Abstract
A high proportion of β-cells die within days of islet transplantation. Reports suggest that induction of hypoxia-inducible factor-1α (HIF-1α) predicts adverse transplant outcomes. We hypothesized that this was a compensatory response and that HIF-1α protects β-cells during transplantation. Transplants were performed using human islets or murine β-cell-specific HIF-1α-null (β-HIF-1α-null) islets with or without treatment with deferoxamine (DFO) to increase HIF-1α. β-HIF-1α-null transplants had poor outcomes, demonstrating that lack of HIF-1α impaired transplant efficiency. Increasing HIF-1α improved outcomes for mouse and human islets. No effect was seen in β-HIF-1α-null islets. The mechanism was decreased apoptosis, resulting in increased β-cell mass posttransplantation. These findings show that HIF-1α is a protective factor and is required for successful islet transplant outcomes. Iron chelation with DFO markedly improved transplant success in a HIF-1α-dependent manner, thus demonstrating the mechanism of action. DFO, approved for human use, may have a therapeutic role in the setting of human islet transplantation.
Keywords: Hypoxia-inducible factor-1α (HIF-1α), β-Cell function, Islet transplantation, Deferoxamine (DFO), Diabetes, Hypoxia
INTRODUCTION
Islet transplantation has the potential to cure type 1 diabetes (47). However, its use as a therapy for type 1 diabetes is limited by relatively poor outcomes for the number of islets transplanted (42,44,48). This is in contrast to results for whole-pancreas transplantation (43,49). In islet transplant recipients who do achieve insulin independence, a gradual return to requiring insulin therapy is common (11,42,48) and occurs more frequently than it does in whole-pancreas recipients.
Many factors influence the outcome of islet transplantation, including donor characteristics and availability, enzyme type and quality, pancreas procurement, preservation, cold ischemia time, the isolation process itself, and islet gene expression patterns (7,8,10,34,40–42). Isolation and purification inevitably devascularize the islets, inducing hypoxia that has a negative impact on β-cell survival and function (5,9). This may contribute to smaller islets performing well in transplantation (23,26,55). After isolation, islets deteriorate within 24–48 h (20). Short-term islet cell death occurs due to nutrient deprivation, metabolic changes, proinflammatory molecules released by islets and acinar tissue, harmful enzymes released by residual acinar tissue, physical islet fragmentation, and recurrent immune attack (6,12,42,43).
Hypoxia is deleterious for cell function, and all multicellular organisms have homeostatic mechanisms to preserve function in the face of decreasing oxygen tension (15,46). In mammals, this includes the hypoxia-inducible factors (HIFs) including HIF-1α. HIF-1α is a transcription factor, 254 and together with its heterodimeric partner ARNT (aryl hydrocarbon receptor nuclear translocator), HIF-1α forms the active HIF-1 transcription factor (15,45). HIF-1 regulates responses to hypoxia (45) and is known to increase cell survival following hypoxic challenge (3,45,53). In other tissues, HIF-1α increases vascular endothelial growth factor (VEGF) (24) and antiapoptotic genes B-cell lymphoma 2 (BCL-2) and B-cell lymphoma-extra large (BCL-xL), while it decreases proapoptotic genes BCL-2 associated X protein (BAX) and BCL2-antagonist/killer 1 (BAK). We recently reported that HIF-1α upregulates beneficial genes in β-cells including glucose transporter 2 (GLUT2) and glucokinase (GCK) (5).
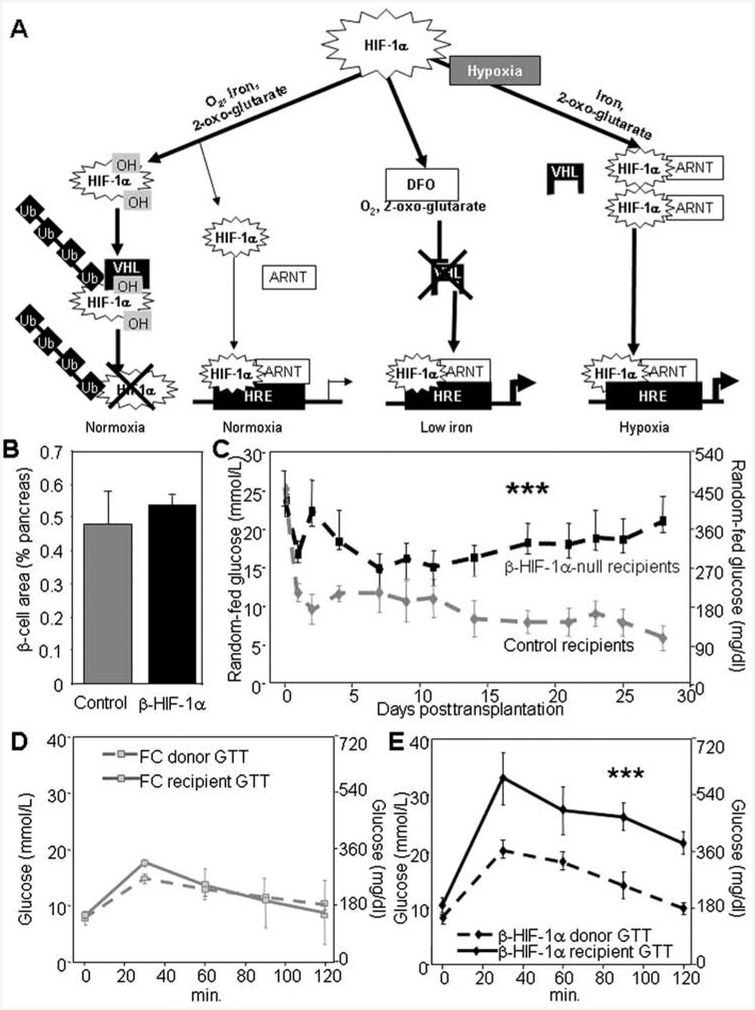
In the basal state, HIF-1α is rapidly proteolyzed. It undergoes hydroxylation on prolyl, and asparagine residues followed by ubiquitination and proteolysis (28). Hydroxylation is dependent on oxygen and iron; thus, hypoxia or iron deprivation stabilizes HIF-1α and protein level increase, as shown in Figure 1A. As well as hypoxia, genetic inactivation of vHL (von Hippel-Lindau protein) or the prolyl hydroxylases, treatment with heavy metals such as cobalt chloride, and iron chelation with deferoxamine (DFO) also increase HIF-1α protein levels (22,52).
Figure 1.
β-Cell hypoxia-inducible factor-1α (HIF-1α) is necessary for optimal islet transplant outcomes. (A) HIF-1α is rapidly degraded under normoxic conditions. In the presence of oxygen, iron, and 2-oxo-glutarate, it is hydroxylated, associates with von Hippel-Lindau protein (VHL), and is ubiquitinated (Ub) and proteolyzed. In the absence of VHL protein or in the setting of decreased oxygen or decreased iron, HIF-1α proteolysis is inhibited, and the protein partners with aryl hydrocarbon receptor nuclear translocator (ARNT) regulate transcription. DFO, deferoxamine; HRE, hypoxia response element. (B) β-Cell area did not differ between β-HIF-1α and floxed-control (FC) mice (n = 6). (C) β-HIF-1α-null islet transplant recipients had higher random-fed glucose values than floxed-control islet recipients (n = 6). (D) Recipients of floxed-control islet transplants exhibited similar glucose tolerance to the FC donors (n = 6). (E) β-HIF-1α-null islet transplant recipients had severely impaired glucose tolerance compared to the glucose tolerance of the β-HIF-1α donors (n = 6). Error bars indicate ±SEM. ***p < 0.001.
There is an early ischemic period immediately following islet transplantation, leading to increased apoptotic β-cell death (54). HIF-1α plays an important cell survival role in hypoxic settings, and islet transplantation creates such an environment. So it is perhaps surprising that a number of studies have reported that increased HIF-1α is associated with poor transplant outcome (27,30,32). However, we hypothesized that high levels of HIF-1α may be correlated with poor outcome in these reports because the induction of HIF-1α was a marker of antecedent islet stress, which itself caused poor outcomes.
Thus, we hypothesized, firstly, that HIF-1α is a beneficial factor in islet transplantation, which is required for successful islet transplantation and, secondly, that increasing HIF-1α in a nontoxic manner would improve islet transplant outcomes. These hypotheses were tested using islets from mice lacking β-cell HIF-1α (β-HIF-1α-null mice) and human islets.
Transplant outcomes were poor for recipients of β-HIF-1α-null islets. Increasing HIF-1α via iron chelation with DFO improved transplant outcomes for both murine and human islets. This improvement was not seen in β-cells lacking HIF-1α, demonstrating that HIF-1α was required for the effect. DFO treatment led to improvement in outcomes for both minimal mass and adequate mass transplant models of human islets. DFO decreased apoptosis 24 h post-transplant and was associated with improved glucose control over the month after transplantation. At 28 days, β-cell mass was greater in the DFO-treated groups. These results show that HIF-1α is not harmful but is in fact beneficial for islet transplant outcomes, that DFO improves outcomes of STOKES ET AL. human islet transplants, and that the mechanism of benefit requires HIF-1α. DFO is approved for human use, and it may have a role in clinical islet transplantation.
MATERIALS AND METHODS
Human studies were approved by the St. Vincent’s Hospital Ethics Committee, and animal studies were approved by the Garvan Institute Animal Ethics Committee. Human pancreatic islets were purified using the modified Ricordi method (36,41).
Animals
Donor β-cell-specific HIF-1α knockout mice (β-HIF-1α-null) were generated using the Cre-lox system. Mice with floxed HIF-1α alleles were bred with mice expressing Cre-recombinase under the control of the rat insulin promoter (RIP-Cre mice) to generate floxed controls or β-HIF-1α-null mice on the C57Bl/6 background (50). Recipient mice were either female C57Bl/6 (mouse transplants) or severe combined immunodeficiency (SCID, human transplants) mice of ages 8–12 weeks. SCID mice were obtained from the Animal Resource Centre (Canning Vale, WA). C57Bl/6 mice were obtained from the Animal Bioresources facility (Moss Vale, NSW).
Diabetes Induction
C57Bl/6 recipient mice were rendered diabetic with intraperitoneal injection of streptozotocin (STZ; Sigma-Aldrich) in 10 mM citrate buffer, at a dose of 200 mg/kg. Diabetes was induced in SCID mice by intravenous alloxan tetrahydrate (Sigma-Aldrich) at 75 mg/kg. Diabetes was defined as blood glucose levels ≥20.0 mmol/L/360 mg/dl for 2 or more consecutive days before transplantation. All mice that were not diabetic at study end had grafts removed to confirm diabetes recurrence and exclude the possibility of β-cell regeneration.
Mouse Islet Isolation, Hypoxia, and DFO Treatment
Islets from β-HIF-1α-null or control mice were isolated and purified as previously reported (5,16). Islets were counted and then cultured with or without DFO (125 μM; Sigma-Aldrich) in Roswell Park Memorial Institute medium (RPMI; Invitrogen), at a density of 200–250 islets per well for 2 h. Where stated, islets were placed in a hypoxic chamber and exposed to 5% oxygen for 2 h prior to preparation for transplantation. Mouse islets were transplanted within 6 h of isolation.
Mouse and Human Islet Transplantation and DFO Culture/Injection
A marginal islet mass (islets from one donor transplanted into one recipient) was transplanted from donor mice with β-cell-specific deletion of HIF-1α (β-HIF-1α-null) or their floxed littermate controls. The minimal 256 mass transplant model was chosen to mimic the human situation and to allow comparison of donor and recipient glucose tolerance tests (GTTs).
Human islets were cultured overnight in either control media or media supplemented with DFO (125 μM) and transplanted into SCID recipients. Human islet transplant groups were (1) supraphysiological mass transplant of 2,000 control cultured islet equivalents (IEQ); (2) minimal mass transplant of 600 IEQ; or (3) 600 IEQ of islets cultured in DFO overnight (125 μM). For the adequate mass experiment, the transplant groups were (1) 2,000 control IEQ; (2) 2,000 IEQ of islets cultured in DFO overnight; or (3) 2,000 IEQ of islets cultured in DFO plus DFO injections (125 nM/g mouse weight) of recipient mice on days 0, 2, and 4 posttransplant. For each human donor, at least one of each of the three transplant groups was performed to avoid the important confounder of interdonor variability. Islets were transplanted beneath the left kidney capsule under isoflurane anesthesia and oxygen and dispersed in a standardized manner.
Graft function was determined by monitoring randomfed blood glucose levels up to 28 days. Left nephrectomy was performed on day 28 to confirm diabetes reoccur-rence. Any mouse that was not frankly diabetic by 48 h postnephrectomy (blood glucose levels ≥20.0 mmol/L for two or more readings) was excluded.
Glucose Tolerance Test (GTT)
Glucose tolerance test (GTT) was performed on the recipient mice on day 25 posttransplant as previously reported (5,16). Mice were fasted overnight. An intra-peritoneal injection of 20% dextrose (Sigma-Aldrich) was administered at a dose of 2 g/kg. Blood samples were taken at the times indicated.
ATP Content
ATP was measured following the indicated oxygen exposure using the Roche Bioluminescence kit according to the manufacturer’s instructions. Results were corrected for total protein.
Glucose Oxidation
Glucose oxidation was measured by assessing the formation of 14CO2 from the metabolism of U-[14C]glucose (Perkin Elmer) as previously reported (14,19).
Human Islets and Interleukin 1ß (IL-1ß) Culture
Islets were cultured with IL-1ß (200 U/ml; R&D Systems, USA) for 2 h, and gene expression was measured by real-time PCR as previously reported (5).
Mouse Primers were as follows: Glut1, acctatggccaaggacacac and ctggtctcaggcaaggaaag; Glut2, cat gctgagctctgctgaag and acagtccaacggatccactc; insulin receptor substrate 2 (Irs2), gtagttcaggtcgcctctgc and ttgggaccaccactcctaag; pancreatic and duodenal homeobox 1 (Pdx1), gaaatccaccaaagctcacg and ttcaacatcactgcca gctc; phosphofructokinase (Pfk), atggcaaagctatcggtgtc and acacagtcccatttggcttc; Bak, cgctacgacacagagttcca and ggtagacgtacagggccaga; Bax, tgcagaggatgattgctgac and gatcagctcgggcactttag; Bcl2, tctgaaggattgatggcaga and catcagccacgcctaaaagt; Bclxl, ccattgctaccaggagaacc and aggagctggtttaggggaaa.
Human primers were as follows: GLUT1, agcct gcaaactcactgctc and cctaccctcaatccacaagc; GLUT2, ggccattactaacacgcattg and agcaccctgctaagcttttg; IRS2, cagtgttttccttttgggtacg and tggctattaaggagggcatc; v-akt murine thymoma viral oncogene homolog 2 (AKT2), acacctctgggtgtttggag and gaggagaaaggccagtaggg; PFK, ttgactgcaggaagaacgtg and gcacacaaatggaatcatcg.
Immunohistochemistry
Kidney grafts were collected and fixed in 10% (phosphate-buffered) formalin. Specimens were paraffin-embedded and 5-μm sections were cut. Antibodies for immunohistochemistry were rabbit anti-insulin (Cell Signaling, Danvers, MA), mouse anti-cluster of differentiation 31 (CD31; Zymed, San Francisco, CA), and anti-mouse and anti-rabbit secondary antibodies (DAKO, Carpinteria, CA). Sections were stained using a DAKO autostainer and detected with a peroxidase substrate containing 3,3-diaminobenzidine (brown) and counterstained with hematoxylin. For CD31 staining, tyramide signal amplification (TSA; DAKO) was used. Images were taken with a Leica DCF420 (Leica, Germany). For immunofluorescence, primary antibodies were rabbit anti-cleaved caspase 3 antibody (R&D Systems, Sydney, Australia) and guinea pig polyclonal anti-insulin antibody (DAKO) diluted 1:100. Secondary antibodies included anti-rabbit cyanine 3 (Cy3), anti-guinea pig Cy2 (Invitrogen), and DAPI (DAKO). Images were taken with a Zeiss inverted microscope (Zeiss) and Axiovision software.
β-Cell area was calculated using ImageJ (NIH free-ware). Freehand circling of insulin-positive cells was performed on every sixth section of each kidney graft from first positive staining through to graft exhaustion to produce a total calculated ß-cell area volume. Apoptosis was assessed by measuring cleaved caspase 3-positive and insulin-positive cell area in four sections spaced equally over the graft of 24-h posttransplant kidneys.
CD31 staining was quantified with ImageJ on CD31-immunostained sections that were not hematoxylin counterstained. Three widely separated sections per graft were used. For normal histological examination, separate sections were CD31 stained and hematoxylin counterstained.
Fluorescence-Activated Cell Sorting (FACS)
Cell cycle analysis was performed using the fluorescein isothiocyanate (FITC) bromodeoxyuridine (BrdU) flow kit (BD Pharmingen, USA). MIN6 cells or isolated mouse islets were pretreated ±125 μM DFO. Cells were then pulsed with 10 μM BrdU for 50 min, with readdition of DFO to the appropriate wells, at 37°C with 5% CO2. Islets were gently dispersed with trypsin. BrdU and 7-amino-actinomycin D (7AAD) staining was performed as per the manufacturer’s instructions. Flow cytometric data were acquired using a FACSCanto (BD Biosciences, USA) and analyzed using FlowJo software (Tree Star). MIN6 cells were obtained from Dr. J. Miyazaki, Physiological Chemistry, Osaka University, Osaka, Japan.
Statistical Analysis
For all figures, error bars indicate ±SEM. SPSS14.0 was used to calculate p values, and unless otherwise specified, unpaired two-tailed t tests with unequal variance were used. A value of p < 0.05 was considered significant. Where multiple comparisons were made, Bonferroni correction was used.
RESULTS
β-Cell HlF-1α Is Required for Successful Islet Transplantation
Using the Cre-lox system, with Cre under the control of the rat insulin promoter (RIP-Cre), and mice with floxed HIF-1α (floxed controls), we generated β-cell-specific HIF-1α knockout mice (β-HIF-1α-null) as previously reported (5,50). As shown in that report, RIP-Cre alone mice have normal glucose tolerance (5). β-HIF-1α-null mice have normal fasting glucose and mildly increased postchallenge levels on glucose tolerance testing (GTT) (5).
Glucose tolerance tests were carried out on islet donor mice, and islets were isolated from the donors (β-HIF-1α-null and floxed controls) at least 24 h later for transplantation into female diabetic C57Bl/6 mice (8–12 weeks of age). As shown in Figure 1B, in separate mice, β-cell area is similar in β-HIF-1α-null and controls, being nonsignificantly higher in β-HIF-1α-null mice. Despite this, recipients of β-HIF-1α-null islets had markedly worse glucose control posttransplantation (Fig. 1C) compared to recipients of floxed-control islets. Average random-fed glucose was 382 ± 58 mg/dl (21.2 ± 3.2 mmol/L) in β-HIF-1α-null recipients versus 194 ± 19 mg/dl (10.8 ± 1.6 mmol/L) in recipients of floxed-control islets (p < 0.001).
Glucose tolerance tests prior to nephrectomy showed that in floxed-control recipients, glucose tolerance did not differ significantly between the donor mouse and the recipients (Fig. 1D). As previously reported, β-HIF-1α-null mice had mildly worse glucose tolerance at baseline compared to floxed controls (Fig. 1E, dashed line). However, in pronounced contrast to floxed-control islet transplant recipients, glucose tolerance in β-HIF-1α-null islet recipients was markedly worse than in their β-HIF-1α-null donors prior to islet isolation (Fig. 1E, solid line) (p < 0.001 vs. their donors). Thus, lack of HIF-1α in β-cells resulted in severe impairment of glucose tolerance posttransplantation.
These results indicated that β-cell HIF-1α was important for successful islet transplantation. We next examined the effect of increasing HIF-1α in human islet transplants using DFO.
DFO Improved Outcomes of Minimal Mass Human Islet Transplantation
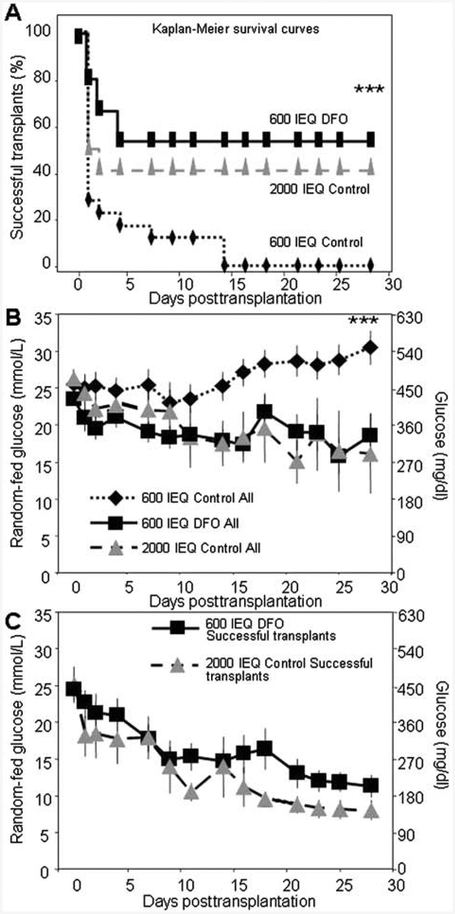
Human islets were transplanted into female diabetic immunodeficient (SCID) (8–12 weeks of age) mice using a minimal mass model of 600 islet equivalents (IEQ) or an adequate mass model of 2,000 IEQ. For the minimal mass model, islets were cultured with or without addition of DFO, and the 2,000 IEQ transplants were control-cultured. For each of the three groups (2,000 IEQ, 600 IEQ, and 600 IEQ + DFO), at least one transplant was performed for each of nine human donors to avoid the important issue of interdonor variability.
Recipients of 2,000 IEQ control cultured human islets were cured (defined arbitrarily as glucose <20 mmol/L) in 42% of cases. As expected, minimal mass transplantation performed poorly, with 600 IEQ of control islets curing 0% of mice at 28 days. In contrast, minimal mass transplant of 600 IEQ islets cultured with DFO had a 53% success rate [p < 0.001 vs. minimal mass control (600 IEQ) and p=ns vs. adequate mass 2,000 IEQ transplants] (Fig. 2A). The random-fed glucose results for the three groups are shown in Figure 2B. Recipients of 600 IEQ + DFO-cultured islets had random-fed glucose levels that were similar to those measured in recipients of 2,000 IEQ transplants. Figure 2C shows the average posttrans-plant glucose for the successful 600 IEQ + DFO and the successful 2,000 IEQ control transplants. This confirms that glucose levels were similar between recipients of 600 IEQ+DFO transplants and those of 2,000 IEQ transplants (n = 9 donors for minimal mass transplants).
Figure 2.
DFO improved outcomes for minimal mass human islet transplants. (A) Diabetes-free survival Kaplan-Meier plots. Minimal mass [600 islet equivalent (IEQ)] transplants of human islets cured 0% of mice by day 28. Adequate mass controls (2,000 IEQ transplants) cured 42% of mice. Transplants of 600 IEQ of DFO-cultured islets from the same donors cured 53% of recipient mice (p < 0.001 vs. 600 IEQ control). (B) The 600 IEQ control transplant recipients had high glucose levels throughout the 28-day period versus the 600 IEQ DFO recipients or 2,000 IEQ controls. The 600 IEQ DFO transplants did not differ significantly from the 2,000 IEQ controls. (C) Average random-fed glucose values posttransplantation for the successful 600 DFO and successful 2,000 IEQ recipient mice. n = 9–12 per group. ***p < 0.001 for 600 IEQ versus 600 IEQ DFO.
DFO Improved Glycemia in Recipients of Adequate Mass Transplants
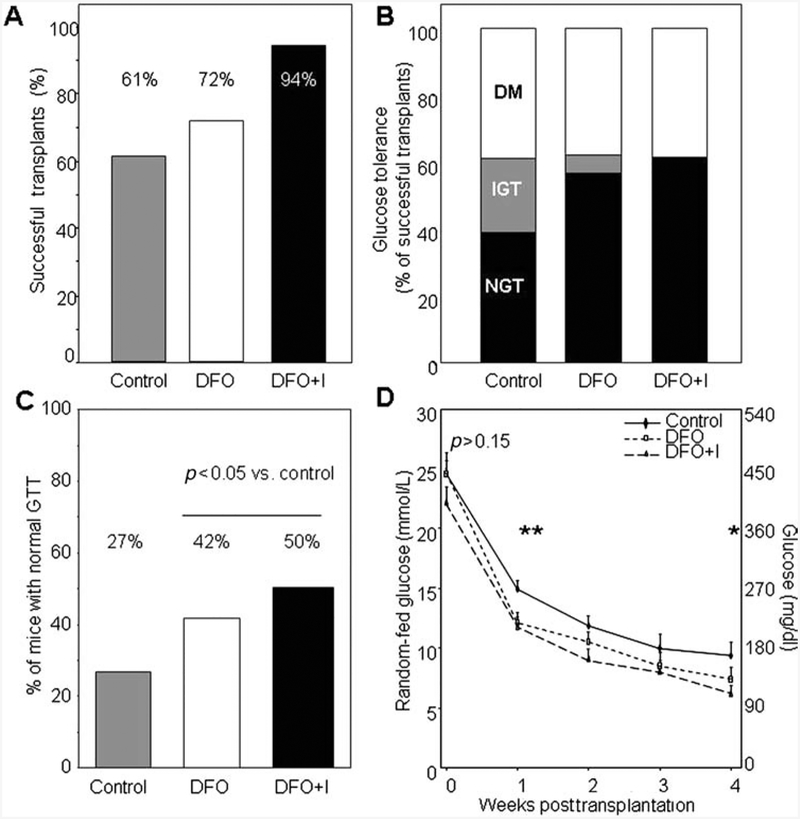
In a separate experiment using eight different human donors, we examined the effects of DFO on adequate mass transplants. The transplant groups were 2,000 IEQ control, 2,000 IEQ cultured with DFO, and 2,000 IEQ cultured with DFO where the female SCID recipient mice (8–12 weeks of age) also received DFO injections (DFO + Injections) on the day of transplant and on days 2 and 4 posttransplant.
The cure rate for controls was 61%, which was slightly lower than that for DFO-treated islets, 72% (Fig. 3A). DFO + Injections cured 94% of mice. In the cured mice, outcomes were better for the DFO and DFO+Injections groups, with more mice having completely normal glucose tolerance [all GTT postload glucose values <145 mg/dl (<8 mmol/L)] and fewer mice having impaired glucose tolerance (IGT) or diabetes (Fig. 3B). IGT was defined as glucose levels of 145–200 mg/dl (8–11 mmol/L) and diabetes as glucose levels of >200 mg/dl (11 mmol/L). Figure 3C shows that with higher proportions of mice achieving the cutoff for cure based on random-fed glucose and higher proportions of the cured mice having normal glucose tolerance, the overall proportion of recipient mice with normal glucose tolerance was 55% higher in the DFO group (27% vs. 42%) and 85% higher in the DFO+Injections group (27% vs. 50%). Overall, mice receiving DFO-treated islets had a significantly higher rate of normal glucose tolerance after transplant compared to controls (p = 0.023 by chi-square) (n = 8 donors for adequate mass transplants). In Figure 3D, random-fed glucose levels were lower in the successful DFO and DFO + Injections transplant recipients than in the controls. To examine whether the effect of DFO was durable, 18 separate cured DFO-transplant recipients were observed up to 85 ± 3 days. All of these mice remained free of diabetes until nephrectomy.
Figure 3.
DFO improved outcomes of adequate mass human islet transplants. (A) 2,000 IEQ of control cultured islets cured 61% of mice; 2,000 IEQ of DFO-cultured islets cured 72% and mice receiving DFO-cultured islets plus DFO injections (DFO+I) of the cured 94% of mice (p = ns). (B) In the cured mice, there was a trend to fewer mice having impaired glucose tolerance (IGT) or diabetes (DM) in the DFO-treated islets and DFO-treated islets plus DFO injection of recipient mice. (C) Overall, a higher percentage of recipients had normal glucose tolerance (NGT) in the two DFO groups versus controls. (D) Random-fed blood glucose levels in successful transplants were lower in the DFO groups than in the control recipients. n = 8–10 per group. *p < 0.05, **p < 0.01. GTT, glucose tolerance test.
HlF-1α Is Required for the Beneficial Effect of DFO
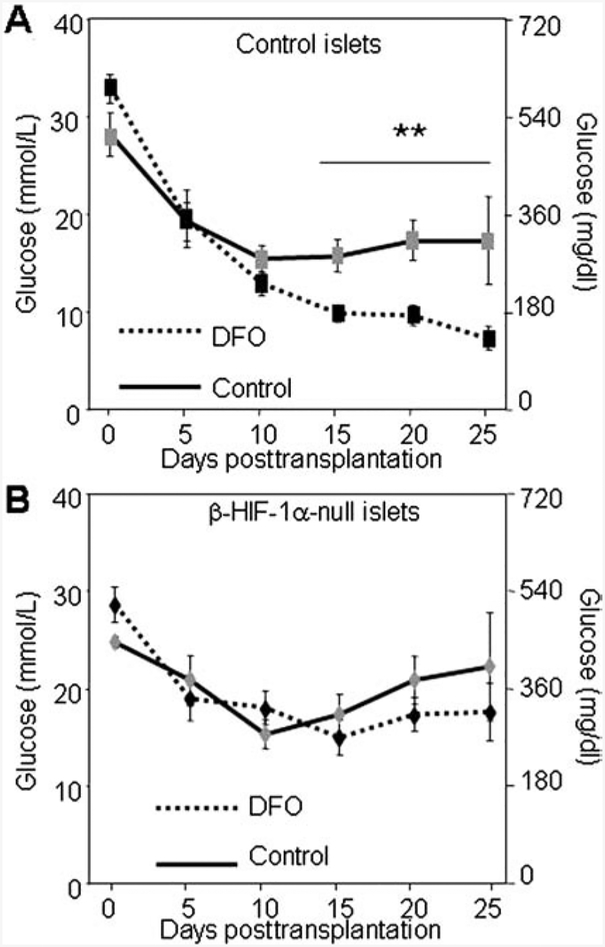
Iron chelation is well known to increase HIF-1α; however, DFO has the potential for other effects via removing iron from other molecules. To determine whether the beneficial effect on transplant outcome was mediated by HIF-1α or was HIF-1α independent, we used β-HIF-1α-null and floxed-control donors in a syngeneic mouse transplant model. Since glucose tolerance was normal in the floxed-control islet recipients (Fig. 1D), and therefore DFO could not improve normal outcomes, we stressed islets from both groups by subjecting them to 5% oxygen exposure for 2 h pretransplantation. When floxed-control islets were treated with DFO during the period of hypoxic exposure, the recipients displayed significantly improved islet transplant outcomes (Fig. 4A). DFO had no effect in transplants of β-HIF-1α-null islets (Fig. 4B), demonstrating that β-cell HIF-1α was required for the benefit. A separate experiment was carried out using a new cohort of β-HIF-1α-null donors ± DFO treatment where the islets were not subjected to hypoxia and verified that there was also no benefit of DFO treatment in β-HIF-1α-null islets when they were unstressed (p > 0.3).
Figure 4.
DFO was ineffective in β-HIF-1α islet transplants. Islets for both groups were exposed to 5% oxygen for 2 h with or without DFO treatment prior to transplantation. (A) Floxed-control recipients had improved glucose values after transplantation when islets were cultured with DFO. (B) No improvement was seen for β-HIF-1α-null transplants when the islets were cultured with DFO. n = 4–6 per group. **p < 0.01 versus control-cultured islets.
Mechanisms of DFO Benefit
DFO Increased ATP Content in Hypoxic Islets and Decreased Apoptosis 24 h Posttransplantation.
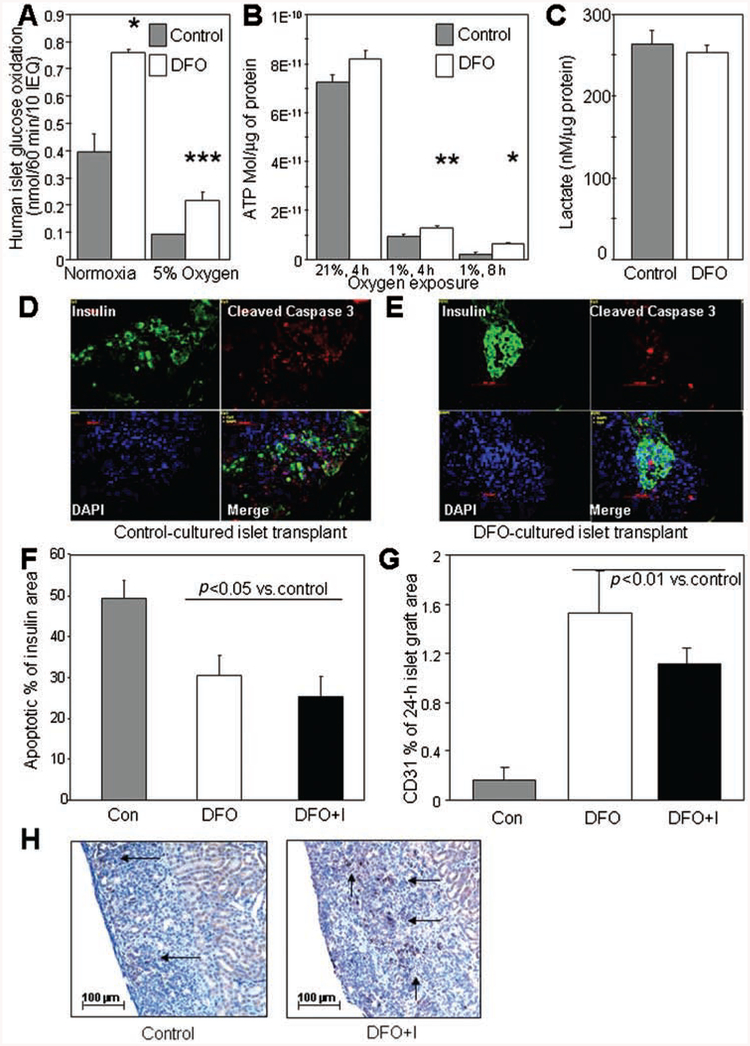
Given that the treatment was only pretransplantation, or in the first week of transplantation in the case of the DFO+Injections group, the most likely mechanism of improved transplant outcome was decreased short-term apoptosis due to improved energy supply. We examined glucose oxidation in human islets treated with DFO at normoxia and hypoxia. Glucose oxidation was significantly increased at 21% oxygen (93% increase, p=0.028) (Fig. 5A). At 5% oxygen, the increase was more pronounced (132% increase, p=0.0008) (Fig. 5A), such that the DFO-treated islets maintained 55% of normoxic control levels of glucose oxidation, whereas control treated islets were able to achieve only 24% of normal levels. We examined ATP content in isolated human islets cultured overnight in media or media supplemented with DFO and then exposed to the oxygen concentration indicated in Figure 5B. DFO did not alter islet ATP concentrations at 21% oxygen, but in human islets exposed to 1% oxygen for 4 h, ATP content was significantly increased by DFO treatment (37% increase, p = 0.0055). In human islets exposed to 1% oxygen for 8 h, a larger proportional increase in ATP was observed (172% increase, p=0.026). Thus, DFO treatment led to substantially better preservation of ATP status during hypoxia. Lactate concentrations were not altered (Fig. 5C).
Figure 5.
Mechanisms of DFO action. (A) Glucose oxidation was significantly increased by DFO treatment of human islets, both at normoxia and at 5% oxygen. (B) The ATP content of islets exposed to 1% oxygen was increased by pretreatment with DFO (white bars) compared to control culture (gray bars). (C) Lactate concentrations were not altered. (D) Cleaved caspase 3 and insulin staining in a control transplant at 24 h. Scale bar: 50 μm. (E) Cleaved caspase 3 and insulin staining in a DFO transplant at 24 h. (F) Quantitation of cleaved caspase 3/insulin+ graft area showed a significant decrease in apoptotic area in the DFO groups versus controls (p < 0.05). (G) CD31 staining was significantly higher in DFO-treated islet grafts than in controls as a proportion of graft area. (H) Representative examples of CD31 staining in the grafts demonstrating loss of CD31 staining in a high proportion of islets. Arrows indicate areas of CD31 staining. **p < 0.01; *p < 0.05. DFO+I, DFO plus injections group.
In a separate series of transplants from four human donors, human islets were transplanted into SCID mice in the following treatment groups: 2,000 IEQ control; 2,000 IEQ DFO; or 2,000 IEQ DFO + Injections. Recipient mice were sacrificed at 24 h for graft collection. Apoptosis was assessed in the grafts by measuring the insulin-positive, cleaved caspase 3-positive area. Sample images are shown in Figure 5D (control) and E (DFO). Cleaved caspase 3-positive cells were measured as a proportion of the insulin-positive area, and the quantification is shown in Figure 5F. There was a 30–40% decrease in apoptotic islet area in the DFO and DFO + Injections groups compared to controls at 24 h posttransplantation (p < 0.05) (n=4 donors for apoptosis and caspase 3 at 24 h).
DFO Treatment Increased CD31 Staining at 24 h
Donor endothelial survival was assessed by CD31 staining in the same human islet grafts removed at 24 h. In the grafts at 24 h posttransplantation, there was significantly greater preservation of CD31 staining in the two DFO-treated groups than in the control transplants, with a >5-fold increase compared to control-cultured islets, p < 0.05 (n = 4 donors for CD31 at 24 h) (Fig. 5G). Images of CD31-stained sections with hematoxylin counterstaining are shown in Figure 5H.
DFO Increased β-Cell Volume 28 Days Posttransplantation
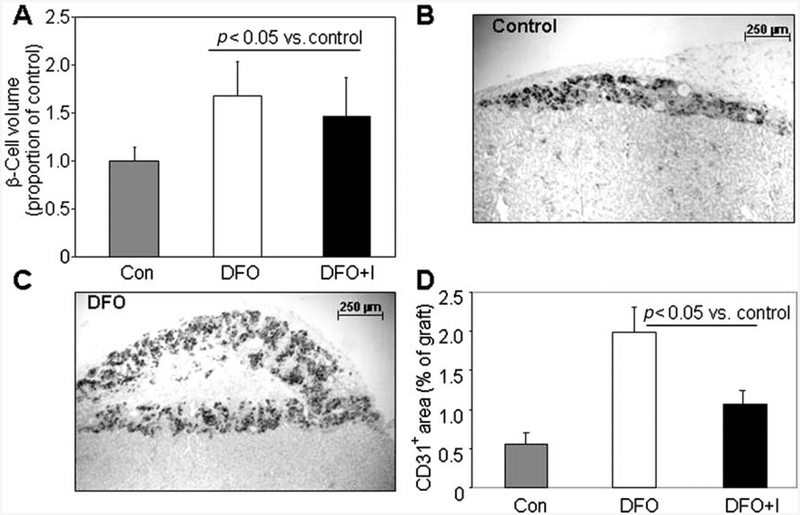
β-Cell graft volume was measured at 28 days posttrans-plantation using the grafts from the experiment shown in Figure 3. Consistent with the increased glucose oxidation and ATP and decrease in apoptosis at 24 h, Figure 6A shows that there was a 50–60% increase in total graft β-cell volume at 28 days in DFO-treated islet grafts compared to controls (n=8 donors for β-cell volume at 28 days). Representative low-power images of grafts for control and DFO are shown in Figure 6B and C. In the 28-day grafts, there was also a significant increase in CD31 staining in the DFO-treated groups (Fig. 6D), again suggesting better preservation of the original vascular endothelium. The percentage of CD31 staining tended to be greater for each group at 28 days than at 24 h (n=8 donors for CD31 at 28 days). To test whether DFO increased β-cell proliferation, cell cycle progression was studied. There was a trend toward a small increase in cells in the G2/M phase following DFO treatment of MIN6 cells (19.6 ± 0.3% vs. 18.1 ± 0.5%, p = 0.08). In primary mouse islets, DFO had no significant effect (9.3 ± 0.5% vs. 8.9 ± 0.5%).
Figure 6.
Mechanisms of DFO action at 28 days posttransplantation. (A) At 28 days posttransplantation, β-cell volume was significantly increased in DFO and DFO + Injections (DFO+I) transplants compared to controls. (B) A low-power image of a control graft at 28 days. (C) A low-power image of a DFO graft at 28 days. (D) CD31 staining was significantly greater at 28 days in the DFO-treated grafts than in the control grafts.
Gene Expression With HIF-1α Deletion and With DFO
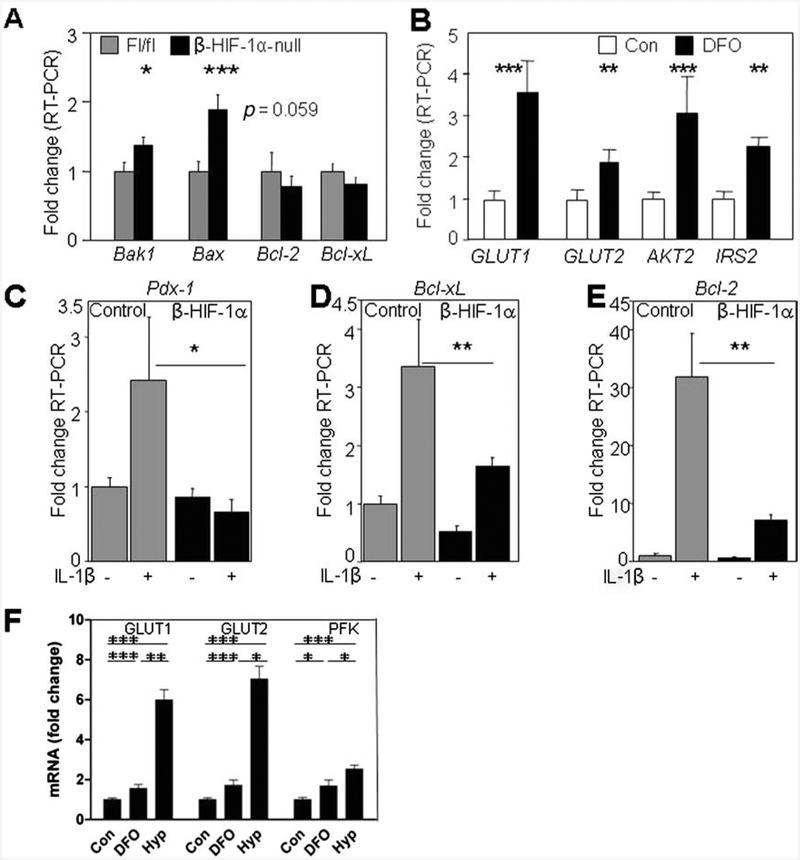
Consistent with the previously reported effects of HIF-1α in other tissues, β-HIF-1α-null islets had altered expression of apoptotic genes. There was increased expression of proapoptotic genes Bax and Bak (Fig. 7A) and a trend to decreased expression of the antiapoptotic Bcl-2.
Figure 7.
Gene expression changes with HIF-1α and DFO. (A) β-HIF-1α-null islets (black bars) showed an increased expression of the proapoptotic genes BCL-2 associated X protein (Bax) and BCL2-antagonist/killer 1 (Bak) and a trend to decreased expression of the antiapoptotic gene B-cell lymphoma 2 (Bcl-2) compared to controls (gray bars) (n = 6 per group). (B) Increased expression of glucose uptake genes, glucose transporter 1 (GLUT1) and GLUT2, and β-cell survival and function factors, v-akt murine thymoma viral oncogene homolog 2 (AKT2) and insulin receptor substrate 2 (IRS2), was present in human islets cultured with DFO (black bars) compared to control cultured islets (white bars). β-Cell survival genes (n = 6 per group) were induced by low-dose IL-1β, but the induction was impaired or absent in β-HIF-1α islets for (C) pancreatic and duodenal homeobox 1 (Pdxl), (D) B-cell lymphoma-extra large (Bcl-xl), and (E) Bcl2. (F) DFO and hypoxia both increased mRNA expression in human islets. *p< 0.05; **p < 0.01; ***p < 0.001.
Gene expression analysis of human islets cultured with or without DFO showed increased expression of the glucose uptake genes GLUT1 and GLUT2 and increased expression of the β-cell survival/function factors AKT2 and IRS2 (Fig. 7B).
To investigate the effect of the immune response on DFO-treated islets and HIF-1α function, isolated mouse islets were cultured with IL-1β. IL-1β is a classic cytokine that is increased in type 1 diabetes and the expression of which is increased in transplanted islets (31). Pdx1 (Fig. 7C) and the antiapoptotic genes Bcl-xL (Fig. 7D) and Bcl2 (Fig. 7E) were induced in the control islets, but this induction was impaired in the β-HIF-1α-null islets, and completely blocked for Pdx1. This suggests that HIF-1α may be important for the regulation of β-cell survival if inflammation develops posttransplantation.
We compared the effects of DFO and hypoxia on gene expression in human islets (Fig. 7F). As shown and consistent with our previous report, DFO increased the expression of Glut1, Glut2, and Pfk. Hypoxia caused significantly greater increases in these genes.
Early Addition of DFO to Human Islet Isolation Procedure
In all of the previous experiments, we tested adding DFO to the culture medium after completion of the isolation procedures. We then examined whether adding DFO immediately after the enzymatic digestion step would improve yield of viable islets. Forty-eight isolations without DFO were compared to 21 isolations with addition of DFO. The DFO isolation donors were nonsignificantly older and lighter. Both of those factors usually associate with lower yield. Despite this, islet yield was significantly greater in the DFO isolations at 4,313 ± 414 IEQ/g pancreas versus 2,438 ± 262 IEQ/g pancreas in control preparations, p = 0.0003. Viability was nonsignificantly higher in the DFO-treated group at 77 ± 2% versus 73 ± 2%.
DISCUSSION
At present, the only cures for people with type 1 diabetes are either whole-pancreas or islet cell transplantation. A normal pancreas is estimated to contain ~1 million islets (13,21,39). From a good islet isolation procedure, 500,000 islets may be purified. Many of these die before transplantation (20), and it is thought that 30–70% of transplanted islets die within 1 week (47,51). If starting with 1 million islets, this would leave an estimated 150,000–250,000 islets or 15–25% of the original number. In normal glucose tolerant individuals, >50% pancreatectomy frequently induces glucose intolerance or diabetes even in the relatively short term, with rates differing between studies: 27–41% (18), 86% (29), and 43% (25). Clearly, the smaller the surviving β-cell mass, the lower the likelihood of successful long-term outcomes, and this undoubtedly contributes to the gradual return to insulin therapy for many of the patients. This is likely to be exacerbated in the longer term by other factors including recurrent autoimmunity, but if insufficient islets are present at baseline, then eventual failure is more likely. Thus, there is an urgent need to improve β-cell survival in the peritransplant period so that long-term function can be improved.
Hypoxia is an inevitable result of devascularization followed by transplantation of islets. The HIF-1 transcription factor coordinates the cellular response to hypoxia. It is induced by stressors including hypoxia, cytokines, and reactive oxygen species. In these studies, we demonstrate that HIF-1α potentiates successful transplant outcome. It is likely that in the previous reports, where increased HIF-1α correlated with poor transplant outcomes, HIF-1α was induced by antecedent islet stress (27,30,32). This stress would be deleterious, and HIF-1α induction was a marker of that stress. The poor transplant outcomes for islets lacking β-cell HIF-1α demonstrated that the effect of HIF-1α is in fact a protective factor. The beneficial effects of DFO required the presence of HIF-1α in β-cells.
Short-term islet cell death after transplantation is predominantly due to apoptosis or necrosis. DFO increased glucose oxidation in islets at normoxia and in islets exposed to hypoxia and was associated with pronounced increase in ATP content. There was decreased apoptosis at 24 h posttransplantation and increased endothelial preservation in human islets at 24 h. At 28 days after transplantation, there was increased β-cell mass. Thus, the mechanism of improved glucose control following short-term treatment with DFO is decreased short-term β-cell loss, leading to greater β-cell mass and function at 28 days. DFO treatment pretransplantation increased mRNA expression and protein translation of protective factors prior to the insult at transplantation, providing improved energy supply following exposure to hypoxia.
In parallel, there was a >5-fold increase in islet endothelial staining at 24 h, with a significant increase persisting at 28 days. Whether loss of CD31 staining at 24 h is due to apoptosis or dedifferentiation, or their combination is unclear (1,38). There was a tendency to greater CD31 staining as a proportion of graft area in all groups at 28 days compared to 24 h, but the substantial difference persisted in the DFO-treated versus control transplants, suggesting that the initial peritrans-plant period is the most important determinant of native endothelial survival in transplants. Increased survival of donor endothelium would potentially improve revascularization. In addition, islet endothelium is highly specialized with increased density and fenestrations, which facilitates delivery of nutrients to islets and of insulin and other hormones to the circulation (1,17). Transgenic VEGF overexpression and deletion or knockdown of thrombospondin-1 both enhance islet vascularization (33,37), and rapamycin is known to inhibit revascularization (4). DFO is a simple drug treatment that increased islet transplant endothelial survival.
Lack of HIF-1α increased proapoptotic gene expression and decreased antiapoptotic genes in islets. Opposing gene expression changes with DFO are likely to have contributed to decreased apoptosis at 24 h, in combination with improved energy supply (ATP). Interestingly, HIF-1α was also needed for normal induction of antiapoptotic genes in the setting of exposure to interleukin-1β. The results are consistent with reports that DFO treatment improved outcomes for transplants into nonobese diabetic (NOD) mice and for allogeneic grafts (2,35) without appearing to alter immunological attack. These results provide a potential mechanism for those benefits.
In summary, HIF-1α is a protective factor in islet transplantation: it is required for successful islet transplant outcomes. Increasing HIF-1 a in a nontoxic manner using DFO improved outcomes for human islets transplanted into SCID mice. If the 50–60% increase in β-cell mass at 28 days translates into the human clinical situation, DFO may improve the proportion of patients who are able to be cured with single-donor transplants and the number of patients who are able to remain insulin-free posttrans-plantation. Since DFO is approved for human use and the doses used in our studies were in the range of the human therapeutic concentrations, DFO could be considered for use in human islet transplantation protocols.
ACKNOWLEDGMENTS:
This work was funded by the Juvenile Diabetes Research Foundation (JDRF), National Health and Medical Research Council (NHMRC) of Australia, and Diabetes Australia Research Trust (DART), and J.E.G was additionally funded by the L’Oreal Australian Women in Science Fellowship. We would like to thank Alice Boulghourjian from the histology core at Garvan, Biological Testing Facility (BTF) at the Garvan and the Animal Bioresources Centre (ABR) at Moss Vale for breeding and maintenance of our mice; Anita Patel and Lindy Williams from NPTU and Lina Mariana from St. Vincent’s Melbourne for preparation of islets. We would also like to thank Dr. Andrew Dwyer (Concord Hospital, Sydney) and Professors Donald Chisholm, Anthony Basten, and Robert Brink (all Garvan Institute) for helpful comments on the manuscript. R.A.S., K.C., N.D., S.M.L., WJ.H, PJ.O, T.L., T.W.K., F.J.G., and J.E.G. researched, discussed, wrote, and reviewed the paper. S.G. discussed the paper. H.T. and J.S. researched the paper. The authors declare no conflict of interest.
REFERENCES
- 1.Bonner-Weir S The microvasculature of the pancreas, with emphasis on that of the islets of Langerhans In: Shepro D, ed. Microvascular research. Amsterdam: Elsevier; 1986: 759–768. [Google Scholar]
- 2.Bradley B; Prowse SJ; Bauling P; Lafferty KJ Desferrioxamine treatment prevents chronic islet allograft damage. Diabetes 35(5):550–555; 1986. [DOI] [PubMed] [Google Scholar]
- 3.Calvani M; Rapisarda A; Uranchimeg B; Shoemaker RH; Melillo G Hypoxic induction of an HIF-1α-dependent bFGF autocrine loop drives angiogenesis in human endothelial cells. Blood 107(7):2705–2712; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cantaluppi V; Biancone L; Romanazzi GM; Figliolini F; Beltramo S; Ninniri MS; Galimi F; Romagnoli R; Franchello A; Salizzoni M Antiangiogenic and immunomodulatory effects of rapamycin on islet endothelium: Relevance for islet transplantation. Am. J. Transplant 6(11):2601–2611; 2006. [DOI] [PubMed] [Google Scholar]
- 5.Cheng K; Ho K; Stokes R; Scott C; Lau SM; Hawthorne WJ; O’Connell PJ; Loudovaris T; Kay T; Kulkarni RN; Okada T; Wang XL; Yim S; Shah YM; Grey S; Biankin A; Kench J; Laybutt DR; Gonzalez FJ; Kahn CR; Gunton JE Hypoxia-inducible factor-1a regulates β-cell function in mouse and human islets. J. Clin. Invest 120(6):2171–2183; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coulombe M; Gill RG The immunobiology of pancreatic islet transplantation. Adv. Exp. Med. Biol 552:154–169; 2004. [PubMed] [Google Scholar]
- 7.Cowley MJ; Weinberg A; Cantley J; Walters SN; Patel A; Jimenez-Vera E; Williams L; Gunton JE; Alexander SI; Kaplan W; Hawthorne WJ; O’Connell PJ; Grey SJ Human islets express a marked pro-inflammatory molecular signature prior to transplantation. Cell Transplant. 21(9):2063–2078; 2012. [DOI] [PubMed] [Google Scholar]
- 8.Deters NA; Stokes RA; Gunton JE Islet transplantation: Factors in short-term islet survival. Arch. Immunol. Ther. Exp 59(6):421–429; 2011. [DOI] [PubMed] [Google Scholar]
- 9.Dionne KE; Colton CK; Yarmush ML Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans. Diabetes 42(1):12–21; 1993. [DOI] [PubMed] [Google Scholar]
- 10.Emamaullee JA; Shapiro AM Factors influencing the loss of β-cell mass in islet transplantation. Cell Transplant. 16(1):1–8; 2007. [PubMed] [Google Scholar]
- 11.Faradji RN; Tharavanij T; Messinger S; Froud T; Pileggi A; Monroy K; Mineo D; Baidal DA; Cure P; Ponte G; Mendez AJ; Selvaggi G; Ricordi C; Alejandro R Long-term insulin independence and improvement in insulin secretion after supplemental islet infusion under exenatide and etanercept. Transplantation 86(12):1658–1665; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giuliani M; Moritz W; Bodmer E; Dindo D; Kugelmeier P; Lehmann R; Gassmann M; Groscurth P; Weber M Central necrosis in isolated hypoxic human pancreatic islets: Evidence for postisolation ischemia. Cell Transplant. 14(1):67–76; 2005. [DOI] [PubMed] [Google Scholar]
- 13.Gray H; Williams PL; Bannister LH Gray’s anatomy: The anatomical basis of medicine and surgery. London: Churchill Livingstone; 1995. [Google Scholar]
- 14.Gremlich S; Nolan C; Roduit R; Burcelin R; Peyot ML; Delghingaro-Augusto V; Desvergne B; Michalik L; Prentki M; Wahli W Pancreatic islet adaptation to fastingis dependent on peroxisome proliferator-activated receptor alpha transcriptional upregulation of fatty acid oxidation. Endocrinology 146:375–382; 2005. [DOI] [PubMed] [Google Scholar]
- 15.Guillemin K; Krasnow MA The hypoxic response: Huffing and HIFing. Cell 89(1):9–12; 1997. [DOI] [PubMed] [Google Scholar]
- 16.Gunton JE; Kulkarni RN; Yim S; Okada T; Hawthorne WJ; Tseng YH; Roberson RS; Ricordi C; O’Connell PJ; Gonzalez FJ; Kahn CR Loss of ARNT/HIF1beta mediates altered gene expression and pancreatic-islet dysfunction in human type 2 diabetes. Cell 122(3):337–349; 2005. [DOI] [PubMed] [Google Scholar]
- 17.Henderson J; Moss M A morphometric study of the endocrine and exocrine capillaries of the pancreas. Q. J. Exp. Physiol 70(3):347–356; 1985. [DOI] [PubMed] [Google Scholar]
- 18.Huang JJ; Yeo CJ; Sohn TA; Lillemoe KD; Sauter RN; Coleman J; Hruban RH; Cameron JL Quality of life and outcomes after pancreaticoduodenectomy. Ann. Surg 231(6):890–898; 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kebede M; Favaloro J; Gunton JE; Laybutt DR; Shaw M; Wong N; Fam BC; Aston-Mourney K; Rantzau C; Zulli A; Proietto J; Andrikopoulos S Fructose-1,6-bisphosphatase overexpression in pancreatic β-cells results in reduced insulin secretion: A new mechanism for fat-induced impairment of β-cell function. Diabetes 57(7):1887–1895; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kin T; Senior P; O’Gorman D; Richer B; Salam A; Shapiro AM Risk factors for islet loss during culture prior to transplantation. Transpl. Int 21(11):1029–1035; 2008. [DOI] [PubMed] [Google Scholar]
- 21.Lacy PE Status of islet cell transplantation. Diabetes Rev. 1:76–92; 1993. [Google Scholar]
- 22.Lee JW; Bae SH; Jeong JW; Kim SH; Kim KW Hypoxia-inducible factor (HIF-1α): Its protein stability and biological functions. Exp. Mol. Med 36:1–12; 2004. [DOI] [PubMed] [Google Scholar]
- 23.Lehmann R; Zuellig RA; Kugelmeier P; Baenninger PB; Moritz W; Perren A; Clavien PA; Weber M; Spinas GA Superiority of small islets in human islet transplantation. Diabetes 56(3):594–603; 2007. [DOI] [PubMed] [Google Scholar]
- 24.Levy AP; Levy NS; Wegner S; Goldberg MA Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J. Biol. Chem 270(22):13333–13340; 1995. [DOI] [PubMed] [Google Scholar]
- 25.Litwin J; Dobrowolski S; Orlowska-Kunikowska E; Sledzinski Z Changes in glucose metabolism after Kausch-Whipple pancreatectomy in pancreatic cancer and chronic pancreatitis patients. Pancreas 36(1):26–30; 2008. [DOI] [PubMed] [Google Scholar]
- 26.MacGregor RR; Williams SJ; Tong PY; Kover K; Moore WV; Stehno-Bittel L Small rat islets are superior to large islets in in vitro function and in transplantation outcomes. Am. J. Physiol. Endocrinol. Metab 290(5):E771–779; 2006. [DOI] [PubMed] [Google Scholar]
- 27.Marshall D; Sabek O; Fraga D; Kotb M; Gaber AO Examination of the molecular signature associated with islet dysfunction. Transplant. Proc 37(2):1311–1312; 2005. [DOI] [PubMed] [Google Scholar]
- 28.Maxwell PH; Wiesener MS; Chang GW; Clifford SC; Vaux EC; Cockman ME; Wykoff CC; Pugh CW; Maher ER; Ratcliffe PJ The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399(6733):271–275; 1999. [DOI] [PubMed] [Google Scholar]
- 29.Menge BA; Tannapfel A; Belyaev O; Drescher R; Muller C; Uhl W; Schmidt WE; Meier JJ Partial pancreatectomy in adult humans does not provoke β-cell regeneration. Diabetes 57(1):142–149; 2008. [DOI] [PubMed] [Google Scholar]
- 30.Miao G; Ostrowski RP; Mace J; Hough J; Hopper A; Peverini R; Chinnock R; Zhang J; Hathout E Dynamic production of hypoxia-inducible factor-1a in early transplanted islets. Am. J. Transplant 6(11):2636–2643; 2006. [DOI] [PubMed] [Google Scholar]
- 31.Montolio M; Biarnes M; Tellez N; Escoriza J; Soler J; Montanya E Interleukin-1beta and inducible form of nitric oxide synthase expression in early syngeneic islet transplantation. J. Endocrinol 192(1):169–177; 2007. [DOI] [PubMed] [Google Scholar]
- 32.Moritz W; Meier F; Stroka DM; Giuliani M; Kugelmeier P; Nett PC; Lehmann R; Candinas D; Gassmann M; Weber M Apoptosis in hypoxic human pancreatic islets correlated with HIF-1α expression. FASEB J. 16:745–747; 2002. [DOI] [PubMed] [Google Scholar]
- 33.Narang AS; Cheng K; Henry J; Zhang C; Sabek O; Fraga D; Kotb M; Gaber AO; Mahato RI Vascular endothelial growth factor gene delivery for revascularization in transplanted human islets. Pharm. Res 21(1):15–25; 2004. [DOI] [PubMed] [Google Scholar]
- 34.Noguchi H; Naziruddin B; Jackson A; Shimoda M; Ikemoto T; Fujita Y; Chujo D; Takita M; Kobayashi N; Onaca N; Levy MF; Matsumoto S Low-temperature preservation of isolated islets is superior to conventional islet culture before islet transplantation. Transplantation 89(1):47–54; 2010. [DOI] [PubMed] [Google Scholar]
- 35.Nomikos IN; Prowse SJ; Carotenuto P; Lafferty KJ Combined treatment with nicotinamide and desferrioxamine prevents islet allograft destruction in NOD mice. Diabetes 35(11):1302–1304; 1986. [DOI] [PubMed] [Google Scholar]
- 36.O’Connell PJ; Hawthorne WJ; Holmes-Walker J; Nankivell BJ; Gunton JE; Patel AT; Walters SN; Pleass HCC; Allen RDM; Chapman JR Clinical islet transplantation in type 1 diabetes mellitusL results of Australia’s first trial. Med. J. Aust 184(5):221–225; 2006. [DOI] [PubMed] [Google Scholar]
- 37.Olerud J; Johansson M; Lawler J; Welsh N; Carlsson PO Improved vascular engraftment and graft function after inhibition of the angiostatic factor thrombospondin-1 in mouse pancreatic islets. Diabetes 57(7):1870; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parr EL; Bowen KM; Lafferty KJ Cellular changes in cultured mouse thyroid glands and islets of Langerhans. Transplantation 30:135–141; 1980. [DOI] [PubMed] [Google Scholar]
- 39.Pipeleers D; Kiekens R; Ling Z; Wilikens A; Schuit F Physiologic relevance of heterogeneity in the pancreatic β-cell population. Diabetologia 37:57–64; 1994. [DOI] [PubMed] [Google Scholar]
- 40.Ponte GM; Pileggi A; Messinger S; Alejandro A; Ichii H; Baidal DA; Khan A; Ricordi C; Goss JA; Alejandro R Toward maximizing the success rates of human islet isolation: Influence of donor and isolation factors. Cell Transplant. 16(6):595–607; 2007. [DOI] [PubMed] [Google Scholar]
- 41.Ricordi C; Lacy PE; Finke EH; Olack BJ; Scharp DW Automated method for isolation of human pancreatic islets. Diabetes 37(4):413–420; 1988. [DOI] [PubMed] [Google Scholar]
- 42.Robertson RP Islet transplantation a decade later and strategies for filling a half-full glass. Diabetes 59(6):1285–1291; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robertson RP; Davis C; Larsen J; Stratta R; Sutherland DE Pancreas and islet transplantation for patients with diabetes. Diabetes Care 23(1):112–116; 2000. [DOI] [PubMed] [Google Scholar]
- 44.Ryan EA; Lakey JR; Paty BW; Imes S; Korbutt GS; Kneteman NM; Bigam D; Rajotte RV; Shapiro AM Successful islet transplantation: Continued insulin reserve provides long-term glycemic control. Diabetes 51(7):2148–2157; 2002. [DOI] [PubMed] [Google Scholar]
- 45.Semenza GL Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu. Rev. Cell. Dev. Biol 15:551–578; 1999. [DOI] [PubMed] [Google Scholar]
- 46.Semenza GL Oxygen homeostasis. Wiley Interdiscip. Rev. Syst. Biol. Med 2(3):336–361; 2010. [DOI] [PubMed] [Google Scholar]
- 47.Shapiro AM; Lakey JR; Ryan EA; Korbutt GS; Toth E; Warnock GL; Kneteman NM; Rajotte RV Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med 343(4):230–238; 2000. [DOI] [PubMed] [Google Scholar]
- 48.Shapiro AM; Ricordi C; Hering BJ; Auchincloss H; Lindblad R; Robertson RP; Secchi A; Brendel MD; Berney T; Brennan DC; Cagliero E; Alejandro R; Ryan EA; DiMercurio B; Morel P; Polonsky KS; Reems JA; Bretzel RG; Bertuzzi F; Froud T; Kandaswamy R; Sutherland DE; Eisenbarth G; Segal M; Preiksaitis J; Korbutt GS; Barton FB; Viviano L; Seyfert-Margolis V; Bluestone J; Lakey JR International trial of the Edmonton protocol for islet transplantation. N. Engl. J. Med 355(13):1318–1330; 2006. [DOI] [PubMed] [Google Scholar]
- 49.Sutherland DER; Gruessner R; Kandswamy R; Humar A; Hering B; Gruessner A β-Cell replacement therapy (pancreas and islet transplantation) for treatment of diabetes mellitus: An integrated approach. Transplant. Proc 36(6):1697–1699; 2004. [DOI] [PubMed] [Google Scholar]
- 50.Tomita S; Ueno M; Sakamoto M; Kitahama Y; Ueki M; Maekawa N; Sakamoto H; Gassmann M; Kageyama R; Ueda N; Gonzalez FJ; Takahama Y Defective brain development in mice lacking the HIF-1α gene in neural cells. Mol. Cell. Biol 23(19):6739–6749; 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toso C; Oberholzer J; Ris F; Triponez F; Bucher P; Demirag A; Andereggen E; Buehler L; Cretin N; Fournier B; Majno P; Hong Y; Lou J; Morel P Factors affecting human islet of Langerhans isolation yields. Transplant. Proc 34(3):826–827; 2002. [DOI] [PubMed] [Google Scholar]
- 52.Triantafyllou A; Liakos P; Tsakalof A; Georgatsou E; Simos G; Bonanou S Cobalt induces hypoxia-inducible factor-1α (HIF-1α) in HeLa cells by an iron-independent, but ROS-, PI-3K- and MAPK-dependent mechanism. Free Radic. Res 40(8):847–856; 2006. [DOI] [PubMed] [Google Scholar]
- 53.Walmsley SR; Print C; Farahi N; Peyssonnaux C; Johnson RS; Cramer T; Sobolewski A; Condliffe AM; Cowburn AS; Johnson N; Chilvers ER Hypoxia-induced neutrophil survival is mediated by HIF-1α-dependent NF-kappaB activity. J. Exp. Med 201(1):105–115; 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang W; Upshaw L; Strong DM; Robertson RP; Reems J Increased oxygen consumption rates in response to high glucose detected by a novel oxygen biosensor system in nonhuman primate and human islets. J. Endocrinol 185(3):445–455; 2005. [DOI] [PubMed] [Google Scholar]
- 55.Williams SJ; Huang HH; Kover K; Moore W; Berkland C; Singh M; Smirnova IV; Macgregor R; Stehno-Bittel L Reduction of diffusion barriers in isolated rat islets improves survival, but not insulin secretion or transplantation outcome. Organogenesis 6(2):115–124; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]