinsic cell death pathway, allows for immune evasion.
Alternatively, tumor cells may escape immune surveillance by creating a microenvironment that silences cells of the immune system (anergy) (36). This can occur through upregulation of immune cell inhibitory molecules—PD1 (37) or CTLA-4 (38)—or alterations in the extracellular milieu (e.g., through acidification of the extracellular space) (9). Changes in extracellular lactate, a key player in this acidification, have been demonstrated to affect cells of both the lymphoid and myeloid lineages. Within myeloid-derived cells, monocytes demonstrate decreased motility in the presence of high extracellular lactate concentrations (contrasted with the increased motility seen in many cancer cells) (39). Lactate also biases tumor-associated macrophages towards a “tumor-friendly” M2 phenotype (40) and downregulates expression of both TNFα and IL-6, both of which help to mediate tumor cell immune escape (39). Similarly, high lactate levels impair IFNγ-producing T cells and NK cells of the lymphoid lineage by decreasing recognition of phenotypically abnormal cells and thus immunosurveillance within tumors (41). The acidic microenvironment created by lactate build-up compounds this issue by impairing the generation of natural killer cells responsible for tumor immunosurveillance (42).
Lactate specifically also impairs proper presentation of tumor cell antigens to members of the adaptive immune system by preventing normal differentiation of monocytes into dendritic cells (43). Similarly, high lactate levels inhibit proper function of CD8+ T cells and promote T cell anergy (44) by impairing lactate exportation (45). Generation of lactate by tumor cells can also deplete extracellular glucose stores to the point of becoming insufficient to sustain the effector functions of local immune cells, such as tumor cell killing (9).
ECM degradation, local invasion, and metastatic potential
As tumor cells proliferate, they must generate new regions for expansion, which involves degradation of the ECM and invasion of local tissues. Decreased extracellular pH promotes de novo actin filament production, essential for cellular migration (46). It may also alter the binding properties of tumor cell surface integrins, improving the ability of tumor cells to bind ECM components and migrate along them (46). Furthermore, acidification of the extracellular space increases the number and size of tumor cell “invadopodia”, themselves responsible for the amoeboid movements that underlie tumor cell migration (47). This relationship of pH to tumor growth has been demonstrated both directly in vitro and indirectly in vivo (48). Additionally, alkalization of the tumor environment in vitro directly inhibits tumor invasion, suggesting that acidification of the local tumor microenvironment is necessary for local invasion.
Decreased extracellular pH also activates proteinases released by tumor cells, such as matrix metalloproteinases-9 (49), cathepsin B, and hyaluronidase-2 (50). These enzymes degrade surrounding matrix, promoting tumor cell invasion, and their inhibition significantly impairs tumor cell invasion in preclinical studies, though clinical trials have been unsuccessful (51). ECM lysis by secreted proteinases may also free growth factors embedded in the matrix, including VEGF, transforming growth factor-β (TGFβ), and fibroblast growth factor-2 (FGF2), which can further promote tumor growth and angiogenesis (52). Acidification of the extracellular milieu in vitro leads to increased expression of hyaluronan and increased expression of tumor-specific varieties of CD44, which is responsible for binding hyaluronan and allowing tumor cell invasion (53,54).
To date, multiple in vitro experiments have implicated lactate or lactate metabolites either directly or indirectly in almost all of the above processes. Additionally, evidence suggests that lactate may help mitigate several of the key steps in the formation of metastatic disease, such as invasion of neighboring vessels (55,56). It is known that lactate formation contributes to the strong ion difference (SID) in vivo, and consequently to acidification of the local tumor environment (5). This in turn both decreases the binding avidity of cell integrins to elements of the surrounding ECM (46) and downregulates the expression of E-cadherins on tumor cells, helping to free them from neighboring cells (57,58). Acidification of the tumor stroma also results in activation of the extracellular proteinases that degrade ECM, such as the matrix metalloproteinases (50), and increases the density and length of “invadopodia”—the foot processes used by migrating cells (47). Lactate specifically appears to upregulate cathepsin B (59) and matrix metalloproteinase-9 (60), the latter of which has been correlated with both survival and the formation of distant metastases in multiple pathologies, including breast (61), ovarian (62), and prostate cancer (63). To that end, direct inhibition of matrix metalloproteinases has prevented metastasis formation in vivo (64). Unfortunately, none of the early-stage clinical trials examining MMP inhibitor use have reported significant survival benefits (65).
Additionally, lactate has been demonstrated to increase the expression of CD44 (53), perhaps due to the colocalization of the lactate transporters MCT1 and MCT4 with CD44 in cultured cells (66). As stated above, CD44 is commonly upregulated in tumor cells (67) and has been associated with both tumor burden and metastasis (68). More importantly, CD44 upregulation has been demonstrated in vitro to potentiate the adherence of metastatic breast and prostate cells to bone marrow endothelium, a key step in the formation of bony metastases (69). CD44 may also underlie the increase in tumor migratory capacity that has been correlated with lactate and lactate metabolites (39,70,71).
Evidence from in vivo experiments has suggested that tumor microenvironment acidification and increased lactate concentrations are necessary for several of the key steps in the formation of tumor metastases. Rizwan et al. demonstrated in a murine model that both LDHA expression levels and overall lactate production correlated with disease severity. Knockdown of LDHA resulted in increased time to first metastasis and longer overall survival (72). Concomitantly, Robey et al. reported that use of alkalinized drinking water decreased matrix proteinase activity in a murine model of breast adenocarcinoma (73). Alkalinization of the tumor microenvironment through this mechanism also decreased the rate of spontaneous metastasis (74) and increased overall survival (73). However, the authors did not report whether the therapeutic benefits were also associated with decreases in lactate concentration. Most recently, Zhao et al. provided evidence suggesting that LDHA/LDH5 overexpression may mediate the epithelial-mesenchymal transition that characterizes metastatic disease (75).
Several clinical studies have also been published over the past two decades correlating tumor sample lactate concentration with the incidence of metastatic disease (Table 1). An early study by Schwickert et al. examined a cohort of patients with cervical cancer and reported significantly higher lactate levels in primary tumor samples harvested from patients with metastatic disease as compared to non-metastatic disease (76). A follow-up study by the same group also reported higher lactate concentrations to be correlated with poorer overall survival and poorer disease-free survival (78). Similar findings have been reported in patients with gastric cancer (81), head and neck cancer (77,79), and colorectal adenocarcinoma (80). These results are similar to in vitro findings which have demonstrated cells with higher lactate production to have greater propensity to metastasize (82).
Table 1. Studies examining serum lactate levels and metastatic disease.
| Study | Methodology | Findings |
|---|---|---|
| Schwickert et al., 1995 (76) | ❖ Cryostat sections of 11 tumor samples from 10 patients with cervical carcinoma of various stages | ❖ Lactate levels significantly higher (P<0.05) in tumor samples from patients with documented metastases |
| ❖ Lactate levels in samples measured and compared between patients with and without clinically-documented metastasis | ❖ Lactate levels significantly higher in viable tumor samples compared to necrotic samples | |
| Walenta et al., 1997 (77) | ❖ Specimens from 15 patients with head and neck cancer | ❖ Sample lactate levels significantly higher in patients with metastatic disease (12.3 vs. 4.7 μmol/g; P<0.005) |
| ❖ Quantitative bioluminescence imaging of lactate levels compared between patients with and without metastatic disease | ❖ Lactate levels spread over a greater range in patients with metastases | |
| Walenta et al., 2000 (78) | ❖ Cryostat sections of 35 tumor samples from 34 patients with cervical carcinoma (most stage II or III) | ❖ Lactate levels were significantly higher (P=0.001) in tumor samples from patients with metastatic disease |
| ❖ Lactate levels in samples measured and compared between patients with and without detectable metastases | ❖ High tumor lactate levels were associated with significantly worse disease-free (60.5 vs. 22.1 months; P=0.014) and overall survival (70.9 vs. 31.0 months; P=0.015) | |
| ❖ Lactate levels correlated with overall and disease-free survival | ||
| Brizel et al., 2001 (79) | ❖ Biopsies from 34 patients with head and neck cancer | ❖ High tumor lactate concentrations correlated with poorer metastasis-free survival at 2 years (90% vs. 25%; P<0.0001) |
| ❖ Tumor samples analyzed for lactate concentration | ❖ High tumor lactate concentrations correlated with poorer 2-year overall survival (90% vs. 35%; P<0.0001) | |
| ❖ Sample lactate concentrations correlated with overall and metastasis-free survival at 2 years | ❖ Median lactate concentration in tumors that metastasized significantly lower than those remaining local (12.9 vs. 4.8 μmol/g; P<0.005) | |
| Walenta et al., 2003 (80) | ❖ 33 cryobiopsy samples from 24 patients with rectal adenocarcinoma | ❖ Lactate levels significantly higher in patients with metastatic disease (13.4 vs. 6.9 μmol/g; P<0.005) |
| ❖ Lactate levels measured and compared between metastatic and nonmetastatic groups | ❖ No significant difference in lactate levels between normal tissue or adenoma and non-metastatic disease | |
| ❖ Nodal involvement did not correlate with lactate levels | ||
| ❖ All patients with metastatic disease had lactate levels greater than 8.0 μmol/g | ||
| Hur et al., 2013 (81) | ❖ Samples from 152 patients having undergone surgery for gastric adenocarcinoma | ❖ PDK-1 levels correlated with overall and progression-free survival |
| ❖ Samples stained for PDK-1 and subjected to glucose uptake and lactate production assays | ❖ In vitro levels of PDK-1 expression correlated with higher lactate production | |
| ❖ 5-fluorouracil-mediated cell killing decreased PDK-1 expression which was correlated with decreased lactate production |
Bone pain and osteolytic bone metastases
One of the chief concerns of metastatic spine disease to the spinal oncology surgeon is mechanical instability of the vertebral column. Progressive destruction of the anterior and middle columns steadily reduces the strength of the vertebral body, and can ultimately result in an incompetent body that is incapable of fully supporting the torso’s mass (83-87). At said point the body begins to fracture and undergo height loss. Regardless of the extent of disease or collapse, 70% of patients will suffer from cancer-associated bone pain (88). Those with the most severe and concerning disease will also experience mechanical, or movement-related pain, which is commonly used as a clinical indicator of potential mechanical instability (89).
The role of lactate in osteolytic bone tumors can then be considered from two perspectives—in terms of its contribution to progressive osteolysis and mechanical instability, and in terms of its contribution to cancer-related bone pain (Figure 2). The latter is far more common and is thought to stem from a combination of acidification of the tumor microenvironment and stretching of the periosteum, which can occur with large or eccentrically-located tumors (88). Lactate is thought to be a major contributor to acidosis of the tumor extracellular microenvironment as lactate export can decrease extracellular pH to the 5.5–7.0 range (13). This low pH is sufficient to activate TRPV1 and ASIC3 ionotropic receptors found on the CGRP+, SP+ small, unmyelinated nociceptive afferents that innervate the bone marrow and periosteum (90-93). Proton release by activated osteoclasts may additionally contribute to activation of these fibers (88). Evidence for this latter mechanism of bone pain is equivocal at best though, as randomized controlled trials of bisphosphonates—inorganic molecules that inhibit osteoclast activity—have provided only weak evidence suggesting that they are effective at relieving oncologic bone pain (94).
Figure 2.
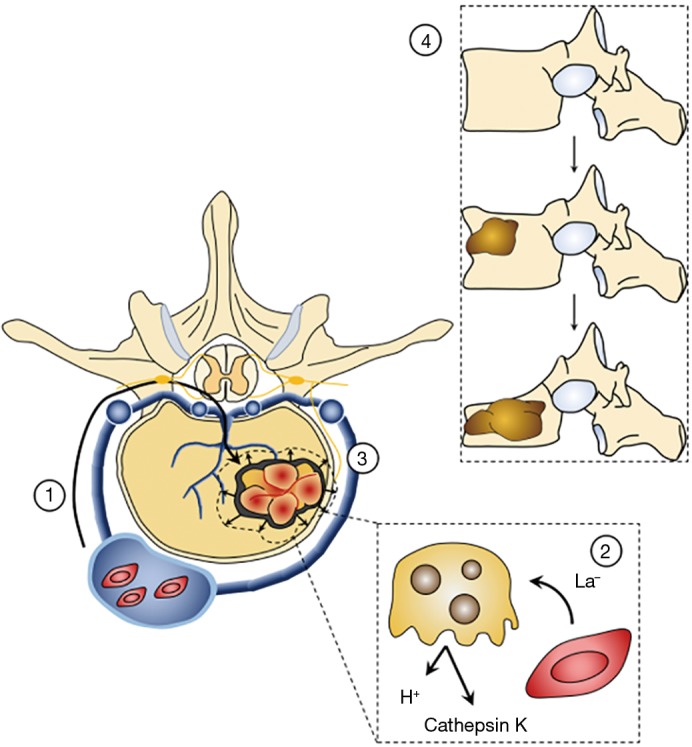
Increased lactate production contributes to progressive osteolysis, bone pain, and mechanical instability in the spine. 1: Circulating tumor cells can move retrogradely through lumbar intersegmental veins and into the vertebral venous plexus (of Batson). From there they progress through basilar veins to seed the vertebral body. Once the tumor cells have instantiated themselves in the local bone, they begin to proliferate. 2: The actively proliferating cells release lactate into the local microenvironment, which can be taken up and used as fuel by osteoclasts. Increased osteoclastic activity results in greater destruction of local bone through acidification and the release of proteases such as cathepsin K. The increased acidity of the bone milieu is thought to activate acid-sensitive receptors, such as ASIC1a and ASIC 1b, on CGRP+ nociceptive afferents that innervate the bone, creating the oncologic pain seen in many patients with bony metastases. 3: Additionally, as the tumor continue to grow, it may compromise the cortical bone and displace the overlying periosteum. The latter is richly innervated by nociceptive afferents, giving rise to mechanical pain. 4: As the lesions continue to increase in size (top to bottom), the structural integrity of the vertebral body is compromised, leading to wedging and eventual vertebral body collapse. This mechanical instability may require surgical intervention and reconstruction (credit Z Pennington).
CGRP+ sensory neurons innervating the bone also appear to play a role in the neuropathic pain experienced by many patients with metastatic disease, possibly through the formation of neuromas at the sensory tips of neurons. These neuromas are thought to underlie the breakthrough pain experienced by many patients with metastatic disease (88), which is notoriously difficult to treat (95). Tumors may also directly injure nociceptive fibers, in part explaining the relative intractability of bone pain to conventional analgesics (95). Along these lines, experiments in a murine model of breast cancer have demonstrated upregulation of acid-sensing receptors—ASIC1a and ASIC1b—in sensory neurons innervating compromised bone, as compared to those innervating unaffected bone (96). These changes have previously been linked to hyperalgesia and allodynia (97). Phenotypic changes are also noted in the machinery of the dorsal horn with increased expression of immediate early genes (96) previously tied to the development of neuropathic pain (98).
Bone remodeling is normally characterized by the balanced activities of osteoblast and osteoclasts, which lay down and resorb bone matrix, respectively (99). In cases of metastatic disease to the spine, these activities can become unbalanced resulting in either net bony deposition, or more commonly, bony destruction, visualized radiographically as osteoblastic and osteolytic lesions, respectively. As the tumor progresses in size and net bone mineral density of the vertebral body decreases, loading of the spine can result in a series of microfractures (88). These destabilizing fractures have two effects: (I) progressive vertebral height loss and potential deformity, and (II) distortion of the overlying periosteum with application of load to the vertebral column (100). Periosteal distortion stretches and activates the nociceptive neurons, creating oncologic pain, which is often excruciating owing to the high density of neuronal input to the periosteum (88).
In vitro experimentation has suggested that lactate may also play a key role in generating this mechanical instability-related pain. Using a human-derived breast adenocarcinoma line, Lemma and colleagues demonstrated that lactate produced by the tumor cells feeds and consequently activates local osteoclasts (82). High lactate production was associated with upregulation of MCT4s and downregulation of MCT1s in tumor cells, consistent with the conclusion that the high extracellular lactate levels results from net lactate export (82). By contrast, osteoclasts demonstrated phenotypic changes more consistent with increased uptake of lactate. Inhibition of MCT1 led to significant decreases in osteoclast-dependent collagen 1 degradation where lactate-feeding increased degradation, suggesting that neoplasm-derived lactate feeds osteoclasts to promote osteolysis (101).
Proposed therapeutic targets
Drugs with the potential to block or alter lactate metabolism in such a way as to halt tumor progression, radiosensitize the tumor, or decrease vertebral column osteolysis may have clinical utility. In their recent review, Muir and Vander Heiden note that much of our understanding of the tumor microenvironment is limited by experimental models though (102). For example, cell cultures replicate neither the exact milieu that makes up the tumor microenvironment, nor do they recreate the three-dimensional relationship a tumor takes on in the in vivo environment. Nonetheless, therapies targeting various “metabolic checkpoints” are currently being explored.
Dichloroacetate, monocarboxylate inhibitors, and LDH inhibitors
To date several interventions directed at addressing lactate metabolism have been implemented with varying degrees of success. Pyruvate dehydrogenase kinase (PDK) inhibitors, originally employed for patients with lactic acidosis, have been tested for their ability to remove inhibition of the pyruvate dehydrogenase complex and allow pyruvate to be shuttled away from LDH and into the citric acid cycle (17). These drugs, notably dichloroacetate (DCA), have been demonstrated to inhibit tumor growth both in vitro and in vivo (103) as well as decrease metastatic progression in vivo (104). A recent phase II clinical trial investigating the use of DCA in refractory breast and non-small cell cancer found adverse effects to be unacceptably high though, leading to early termination (NCT01029925). By contrast, a subsequent phase 1 study in glioblastoma found it to be reasonably well tolerated (105). Other phase 1 studies in head and neck cancer (NCT01163487), squamous cell cancer (NCT01386632) and metastatic solid tumors (NCT00566410) have been conducted but have failed to report therapeutic benefit.
LDHA/LDH5 and lactate transporters (e.g., the MCT family) have also been targeted. In vitro experiments have demonstrated that LDHA knockdown impairs tumor growth (16,106) and in vivo experiments have confirmed that animals injected with LDHA-deficient tumors have significantly improved survival relative to control animals (16). Small molecule inhibition of LDHA was similarly effective at inhibiting tumor progression in vitro (18,107-112) and in vivo (20). As of yet, none of the small molecule inhibitors has progressed to the point of being a clinically viable treatment and no clinical trials have been registered (113), possibly due to the bidirectional, near equilibrium nature of LDH, regardless of isoform.
MCT inhibitors have demonstrated somewhat greater success. MCT1 inhibitors (e.g., AZD3965) have shown anti-tumor activity in vitro, significantly impairing lactate production and leading to massive tumor cell die off (14). Recently, in vitro and in vivo results of the inhibitor AR-C155858 have demonstrated mixed results with regard to its effects on tumor growth (114,115). Curiously, AR-C155858 demonstrates higher MCT1 affinity than AZD3965 (116,117), though it is the latter which has demonstrated more success in vivo (118,119) and is currently being tested in a phase 1 trial (NCT01791595). This contradictory result may be secondary to tumor adaptation as has been demonstrated with other MCT1 inhibitors. In these studies MCT1 inhibition led to MCT4 upregulation and “tumor escape,” which may prove problematic as MCT inhibitors continue to be moved along the pathway to clinical utilization (120). Our personal experience with several MCT inhibitors in mice demonstrated them to be ineffective at tumor control, and in some cases toxic, as some older inhibitors [e.g., α-cyano-4-hydroxycinnamate (CHC)] had low target specificity and multiple off-target effects, including inhibition of pyruvate transporters (personal communication with Andrew Halestrap, University of Bristol, 2013).
Although drugs that seek to lower lactate are promising, care must be taken to consider the model used and the various roles lactate has in normal metabolism. For example, pertinent to the spine, oligodendrocytes rely heavily on lactate as fuel (121) and as a precursor for making lipids. Murine and human oligodendrocytes highly express MCT1, the inhibition of which results in significant axonal damage in vitro and in vivo (122). Additionally, reduction of MCT1 expression is seen in both patients with amyotrophic lateral sclerosis (ALS) and animal models of ALS, suggesting a critical role for lactate in neuronal function. Therapies that non-specifically block lactate flux may therefore have profound deleterious effects on central nervous system white matter (123).
Finally, it should be emphasized that both LDH and MCTs readily operate in both directions. MCTs function via diffusion, while lactate-pyruvate interconversion is a near-equilibrium reaction regardless of LDH subtype (124). Although traditional thinking teaches that MCT1s favor import and MCT4s favor export, in actuality, both isotypes perform both activities (5). To this end, MCT1s may appear to favor import as they are commonly upregulated in tissues that consume lactate, as compared to MCT4s, which are preferentially upregulated in lactate-exporting tissues (5). Newsholme has similarly pointed out that LDH subtype is likely of minimal importance given that it does not alter the net free energy change of lactate-pyruvate interconversion (125). In summary, while LDH and MCT inhibitors offer tremendous potential, care should be used in experimental trials in light of their bidirectional nature.
Radioresistance
Several groups, including those of Quennet et al. (126) and Sattler et al. (127) have demonstrated that in vivo lactate concentrations directly correlate with tumor response to fractionated irradiation. Lactate is proposed to confer this radioresistance indirectly by means of pyruvate generation (127). Pyruvate is a potent free radical scavenger and so may prevent accumulation of these species, which mediates DNA damage and cell death (128). Along these lines, use of inhibitors that decrease pyruvate levels should increase lesion radiosensitivity. This has been demonstrated in vivo using MCT1 inhibitors in murine models of small cell (129) and non-small cell lung cancer (14). Most recently, Corbet et al. published the results of an experiment examining the effects of a mitochondrial pyruvate transporter on radiosensitivity in a murine model of cervical carcinoma (130). Blockage of this transporter decreased tumor cell lactate uptake and resulted in cell killing as opposed to cell senescence, which was seen with application of an MCT1 inhibitor (AR-C155858). Further evidence is required to evaluate the utility of this new clinical target.
Conclusions
Though held as a metabolic waste product for the better part of a century, lactate has steadily come to be appreciated as an essential component of tumor carcinogenesis. Both in vitro and in vivo work has demonstrated it to play key roles in tumor growth, angiogenesis, the epithelial-to-mesenchymal transition, and the formation of painful osteolytic metastases. Due to its relatively late appearance in the cancer literature, clinical interventions aimed at addressing dysregulated lactate metabolism are currently undergoing preclinical and early clinical investigation. Early preclinical results have been promising and suggest that restoration of normal lactate homeostasis may inhibit the formation of bony metastasis, a potential boon for the spinal oncologist. Additionally, blockage of lactate exportation may increase tumor radiosensitivity and thereby provide potential interventions for patients with mechanically stable metastatic spine disease who are too frail to undergo surgical intervention. Much additional research is necessary before any changes in clinical standard of care can occur. But success in these endeavors may present spinal surgeons with an option for prophylaxis against progressive spinal instability—an intervention which is currently unavailable.
Acknowledgments
None.
Footnotes
Conflicts of Interest: ML Goodwin: Consultant for ROM3, Augmedics; DM Sciubba: Consultant for Orthofix, Globus, K2M, Medtronic, Stryker, Baxter. The other authors have no conflicts of interest to declare.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 2.Budczies J, von Winterfeld M, Klauschen F, et al. The landscape of metastatic progression patterns across major human cancers. Oncotarget 2015;6:570-83. 10.18632/oncotarget.2677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fornasier VL, Horne JG. Metastases to the vertebral column. Cancer 1975;36:590-4. [DOI] [PubMed] [Google Scholar]
- 4.Klimo P, Jr, Thompson CJ, Kestle JR, et al. A meta-analysis of surgery versus conventional radiotherapy for the treatment of metastatic spinal epidural disease. Neuro Oncol 2005;7:64-76. 10.1215/S1152851704000262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferguson BS, Rogatzki MJ, Goodwin ML, et al. Lactate metabolism: historical context, prior misinterpretations, and current understanding. Eur J Appl Physiol 2018;118:691-728. 10.1007/s00421-017-3795-6 [DOI] [PubMed] [Google Scholar]
- 6.Heron M, Anderson RN. Changes in the Leading Cause of Death: Recent Patterns in Heart Disease and Cancer Mortality. NCHS Data Brief 2016;(254):1-8. [PubMed] [Google Scholar]
- 7.Jeggo PA, Pearl LH, Carr AM. DNA repair, genome stability and cancer: a historical perspective. Nat Rev Cancer 2016;16:35-42. 10.1038/nrc.2015.4 [DOI] [PubMed] [Google Scholar]
- 8.Seyfried TN, Flores RE, Poff AM, et al. Cancer as a metabolic disease: implications for novel therapeutics. Carcinogenesis 2014;35:515-27. 10.1093/carcin/bgt480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liberti MV, Locasale JW. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem Sci 2016;41:211-8. 10.1016/j.tibs.2015.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu XD, Shao SX, Jiang HP, et al. Warburg effect or reverse Warburg effect? A review of cancer metabolism. Oncol Res Treat 2015;38:117-22. 10.1159/000375435 [DOI] [PubMed] [Google Scholar]
- 11.Warburg O, Wind F, Negelein E. The Metabolism of Tumors in the Body. J Gen Physiol 1927;8:519-30. 10.1085/jgp.8.6.519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warburg O, Minami S. Versuche an überlebendem carcinom-gewebe. Klinische Wochenschrift 1923;2:776-7. 10.1007/BF01712130 [DOI] [Google Scholar]
- 13.San-Millán I, Brooks GA. Reexamining cancer metabolism: lactate production for carcinogenesis could be the purpose and explanation of the Warburg Effect. Carcinogenesis 2017;38:119-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sonveaux P, Végran F, Schroeder T, et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest 2008;118:3930-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodwin ML, Gladden LB, Nijsten MWN, et al. Lactate and cancer: revisiting the warburg effect in an era of lactate shuttling. Front Nutr 2015;1:27. 10.3389/fnut.2014.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 2006;9:425-34. 10.1016/j.ccr.2006.04.023 [DOI] [PubMed] [Google Scholar]
- 17.Vander Heiden MG. Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov 2011;10:671-84. 10.1038/nrd3504 [DOI] [PubMed] [Google Scholar]
- 18.Fiume L, Manerba M, Vettraino M, et al. Impairment of aerobic glycolysis by inhibitors of lactic dehydrogenase hinders the growth of human hepatocellular carcinoma cell lines. Pharmacology 2010;86:157-62. 10.1159/000317519 [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Yang Z, Chen Z, et al. Effects of the suppression of lactate dehydrogenase A on the growth and invasion of human gastric cancer cells. Oncol Rep 2015;33:157-62. 10.3892/or.2014.3600 [DOI] [PubMed] [Google Scholar]
- 20.Le A, Cooper CR, Gouw AM, et al. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A 2010;107:2037-42. 10.1073/pnas.0914433107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bielenberg DR, Zetter BR. The Contribution of Angiogenesis to the Process of Metastasis. Cancer J 2015;21:267-73. 10.1097/PPO.0000000000000138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maccauro G, Spinelli MS, Mauro S, et al. Physiopathology of spine metastasis. Int J Surg Oncol 2011;2011:107969. 10.1155/2011/107969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coman DR, deLong RP. The role of the vertebral venous system in the metastasis of cancer to the spinal column; experiments with tumor-cell suspensions in rats and rabbits. Cancer 1951;4:610-8. [DOI] [PubMed] [Google Scholar]
- 24.Weidner N, Semple JP, Welch WR, et al. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med 1991;324:1-8. 10.1056/NEJM199101033240101 [DOI] [PubMed] [Google Scholar]
- 25.Trabold O, Wagner S, Wicke C, et al. Lactate and oxygen constitute a fundamental regulatory mechanism in wound healing. Wound Repair Regen 2003;11:504-9. 10.1046/j.1524-475X.2003.11621.x [DOI] [PubMed] [Google Scholar]
- 26.Sauer LA, Dauchy RT. Regulation of lactate production and utilization in rat tumors in vivo. J Biol Chem 1985;260:7496-501. [PubMed] [Google Scholar]
- 27.Iommarini L, Porcelli AM, Gasparre G, et al. Non-Canonical Mechanisms Regulating Hypoxia-Inducible Factor 1 Alpha in Cancer. Front Oncol 2017;7:286. 10.3389/fonc.2017.00286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsumoto K, Imagawa S, Obara N, et al. 2-Oxoglutarate downregulates expression of vascular endothelial growth factor and erythropoietin through decreasing hypoxia-inducible factor-1alpha and inhibits angiogenesis. J Cell Physiol 2006;209:333-40. 10.1002/jcp.20733 [DOI] [PubMed] [Google Scholar]
- 29.Semenza GL, Jiang BH, Leung SW, et al. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem 1996;271:32529-37. 10.1074/jbc.271.51.32529 [DOI] [PubMed] [Google Scholar]
- 30.Ullah MS, Davies AJ, Halestrap AP. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. J Biol Chem 2006;281:9030-7. 10.1074/jbc.M511397200 [DOI] [PubMed] [Google Scholar]
- 31.Manning Fox JE, Meredith D, Halestrap AP. Characterisation of human monocarboxylate transporter 4 substantiates its role in lactic acid efflux from skeletal muscle. J Physiol (Lond) 2000;529 Pt 2:285-93. 10.1111/j.1469-7793.2000.00285.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011;473:298-307. 10.1038/nature10144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vivier E, Ugolini S, Blaise D, et al. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol 2012;12:239-52. 10.1038/nri3174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peter ME, Hadji A, Murmann AE, et al. The role of CD95 and CD95 ligand in cancer. Cell Death Differ 2015;22:549-59. 10.1038/cdd.2015.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fulda S. Regulation of cell death in cancer-possible implications for immunotherapy. Front Oncol 2013;3:29. 10.3389/fonc.2013.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vinay DS, Ryan EP, Pawelec G, et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin Cancer Biol 2015;35 Suppl:S185-98. 10.1016/j.semcancer.2015.03.004 [DOI] [PubMed] [Google Scholar]
- 37.Sun C, Mezzadra R, Schumacher TN. Regulation and Function of the PD-L1 Checkpoint. Immunity 2018;48:434-52. 10.1016/j.immuni.2018.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu P, Liu Q, Deng G, et al. The prognostic value of cytotoxic T-lymphocyte antigen 4 in cancers: a systematic review and meta-analysis. Sci Rep 2017;7:42913. 10.1038/srep42913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goetze K, Walenta S, Ksiazkiewicz M, et al. Lactate enhances motility of tumor cells and inhibits monocyte migration and cytokine release. Int J Oncol 2011;39:453-63. [DOI] [PubMed] [Google Scholar]
- 40.Colegio OR, Chu N, Szabo AL, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 2014;513:559-63. 10.1038/nature13490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brand A, Singer K, Koehl GE, et al. LDHA-Associated Lactic Acid Production Blunts Tumor Immunosurveillance by T and NK Cells. Cell Metab 2016;24:657-71. 10.1016/j.cmet.2016.08.011 [DOI] [PubMed] [Google Scholar]
- 42.Müller B, Fischer B, Kreutz W. An acidic microenvironment impairs the generation of non-major histocompatibility complex-restricted killer cells. Immunology 2000;99:375-84. 10.1046/j.1365-2567.2000.00975.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gottfried E, Kunz-Schughart LA, Ebner S, et al. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood 2006;107:2013-21. 10.1182/blood-2005-05-1795 [DOI] [PubMed] [Google Scholar]
- 44.Calcinotto A, Filipazzi P, Grioni M, et al. Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes. Cancer Res 2012;72:2746-56. 10.1158/0008-5472.CAN-11-1272 [DOI] [PubMed] [Google Scholar]
- 45.Fischer K, Hoffmann P, Voelkl S, et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood 2007;109:3812-9. 10.1182/blood-2006-07-035972 [DOI] [PubMed] [Google Scholar]
- 46.Webb BA, Chimenti M, Jacobson MP, et al. Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer 2011;11:671-7. 10.1038/nrc3110 [DOI] [PubMed] [Google Scholar]
- 47.Busco G, Cardone RA, Greco MR, et al. NHE1 promotes invadopodial ECM proteolysis through acidification of the peri-invadopodial space. FASEB J 2010;24:3903-15. 10.1096/fj.09-149518 [DOI] [PubMed] [Google Scholar]
- 48.Estrella V, Chen T, Lloyd M, et al. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res 2013;73:1524-35. 10.1158/0008-5472.CAN-12-2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Putney LK, Barber DL. Expression profile of genes regulated by activity of the Na-H exchanger NHE1. BMC Genomics 2004;5:46. 10.1186/1471-2164-5-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bourguignon LYW, Singleton PA, Diedrich F, et al. CD44 interaction with Na+-H+ exchanger (NHE1) creates acidic microenvironments leading to hyaluronidase-2 and cathepsin B activation and breast tumor cell invasion. J Biol Chem 2004;279:26991-7007. 10.1074/jbc.M311838200 [DOI] [PubMed] [Google Scholar]
- 51.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science 2002;295:2387-92. 10.1126/science.1067100 [DOI] [PubMed] [Google Scholar]
- 52.Shuman Moss LA, Jensen-Taubman S, Stetler-Stevenson WG. Matrix metalloproteinases: changing roles in tumor progression and metastasis. Am J Pathol 2012;181:1895-9. 10.1016/j.ajpath.2012.08.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stern R, Shuster S, Neudecker BA, et al. Lactate stimulates fibroblast expression of hyaluronan and CD44: the Warburg effect revisited. Exp Cell Res 2002;276:24-31. 10.1006/excr.2002.5508 [DOI] [PubMed] [Google Scholar]
- 54.Edward M, Gillan C, Micha D, et al. Tumour regulation of fibroblast hyaluronan expression: a mechanism to facilitate tumour growth and invasion. Carcinogenesis 2005;26:1215-23. 10.1093/carcin/bgi064 [DOI] [PubMed] [Google Scholar]
- 55.Sahai E. Illuminating the metastatic process. Nat Rev Cancer 2007;7:737-49. 10.1038/nrc2229 [DOI] [PubMed] [Google Scholar]
- 56.Paul CD, Mistriotis P, Konstantopoulos K. Cancer cell motility: lessons from migration in confined spaces. Nat Rev Cancer 2017;17:131-40. 10.1038/nrc.2016.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Y, Lin S, Chen Y, et al. LDH-Apromotes epithelial-mesenchymal transition by upregulating ZEB2 in intestinal-type gastric cancer. Onco Targets Ther 2018;11:2363-73. 10.2147/OTT.S163570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiang F, Ma S, Xue Y, et al. LDH-A promotes malignant progression via activation of epithelial-to-mesenchymal transition and conferring stemness in muscle-invasive bladder cancer. Biochem Biophys Res Commun 2016;469:985-92. 10.1016/j.bbrc.2015.12.078 [DOI] [PubMed] [Google Scholar]
- 59.Rozhin J, Sameni M, Ziegler G, et al. Pericellular pH affects distribution and secretion of cathepsin B in malignant cells. Cancer Res 1994;54:6517-25. [PubMed] [Google Scholar]
- 60.Matsubara T, Diresta GR, Kakunaga S, et al. Additive Influence of Extracellular pH, Oxygen Tension, and Pressure on Invasiveness and Survival of Human Osteosarcoma Cells. Front Oncol 2013;3:199. 10.3389/fonc.2013.00199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li H, Qiu Z, Li F, Wang C. The relationship between MMP-2 and MMP-9 expression levels with breast cancer incidence and prognosis. Oncol Lett 2017;14:5865-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li LN, Zhou X, Gu Y, et al. Prognostic value of MMP-9 in ovarian cancer: a meta-analysis. Asian Pac J Cancer Prev 2013;14:4107-13. 10.7314/APJCP.2013.14.7.4107 [DOI] [PubMed] [Google Scholar]
- 63.Schveigert D, Valuckas KP, Kovalcis V, et al. Significance of MMP-9 expression and MMP-9 polymorphism in prostate cancer. Tumori 2013;99:523-9. 10.1177/030089161309900414 [DOI] [PubMed] [Google Scholar]
- 64.Rofstad EK, Mathiesen B, Kindem K, et al. Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice. Cancer Res 2006;66:6699-707. 10.1158/0008-5472.CAN-06-0983 [DOI] [PubMed] [Google Scholar]
- 65.Overall CM, López-Otín C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer 2002;2:657-72. 10.1038/nrc884 [DOI] [PubMed] [Google Scholar]
- 66.Slomiany MG, Grass GD, Robertson AD, et al. Hyaluronan, CD44, and emmprin regulate lactate efflux and membrane localization of monocarboxylate transporters in human breast carcinoma cells. Cancer Res 2009;69:1293-301. 10.1158/0008-5472.CAN-08-2491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Senbanjo LT, Chellaiah MA. CD44: A Multifunctional Cell Surface Adhesion Receptor Is a Regulator of Progression and Metastasis of Cancer Cells. Front Cell Dev Biol 2017;5:18. 10.3389/fcell.2017.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McFarlane S, Coulter JA, Tibbits P, et al. CD44 increases the efficiency of distant metastasis of breast cancer. Oncotarget 2015;6:11465-76. 10.18632/oncotarget.3410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Draffin JE, McFarlane S, Hill A, et al. CD44 potentiates the adherence of metastatic prostate and breast cancer cells to bone marrow endothelial cells. Cancer Res 2004;64:5702-11. 10.1158/0008-5472.CAN-04-0389 [DOI] [PubMed] [Google Scholar]
- 70.Baumann F, Leukel P, Doerfelt A, et al. Lactate promotes glioma migration by TGF-beta2-dependent regulation of matrix metalloproteinase-2. Neuro-oncology 2009;11:368-80. 10.1215/15228517-2008-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guedes M, Araújo JR, Correia-Branco A, et al. Modulation of the uptake of critical nutrients by breast cancer cells by lactate: Impact on cell survival, proliferation and migration. Exp Cell Res 2016;341:111-22. 10.1016/j.yexcr.2016.01.008 [DOI] [PubMed] [Google Scholar]
- 72.Rizwan A, Serganova I, Khanin R, et al. Relationships between LDH-A, lactate, and metastases in 4T1 breast tumors. Clin Cancer Res 2013;19:5158-69. 10.1158/1078-0432.CCR-12-3300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Robey IF, Nesbit LA. Investigating mechanisms of alkalinization for reducing primary breast tumor invasion. Biomed Res Int 2013;2013:485196. 10.1155/2013/485196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Robey IF, Baggett BK, Kirkpatrick ND, et al. Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res 2009;69:2260-8. 10.1158/0008-5472.CAN-07-5575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao J, Huang X, Xu Z, et al. LDHA promotes tumor metastasis by facilitating epithelial mesenchymal transition in renal cell carcinoma. Mol Med Rep 2017;16:8335-44. 10.3892/mmr.2017.7637 [DOI] [PubMed] [Google Scholar]
- 76.Schwickert G, Walenta S, Sundfør K, et al. Correlation of high lactate levels in human cervical cancer with incidence of metastasis. Cancer Res 1995;55:4757-9. [PubMed] [Google Scholar]
- 77.Walenta S, Salameh A, Lyng H, et al. Correlation of high lactate levels in head and neck tumors with incidence of metastasis. Am J Pathol 1997;150:409-15. [PMC free article] [PubMed] [Google Scholar]
- 78.Walenta S, Wetterling M, Lehrke M, et al. High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res 2000;60:916-21. [PubMed] [Google Scholar]
- 79.Brizel DM, Schroeder T, Scher RL, et al. Elevated tumor lactate concentrations predict for an increased risk of metastases in head-and-neck cancer. Int J Radiat Oncol Biol Phys 2001;51:349-53. 10.1016/S0360-3016(01)01630-3 [DOI] [PubMed] [Google Scholar]
- 80.Walenta S, Chau T, Schroeder T, et al. Metabolic classification of human rectal adenocarcinomas: a novel guideline for clinical oncologists? J Cancer Res Clin Oncol 2003;129:321-6. 10.1007/s00432-003-0450-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hur H, Xuan Y, Kim YB, et al. Expression of pyruvate dehydrogenase kinase-1 in gastric cancer as a potential therapeutic target. Int J Oncol 2013;42:44-54. 10.3892/ijo.2012.1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lemma S, Di Pompo G, Porporato PE, et al. MDA-MB-231 breast cancer cells fuel osteoclast metabolism and activity: A new rationale for the pathogenesis of osteolytic bone metastases. Biochim Biophys Acta Mol Basis Dis 2017;1863:3254-64. 10.1016/j.bbadis.2017.08.030 [DOI] [PubMed] [Google Scholar]
- 83.Alkalay R, Adamson R, Miropolsky A, et al. Female Human Spines with Simulated Osteolytic Defects: CT-based Structural Analysis of Vertebral Body Strength. Radiology 2018;288:436-44. 10.1148/radiol.2018171139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alkalay RN, Harrigan TP. Mechanical assessment of the effects of metastatic lytic defect on the structural response of human thoracolumbar spine. J Orthop Res 2016;34:1808-19. 10.1002/jor.23154 [DOI] [PubMed] [Google Scholar]
- 85.Whyne CM, Hu SS, Lotz JC. Parametric finite element analysis of vertebral bodies affected by tumors. J Biomech 2001;34:1317-24. 10.1016/S0021-9290(01)00086-0 [DOI] [PubMed] [Google Scholar]
- 86.Whyne CM, Hu SS, Lotz JC. Burst fracture in the metastatically involved spine: development, validation, and parametric analysis of a three-dimensional poroelastic finite-element model. Spine 2003;28:652-60. 10.1097/01.BRS.0000051910.97211.BA [DOI] [PubMed] [Google Scholar]
- 87.Taneichi H, Kaneda K, Takeda N, et al. Risk factors and probability of vertebral body collapse in metastases of the thoracic and lumbar spine. Spine (Phila Pa 1976) 1997;22:239-45. 10.1097/00007632-199702010-00002 [DOI] [PubMed] [Google Scholar]
- 88.Yoneda T, Hiasa M, Nagata Y, et al. Acidic microenvironment and bone pain in cancer-colonized bone. Bonekey Rep 2015;4:690. 10.1038/bonekey.2015.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fisher CG, DiPaola CP, Ryken TC, et al. A Novel Classification System for Spinal Instability in Neoplastic Disease: An Evidence-Based Approach and Expert Consensus From the Spine Oncology Study Group. Spine (Phila Pa 1976) 2010;35:E1221-9. 10.1097/BRS.0b013e3181e16ae2 [DOI] [PubMed] [Google Scholar]
- 90.Hiasa M, Okui T, Allette YM, et al. Bone Pain Induced by Multiple Myeloma Is Reduced by Targeting V-ATPase and ASIC3. Cancer Res 2017;77:1283-95. 10.1158/0008-5472.CAN-15-3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mach DB, Rogers SD, Sabino MC, et al. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience 2002;113:155-66. 10.1016/S0306-4522(02)00165-3 [DOI] [PubMed] [Google Scholar]
- 92.Jimenez-Andrade JM, Mantyh WG, Bloom AP, et al. A phenotypically restricted set of primary afferent nerve fibers innervate the bone versus skin: therapeutic opportunity for treating skeletal pain. Bone 2010;46:306-13. 10.1016/j.bone.2009.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wakabayashi H, Wakisaka S, Hiraga T, et al. Decreased sensory nerve excitation and bone pain associated with mouse Lewis lung cancer in TRPV1-deficient mice. J Bone Miner Metab 2018;36:274-85. 10.1007/s00774-017-0842-7 [DOI] [PubMed] [Google Scholar]
- 94.Porta-Sales J, Garzón-Rodríguez C, Llorens-Torromé S, et al. Evidence on the analgesic role of bisphosphonates and denosumab in the treatment of pain due to bone metastases: A systematic review within the European Association for Palliative Care guidelines project. Palliat Med 2017;31:5-25. 10.1177/0269216316639793 [DOI] [PubMed] [Google Scholar]
- 95.Luger NM, Sabino MAC, Schwei MJ, et al. Efficacy of systemic morphine suggests a fundamental difference in the mechanisms that generate bone cancer vs inflammatory pain. Pain 2002;99:397-406. 10.1016/S0304-3959(02)00102-1 [DOI] [PubMed] [Google Scholar]
- 96.Nagae M, Hiraga T, Yoneda T. Acidic microenvironment created by osteoclasts causes bone pain associated with tumor colonization. J Bone Miner Metab 2007;25:99-104. 10.1007/s00774-006-0734-8 [DOI] [PubMed] [Google Scholar]
- 97.Duan B, Wu L, Yu Y, et al. Upregulation of acid-sensing ion channel ASIC1a in spinal dorsal horn neurons contributes to inflammatory pain hypersensitivity. J Neurosci 2007;27:11139-48. 10.1523/JNEUROSCI.3364-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bojovic O, Bramham CR, Tjølsen A. Stimulation-induced expression of immediate early gene proteins in the dorsal horn is increased in neuropathy. Scand J Pain 2016;10:43-51. 10.1016/j.sjpain.2015.09.002 [DOI] [PubMed] [Google Scholar]
- 99.Raggatt LJ, Partridge NC. Cellular and molecular mechanisms of bone remodeling. J Biol Chem 2010;285:25103-8. 10.1074/jbc.R109.041087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Martin CD, Jimenez-Andrade JM, Ghilardi JR, et al. Organization of a unique net-like meshwork of CGRP+ sensory fibers in the mouse periosteum: implications for the generation and maintenance of bone fracture pain. Neurosci Lett 2007;427:148-52. 10.1016/j.neulet.2007.08.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.David Roodman G, Silbermann R. Mechanisms of osteolytic and osteoblastic skeletal lesions. Bonekey Rep 2015;4:753. 10.1038/bonekey.2015.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Muir A, Vander Heiden MG. The nutrient environment affects therapy. Science 2018;360:962-3. 10.1126/science.aar5986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bonnet S, Archer SL, Allalunis-Turner J, et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell 2007;11:37-51. 10.1016/j.ccr.2006.10.020 [DOI] [PubMed] [Google Scholar]
- 104.Kolesnik DL, Pyaskovskaya ON, Boychuk IV, et al. Effect of dichloroacetate on Lewis lung carcinoma growth and metastasis. Exp Oncol 2015;37:126-9. 10.31768/2312-8852.2015.37(2):126-129 [DOI] [PubMed] [Google Scholar]
- 105.Dunbar EM, Coats BS, Shroads AL, et al. Phase 1 trial of dichloroacetate (DCA) in adults with recurrent malignant brain tumors. Invest New Drugs 2014;32:452-64. 10.1007/s10637-013-0047-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shim H, Dolde C, Lewis BC, et al. c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proc Natl Acad Sci U S A 1997;94:6658-63. 10.1073/pnas.94.13.6658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Manerba M, Vettraino M, Fiume L, et al. Galloflavin (CAS 568-80-9): a novel inhibitor of lactate dehydrogenase. Chem Med Chem 2012;7:311-7. 10.1002/cmdc.201100471 [DOI] [PubMed] [Google Scholar]
- 108.Farabegoli F, Vettraino M, Manerba M, et al. Galloflavin, a new lactate dehydrogenase inhibitor, induces the death of human breast cancer cells with different glycolytic attitude by affecting distinct signaling pathways. Eur J Pharm Sci 2012;47:729-38. 10.1016/j.ejps.2012.08.012 [DOI] [PubMed] [Google Scholar]
- 109.Fiume L, Vettraino M, Carnicelli D, et al. Galloflavin prevents the binding of lactate dehydrogenase A to single stranded DNA and inhibits RNA synthesis in cultured cells. Biochem Biophys Res Commun 2013;430:466-9. 10.1016/j.bbrc.2012.12.013 [DOI] [PubMed] [Google Scholar]
- 110.Granchi C, Roy S, Giacomelli C, et al. Discovery of N-hydroxyindole-based inhibitors of human lactate dehydrogenase isoform A (LDH-A) as starvation agents against cancer cells. J Med Chem 2011;54:1599-612. 10.1021/jm101007q [DOI] [PubMed] [Google Scholar]
- 111.Granchi C, Roy S, De Simone A, et al. N-Hydroxyindole-based inhibitors of lactate dehydrogenase against cancer cell proliferation. Eur J Med Chem 2011;46:5398-407. 10.1016/j.ejmech.2011.08.046 [DOI] [PubMed] [Google Scholar]
- 112.Wang Z, Wang D, Han S, et al. Bioactivity-guided identification and cell signaling technology to delineate the lactate dehydrogenase A inhibition effects of Spatholobus suberectus on breast cancer. PLoS One 2013;8:e56631. 10.1371/journal.pone.0056631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Granchi C, Paterni I, Rani R, et al. Small-molecule inhibitors of human LDH5. Future Med Chem 2013;5:1967-91. 10.4155/fmc.13.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Guan X, Bryniarski MA, Morris ME. In vitro and In vivo Efficacy of the Monocarboxylate Transporter 1 Inhibitor AR-C155858 in the Murine 4T1 Breast Cancer Tumor Model. AAPS J 2018;21:3. 10.1208/s12248-018-0261-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pivovarova AI, MacGregor GG. Glucose-dependent growth arrest of leukemia cells by MCT1 inhibition: Feeding Warburg's sweet tooth and blocking acid export as an anticancer strategy. Biomed Pharmacother 2018;98:173-9. 10.1016/j.biopha.2017.12.048 [DOI] [PubMed] [Google Scholar]
- 116.Curtis NJ, Mooney L, Hopcroft L, et al. Pre-clinical pharmacology of AZD3965, a selective inhibitor of MCT1: DLBCL, NHL and Burkitt's lymphoma anti-tumor activity. Oncotarget 2017;8:69219-36. 10.18632/oncotarget.18215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nancolas B, Sessions RB, Halestrap AP. Identification of key binding site residues of MCT1 for AR-C155858 reveals the molecular basis of its isoform selectivity. Biochem J 2015;466:177-88. 10.1042/BJ20141223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Polański R, Hodgkinson CL, Fusi A, et al. Activity of the monocarboxylate transporter 1 inhibitor AZD3965 in small cell lung cancer. Clin Cancer Res 2014;20:926-37. 10.1158/1078-0432.CCR-13-2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Noble RA, Bell N, Blair H, et al. Inhibition of monocarboxyate transporter 1 by AZD3965 as a novel therapeutic approach for diffuse large B-cell lymphoma and Burkitt lymphoma. Haematologica 2017;102:1247-57. 10.3324/haematol.2016.163030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Quanz M, Bender E, Kopitz C, et al. Preclinical Efficacy of the Novel Monocarboxylate Transporter 1 Inhibitor BAY-8002 and Associated Markers of Resistance. Mol Cancer Ther 2018;17:2285-96. 10.1158/1535-7163.MCT-17-1253 [DOI] [PubMed] [Google Scholar]
- 121.Sánchez-Abarca LI, Tabernero A, Medina JM. Oligodendrocytes use lactate as a source of energy and as a precursor of lipids. Glia 2001;36:321-9. 10.1002/glia.1119 [DOI] [PubMed] [Google Scholar]
- 122.Lee Y, Morrison BM, Li Y, et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 2012;487:443-8. 10.1038/nature11314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rinholm JE, Hamilton NB, Kessaris N, et al. Regulation of oligodendrocyte development and myelination by glucose and lactate. J Neurosci 2011;31:538-48. 10.1523/JNEUROSCI.3516-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xie J, Wu H, Dai C, et al. Beyond Warburg effect--dual metabolic nature of cancer cells. Sci Rep 2014;4:4927. 10.1038/srep04927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Newsholme EA. Enzymes, Energy and Endurance: Some Provocative Thoughts. In: Poortmans JR, editor. Principles of Exercise Biochemistry. Brussels: Karger Publishers, 2004:1-35. [Google Scholar]
- 126.Quennet V, Yaromina A, Zips D, et al. Tumor lactate content predicts for response to fractionated irradiation of human squamous cell carcinomas in nude mice. Radiother Oncol 2006;81:130-5. 10.1016/j.radonc.2006.08.012 [DOI] [PubMed] [Google Scholar]
- 127.Sattler UGA, Meyer SS, Quennet V, et al. Glycolytic metabolism and tumour response to fractionated irradiation. Radiother Oncol 2010;94:102-9. 10.1016/j.radonc.2009.11.007 [DOI] [PubMed] [Google Scholar]
- 128.Barker HE, Paget JTE, Khan AA, et al. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer 2015;15:409-25. 10.1038/nrc3958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bola BM, Chadwick AL, Michopoulos F, et al. Inhibition of monocarboxylate transporter-1 (MCT1) by AZD3965 enhances radiosensitivity by reducing lactate transport. Mol Cancer Ther 2014;13:2805-16. 10.1158/1535-7163.MCT-13-1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Corbet C, Bastien E, Draoui N, et al. Interruption of lactate uptake by inhibiting mitochondrial pyruvate transport unravels direct antitumor and radiosensitizing effects. Nat Commun 2018;9:1208. 10.1038/s41467-018-03525-0 [DOI] [PMC free article] [PubMed] [Google Scholar]