Abstract
Neurons show diverse firing patterns. Even neurons belonging to a single chemical or morphological class, or the same identified neuron, can display different types of electrical activity. For example, motor neuron MN5, which innervates a flight muscle of adult Drosophila, can show distinct firing patterns under the same recording conditions. We developed a two-dimensional biophysical model and show that a core complement of just two voltage-gated channels is sufficient to generate firing pattern diversity. We propose Shab and DmNaυ to be two candidate genes that could encode these core currents, and find that changes in Shab channel expression in the model can reproduce activity resembling the main firing patterns observed in MN5 recordings. We use bifurcation analysis to describe the different transitions between rest and spiking states that result from variations in Shab channel expression, exposing a connection between ion channel expression, bifurcation structure, and firing patterns in models of membrane potential dynamics.
Keywords: Bifurcation, Electrophysiology, Excitability, Firing pattern, Gene expression, Ion channels
1. Introduction
The brain contains a variety of neurons displaying different types of electrical activity (Connors and Gutnick 1990; Tan et al. 2007). Even within neuronal classes, firing patterns are variable. For example, cortical neurons are often classified based on their electrophysiological profiles, which include regular spiking (RS) and fast spiking (FS) (Connors and Gutnick 1990; Steriade 2001). Many FS neurons express γ -aminobutyric acid (GABA) and show a non-pyramidal morphology, while most RS neurons are glutamatergic pyramidal or spiny stellate cells, supporting the idea that neurons of a chemical or morphological class can also be distinguished by their activity (Connors and Gutnick 1990; Steriade 2001). However, under certain stimulation conditions, the same recorded neuron can transition between RS and FS profiles (Steriade et al. 1998).
Variability is also seen in recordings from invertebrate neurons. For example, cultured Drosophila embryonic neurons respond to current stimulation with graded peaks, single action potentials (APs), or multiple APs (Peng and Wu 2007; Saito and Wu 1991). Multiple AP firing patterns are further divided into adaptive, tonic, delayed, and damping firing (Peng and Wu 2007). Recordings from flight motor neuron 5 (MN5) in adult Drosophila show at least four distinct responses to current stimulation, including single APs, tonic, and delayed firing (Duch et al. 2008). How can the same identified neuron produce such diverse electrical activity?
Neuronal activity is primarily mediated by channels that facilitate transmembrane movement of ions, such as sodium (Na+) and potassium (K+) (Hille 2001). Ion channels, particularly those permeable to K+, are diverse (Coetzee et al. 1999; Jan and Jan 1990; Salkoff et al. 1992). Functional pore-forming (α) subunits of voltage-gated K+ (Kv) channels are encoded by 4 gene subfamilies: Kv1 (Shaker), Kv2 (Shab), Kv3 (Shaw), and Kv4 (Shal) (Jan and Jan 1997; Salkoff et al. 1992). Kυ genes encode channels with distinct voltage sensitivities and kinetics (Coetzee et al. 1999; Salkoff et al. 1992). Diversity is increased by splice variants (Baro et al. 2001; Timpe et al. 1988), heteromultimeric channels (Isacoff et al. 1990), and modulatory beta subunits (Heinemann et al. 1996). Consequently, determining the contribution of a specific ion channel gene to neuronal excitability is rarely clear-cut experimentally (Trimmer and Rhodes 2004).
Modeling provides a unique opportunity to explore the contribution of specific ionic currents to the variety of firing patterns neurons can display (Günay et al. 2008; Prinz et al. 2003). We developed a two-dimensional biophysical model to simulate membrane potential dynamics of Drosophila MN5. The model includes voltage-gated Na+ and K+ currents and a leak current. The parameters for the Na+ and K+ currents were chosen to represent channels encoded by the DmNaυ (Lin et al. 2009; O’Donnell Olson et al. 2008) and Shab (Tsunoda and Salkoff 1995b) genes, respectively. Elimination of Shab currents prevents repetitive firing in Drosophila neurons, showing it is a major player in the regulation of neural activity patterns (Peng and Wu 2007). Using the model, we studied the range of firing behaviors produced by varying Shab channel expression. We find that small changes in Shab expression produce the primary firing patterns recorded from MN5, including delayed firing which is thought to require the presence of a transient (A-type) K+ current encoded by the Shal gene (Choi et al. 2004; Ping et al. 2011; Schaefer et al. 2010). Thus, we show that a core complement of just two ion currents is sufficient to produce a wide range of firing behaviors, and further identify two candidate genes, Shab and DmNaυ, that may encode these core currents. We use bifurcation analysis to describe the qualitatively different transitions between rest and spiking states underlying distinct firing profiles, thereby linking ion channel expression, bifurcation structure, and diversity of firing patterns in a minimal model of an identified motor neuron.
2. Methods
2.1. Electrophysiology
Adult Drosophila melanogaster were dissected, as described previously (Duch et al. 2008; Ryglewski and Duch 2009). Recordings were made either from flies with targeted expression of GFP in a subset of motor neurons (C380-GAL4; UAS-mCD8-GFP; Cha-GAL80 (Sanyal et al. 2003), or from control flies (strain w1118). No systematic differences were found between flies with or without GFP expression. Preparations were mounted onto a Zeiss fluorescence microscope (Axio-scope 2FS) and perfused with standard solution (Jan and Jan 1976) composed of (in mM): 128 NaCl, 2 KCl, 1.8 CaCl2, 4 MgCl2, 5 HEPES, and ~35 sucrose adjusted as necessary to a final osmolality of 295 mOsm/kg. pH was adjusted with 1 M NaOH to 7.2. Access to MN5 was achieved by removing the ganglionic sheath surrounding the cell with a 0.5 MΩ-resistance patch pipette filled with 2 % protease diluted in buffer. All chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
MN5 could be identified unambiguously either following expression of UAS-GFP under the control of the motor neuron driver C380-GAL4 (Ryglewski et al. 2012; Sanyal et al. 2003), or by Dye I injection into the target muscle fiber of MN5 12 hours before dissection to allow enough time for retrograde dye diffusion into MN5 somata (for w1118 flies without GFP expression). The Dye I injection technique is described in Ryglewski and Duch (2012).
After cells were exposed and the area washed, MN5 was recorded with a 5.8–6.5 MΩ-resistance patch pipette pulled from borosilicate glass (1.5 mmOD/1 mm ID no filament, World Precision Instruments) with a Narishige vertical puller. Pipettes were filled with internal solution consisting of (in mM): 140 Kgluconate, 2 MgCl2,11 EGTA, 10 HEPES, 2 MgATP. As needed, pH was adjusted to 7.2 with 1M KOH and osmolality adjusted to 300 mOsm/kg with glucose. Electrical activity of MN5 was recorded in situ using somatic patch clamp, as described previously (Duch et al. 2008; Ryglewski and Duch 2009; Ryglewski et al. 2012). Briefly, a gigaseal was first obtained and then negative pressure was applied to enter the whole-cell configuration. Recordings were made at room temperature (~22 °C), amplified with an Axopatch 200B, digitized at 20 kHz with a Digidata 1322A, and stored and analyzed with pCLAMP 10.2 software (all from Molecular Devices). Whole-cell capacitance was 127 ± 16 pF and membrane input resistance was 97 ± 31 MΩ. MN5 was stimulated with square pulse injections of current between 100 pA and 2 nA.
2.2. Neuron model
2.2.1. General expressions for currents
Channel-mediated currents are the result of electrical drift and diffusion. Since the commonly used conductance-based (CB) model does not incorporate diffusion, we use the electrodiffusion (ED) model formulation derived by Endresen et al. (2000) and later expanded by Herrera-Valdez (2012). The CB model is a linear approximation of the ED formulation, the latter having several advantages over the former, as discussed in detail in Herrera-Valdez (2012). Briefly, experimental data from a variety of neurons show current-voltage (I–V) relationships best fit by a hyperbolic sine (Baranauskas and Martina 2006; Hardie and Minke 1994; Neher 1971), in agreement with the ED derivation. Furthermore, data from current recordings can be directly incorporated into the model without additional calculations of conductance as required by the CB model (Herrera-Valdez 2012).
The whole-cell membrane current carried by a channel permeable to ion s has the form
| (1) |
The parameter ās represents the maximum amplitude of the current through the open channel (Endresen et al. 2000; Herrera-Valdez 2012) and is a multiple of the number of channels in the membrane. Therefore, ās can be regarded as an indicator of channel expression. The proportion of open channels is represented by p, typically written as a product of gating variables, each taking values between zero and one (Ermentrout and Terman 2010; Izhikevich 2007). A full list of parameters and constants can be found in Table 1.
Table 1.
Parameters and constants
| Name | Value | Units | Description |
|---|---|---|---|
| q | 1.60217733 × 10−19 | C | Elementary charge |
| K | 1.38065812 × 10−20 | mJ/K | Boltzmann’s constant |
| T | 273.15 + 22 | K | Absolute temperature (corresponds to room temperature at 22 °C) |
| 25.43 | mV | Boltzmann’s potential | |
| RIn | 100 | MΩ | Input resistance |
| Cm | 130 | pF | Membrane capacitance |
| υNa | 70 | mV | Reversal potential for Na+ |
| υK | −90 | mV | Reversal potential for K+ |
| υL | −60 | mV | Reversal potential for leak |
| āNa | 13 | nA | Maximal amplitude for DmNaυ current |
| āK | [13, 65] | nA | Maximal amplitude for Shab current |
| āL | 0.5 | nA | Constant amplitude for leak current |
| 1 | Normalized amplitude for DmNaυ current | ||
| [1,5] | Normalized amplitude for Shab current | ||
| 0.04 | Normalized amplitude for leak current | ||
| 100 | nA/nF | Scaling factor for physiologically relevant dυ/dt | |
| υm | −28 | mV | Half-activation for DmNaυ (Lin et al. 2009; O’Donnell Olson et al. 2008) |
| υw | −1 | mV | Half-activation for Shab (Tsunoda and Salkoff 1995b) |
| ηm | 2 | Gating charge of activation for DmNaυ (estimated from Lin et al. 2009) | |
| ηw | 2 | Gating charge of activation for Shab (Tsunoda and Salkoff 1995b) | |
| τ̄w | 5 or 10 | ms | Maximum time constant for Shab (estimated from Tsunoda and Salkoff 1995b) |
| σw | 0.3 or 0.7 | Symmetry of time constant for Shab (estimated from Tsunoda and Salkoff 1995b) | |
2.2.2. Membrane potential and specific currents
The dynamics of the membrane potential can be written as
| (2) |
where Is, IN, Ik, and IL represent, respectively, an externally applied stimulus current, Na+, K+, and leak currents. Na+ channels are modeled after those encoded by the DmNaυ (para) gene expressed in Drosophila neurons (Lin et al. 2009; O’Donnell Olson et al. 2008). K+ channels are based on those encoded by the shab gene, the primary contributor to the delayed rectifier current in Drosophila muscle and neurons (Tsunoda and Salkoff 1995b). IL represents currents mediated by electrogenic pumps and channels with relatively small contributions to the change in membrane potential (Chay and Kang 1988; Herrera-Valdez and Lega 2011; Hodgkin and Huxley 1952b; Sonders and Amara 1996).
The currents in the model are:
| (3) |
| (4) |
| (5) |
The terms represent the maximal whole-membrane amplitudes of the Shab, DmNaυ, and leak currents, respectively. Activation of K+ (Shab) channels is represented by the gating variable w as given by:
| (6) |
where
| (7) |
is a function that describes the steady-state balance between opening and closing rates in the gating mechanism (Endresen et al. 2000). Of note, σw specifies the symmetry of the voltage-dependence of the time constant (Herrera-Valdez 2012; Willms et al. 1999) and can be used to control the delay in gating for any change in υ. The steady state of the gate is a function of υ given by
| (8) |
Values for the parameters υw and ηw were taken directly from data in Tsunoda and Salkoff (1995b), while σw was found by modeling the voltage clamp and fitting it to recordings in Tsunoda and Salkoff (1995b) (Online Resource 1). Shab channels inactivate very slowly, so the inactivation variable is assumed to be equal to one (Av-Ron et al. 1993; Connor and Stevens 1971). Activation of Na+ (DmNaυ) channels m is fast and assumed to be at steady state (Av-Ron et al. 1991; Rinzel 1985), given by an equation of the same form as Eq. (8). The time courses of Na+ channel inactivation and delayed rectifier K+ channel activation follow an almost linear relationship (Av-Ron et al. 1991; Rinzel 1985). As a consequence, the inactivation of DmNaυ channels is approximated by (1 – w) (Av-Ron et al. 1991; Rinzel 1985). Therefore, the state variables of the model are υ and w, as given by Eqs. (2) and (6), respectively. The parameter values used to model each current can be found in Table 1.
2.2.3. Rescaling and parameter estimation
Rescaling of the model and parameter estimation was described in detail previously (Herrera-Valdez 2012). Briefly, all maximum current amplitudes were divided by a normalizing amplitude ā, thereby expressing the amplitudes as ratios of the form as = ās/ā. The choice ā = āN yields
| (9) |
and allows investigation, in particular, of the effects of changing the number of K+ (Shab) channels relative to the number of Na+ (DmNaυ) channels. As a rule of thumb obtained from analysis herein and previous analyses of the model (Herrera-Valdez 2012), the ratio aK = āK/āN should be between 1 and 5 when modeling neurons with similar Cm as MN5 and K+ channels with gating properties similar to those of the Shab channel. Values of aK < 1 typically produce only depolarization block in response to current injection, while aK > 5 tends to produce spiking only with non-physiological levels of injected current and at a frequency exceeding that observed in recordings.
If we let ξ = āN/Cm, Eq. (2) can be written as:
| (10) |
where J = Is/āN. The constant ξ thus acts as a scaling factor to ensure the model produces APs with a dv/dt similar to MN5 recordings. Note that ξ can be adjusted to match experimentally measured dv/dt in other neurons. The value of ξ was set to the average maximum dv/dt from 52 MN5 recordings (~ 100). The average Cm was also taken directly from recordings. Thus, ān = ξ * Cm.
The maximum amplitude of the leak current, āL, was estimated by taking into account the input resistance, RIn, as obtained from MN5 recordings, and calculating āL ≈ 2υB/RIn (Herrera-Valdez 2012). Importantly, although the maximum dv/dt, Cm, and RIn did vary some across recordings, our focus was to explore the effects of varying ion channel expression, rather than a change in these parameters. Therefore, a single value, close to the average of each of these measurements as obtained from recordings, was chosen as representative and used for all simulations (see Table 1).
2.2.4. Steady-state current and bifurcation analysis
To assess some of the main computational properties of a model neuron like the one specified in Eqs. (2)–(8), the gating variable w can be replaced by its steady state (i.e. setting dw/dt = 0) to obtain the total steady-state current as a function of υ:
| (11) |
The system is at steady state if I∞(υ) subtracted from the stimulation current IS equals zero. The set of υ-values that correspond to the fixed points of the system is the set of zeros of IS – I∞(υ), that is V* = {υ* : IS – I∞(υ) = 0}. The monotonicity, or lack thereof, in the curve I∞(υ) is an indicator of some of the steady-state bifurcations that can be observed as a function of IS. Note that IS – I∞(υ) gives the number, but not the type, of fixed points of the system, and it does not help to determine if there are limit cycles.
To study bifurcations due to changes in the type or stability of fixed points, we calculated the two eigenvalues corresponding to each fixed point for different values of IS. In these kinds of models, and for parameter ranges that overlap with measures from biophysical experiments, the system Eqs. (2)–(8) is expected to have at least one asymptotically stable attractor (Ermentrout and Terman 2010; Izhikevich 2007; Rinzel and Ermentrout 1989). For instance, if all the fixed points are unstable, there is no resting membrane potential and a limit cycle is expected to exist. Since the system is meant to represent a neuron, we set thresholds of 30 mV for the amplitude of oscillations and 10 mV/ms for the maximum dv/dt to detect repetitive spiking. The term rheobase is often used in the neuroscience literature to refer to the smallest current required to elicit as few as a single spike with a long (typically 100 s of milliseconds, but theoretically infinite) stimulus of constant amplitude (Fleshman et al. 1981). In our analysis, we are interested in finding the minimum constant stimulus amplitude that triggers repetitive spiking (stable limit cycles). Therefore, we will not use the term rheobase and will instead refer to such a minimum stimulation as cycle trigger current, or Icyc in short. Note that using ever-smaller current steps or pulses of longer duration reveals that rheobase is rarely defined as a single unique value (Izhikevich 2007). To address this issue, and keeping in mind a practical connection with experiments, the values of Icyc are reported with a resolution of 1 pA pulse amplitude and 400 ms pulse duration.
2.3. Simulations
All numerical simulations were obtained using Python 2.7 on Lenovo T400 laptops with Intel(R) Core(TM)2 Duo T9600 CPUs at 2.8 GHz running Linux Kubuntu 11. Simulations were performed using the solver odeint contained in the Python module scipy.integrate (Jones et al. 2001), which uses lsoda from the Fortran library odepack. Depending on the numerics of the simulation, the solver uses Adams’ method for nonstiff problems, or a method based on backward differentiation formulas for stiff problems. In addition, odeint allows time-step control to test numerical schemes and precision (simulations not shown). The accuracy of the simulations was tested using different time steps and relative tolerances, and it was concluded that the default parameter values for integration give accurate results in an optimal amount of simulation time. The default time step chosen for the time axis of the simulations was 0.025 ms. Figures were produced with the Python module matplotlib (Hunter et al. 2008), except for bifurcation diagrams showing the stable and unstable periodic branches, which were generated using XPPAUT (Ermentrout 2006).
3. Results
3.1. MN5 shows distinct firing patterns indicative of different bifurcations of the steady state
MN5 innervates the dorsal longitudinal flight muscle of adult Drosophila (Fernandes and Keshishian 1998; Ikeda and Koenig 1988). We recorded from a total of 52 MN5s, 48 (92 %) of which fired in response to current injection. However, not all neurons showed the same firing pattern under the same recording conditions (Fig. 1). Different firing patterns had distinct incidences, and contained features which are consistent with model behaviors that indicate the type of bifurcation producing the transition into or out of spiking.
Fig. 1.
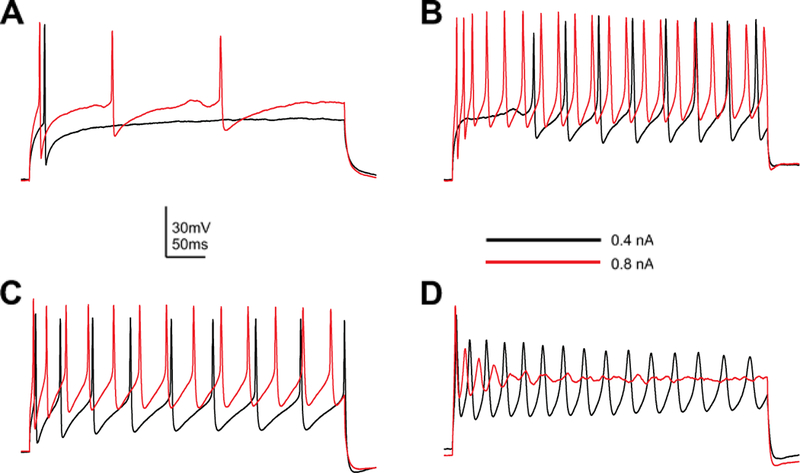
Different electrophysiological profiles recorded from Drosophila MN5. Intracellular recordings were made in whole-cell patch configuration from MN5 in wild-type adult Drosophila. At least four different firing behaviors can be observed in response to a 500 ms square-pulse current injection of 0.4 nA (black traces) or 0.8 nA (red traces). (A) Low-amplitude current stimulation elicits a single action potential (black trace), while increased stimulation elicits repetitive spiking that adapts in frequency (red trace). (B) Repetitive firing is elicited by low-amplitude stimulation, but commences only after a delay (black trace). Increasing the current amplitude eliminates the delay and increases the firing frequency (red trace). (C) Repetitive firing without a significant delay is induced even by low-amplitude current injection (black trace), while increasing the amplitude increases the firing frequency (red trace). (D) Low-amplitude stimulation produces a large initial spike, followed by triangular-shaped spikes which diminish in amplitude and broaden as stimulation continues (black trace). Larger stimulation amplitudes induce dampening oscillations that end in depolarization block (red trace)
In response to low-level current injection, 20 of 52 (38 %) MN5s fired a single AP followed by sustained depolarization (Fig. 1(A), black trace). The return to a resting, though depolarized, state is in agreement with a dynamical system that has at least one stable fixed point while current is being injected. This fixed point is probably a node, since the convergence toward rest is not oscillatory (Guckenheimer and Holmes 1990; Izhikevich 2007). The lack of subthreshold oscillations, both transitioning into and out of spiking, is characteristic of a model neuron whose membrane potential is near a saddle-node (SN), rather than an Adronov-Hopf (AH), bifurcation (Guckenheimer and Holmes 1990; Izhikevich 2007). With increasing current, MN5s that previously fired a single AP also fired additional APs with spike frequency adaptation (Fig. 1(A), red trace). Subthreshold oscillations occur prior to the second and third spikes, which is characteristic of systems near an AH bifurcation. However, the initially low firing rate is still suggestive of a SN bifurcation.
In response to low levels of current, 2 of 52 (4 %) of MN5s exhibited repetitive firing, but only after a delay of tens to hundreds of milliseconds (Fig. 1(B), black trace). Although few neurons showed delayed firing, it is interesting to note the presence of this firing pattern in adult motor neurons, since it is one of the prominent patterns in larval motor neurons (Choi et al. 2004; Schaefer et al. 2010). Long delays to first spike are typically observed when the membrane potential of a model neuron is near a SN bifurcation. However, the small and slow oscillation of increasing amplitude prior to the first spike indicates a system that may instead be near an AH bifurcation (Guckenheimer and Holmes 1990; Izhikevich 2007). Alternatively, the subthreshold oscillation could be the result of noise. With larger current, the firing frequency increased and there was no delay to first spike (Fig. 1(B), red trace). These behaviors do not distinguish between a system transitioning into spiking through either an AH or SN bifurcation, as they could occur in both cases.
Even at low levels of current injection, 22 of 52 (42 %) of MN5s transitioned into spiking very quickly and without a long delay to first spike (Fig. 1(C), black trace). The lack of delay is consistent with a model system that is not near a SN bifurcation (Izhikevich 2007), but this cannot be ruled out since fast spiking can also occur in systems that transition through SN but are stimulated beyond the bifurcation point. A larger current pulse produced faster firing with a negligible decrease in the latency to first spike (Fig. 1(C), red trace), again not leading to a clear prediction about the representative dynamical system.
Finally, 4 of 52 (4 %) showed repetitive firing in response to low current, but with APs that decreased in amplitude and broadened during stimulation (Fig. 1(D), black trace). With more current, the response reduced to a single AP followed by dampening oscillations that ended in depolarization block (Fig. 1(D), red trace). These behaviors correspond to a model membrane whose potential is spiraling towards a stable focus point and thus transitioning out of spiking through an AH bifurcation (Guckenheimer and Holmes 1990; Izhikevich 2007).
The differences in the electrophysiological profiles described above indicate topological non-equivalence between the underlying dynamical systems (Izhikevich 2007), and could be due to differences in the levels of ion channel expression. Specifically, we hypothesized that many of the firing profiles observed in MN5 could be generated by changes in the expression of Shab K+ channels. We focused on Shab channels since they are the primary carrier of delayed rectifier K+ currents in Drosophila neurons (Tsunoda and Salkoff 1995b) and strongly influence repetitive firing (Peng and Wu 2007).
3.2. Shab channel expression is a determinant of the steady-state current-voltage relationship
Shab channel expression is represented in the model by the parameter aK, which specifies the maximal amplitude of the Shab current relative to the Na+ (DmNaυ) current. We examined the steady-state current-voltage relationship, I∞ (υ) in (Eq. 11), for aK between 1 (equal maximum current amplitudes) and 5 (Shab current amplitude 5 times larger than the DmNaυ current amplitude). The value of aK influences both the shape of the steady-state curve as a function of v and the number of zero crossings (Fig. 2). Recall that the zero crossings of I∞ (υ) occur at the υ-values of the fixed points of the system in the absence of stimulation (vertical dotted line). The curves are non-monotonic with 3 zero crossings for aK = 1, 2 and monotonic with a unique zero crossing for aK = 3, 4, 5. In other words, the number of fixed points decreases from 3 to 1 as the expression of Shab increases, thereby producing topologically non-equivalent membranes with potentially different spike-generating mechanisms.
Fig. 2.
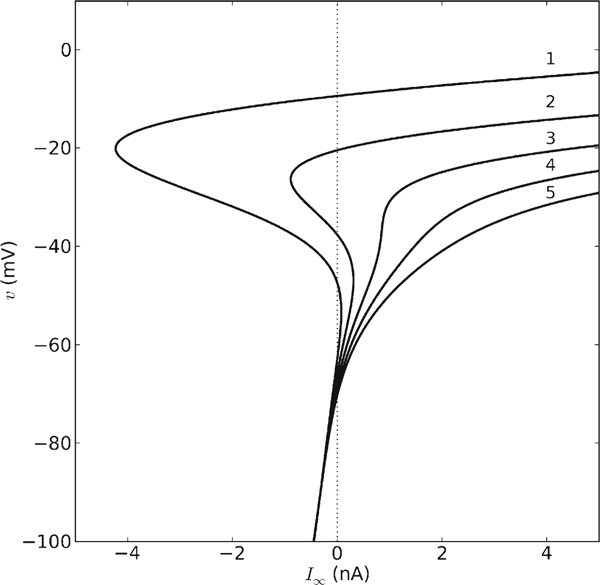
Steady-state curves resulting from variation in Shab channel expression. The steady-state current I∞ (υ) was calculated according to Eq. (11) for different maximum amplitudes of the Shab current relative to the DmNaυ current (aK). The value of aK was varied between 1 and 5, as indicated above the corresponding curve
If current is injected (IS > 0), the fixed points are found by examining the crossings at the injected current value (i.e. moving the vertical dotted line in Fig. 2 to the specified IS). Membranes with different Shab expression levels may be topologically equivalent with respect to the number of fixed points, yet the fixed points may be found at different υ values. Thus, two membranes could display different transitions into repetitive spiking for IS close to that which produces repetitive firing (Icyc), or similar spiking behavior for IS larger than their respective Icyc.
3.3. Shab channel expression is a determinant of the type and stability of fixed points
We analyzed the type and local stability of the fixed points as a function of IS for aK in {1, 2, 3, 4, 5} (Fig. 3). Each curve corresponding to a single aK contains several types of fixed points, indicating that increasing IS induces sequences of qualitative changes in the behavior of the system. Furthermore, these sequences can differ depending on the value of aK, even between membranes with the same number of fixed points.
Fig. 3.
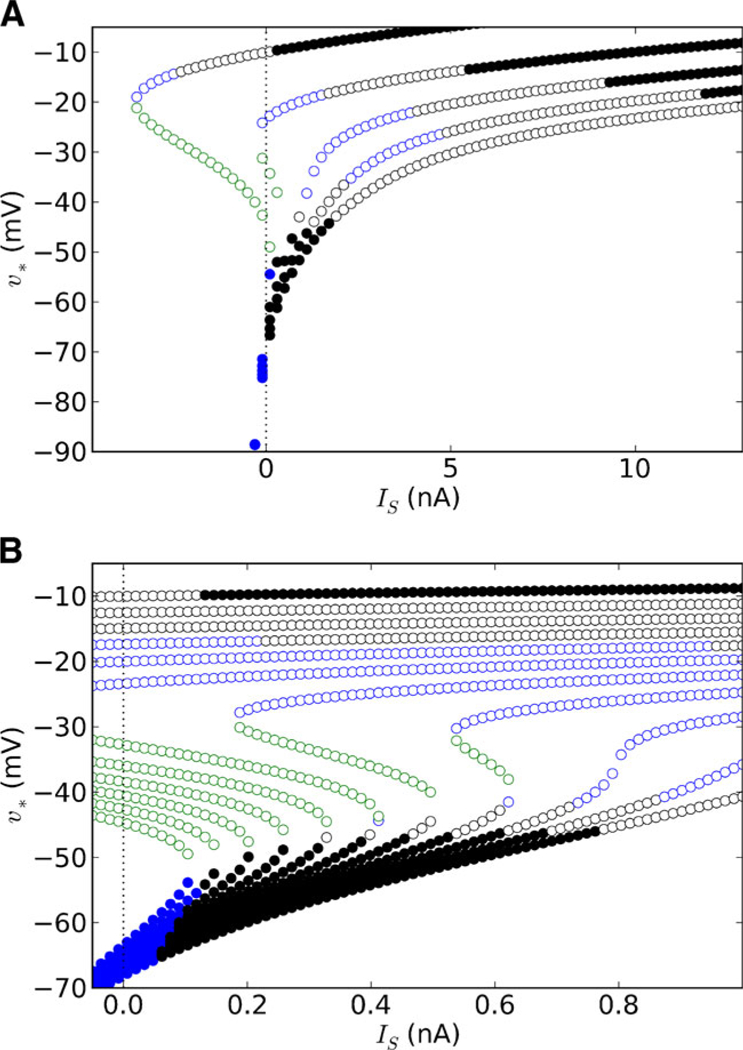
Bifurcation diagrams for different levels of Shab channel expression. Curves are comprised of the υ-values of the fixed points (υ*) as a function of the current stimulation amplitude (Is). Note that the shapes of these curves are identical to the shapes of the I∞(υ) curves (Fig. 2), since the curve of υ-values is obtained by calculating the zero crossings of Is – I∞(υ) using different values of Is. Dashed line marks where Is = 0, for ease of identifying the fixed points in the absence of stimulation. Shab channel expression (aK) was varied between 1 and 5 (as indicated) in steps of 1.0 (A), or between 1 and 3 in steps of 0.2 (B). Open circles represent unstable fixed points, while filled circles represent asymptotically stable fixed points. Black, blue, and green circles represent, respectively, foci, nodes, and saddles. Parameters: υw = −1, ηw = 2, σw = 0.7, τ̄w = 10 ms
For example, when aK = 1 and Is is small, the lower branch of the curve up to the knee point includes only stable nodes, while the middle and upper branches contain saddles and unstable foci, respectively (Fig. 3(A), (B), left-most curves). As IS increases, the number of fixed points decreases from 3 to 1 through a SN bifurcation, leaving an unstable focus as the only fixed point for small IS and a stable focus for larger IS. In contrast, when aK = 1.4 or 1.6, the system also has 3 fixed points for small IS, but the stable nodes in the lower branch become stable foci prior to the SN bifurcation (Fig. 3(B), third and fourth curves from left). Increasing aK to between 1.8 and 2.4 (Fig. 3(B), fifth through eighth curves from left) causes the stable foci in the lower branch to become unstable, indicating that an AH bifurcation will occur before the SN bifurcation. A neuron with a non-monotonic IS-υ* relationship may therefore have an Icyc that does not correspond to the SN bifurcation. This means that I∞ (υ) cannot always be used as a predictor of the type of transition between rest and repetitive spiking (Izhikevich 2007). Furthermore, although the membranes for aK between 1 and 2.4 are topologically equivalent, the spike generating mechanisms for select membranes could be different due to the distinct bifurcation sequences.
For aK ≥ 2.6, the curves are all monotonic and have the same sequence of changes in the type and stability of the fixed points (Fig. 3(B), last three curves from right). Therefore, these membranes are expected to transition into spiking through the same mechanism. However, the υ-value at which these bifurcations occur is clearly different, with membranes characterized by larger aK’s requiring, for example, more current to produce the transition from stable to unstable foci (i.e. AH bifurcation) in the lower branch. Importantly, as seen previously, an AH bifurcation also occurs for some membranes characterized by a non-monotonic I – V relationship. Therefore, topologically non-equivalent membranes could transition into spiking through the same mechanism.
3.4. Transitions into repetitive spiking vary with Shab channel expression
We characterized the transitions from rest to repetitive spiking for aK between 1 and 3. The model membrane was stimulated by two 400 ms square pulses, the first 1 pA below Icyc and the second at Icyc. This difference in amplitudes is not meant to reproduce experimental current steps, which often differ by hundreds of pA. Rather, we use this small change in amplitude to see what happens as the membrane moves through the bifurcation. In this reduced system spiking is generated in one of two main ways, depending on the value of aK. Interestingly, we expected spiking would be generated by either a SN or AH bifurcation, but instead observed that spiking is produced by either a SN or a fold limit cycle (FLC) bifurcation.
For membranes characterized by aK ≥ 1.6, even if topologically non-equivalent, the transition into spiking is through a FLC bifurcation. To illustrate, when aK = 2.0 or 3.0, the membrane responds to current stimulation below Icyc with a single spike early in the pulse (Fig. 4(A1), (B1), black traces). Phase plane analysis shows that for aK = 3.0, the membrane has one fixed point (Fig. 4(A2)), while for aK = 2.0 the membrane has three (Fig. 4(B2)), in the absence of stimulation. In both cases, the fixed point characterizing the resting membrane potential is a stable node. Stimulation converts the node to a focus (Fig. 4(A3), (B3)), but it remains stable, causing the membrane to oscillate back toward a resting (yet depolarized) state for the remainder of the stimulus. As IS increases to Icyc, repetitive spiking is induced (Fig. 4(A1), (B1), gray traces), but the number and stability of fixed points does not change for either membrane (Fig. 4(A4), (B4)). Repetitive spiking begins when both systems are pushed towards the basin of attraction of a stable limit cycle, which emerges within an IS window, thus indicating the presence of a bistable regime. (A bifurcation diagram showing the stable and unstable periodic solution branches for the example of aK = 2.0 can be seen in Online Resource 2.) The emergence (or disappearance) of limit cycles in this way constitutes a FLC bifurcation, and is characteristic of systems near AH bifurcations (Izhikevich 2007). Indeed, for aK = 2.0 or 3.0, an AH bifurcation occurs when IS increases beyond Icyc, as seen in the fixed point curves when the focus points in the lower branch lose stability (Fig. 3(B)), but it does not cause the transition into repetitive spiking under this parameter regime.
Fig. 4.
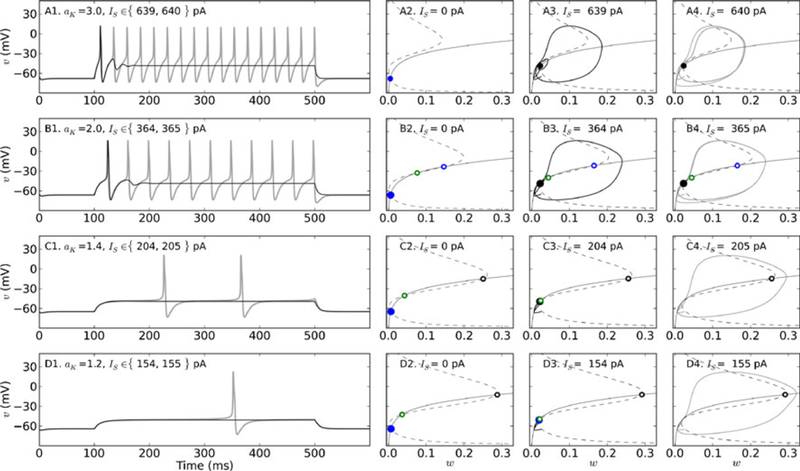
Transitions from rest to repetitive spiking for different levels of Shab channel expression. Membrane potential dynamics, nullclines, and trajectories in phase space for aK = 3.0 (A1–A4), 2.0 (B1–B4), 1.4 (C1-C4)), or 1.2 (D1-D4). A1, B1, C1, D1: Responses to two 400 ms square pulses of current, the first at 1 pA below (black traces) and the second at Icyc (gray traces). A2, B2, C2, D2: Nullclines Icyc for υ (dashed) and w (solid) are plotted in phase space in the absence of stimulation (Is = 0). Intersections of the nullclines are marked with circles to indicate stable (filled) or unstable (open) fixed points. Nodes are blue, saddle points are green, and foci are black. If there is more the one fixed point, the one located at the lowest membrane potential has the larger marker size. A3, B3, C3, D3: Nullclines and trajectories (solid black) of the system in phase space corresponding to the response to current stimulation 1 pA below Icyc. Nullclines and fixed points are plotted as described above. A4, B4, C4, D4: Nullclines and trajectories of the system in phase space corresponding to the response to current stimulation at Icyc. Parameters: σw = 0.7,. τ̄w = 10 ms, with initial conditions (w0, υ0) = (0.025, −65). See Table 1 for all other parameters
Repetitive spiking is instead generated by a SN bifurcation in membranes with aK ≤ 1.4. For example, when aK = 1.2 or 1.4, current stimulation below Icyc elicits a sustained depolarization of the membrane potential, but no spiking (Fig. 4(C1), (D1), black traces). Both systems have 3 fixed points in the absence of stimulation (Fig. 4(C2), (D2)). Stimulation just below Icyc brings the stable fixed point characterizing the resting potential very close to the saddle point, but three fixed points still remain (Fig. 4(C3)). When IS increases to Icyc, the two converging points annihilate each other, leaving a single fixed point (Fig. 4(C4)). Thus, both membranes transition into repetitive spiking through a SN bifurcation, producing a long delay to first spike and a subsequently low firing frequency (Fig. 4(C1), gray trace). Note that although only one spike is produced during the 400 ms stimulation period when aK = 1.2, the neuron can be seen to be repetitively spiking if longer current pulses are used (not shown).
The effects of varying aK within the interval [1,3] on the steady state I – V curve, the bifurcation type, Icyc, delay to first spike, and interspike interval (ISI) associated with the transition into repetitive spiking, are summarized in Table 2.
Table 2.
Effects of varying aK on transition into repetitive spiking, (τ̄w, σw) = (10, 0.7)
| aK | I∞(υ) curve | Bifurcation | Icyc (pA) | delay (ms) | ISI (ms) |
|---|---|---|---|---|---|
| 1.0 | Non-monotonic | Saddle-node | 112 | >300 | >300 |
| 1.2 | Non-monotonic | Saddle-node | 155 | >200 | >200 |
| 1.4 | Non-monotonic | Saddle-node | 205 | >100 | >100 |
| 1.6 | Non-monotonic | Fold limit cycle | 259 | <50 | <100 |
| 1.8 | Non-monotonic | Fold limit cycle | 312 | <50 | <50 |
| 2.0 | Non-monotonic | Fold limit cycle | 365 | <25 | <50 |
| 2.2 | Non-monotonic | Fold limit cycle | 418 | <25 | <50 |
| 2.4 | Non-monotonic | Fold limit cycle | 472 | <25 | <50 |
| 2.6 | Monotonic | Fold limit cycle | 527 | <25 | <50 |
| 2.8 | Monotonic | Fold limit cycle | 583 | <25 | <50 |
| 3.0 | Monotonic | Fold limit cycle | 640 | <10 | <25 |
3.5. Spiking behaviors vary for different combinations of Shab channel expression and current stimulation
To map firing patterns in the model to those seen in MN5, the model membrane was again stimulated with two 400 ms square pulses of current. In these simulations, current amplitudes were sometimes beyond Icyc and differed by 100 pA to be of the same order of magnitude as current steps used in MN5 recordings. Overall, the results show that certain firing patterns can only be observed for a small range of aK, while other patterns can be produced over a larger range.
For instance, to observe a profile like that seen in Fig. 1(A), in which small current stimulation produces a single early spike while larger amplitudes elicit repetitive firing, the transition into spiking for this system must be through a FLC bifurcation. The single spike (Fig. 5(A), black trace) is produced when the membrane is pushed away from a stable node, but spirals back towards a stable focus point. Increasing the current by 100 pA pushes the membrane into the basin of attraction for the stable limit cycle and now the neuron spikes repetitively (Fig. 5(A), gray trace). This profile is only observed when aK ≥ 1.8. Values below this either cause the membrane to go directly from a graded response (no AP) to repetitive spiking (aK = 1.6; not shown), or to transition through a SN bifurcation, producing late spiking.
Fig. 5.
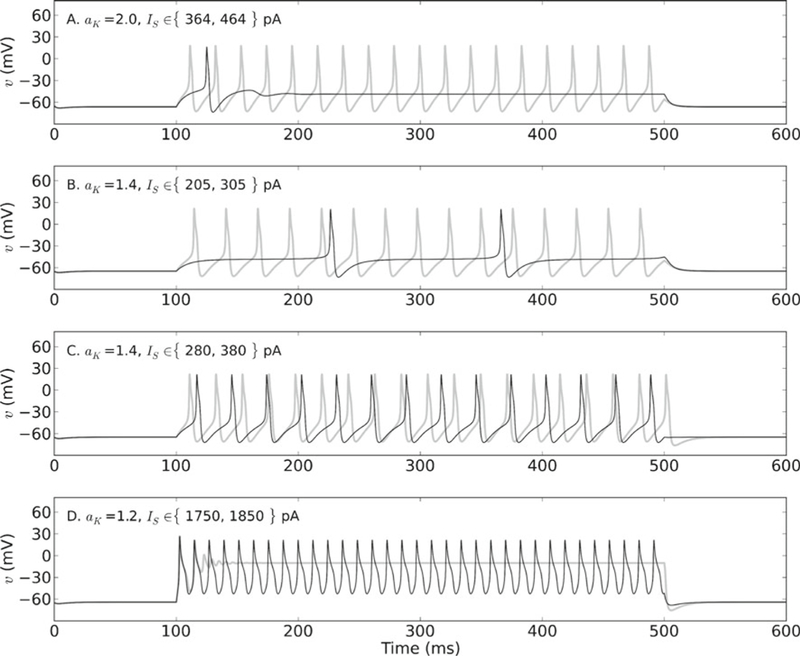
Firing behaviors produced by different combinations of Shab channel expression and stimulation amplitude. (A) Stimulating the membrane just below Icyc when aK = 2.0 causes a single spike followed by a depolarization that is sustained for the duration of the current pulse (black trace). Increasing the current amplitude by 100 pA elicits relatively high-frequency repetitive spiking (gray trace). Compare these responses to those of MN5 in Fig. 1(A). (B) Stimulating at Icyc when aK = 1.4 produces low-frequency repetitive firing with a long delay to first spike (black trace). Increasing the current by 100 pA abolishes the long delay and elicits higher-frequency repetitive firing (gray trace). Compare to MN5 recordings in Fig. 1(B). (C) Stimulating with an amplitude beyond Icyc when aK = 1.4 induces repetitive firing that commences shortly after stimulus onset. Increasing the current by 100 pA decreases the already short spike latency and increases the firing frequency. Compare to MN5 responses in Fig. 1(C). (D) Stimulating with a current well beyond Icyc when aK = 1.2 produces fast-onset repetitive spiking, but APs diminish slightly in amplitude after the first spike and have a triangular shape. Increasing the current by 100 pA produces an initial spike, but subsequent oscillations decrease in amplitude until full depolarization block is reached. Compare to MN5 responses in Fig. 1(D). Parameters same as in Fig. 4
One feature of the firing in Fig. 1(A) that we could not reproduce well was spike frequency adaptation. Stimulating single-spiking model neurons with small steps of current just below Icyc produced what is referred to as an adapting or accommodating profile (Mo et al. 2002), in which more than one spike is elicited, but spiking stops prior to the end of the current pulse (Online Resource 3). In some cases, a small decrease in the firing frequency was observed as spiking progressed (e.g. aK = 1.8; Online Resource 3, C1). However, this process was not reproducible for experimentally-realistic current steps and was not due to true adaptation, since adaptation requires at least 3 variables (Guckenheimer et al. 1997).
Reproducing the profile seen in Fig. 1(B), characterized by a long delay (≥~ 100 ms) to first spike at low current amplitudes and tonic firing with no delay at higher amplitudes, requires that the system go through a SN bifurcation. The long delay (Fig. 5(B), black trace) is produced as the trajectory of the system passes close to the region where fixed points were lost in the bifurcation (Izhikevich 2007). When stimulation increases by 100 pA, the delay is no longer present and the firing rate increases (Fig. 5(B), gray trace) as the system traverses a trajectory farther from the aforementioned region. For the parameter regime examined here, the membrane transitions through a SN bifurcation when aK is between 1.0 and 1.4 (inclusive). Thus, delayed firing is produced only for a small range of aK. The delayed firing for this regime also differs from that in MN5 in that the firing rate following the delay is arbitrarily low and the ISI is similar in duration to the delay. Delayed firing with an ISI shorter than the delay is achievable with the model, and is addressed in detail in Section 3.6.
The firing profile seen in Fig. 1(C), in which tonic firing commences just after stimulus onset, even in response to low levels of current, can be observed in the model for a large range of aK. For values of aK ≥ 1.6, fast-onset tonic firing is produced at Icyc (i.e. at the FLC bifurcation). If aK is between 1.0 and 1.4, however, current amplitudes larger than Icyc must be used to push the trajectory away from the region where fixed points were lost during the SN bifurcation (Fig. 5(C)).
The firing profile in Fig. 1(D), in which current stimulation produces broadening spikes and depolarization block at larger amplitudes, can also be seen over a large range of aK. All values of aK used herein produce similar triangular-like spikes in response to current stimulation well beyond Icyc (Fig. 5(D), black trace), progressing with dampening oscillations towards depolarization block for larger stimulus amplitudes (Fig. 5(D), gray trace). This transition out of spiking is produced as the membrane oscillates back toward a stable focus point.
3.6. Altering channel activation kinetics produces elevated potentials on which spikes ride
Variation in aK generates the main firing profiles of MN5, but does not reproduce other detailed features of the electrical activity. For example, repetitive spiking in MN5 is characterized by spikes which ride on elevated membrane potentials during current stimulation. Although this feature may result from morphological properties of MN5, such as the structure of the dendritic tree, we were interested in how we could reproduce this aspect of firing in our isopotential model. We predicted that such potentials would be produced by right-shifting the peak of the voltage dependence and adjusting the maximum time constant of Shab activation. This is done by varying the parameters σw and τ̄w, respectively. To stay within the range of experimentally observed variation in Shab current activation (Ryan et al. 2008), we set σw = 0.3, which causes a right-shift in the peak of τw relative to υw (Willms et al. 1999). To adjust the width of the APs to be ~2.5 ms, we also decreased τ̄w to 5 ms. As discussed previously, the model configuration is such that changing the time course of Shab activation also alters DmNaυ inactivation. Therefore, effects observed under this parameter regime potentially result from changes in the kinetics of both voltage-gated currents.
Simulations and bifurcation analysis were done for aK between 1 and 5. Under the new parameter regime, the type and stability, but not the location, of the fixed points is changed relative to the previous regime (Online Resource 4). As expected, the altered time course of channel activation produces an elevated membrane potential of ~10–20 mV that persists for the duration of the pulse and on which spikes ride (Fig. 6). Elevated potentials are observed for all aK in the explored range. Additionally, the spikes are smaller in amplitude and the firing frequency is often higher under this regime (compare to Fig. 5).
Fig. 6.
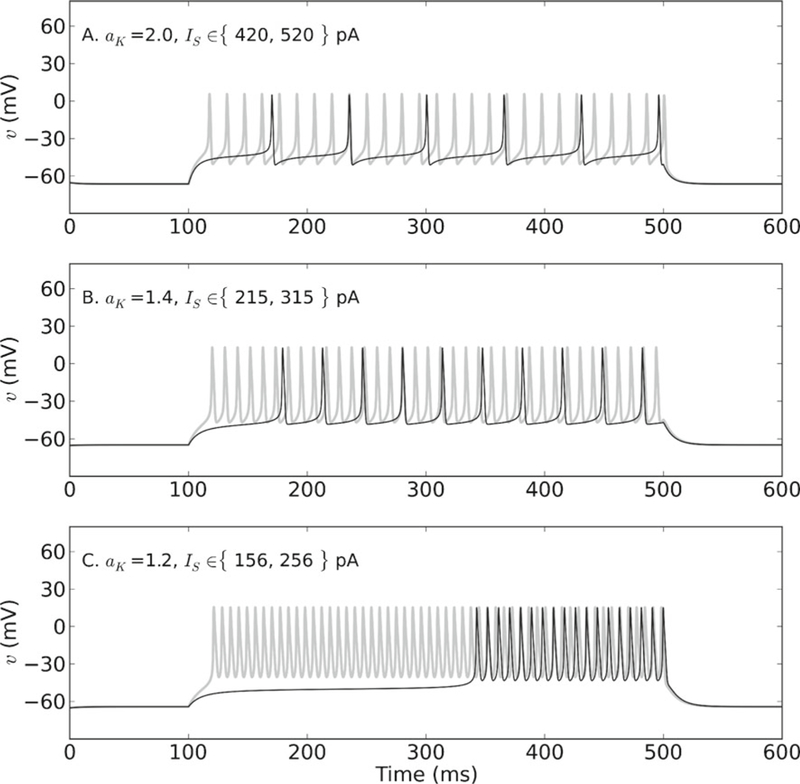
Elevated potentials and delayed firing profiles produced by variation in Shab channel activation and expression. Slower Shab channel activation results in the production of elevated potentials on which spikes ride for each aK. Several different delayed firing profiles are also produced by varying aK. Stimulating the membrane a little beyond Icyc when aK = 2.0 elicits repetitive spiking with a delay to first spike and a subsequent ISI of similar duration (black trace). Increasing the current amplitude by 100 pA decreases the spike latency and elicits higher-frequency spiking (gray trace). Spike shape is similar to MN5 spikes. (B) Stimulating beyond Icyc when aK = 1.4 produces a delay to first spike longer than the subsequent ISI (black trace). Increasing the current by 100 pA abolishes the long delay and elicits higher-frequency repetitive firing (gray trace). Note similarity to delayed firing profile of MN5 in Fig. 1(B). However, spikes in this case have different concavity to those recorded from MN5. (C) Stimulating the membrane just above Icyc when aK = 1.2 produces a much longer delay to first spike than the ISI (black trace). Increasing the current amplitude eliminates the long delay and increases the firing frequency (gray trace). Again, this profile is similar to that in Fig. 1(B). However, the spike shape is worse than that seen when aK = 1.4. Parameters: σw = 0.3, τ̄w = 5 ms
We also noticed a type of delayed firing not seen in the previous parameter regime. When aK = 1.2 or 1.4, repetitive spiking is characterized by an ISI smaller than the delay to first spike (Fig. 6(B), (C), black traces). Increasing the current by 100 pA eliminates the long delay and increases the firing frequency (gray traces). Overall, these membrane responses appear more similar to the delayed firing profile observed in MN5 (compare to Fig. 1(B)). The responses differ from MN5 recordings, however, with respect to spike shape. When aK = 1.2, the spikes are concave up during the slow depolarization phase and concave down during the upstroke and repolarization (Fig. 6(C), black trace). This is in contrast to the spikes in the previous parameter regime, which look more like MN5 spikes (compare to Fig. 5). When aK = 1.4, the spike shape improves, but the difference between the delay and the ISI is smaller (Fig. 6(B), black trace). As aK increases to 2.0, a spike shape resembling that of MN5 is recovered, but there is no long delay to first spike, and the spike latency and ISI are approximately equal in duration (Fig. 6(A), black trace). Thus, changes in aK in this regime reveal a trade-off between spike shape and delayed firing.
4. Discussion
One of the current challenges in neurophysiology is to determine what electrical properties are conferred by the wide array of ion channels expressed in neurons. How much redundancy is present? Which currents are necessary or sufficient for producing distinct types of firing? Our results demonstrate that a core complement of just two ion channels may be sufficient to generate the diversity of firing patterns observed in recordings. Since this diversity depends in part on the kinetics of the channels, we further propose that there are a few combinations of channels that are suitable to produce the basic electrophysiological properties of a cell. For MN5, we propose that the core complement of channels could be those encoded by the Shab and DmNaυ genes. Furthermore, in our model, the relative maximal amplitudes of the core currents for which the different firing patterns are produced vary no more than one order of magnitude. As a consequence, the model predicts that the range of relative expression between Shab and DmNaυ is constrained to a well-defined, and not very large, interval.
4.1. Changes in Shab channel expression produce diverse firing patterns
For this reduced model, small variations in Shab channel expression have profound effects on the transition from rest to repetitive spiking. When Shab channel activation is fast and the density is low, the membrane transitions into spiking through a SN bifurcation, producing long delays to first spike and low initial firing frequencies. In contrast, for the same activation kinetics, increasing Shab channel expression leads to the emergence of a stable limit cycle through a FLC bifurcation, producing a bistable membrane. Current stimulation of sufficient amplitude pushes the membrane into the basin of attraction for the limit cycle, producing repetitive spiking with little to no delay to first spike and fast firing frequencies.
Although experiments to date have not indicated the existence of bistability in MN5, many recorded neurons may not appear to be bistable, even though their membrane is characterized by two stable attractors. The basin of attraction for one of the attractors could be very small, and thus revealing its presence would require a specific combination of initial conditions and small current steps. Furthermore, the fact that hitting upon these conditions is unlikely could cause experimenters occasionally observing this bistability to discard these neurons as ‘unhealthy’, just as some researchers did historically when finding neurons which fire only a single spike (Hodgkin 1948).
It is of particular interest that the model can generate long delays to first spike without a fast inactivating (A-type) current, contrary to the idea that A-type currents, specifically those encoded by Shal, are necessary to produce delayed firing in Drosophila motor neurons (Choi et al. 2004; Ping et al. 2011; Schaefer et al. 2010). Instead, long delays were produced in the model by setting Shab channel expression low so that the neuron transitions into spiking through a SN bifurcation. Our results do not dispute the involvement of Shal/Kv4-mediated currents in generating delays to first spike; experimental evidence that they play a role in both Drosophila and mammalian (Kim et al. 2005; Shibata et al. 2000) neurons is strong. Rather, we propose that low Shab channel expression is another way in which neurons can produce long delays, potentially compensating for decreased or absent Shal/Kv4-mediated currents. We are not aware of any studies which have demonstrated such a relationship between Shab and Shal expression, but this prediction is testable by manipulating currents encoded by each gene using a combination of genetic and pharmacological techniques and recording the resulting firing patterns.
4.2. How do variable firing responses in our model relate to MN5 firing patterns as observed during behavior?
MN5 innervates the dorsal longitudinal flight muscle (DLM), which is predominantly used in flight and male courtship song. The DLM is a stretch-activated asynchronous flight muscle, where MN AP are not synchronized with each muscle contraction (Machin and Pringle 1959). Instead, during flight MNs fire single AP at tonic frequencies of 8 to 20 Hz at approximately every 10th to 20th wing beat to fuel the muscle with intracellular Ca2+ (Dickinson and Tu 1997). During sustained flight MN1– 5 all fire tonically; they do not show doublets or triplets of spikes and they never fire simultaneously. However, the firing frequencies of all five MNs are modulated simultaneously (Levine and Wyman 1973). The conclusion has been that all flight MNs receive common excitatory drive that is translated into tonic firing at set frequencies (Harcombe and Wyman 1977), which are changed simultaneously in MN1–5 when the power output of the muscle is changed, as during lift (Gordon and Dickinson 2006). Sustained flight thus requires constant tonic MN firing with low spike time precision and slow modulations in firing frequency, depending on power output demands.
In contrast, DLM MNs show different firing patterns during male courtship song. Courtship song is characterized by an initial tone burst with muscle contractions at 160Hz (sine song) during which MNs fire at much lower tonic frequencies than during flight, and some DLM units remain silent (Ewing 1977). Sine song is followed by pulse song, which consists of muscle contraction pulses of 3 ms duration that are separated by interpulse intervals of highly accurate and species-specific durations (34 ms in Drosophila melanogaster). Pulse song is characterized by accurately timed MN firing patterns. Consequently, MN5 shows very different firing patterns during flight (tonic firing) and male courtship song (pulse firing). Since both behaviors are thought to share largely similar pre-motor circuitry, MN5 intrinsic properties may contribute to both firing patterns. In fact, ion channel mutations alter pulse song characteristics in Drosophila (Schilcher 1976), though it remains unclear whether this is a result of altered MN excitability or of effects elsewhere in the circuit.
Our reduced two-dimensional model indicates that small changes in MN current amplitude and activation kinetics produce qualitatively different transitions between rest and spiking. One possibility may be that during behavior such small changes result from different modulatory states. At least for Drosophila flight, it is known that biogenic amines like octopamine and tyramine have significant effects on take-off likelihood and flight maintenance (Brembs et al. 2007). An important future challenge will be to test whether different transitions from rest to spiking may result from modulatory input as occurring during normal behaviors such as flight and courtship song.
4.3. Is Shab channel expression a unique solution for producing diverse firing patterns?
Just because our model predicts that changes in Shab channel expression can produce an array of firing patterns does not mean that this is the way that MN5 (or any other neuron) produces these patterns. Changes in the expression of other ion channels could potentially also do the job. Indeed, experimental work has shown that neurons can generate the same firing pattern with disparate channel expression profiles, demonstrating that there exist functionally equivalent combinations (Schulz et al. 2006).
However, several lines of evidence indicate that changes in Shab channel expression might be a preferred strategy for diversifying firing patterns, as opposed to changes in the expression of other K+ channels. In Drosophila, voltage-gated K+ channels are encoded primarily by four genes: Shaker, Shal, Shaw, and Shab (Salkoff et al. 1992; Tsunoda and Salkoff 1995a). Shaw, thought to encode delayed rectifier K+ currents based on studies in oocytes, was shown in cultured Drosophila neurons to instead encode a leak current. Shaw channels have short opening times and very low voltage-sensitivity (Tsunoda and Salkoff 1995b). Thus, Shaw would likely make a poor candidate for regulating firing patterns.
Shaker and Shal encode voltage-dependent currents, which activate and inactivate rapidly (Ryglewski and Duch 2009; Salkoff et al. 1992). Although they have distinct voltage sensitivities, transient potassium channels encoded by both these genes are maximally active in a voltage range left-shifted relative to Shab channels (Covarrubias et al. 1991; Tsunoda and Salkoff 1995b). Their hyperpolarized voltage sensitivity, in combination with their short open times, mean that Shaker and Shal channels are less likely to be open in the relevant voltage range for generating repetitive firing than Shab channels. It is possible that modeling changes in the expression of Shaker and Shal channels could produce diverse firing patterns, but this would likely require current injection outside the physiological range.
In contrast, variation of Shab expression in the model reproduces MN5 firing patterns for values of injected current in agreement with those used in experiments. Experimental work also supports the idea that changes in Shab expression can have profound effects on the ability of neurons to produce certain types of repetitive firing (Peng and Wu 2007). Of course, it might be that some of the specific transitions predicted by the model will not be observed, even if the neuron expresses the ‘right’ number of Shab channels. To observe some of these transitions would require that the state of the neuron be very close to the bifurcation point associated with Icyc, and then that the precise amount of current was injected when the neuron was in this state. However, in general, we predict that, neurons expressing low levels of Shab would be more likely to display long delays to first spike and low firing frequencies than neurons expressing higher levels of Shab.
4.4. Natural variation in channel expression is observed in many types of neurons
In our model, a maximum of a 3 fold change in Shab channel expression reproduced the diversity of MN5 firing behaviors, but is such variation realistic? Research, especially within the last 5–10 years, has demonstrated that neurons show considerable variability in ion channel expression. In MNs of the crustacean stomatogastric (STG) nervous system, Shab mRNA can vary 2–4 fold (Schulz et al. 2006, 2007). In particular, gastric mill neurons show a 4 fold variation in Shab mRNA, yet remarkably low levels of para (DmNaυ) expression (Schulz et al. 2007), indicating that the ratio of Shab to DmNav channels (aK) could reach 3 or larger. Variation in channel expression is also seen in other parts of the crustacean nervous system. Large cell MNs within the cardiac ganglion show a more than 3–9 fold variation in mRNA for a variety of ion channels genes, including Shab, and as much as a 56 fold variation in para mRNA (Tobin et al. 2009).
Similar variation in ion channel expression is observed in mammalian systems. In rats, hippocampal pyramidal cells display a 10 fold variation in the ratio of K+ to Ca2+ mRNA (Eberwine et al. 1992). In mice, dopaminergic neurons within the substantia nigra show a 5–10 fold variation in mRNA encoding for a transient (A-type) potassium channel, and an 8 fold variation in A-type charge density (pC/pF) (Liss et al. 2001). Purkinje neurons in the mouse cerebellum show a 5 fold variation in Na+ or Ca2+ current density (pA/pF) (Swensen and Bean 2005).
Although we are not aware of studies which have measured cell-to-cell variability of Shab expression in Drosophila MN5, previous work gives us an estimate of the extent to which other K+ currents vary in this neuron. Recordings from 57 adult Drosophila show that total peak transient K+ current in MN5 can vary nearly 4 fold (Ryglewski and Duch 2009). Furthermore, measures of current densities in embryonic neurons can give us a rough estimate of the ratio of Shab to para/DmNaυ channels (aK) in the Drosophila nervous system. Embryonic MNs show a Shab-encoded current density of 60 pA/pF, while para-encoded currents are around 30 pA/pF (Baines et al. 2001). Therefore, the estimated aK is 2, supporting the use in our model of aK ≥ 1.
4.5. Changes in Shab activation produce spikes riding on elevated potentials
Altering the rate of Shab current activation by changing the maximum time constant and right-shifting its voltage dependence caused the minimum membrane potential during repetitive spiking to shift up by tens of mVs. Variable activation times have been demonstrated for splice variants of several ion channels (Murbartián et al. 2004; Pan et al. 2001; Saito et al. 1997), and for RNA-edited forms of the Shab channel (Ryan et al. 2008). The rate of K+ channel activation can also vary due to post-translational mechanisms such as phosphorylation (Schulz et al. 2008), glycosylation (Watanabe et al. 2003), or association with accessory (β) channel subunits (Heinemann et al. 1996; Pongs and Schwarz 2010).
As mentioned previously, it is important to recognize that the formulation of the 2-dimensional model means that a change in the activation kinetics of Shab also changes the inactivation kinetics of DmNaυ. Thus, the differences in electrical responses between the two examined parameter regimes could result from a change in the kinetics of both voltage-gated currents. To tease out their relative contributions, future work could include expanding the model to 3 dimensions, where the inactivation of DmNaυ channels is a separate variable. In addition, expansions of the model could include incorporating multiple compartments that would allow us to test how the morphological structure of MN5 contributes to the production of elevated potentials.
4.6. Advantages of the model
A number of minimal models of membrane potential have been proposed previously, but many of these models are not ideal for studying the contribution of ion channel expression to the diversity of firing patterns. For example, the FitzHugh-Nagumo model (Fitz-Hugh 1961; Nagumo et al. 1962), which is a simplification of the classical Hodgkin-Huxley formalism (Hodgkin and Huxley 1952a), is a phenomenological model with equations that do not include terms for channels or channel conductances. The same is true of the later Hindmarsh-Rose model (Hindmarsh and Rose 1984). Although these models are capable of generating different firing patterns, and even bursting in the case of Hindmarsh-Rose, their formulations do not allow one to ask questions about the physiological mechanisms, including differences in ion channel expression, that may produce distinct firing.
Integrate-and-fire models and related formulations using artificial membrane potential resets are computationally inexpensive and can reproduce an impressive diversity of firing patterns seen in cortical neurons (Izhikevich 2003). However, artificial resets at best only capture the dynamics of excitability, but fail to provide biophysical explanations. In contrast, all the parameters in our model can be measured directly from neurons because they have a biophysical correlate. Furthermore, our results relate a measurable parameter, the maximal amplitude of a ionic current encoded by a specific gene, to different firing patterns, thereby offering a biophysical explanation for firing pattern diversity and leading to a number of testable predictions.
Conductance-based (CB) models, based on the standard Hodgkin-Huxley formalism (Hodgkin and Huxley 1952a), are more realistic than the models mentioned above, since they do not include artificial resets, include a number of experimentally measurable parameters, and include channel conductances that can be varied to investigate the role of specific currents. CB models have been used to explore diverse electrical activity in neurons (Arhem and Blomberg 2007; Golomb et al. 2007; Prescott et al. 2008). Arhem and Blomberg (2007) used such a model to relate changes in Na+ and K+ channel density to different patterns of repetitive firing, including distinct frequencies and long delays to first spike. Our model is mathematically related to the CB/HH formulation, but incorporates electrodiffusion, which constitutes a theoretical improvement in describing ion movement across the membrane (Endresen et al. 2000; Herrera-Valdez 2012). In addition, while the Arhem and Blomberg model had 4 dimensions, ours was able to reproduce diverse firing behaviors with just 2 dimensions, making it both biophysical and computationally inexpensive.
A 2-dimensional CB/HH model that can reproduce all three of Hodgkin’s excitability classes (Hodgkin 1948) was presented by Prescott et al. (2008). Their results showed that varying the half-activation potential of the slow K+ current was sufficient to convert neuronal firing behavior between the three excitability classes. However, the authors admit that this variation in half-activation is more “drastic” than would likely be seen in a single type of channel. They split the slow current into two separate currents with distinct voltage sensitivities, and hypothesize that these are differentially expressed by neurons displaying different excitability classes. In our model, it was not necessary to vary half-activation or move to 3 dimensions by incorporating additional populations of currents. The slow current in our model was formulated to represent one type of K+ current encoded by a single gene (Shab), and differential expression of Shab was sufficient to produce a wide range of firing behaviors.
Importantly, the construction of our model allows extensions to study neurons in other parts of the Drosophila nervous system or different developmental stages, as well as neurons in other animals, provided the parameters and core complement of membrane channels are adjusted according to available data from the neurons of interest.
Supplementary Material
Acknowledgements
MAHV, SDB, SR, CD, and SC were supported in part by the National Science Foundation (NSF IIS 0613404). MAHV and ECM were supported in part by the Building Research Infrastructure and Capacity (BRIC) program at UPR-Cayey (P20 MD006144) through the National Institute of Minority Health and Health Disparities. Support for SDB was also provided by the Interdisciplinary Graduate Program in Neuroscience at Arizona State University. Additional support for SR was provided by the German Research Foundation (DFG RY 117/1-1). We thank Cengiz Gunay, Martin Strube, and Joceline Lega for their challenging and insightful feedback. We also thank two anonymous reviewers whose suggestions greatly improved this paper.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10827-012-0416-6) contains supplementary material, which is available to authorized users.
Contributor Information
Marco A. Herrera-Valdez, Institute of Interdisciplinary Research, University of Puerto Rico at Cayey, 205 Antonio R. Barcelo Ave., Cayey, PR 00736, USA Department of Mathematics and Physics, University of Puerto Rico at Cayey, Cayey, PR 00736, USA; Department of Mathematics, University of Arizona, Tucson, AZ 85721, USA; Evelyn F. McKnight Brain Institute, University of Arizona, Tucson, AZ 85724, USA.
Erin C. McKiernan, Institute of Interdisciplinary Research, University of Puerto Rico at Cayey, 205 Antonio R. Barcelo Ave., Cayey, PR 00736, USA
Sandra D. Berger, School of Life Sciences, Arizona State University, Tempe, AZ 85287, USA
Stefanie Ryglewski, School of Life Sciences, Arizona State University, Tempe, AZ 85287, USA; Johannes Gutenberg University Mainz, Institute for Zoology/Neurobiology, 55099 Mainz, Germany.
Carsten Duch, School of Life Sciences, Arizona State University, Tempe, AZ 85287, USA; Johannes Gutenberg University Mainz, Institute for Zoology/Neurobiology, 55099 Mainz, Germany.
Sharon Crook, School of Mathematical and Statistical Sciences, Arizona State University, Tempe, AZ 85287, USA.
References
- Arhem P, & Blomberg C (2007). Ion channel density and threshold dynamics of repetitive firing in a cortical neuron model. Biosystems, 89(1–3), 117–125. [DOI] [PubMed] [Google Scholar]
- Av-Ron E, Parnas H, Segel L (1991). A minimal biophysical model for an excitable and oscillatory neuron. Biological Cybernetics, 65(6), 487–500. [DOI] [PubMed] [Google Scholar]
- Av-Ron E, Parnas H, Segel LA (1993). A basic biophysical model for bursting neurons. Biological Cybernetics, 69, 87–95. [DOI] [PubMed] [Google Scholar]
- Baines R, Uhler J, Thompson A, Sweeney S, Bate M (2001). Altered electrical properties in Drosophila neurons developing without synaptic transmission. Journal of Neuroscience, 21(5), 1523–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranauskas G, & Martina M (2006). Sodium currents activate without a hodgkin and huxley-type delay in central mammalian neurons. Journal of Neuroscience, 26(2), 671–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baro D, Quinones L, Lanning C, Harris-Warrick R, Ruiz M (2001). Alternate splicing of the Shal gene and the origin of IA diversity among neurons in a dynamic motor network. Neuroscience, 106(2), 419–432. [DOI] [PubMed] [Google Scholar]
- Brembs B, Christiansen F, Pflüger H, Duch C (2007). Flight initiation and maintenance deficits in flies with genetically altered biogenic amine levels. Journal of Neuroscience, 27(41), 11122–11131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chay T, & Kang H (1988). Role of single-channel stochastic noise on bursting clusters of pancreatic beta-cells. Biophysical Journal, 54(3), 427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Park D, Griffith L (2004). Electrophysiological and morphological characterization of identified motor neurons in the Drosophila third instar larva central nervous system. Journal of Neurophysiology, 91(5), 2353–2365. [DOI] [PubMed] [Google Scholar]
- Coetzee W, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, et al. (1999). Molecular diversity of K+ channels. Annals of New York Academy of Sciences, 868, 233–285. [DOI] [PubMed] [Google Scholar]
- Connor J, & Stevens C (1971). Inward and delayed outward membrane currents in isolated neural somata under voltage clamp. Journal of Physiology, 213(1), 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors B, & Gutnick M (1990). Intrinsic firing patterns of diverse neocortical neurons. Trends in Neurosciences, 13(3), 99–104. [DOI] [PubMed] [Google Scholar]
- Covarrubias M, Wei A, Salkoff L (1991). Shaker, Shal, Shab, and Shaw express independent K-current systems. Neuron, 7(5), 763–773. [DOI] [PubMed] [Google Scholar]
- Dickinson M, & Tu M (1997). The function of dipteran flight muscle. Comparative Biochemistry and Physiology Part A: Physiology, 116(3), 223–238. [Google Scholar]
- Duch C, Vonhoff F, Ryglewski S (2008). Dendrite elongation and dendritic branching are affected separately by different forms of intrinsic motoneuron excitability. Journal of Neurophysiology, 100(5), 2525–2536. [DOI] [PubMed] [Google Scholar]
- Eberwine J, Yeh H, Miyashiro K, Cao Y, Nair S, Finnell R, et al. (1992). Analysis of gene expression in single live neurons. PNAS, 89(7), 3010–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endresen LP, Hall K, Hoye JS, Myrheim J (2000). A theory for the membrane potential of living cells. European Journal of Biophysics, 29, 90–103. [DOI] [PubMed] [Google Scholar]
- Ermentrout B (2006). PPAUT. Scholarpedia, 2(1), 1399. [Google Scholar]
- Ermentrout G, &Terman D (2010). Mathematical Foundations of Neuroscience. Springer. [Google Scholar]
- Ewing A (1977). The neuromuscular basis of courtship song in Drosophila: the role of the indirect flight muscles. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 119(3), 249–265. [Google Scholar]
- Fernandes J, & Keshishian H (1998). Nerve-muscle interactions during flight muscle development in Drosophila. Development, 125(9), 1769–1779. [DOI] [PubMed] [Google Scholar]
- Fitz-Hugh R (1961). Impulses and physiological states in theoretical models of nerve membrane. Biophysical Journal, 1, 445–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshman J, Munson J, Sypert G, Friedman W (1981). Rheobase, input resistance, and motor-unit type in medial gastrocnemius motoneurons in the cat. Journal of Neurophysiology, 46(6), 1326–1338. [DOI] [PubMed] [Google Scholar]
- Golomb D, Donner K, Shacham L, Shlosberg D, Amitai Y, Hansel D (2007). Mechanisms of firing patterns in fast-spiking cortical interneurons. PLoS Computational Biology, 3(8), e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S, & Dickinson M (2006). Role of calcium in the regulation of mechanical power in insect flight. PNAS, 103(11), 4311–4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guckenheimer J, Harris-Warrick R, Peck J, Willms A (1997). Bifurcation, bursting, and spike frequency adaptation. Journal of Computational Neuroscience, 4(3), 257–277. [DOI] [PubMed] [Google Scholar]
- Guckenheimer J, & Holmes P (1990). Nonlinear oscillations, dynamical systems, and bifurcations of vector fields. Springer. [Google Scholar]
- Günay C, Edgerton J, Jaeger D (2008). Channel density distributions explain spiking variability in the globus pallidus: a combined physiology and computer simulation database approach. Journal of Neuroscience, 28(30), 7476–7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harcombe E, & Wyman R (1977). Output pattern generation by Drosophila flight motoneurons. Journal of Neurophysiology, 40(5), 1066–1077. [DOI] [PubMed] [Google Scholar]
- Hardie R, & Minke B (1994). Spontaneous activation of light-sensitive channels in Drosophila photoreceptors. Journal of general physiology, 103(3), 389–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann S, Rettig J, Graack H, Pongs O (1996). Functional characterization of Kv channel β-subunits from rat brain. Journal of Physiology, 493(3), 625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera-Valdez M (2012). Membranes with the same ion channel populations but different excitabilities. PLoS ONE, 7(4), e34636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera-Valdez MA, & Lega J (2011). Reduced models for the pacemaker dynamics of cardiac cells. Journal ofTheoretical Biology, 270(1), 164–176. [DOI] [PubMed] [Google Scholar]
- Hille B (2001). Ionic channels of excitable membranes (3rd ed.). Sinauer. [Google Scholar]
- Hindmarsh J, & Rose R (1984). A model of neuronal bursting using three coupled first order differential equations. Proceedings of the Royal Society of London. Series B. Biological Sciences, 221(1222), 87–102. [DOI] [PubMed] [Google Scholar]
- Hodgkin A (1948). The local electric changes associated with repetitive action in a nonmedulated axon. Journal of Physiology, 107, 165–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A, & Huxley A (1952a). A quantitative description of membrane current and its application to conduction and excitation in nerve. Journal of Physiology, 117, 500–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A, & Huxley A (1952b). The components of membrane conductance in the giant axon of Loligo. Journal of Physiology, 116(4), 473–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter J, Dale D, Droettboom M (2008). Matplotlib: a Python 2D plotting library. http://matplotlib.sourceforge.net/.
- Ikeda K, & Koenig J (1988). Morphological identification of the motor neurons innervating the dorsal longitudinal flight muscle of Drosophila melanogaster. Journal of Comparative Neurology, 273(3), 436–444. [DOI] [PubMed] [Google Scholar]
- Isacoff E, Jan Y, Jan L, et al. (1990). Evidence for the formation of heteromultimeric potassium channels in xenopus oocytes. Nature, 345(6275), 530–534. [DOI] [PubMed] [Google Scholar]
- Izhikevich E (2007). Dynamical systems in neuroscience. MIT Press. [Google Scholar]
- Izhikevich EM (2003). Simple model of spiking neurons. IEEE Transactions on Neural Networks, 14(6), 1569–1572. [DOI] [PubMed] [Google Scholar]
- Jan L, & Jan Y (1976). Properties of the larval neuromuscular junction in Drosophila melanogaster. Journal of Physiology, 262(1), 189–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan L, & Jan Y (1990). How might the diversity of potassium channels be generated? Trends in Neurosciences, 13(10), 415–419. [DOI] [PubMed] [Google Scholar]
- Jan L, & Jan Y (1997). Voltage-gated and inwardly rectifying potassium channels. Journal of Physiology, 505(2), 267–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E, Oliphant T, Peterson P, et al. (2001). SciPy: open source scientific tools for Python. http://www.scipy.org/.
- Kim J, Wei D, Hoffman D (2005). Kv4 potassium channel subunits control action potential repolarization and frequency-dependent broadening in rat hippocampal CA1 pyramidal neurones. Journal of Physiology, 569(1), 41–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J, & Wyman R (1973). Neurophysiology of flight in wildtype and a mutant Drosophila. PNAS, 70(4), 1050–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Wright D, Muraro N, Baines R (2009). Alternative splicing in the voltage-gated sodium channel DmNav regulates activation, inactivation, and persistent current. Journal of Neurophysiology, 102(3), 1994–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liss B, Franz O, Sewing S, Bruns R, Neuhoff H, Roeper J (2001). Tuning pacemaker frequency of individual dopaminergic neurons by Kv4.3L and KChip3.1 transcription. The EMBO Journal, 20(20), 5715–5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machin K, & Pringle J (1959). The physiology of insect fibrillar muscle. ii. mechanical properties of a beetle flight muscle. Proceedings of the Royal Society of London. Series B. Biological Sciences, 151(943), 204–225. [DOI] [PubMed] [Google Scholar]
- Mo Z, Adamson C, Davis R (2002). Dendrotoxin-sensitive k+ currents contribute to accommodation in murine spiral ganglion neurons. The Journal of physiology, 542(3), 763–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murbartián J, Arias J, Perez-Reyes E, (2004). Functional impact of alternative splicing of human T-type Cav3.3 calcium channels. Journal of Neurophysiology, 92(6), 3399–3407. [DOI] [PubMed] [Google Scholar]
- Nagumo J, Arimoto S, Yoshizawa S (1962). An active pulse transmission line simulating nerve axon. Proceedings of the IRE, 50(10), 2061–2070. [Google Scholar]
- Neher E (1971). Two fast transient current components during voltage clamp on snail neurons. Journal of General Physiology, 58(1), 36–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell Olson R, Liu Z, Nomura Y, Song W, Dong K (2008). Molecular and functional characterization of voltage-gated sodium channel variants from Drosophila melanogaster. Insect Biochemistry and Molecular Biology, 38(5), 604–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z, Selyanko A, Hadley J, Brown D, Dixon J, McKinnon D (2001). Alternative splicing of KCNQ2 potassium channel transcripts contributes to the functional diversity of M-currents. Journal of Physiology, 531(2), 347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng I-F, & Wu C-F (2007). Differential contributions of Shaker and Shab K+ currents to neuronal firing patterns in Drosophila. Journal of Neurophysiology, 97(1), 780–794. [DOI] [PubMed] [Google Scholar]
- Ping Y, Waro G, Licursi A, Smith S, Vo-Ba D, Tsunoda S (2011). Shal/Kv4 channels are required for maintaining excitability during repetitive firing and normal locomotion in Drosophila. PloS ONE, 6(1), e16043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongs O, & Schwarz J (2010). Ancillary subunits associated with voltage-dependent K+ channels. Physiological Reviews, 90(2), 755–796. [DOI] [PubMed] [Google Scholar]
- Prescott S, De Koninck Y, Sejnowski T (2008). Biophysical basis for three distinct dynamical mechanisms of action potential initiation. PLoS Computational Biology, 4(10), e1000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz A, Billimoria C, Marder E (2003). Alternative to hand-tuning conductance-based models: construction and analysis of databases of model neurons. Journal of Neurophysiology, 90(6), 3998–4015. [DOI] [PubMed] [Google Scholar]
- Rinzel J (1985). Excitation dynamics: insights from simplified membrane models. Federation Proceedings, 44(15), 2944–2946. [PubMed] [Google Scholar]
- Rinzel J, & Ermentrout G (1989). Analysis of neural excitability and oscillations In Koch C & Segev I (Eds.), Methods in neuronal modelling: from synapses to networks (pp. 251–291). Cambridge, MA: MIT Press. [Google Scholar]
- Ryan M, Maloney R, Reenan R, Horn R (2008). Characterization of five RNA editing sites in Shab potassium channels. Channels (Austin, Tex.), 2(3), 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryglewski S, & Duch C (2009). Shaker and Shal mediate transient calcium-independent potassium current in a Drosophila flight motoneuron. Journal of Neurophysiology, 102(6), 3673–3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryglewski S, & Duch C (2012). Preparation of Drosophila central neurons for in situ patch clamping. Journal of Visualized Experiments, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryglewski S, Lance K, Levine R, Duch C (2012). Cav2 channels mediate low and high voltage-activated calcium currents in drosophila motoneurons. Journal of Physiology, 590(4), 809–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Nelson C, Salkoff L, Lingle C, (1997). A cysteine-rich domain defined by a novel exon in a Slo variant in rat adrenal chromaffin cells and PC12 cells. Journal of Biological Chemistry, 272(18), 11710–11717. [DOI] [PubMed] [Google Scholar]
- Saito M, & Wu C (1991). Expression of ion channels and mutational effects in giant Drosophila neurons differentiated from cell division-arrested embryonic neuroblasts. Journal of Neuroscience, 11(7), 2135–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkoff L, Baker K, Butler A, Covarrubias M, Pak M, Wei A (1992). An essential set of K+ channels conserved in flies, mice and humans. Trends in Neurosciences, 15(5), 161–166. [DOI] [PubMed] [Google Scholar]
- Sanyal S, Narayanan R, Consoulas C, Ramiswami M (2003). Evidence for cell autonomous ap1 function in regulation of drosophila motor-neuron plasticity. BMC Neuroscience, 4, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer J, Worrell J, Levine R (2010). Role of intrinsic properties in Drosophila motoneuron recruitment during fictive crawling. Journal of Neurophysiology, 104(3), 1257–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilcher F.v. (1976). The behavior of cacophony, a courtship song mutant in drosophila melanogaster. Behavioral Biology, 17(2), 187–196. [DOI] [PubMed] [Google Scholar]
- Schulz D, Goaillard J, Marder E (2006). Variable channel expression in identified single and electrically coupled neurons in different animals. Nature Neuroscience, 9(3), 356–362. [DOI] [PubMed] [Google Scholar]
- Schulz D, Goaillard J, Marder E (2007). Quantitative expression profiling of identified neurons reveals cell-specific constraints on highly variable levels of gene expression. PNAS, 104(32), 13187–13191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz D, Temporal S, Barry D, Garcia M (2008). Mechanisms of voltage-gated ion channel regulation: from gene expression to localization. Cellular and Molecular Life Sciences, 65(14), 2215–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata R, Nakahira K, Shibasaki K, Wakazono Y, Imoto K, Ikenaka K (2000). A-type K+ current mediated by the Kv4 channel regulates the generation of action potential in developing cerebellar granule cells. Journal of Neuroscience, 20(11), 4145–4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonders M, & Amara S (1996). Channels in transporters. Current Opinion in Neurobiology, 6(3), 294–302. [DOI] [PubMed] [Google Scholar]
- Steriade M (2001). The intact and sliced brain. MIT Press. [Google Scholar]
- Steriade M, Timofeev I, Durmuller N, Grenier F (1998). Dynamic properties of corticothalamic neurons and local cortical interneurons generating fast rhythmic (30–40 Hz) spike bursts. Journal of Neurophysiology, 79(1), 483–490. [DOI] [PubMed] [Google Scholar]
- Swensen A, & Bean B (2005). Robustness of burst firing in dissociated Purkinje neurons with acute or long-term reductions in sodium conductance. Journal of Neuroscience, 25(14), 3509–3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M, Theeuwes H, Feenstra L, Borst J (2007). Membrane properties and firing patterns of inferior colliculus neurons: an in vivo patch-clamp study in rodents. Journal of neurophysiology, 98(1), 443–453. [DOI] [PubMed] [Google Scholar]
- Timpe L, Jan Y, Jan L (1988). Four cdna clones from the shaker locus of drosophila induce kinetically distinct a-type potassium currents in xenopus oocytes. Neuron, 1(8), 659–667. [DOI] [PubMed] [Google Scholar]
- Tobin A, Cruz-Bermúdez N, Marder E, Schulz D (2009). Correlations in ion channel mRNA in rhythmically active neurons. PLoS ONE, 4(8), e6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimmer J, & Rhodes K (2004). Localization of voltage-gated ion channels in mammalian brain. Annual Reviews of Physiology, 66, 477–519. [DOI] [PubMed] [Google Scholar]
- Tsunoda S, & Salkoff L (1995a). Genetic Analysis of Drosophila Neurons: Shal, Shaw, and Shab Encode Most Embryonic Potassium Currents. Journal of Neuroscience, 15(3), 1741–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda S, & Salkoff L (1995b). The major delayed rectifier in both Drosophila neurons and muscle is encoded by Shab. Journal of Neuroscience, 15(7), 5209–5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe I, Wang H, Sutachan J, Zhu J, Recio-Pinto E, Thornhill W (2003). Glycosylation affects rat Kv1.1 potassium channel gating by a combined surface potential and cooperative subunit interaction mechanism. Journal ofPhysiology, 550(1), 51–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willms A, Baro D, Harris-Warrick R, Guckenheimer J (1999). An improved parameter estimation method for Hodgkin-Huxley models. Journal of Computational Neuroscience, 6(2), 145–168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


