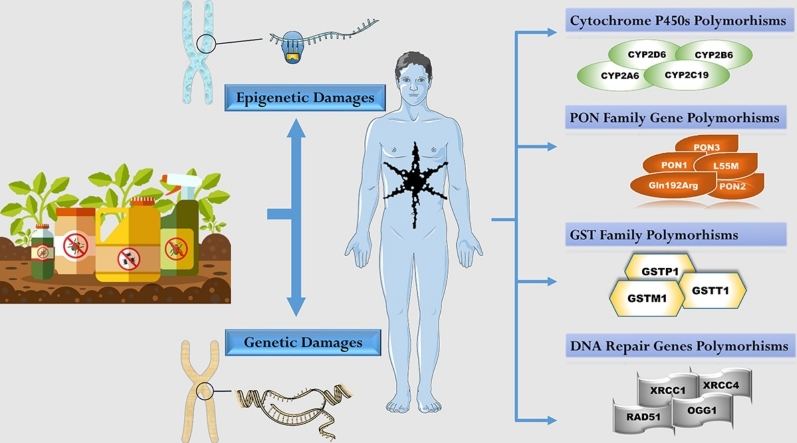
Graphical abstract
Keywords: Pesticides, Carcinogens, Genetic polymorphisms, Gene-environment interactions, Occupational health
Highlights
-
•
Genetic polymorphisms may influence pesticides-induced oxidative damage.Pesticides modulate immune-system cells functionality, leading to the onset of a dangerous pro-inflammatory microenvironment.
-
•
DNA repair genes, Cytochrome P450 s, PON and GST genes have a key role in the metabolism of xenobiotics.
-
•
Many workers are professionally exposed to pesticides with potential health consequences.
Abstract
Occupational and environmental exposure to pesticides may induce harmful effects on human health by promoting the development of a wide range of disorders. Some of the most recently hypothesized mechanisms are oxidative stress and epigenetic modifications, however biological effects seem to be modulated mainly by the occurrence of genetic polymorphisms. The susceptibility to exposure can be evaluated by studying the most common polymorphisms of genes involved in the metabolism of organophosphorus compounds (cytochrome P450, glutathione transferase, acetyltransferases or paraoxonase 1). The aim of this article is to review recent literature data concerning the influence of genetic polymorphisms on pesticides-induced oxidative damage.
1. Introduction
In the last decades, several epidemiological and observatio [15]. Although in recent years the environmental emission of these substances has been reduced and specific guidelines regulate their use, even today a significant fraction of the population is exposed to the risk of environmental pollutants, especially as regards direct and indirect exposure to pesticidesnal studies demonstrated that occupational and environmental exposure to several factors, including benzene, heavy metals, fibers, chemicals, and pesticides may induce detrimental effects on human health by promoting the development of a wide range of disorders, including infectious diseases, immunological diseases, reproductive and neurodegenerative diseases, hematological malignancies and solid tumors [[1], [2], [3], [4], [5]]. Although in recent years the environmental emission of these substances has been reduced and specific guidelines regulate their use, even today a significant fraction of the population is exposed to the risk of environmental pollutants, especially as regards direct and indirect exposure to pesticides [6].
The term pesticide includes a wide and miscellaneous category of chemicals conceived to prevent and defeat weeds or pests. Although their efficacy has contributed to the wide use, occupational and environmental exposure is a threat to the public health. Subjects professionally exposed to pesticides include farmers, gardeners and people working in the production, transportation and sales of these compounds. Some categories of workers (e.g. greenhouses workers) may be exposed to high concentrations of pesticides with potential health consequences [7,8].
Numerous studies confirm the hypotheses according to which exposure to pesticides can produce detrimental effect on respiratory or reproductive system and bring to the development of chronic diseases or cancer. Mutagenic mechanisms affecting oncogenes have been better investigated in vitro, although, also in humans, there is evidence of the association between exposure to pesticides and the presence of mutations in key genes responsible of cancer development and progression [9]. Still, it has been observed that the chromosome translocation t (14; 18) has a higher prevalence in farmers exposed to pesticides, with a greater risk of developing non-Hodgkin's lymphoma (NHL) [10]. Numerous pesticides have been classified by IARC in group 2A as probably carcinogenic to humans [11]. Acute exposure symptoms are easily identified, but the link between real exposure and the development of chronic pesticides-related illness is not easy to assess [5,[12], [13], [14], [15], [16]].
Recent studies have suggested oxidative stress as one of the mechanisms for the adverse health effects of pesticides exposure; the alteration of the physiological balance bring to the excess of oxidant species, resulting in severe damage to cellular components and macromolecules, especially the DNA [[17], [18], [19], [20], [21]]. Moreover, pesticides exposure may induce the modulation of cells functionality, especially that of the immune system (e.g. macrophages, lymphocytes, etc.), leading to the onset of a pro-inflammatory microenvironment responsible for the accumulation of genetic damages and, in turn, for the accumulation of altered cells that were not correctly removed by the immune system [22,23]. Indeed, it is well known that macrophages alterations, and in particular the abnormal secretion of several secreted proteins, play a key role in tumorigenesis and autoimmune diseases [[24], [25], [26], [27], [28]].
Furthermore, recent evidence showed that pesticides are able to induce both genetic and epigenetic alterations in the exposed individuals [29,30]. In particular, it was demonstrated that pesticides are associated to a wide range of genetic polymorphism affecting key genes involved in the regulation of cell cycle, the redox status and drug metabolism [31,32]. On the other hand, emerging evidence showed that pesticides may regulate gene expression through DNA methylation and microRNAs (miRNAs) de-regulation. On this matter, many bioinformatics data have been generated. Moreover, new computational approaches have been developed to identify in silico specific miRNAs or methylation hotspots associated with the presence of disease or to be used as bio-indicators of environmental exposure to pesticides [5,30,[33], [34], [35], [36], [37], [38], [39], [40], [41], [42]].
Other studies demonstrated that some pesticides may induce modifications in the composition of gut microbiota and that the microbiota itself may alterthe response of the organism and the metabolism of pesticides by enhancing or inhibiting the activity of cytochrome enzymes [43]. Of note, gut microbiota is involved in the regulation of several physiological and pathological processes, including cancer [44,45] and neurodegenerative diseases [46]. Therefore, there is raising evidence that modifications of microbiota induced by the exposure to pesticides may represent a trigger for the development of several diseases [47].
Although oxidative stress, epigenetic modifications and gut microbiota modulation represent key pathogenic mechanisms induced by pesticides exposure, their biological effects seem to be modulated mainly by the occurrence of genetic polymorphisms. In particular, some individuals could be more susceptible to pesticide-induced oxidative stress because of the influence of genetic polymorphisms. The susceptibility to exposure can be evaluated by studying the most common polymorphisms of cytochrome P450, of glutathione transferases (including GSTM1, GSTP1, GSTT1); of acetyltransferases (NAT2) and paraoxonases (mostly PON1), which are mainly involved in the metabolism of organophosphorus compounds [48]. In the last years, several Authors focused their attention on glutathione-s-transferases (GSTs) family and paraoxonase (PON1, PON2, and PON3) family and literature data are continually being updated in this field [[49], [50], [51], [52], [53], [54]].
Genetic heritage may increase susceptibility for the development of acute or chronic diseases, especially in subjects already exposed to other pollutants [52,[55], [56], [57]]. This article aims to review recent literature data concerning the influence of genetic polymorphisms on pesticides-induced oxidative damage.
2. Methods
The material for this article was collected by examining the literature available in the PubMed and Scopus database. A search was performed using in combination the following keywords: pesticides AND polymorphisms OR pesticides AND genetic AND polymorphisms. The research was based on original research work and review documents related to the topic published mainly in the last 10 years. The relevance of the subject and the eligibility of all publications detected was evaluated basing on titles and abstracts. The reference lists of selected articles were further screened for relevance. Non-English studies were not taken into consideration.
2.1. Polymorphisms
Genetic polymorphism is the presence in a population of two or more alleles in a particular locus with a frequency greater than 1%. Individual differences in response to xenobiotics may result from the presenceof certain polymorphisms in genes that encode DNA repairing enzymes or phase I/II detoxificationenzymes. The occurrence of some allelic variants can protect a subject exposed to the effects of xenobiotics, or on the contrary make him more vulnerable. The ability to repair DNA is closely correlated with genetic polymorphism in genes that encode DNA repair enzymes. Genetic polymorphisms could therefore be associated with different levels of risk within the general population, since the proteins encoded by the various genotypes alter the biotransformation of the substrates. The impact of genetic polymorphisms concerning the biotransformation mechanisms of OPs or other endogenous and exogenous factors is today the focus of the scientific debate, with a view to better understanding pesticide metabolism, highlighting interindividual differences in metabolizing particular pesticides. This could be the reason behind a differential toxicity to exposure than normally expected. Studies on more extensive samples, even differentiated for ethnic groups, would be useful in this sense.
2.2. Cytochrome P450s
Cytochrome P450s (CYPs) family genes encode proteins that catalyze the biotransformation of several xenobiotic. Environmental toxic chemicals and carcinogens, such as some pesticides, can be converted into bioactive compounds capable to exert their toxic or carcinogenic effects. CYPs are one of the main enzyme systems in xenobiotic metabolism, playing a key role in activation of many chemicals. On the other hand, biotransformation can lead to a complete detoxification of substances, to facilitate their excretion. Individual's capability to metabolize toxicants can be influenced by genetic polymorphisms of CYP enzymes. The study of variant alleles in CYP enzymes may uncover susceptibility biomarkersto environmental toxicity [58].
A recent study reviewed literature about correlation between exposure to organochlorines pesticides and CYP polymorphisms. The aryl hydrocarbon receptor pathway seems to be one mechanism of pathogenesis mediating induction of CYP metabolism. Variant alleles lead to altered metabolism of toxic compounds manifesting their genotoxic or carcinogenic potential [59].
In an Egyptian sample of 30 subjects exposed to OP, the association of the genetic polymorphisms of PON1 Q192R and CYP2D6 G1934A and the enzymatic activity of PON 1 and the pseudo-cholinesterase enzyme (PChE) was evaluated. The Authors found that serum PChE enzyme levels were significantly reduced in patients with chronic exposure; moreover, the CYP2D6 1934A allele was significantly increased in the same subjects. These changes were associated with symptoms such as fatigue and motor weakness, headaches, tearing, depression, paresthesia and sleep disorders [49].
Some authors determined the frequency of CYP2B6 and CYP2C19 polymorphisms in a cohort of pesticide applicators exposed to OP (chlorpyrifos), reporting a similar frequency with European and North American population. The prevalence of polymorphisms in enzymes involved in OP detoxification may facilitate the emergence of pathologies as a consequence of occupational exposure [60,61].
2.3. PON
PON family genes are located in region 21.3–22.1 (7q21.3–22.1) of the chromosome 7. The family include three proteins (PON1, PON2, PON3) exhibiting different activities. PON 1 plays a defense role against atherosclerosis due to its esterase and lipoprotein antioxidant activity. PON2 and PON 3 functions are less clear well than PON 1. They showed antioxidant and antiatherosclerotic properties like PON 1 [62].
In the liver PON1 hydrolyzes organophosphates, which have been previously activated by cytochrome P450; polymorphisms in PON1 can affect the efficacy of the response to DNA damage in individuals exposed to OP [63].
The PON1 192 Q(Gln)/R(Arg) polymorphism is responsible for a remarkable difference in the enzyme activity: the R variant has a higher paraoxonase activity, but it seems to be less efficient in protecting against LDL oxidation compared to PON1192 Q. Hypertension, coronary artery disease, stroke and Parkinson’s disease, have been associated with R genotype, while the Q variant seems to provide better health conditions [64].
A recent study investigated whether there was a correlation between DNA methylation patterns in blood cells, prenatal exposure to pesticides and PON1 Q192R genotypic expression focusing on cardio-metabolic diseases in children. The authors observed that the presence of PON1 192R allele, in exposed to pesticides was associated with a differentDNA methylation profile compared to subjects with a PON1 192QQ genotype and not exposed, assuming that DNA methylation may be one of the mechanisms responsible for the risk of cardio-metabolic events in children carrying the PON1 192R allele prenatally exposed to pesticides. The main limitation of this research is the non-identification of the individual pesticides to which the subjects were exposed [65].
In a Turkish sample of 54 subjects exposed to OP (Chlorpyrifos, Trichlorfon, Parathion and Malathion) the association of the genetic polymorphism of PON1 Q192R and the enzymatic activity of PON 1 and of the enzyme acetylcholinesterase (AChE) was evaluated. The Authors found the following genotypic frequencies of the PON1 192 Q / R polymorphism: 56.5% QQ, 34.2% QR and 9.3% of RR genotypes. Subjects with the PON1 192 R (+) genotype (QR + RR genotypes) showed a higher PON1 activity than the 192 R (-) (QQ) genotype. Then the activity of the AChE enzyme was investigated, showing a significant difference only in the group exposed to OP.Thus, the Authors conclude that the enzymatic activities of PON1 and AChE are different between exposed and not exposed, increasing in relation to the genotypic profile of the exposed subject [51]. Furthermore, a study carried out on placental villous samples showed that OPs are able to modulate the gene encoding the placental butyryl-cholinesterase (BChE), this up-regulation represent an adaptative response to environmental insults [66].
Another study evaluated the correlation between exposure to organophosphorus pesticides and the presence of PON1 L55 M in the context of Parkinson's disease (PD). High exposures have been associated with faster progression of motor, depressive and cognitive symptoms, also taking into accountthe distance from the site of application of the OPs. Previous study demonstrated a significant association between the L55 M polymorphism and the risk of developing PD after exposure to OP [67,68]. PON1 plays a key role in the metabolism of OPs but in detail it is not clear which is the mechanism underlying cognitive decline [69,70].
2.4. GST
Glutathione S-tranferases (GSTs) are enzymes involved in phase II drug metabolization participating in detoxification of xenobiotics or endogenous compounds. GSTs have a key role in the interaction between Glutathione (GSH) and the substrate. Genetic differences in expression and activity of these enzymes are due to polymorphic alleles. These polymorphisms alter GSTs activity and consequently susceptibility for toxic compounds [71].
A case-control study investigated the role of the presence of GSTM1 and GSTT1 polymorphisms in fetal growth restriction (FGR) with regard to organochlorine pesticides exposure. Organochlorine pesticides (OCPs) and oxidative stress are reported as associated with adverse reproductive outcomes. Since the glutathione S-transferase (GST) family acts as a detoxifier of numerous toxins, including organochlorine pesticides, prenatal exposure to OCPS can alter fetal growth. The authors found that the frequency of the GSTM1- / GSTT1- (null) genotype was significantly higher in FGR cases than in controls, suggesting that exposure to pesticides in pregnant women may be a risk factor for development of "idiopathic" FGR, a risk increased by the presence of the GST polymorphism [72]. A previous study had already highlighted the risk of exposure to organochlorine pesticides in women, increasing the biomarkers of oxidative stress and the risk of preterm birth [73].
A study conducted by Gómez-Martín et al. evaluated the influence of the polymorphisms PON1, GSTM1 and GSTT1 in relation to the levels of N7-methyldeoxyguanosine (N7-MedG), used as a DNA damage marker, in 36 farmers professionally exposed to pesticides. N7-MedG levels were higher during periods of exposure to high doses of pesticides compared to the period of least exposure. Furthermore, the Authors showed that the levels of erythrocyte acetylcholinesterase and plasma cholinesterase were significantly decreased during the period of high exposure. In conclusion, the increased exposure to pesticides increases the levels of DNA alkylation, as proof of the genotoxicity of pesticides in humans. Additionally, subjects with genotypes such as null for GSTM1 and PON1 192R allele appear to have a higher risk of genotoxic DNA damage [54].
Exposure to pesticides causes an alteration of oxidative stress biomarkers and this alteration is also affected by the genetic structure of individuals. About that, a study conducted in Brazil studied numerous polymorphisms (OGG1, XRCC1 and XRCC4, PON, GSTT1, GSTM1, GSTP1, CYP2A6, RAD51) in relation to markers of oxidative stress, remarking that agricultural operators exposed to pesticides showed an altered antioxidant enzymes activity, which in the long term can promote the onset of chronic diseases [74]. Same Authors in a more recent study highlighted the central role played by OGG1 and XRCC1 in the repair mechanisms in a sample of 121 exposed farmers compared to unexposed. The exposure concerned both pesticide mixtures and nicotine, since they are workers on tobacco plantations. Polymorphisms of PON1 and SOD2 can alter the process of metabolizing xenobiotics. Such a genetic background, in professionally exposed subjects, increases cellular damage and alters DNA repair capacity [75]. A further study conducted in Brazil underlined the importance of using PPE. In 120 pesticide-exposed blood samples of farmers Authors first, assessed the presence of GST polymorphisms comparing to 115 control samples, in relation to poisoning events after exposure. Subjects with the GSTM1 genotype showed more intoxication events after exposure to pesticides, although only 45% of them used PPE. However, this association was not statistically significant. The Authors therefore conclude by stating that although no statistical evidence was found between the presence of polymorphisms and the poisoning events caused by pesticides, an important finding emerged regarding the correct use of PPE in the prevention of such events [53].
Other studies have investigated the correlations between exposure to pesticides, presence of mutated GST alleles and specific pathologies: Siddarth et al. studied how polymorphisms of xenobiotic metabolizing enzymes increase pesticide accumulation aggravating renal dysfunction, demonstrated by a significant decrease in the estimated glomerular filtration rate [76]; a case-control study conducted by Sharma et al. suggested a possible correlation with higher risk to develop urinary bladder cancer [77]; Pinhel et al. suggested that GSTT1/GSTM1 polymorphisms could promote develop of Parkinson’s disease concurrently to pesticides exposure [78]; Fortes et al. describe GSTM1 null genotypeas a risk modifier for developing cutaneous melanoma [79]; moreover an Indian study showed that GSTT1 null genotype may represent a protection factor against coronary artery disease while, on the other hand, the GSTM1 null genotype can play a key role in the development of the disease as it results in a total absence of the activity of this detoxification mechanism [80]. All these studies reach similar conclusions, or that the combination of these two elements, genetic polymorphisms and pesticides exposure, significantly modifies the risk of development of many diseases.
2.5. DNA repair gene polymorphisms
The x-ray repair cross complementing group 1 (XRCC1) protein and the 8-oxoguanine glycosylase 1 (OGG1) genes are involved in Base Excision Repair (BER) pathway, playing a key role in repairing DNA damage caused by environmental factors. Mutation in XRCC1 is directly proportional with the number of chromosomal aberrations [81]. OGG1 is located on chromosome 3p25 and its inactivation is associated with a reduced production of cytokines and chemokines leading to acute inflammation, conferring resistance to organ damage [82].XRCC1 gene is located on chromosome 19q13.2, and interacting with OGG1 can increase its glycosylase activity [83]. Polymorphisms in these genes can significantly alter DNA damage fixing system.
Other authors evaluated the effect of the haplotypes of the PON1, XRCC1 and hOGG1 genes on DNA damage of lymphocytes collected from farmers. The results demonstrate effective DNA repair for people with the Gln/Gln (PON1) + Arg/Trp (XRCC1) phenotype. Furthermore, the evaluation of the PON1 and hOGG1 genes showed an effective repair for the Gln/Arg + Cys/Cys or Ser/Cys phenotypes, respectively. Both combinations of the polymorphic PON1 gene involved in the metabolism of xenobiotics and related genes encoding enzymes involved in BER-like repair can modulate DNA damage resulting from exposure to pesticides. These results indicate that metabolizing capacity should be considered along with DNA damage repairing pathways when evaluating the individual susceptibility after exposure to xenobiotics [84].
Another study conducted by Rohr et al. on lymphocytes from pesticide-exposed vineyard workers, evaluated both the separate and combined effects of polymorphisms in the PON1 (Gln192Arg), XRCC1 (Arg194Trp) and hOGG1 (Ser326Cys) genes on induction of lymphocyte damage. Authors showed significantly higher DNA damage in the exposed group when a less efficient OGG1Cys allele was present independently of the PON1 genotype. Analysis of the compared effect of XRCC1 and PON1 genotypes in the exposed group suggested that, among the poorly metabolizing PON1Gln/ Gln individuals, the XRCC1 Arg/Trp genotype has a protective effect with respect to MN formation. This indicates that enhanced XRCC1 function may provide some protection from the enhanced genotoxic risk associated with inefficient xenobiotic detoxification in the studied population.These results suggest the important role of enzyme polymorphism in response to DNA damage from pesticide exposure [64]. In contrast to these studies, other Authors denied the significant role of polymorphisms in DNA repair genes in pesticide-exposed populations. A significant increase in the presence of DNA damage in the cells of people exposed to pesticides was observed. However, no differences were found concerning hematological and lipid parameters or significant differences in DNA damage in relation to the different polymorphic variants of PON1, OGG1, XRCC1 and XRCC4 genes [85].
3. Conclusion
Genetic polymorphisms can alter the effects of individual exposure to xenobiotics such as pesticides. Further research on the variability of many DNA repair genes and their combinations is of priority importance to assess their actual role as determinants of pesticide toxicity. Studies about the interaction between DNA repair genes and xenobiotic metabolism genes could also be the key to better understand the pathogenesis of pesticide-induced diseases. A good knowledge of these complex mechanisms could prospectively lead to the development of screening tests that would allow large-scale detection of susceptibility to exposure to pesticides or other xenobiotics. Improvement of preventive actions aimed to protection of most vulnerable subjects in both occupational and environmental settings may be the goal for the near future (Table 1).
Table 1.
Genetic polymorphisms associated with human diseases in pesticide-exposed populations.
| Author | Polymorphic gene | Pesticides | Associated diseases | Ref. |
|---|---|---|---|---|
| Kahl et al., 2018 | PON1 / SOD2 / OGG1 / XRCC1 / XRCC4 | Pesticide mixtures (Carbamates, Organophosphates, Pyrethroids and Organochlorines) | Participants who presented a chronic health condition (diabetes, metabolic, kidney or heart diseases, and cancers), were excluded | [75] |
| Kahl et al. 2018 | PON1 / OGG1 / XRCC1 | Pesticides mixtures (Not defined) | Participants who presented a chronic health condition (diabetes, cardiovascular and neurological disease, cancer) were not included | [81] |
| Declerck et al. 2017 | PON | Not defined | Cardio-metabolic disease | [65] |
| Paul et al. 2017 | PON | Organophosphate | Parkinson’s Disease and related symptoms | [69] |
| Docea et al. 2017 | CYP | Organochlorine | Cancer/Infertility/Neurological disorders | [59] |
| Fortes et al. 2016 | GSTM1 / GSTT1 | Pyrethroid | Melanoma | [79] |
| Khattab et al. 2016 | PON1 / CYP2D6 | Organophosphates | fatigue and motor weakness/headaches/tearing / depression/paresthesia/sleep disorders | [49] |
| Adad et al. 2015 | PON1 / OGG1 / XRCC1 / XRCC4 | Organophosphates / Organic Copper / Triazines / Biological Insecticide (Abamectin) | Cance/Onco-hematological cancer | [85] |
| Gómez-Martín et al. 2015 | PON1 / GSTM1 / GSTT1 | Organophosphates (Chlorpyrifos, Dimetoate, Malathion, Pirimiphos-methyl, Fosmet, Fenamifos / N-methylcarbamates (methomyl, oxamyl, formetanate)/ Pyrethroids (tralomethrin, cypermethrin, acrynathrin)/Neonicotinoids (imidacloprid) / Abamectine/Dithiocarbamates fungicides (macozeb, zineb) | Data not available | [54] |
| Sunay et al. 2015 | PON1 | Organophosphates (Chlorpyrifos, Trichlorfon, Parathion and Malathion) | Cardiovascular diseases/Diabetes/Cancer | [51] |
| Godoy et al. 2014 | GSTM1 / GSTT1 | Glyphosate / 2,4-Dichlorophenoxyacetic acid / Parathion /Abamectin /Thiophanate methyl / Cyproconazole | Headache/nausea/dizziness/fainting/tremors/ excessive salivation convulsions/irritation/pain | [53] |
| Siddarth et al 2014 | GST | Organochlorine | Chronic Kidney Disease | [76] |
| Lee et al. 2013 | PON1 | Organophosphate | Parkinson’s Disease | [68] |
| Sharma et al. 2013 | GSTM1 / GSTT1 | Organochlorine | Urinary Bladder Cancer | [77] |
| Pinhel et al. 2013 | GSTM1 / GSTT1 | Not defined | Parkinson’s Disease | [78] |
| Sharma et al.2012 | GSTM1 / GSTT1 | Organochlorine | Fetal growth restriction | [72] |
| Da Silva et al. 2012 | OGG1 / XRCC1 / XRCC4 / PON / GSTT1 / GSTM1 / GSTP1 / CYP2A6 / RAD51 | Pesticides mixtures (Not defined) | Data not available | [74] |
| Ellison et al. 2012 | CYP2B6 / CYP2C19 | Organophosphate | Neurobehavioral deficits | [60] |
| Rohr et al. 2011 | PON1 / XRCC1 / OGG1 | Bipyridyl/Organophosphates/Copper Sulphate/Carbamates/Others | Coronary artery disease/Stroke/ Hypercholesterolemia/Parkinson’s disease/ Hypertension | [64] |
| Manthripragada et al. 2010 | PON1 | Organophosphate | Parkinson’s Disease | [67] |
| Rohr et al. 2006 | PON1 / XRCC1 / OGG1 | Not defined | Parkinson disease/Reproductive disorders | [84] |
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Fenga C., Gangemi S., Di Salvatore V., Falzone L., Libra M. Immunological effects of occupational exposure to lead. Mol. Med. Rep. 2017;15:3355–3360. doi: 10.3892/mmr.2017.6381. [DOI] [PubMed] [Google Scholar]
- 2.Garozzo A., Falzone L., Rapisarda V., Marconi A., Cinà D., Fenga C., Spandidos D.A., Libra M. The risk of HCV infection among health-care workers and its association with extrahepatic manifestations. Mol. Med. Rep. 2017;15:3336–3339. doi: 10.3892/mmr.2017.6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polo A., Crispo A., Cerino P., Falzone L., Candido S., Giudice A., De Petro G., Ciliberto G., Montella M., Budillon A., Costantini S. Environment and bladder cancer: molecular analysis by interaction networks. Oncotarget. 2017;8 doi: 10.18632/oncotarget.18222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rapisarda V., Salemi R., Marconi A., Loreto C., Graziano A.C., Cardile V., Basile M.S., Candido S., Falzone L., Spandidos D.A., Fenga C., Libra M. Fluoro-edenite induces fibulin-3 overexpression in non-malignant human mesothelial cells. Oncol. Lett. 2016;12:3363–3367. doi: 10.3892/ol.2016.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falzone L., Marconi A., Loreto C., Franco S., Spandidos D.A., Libra M. Occupational exposure to carcinogens: benzene, pesticides and fibers (Review) Mol. Med. Rep. 2016;14:4467–4474. doi: 10.3892/mmr.2016.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Babut M., Arts G.H., Barra Caracciolo A., Carluer N., Domange N., Friberg N., Gouy V., Grung M., Lagadic L., Martin-Laurent F., Mazzella N., Pesce S., Real B., Reichenberger S., Roex E.W.M., Romijn K., Röttele M., Stenrød M., Tournebize J., Vernier F., Vindimian E. Pesticide risk assessment and management in a globally changing world—report from a European interdisciplinary workshop. Environ. Sci. Pollut. Res. 2013;20:8298–8312. doi: 10.1007/s11356-013-2004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suratman S., Edwards J.W., Babina K. Organophosphate pesticides exposure among farmworkers: pathways and risk of adverse health effects. Rev. Environ. Health. 2015;30:65–79. doi: 10.1515/reveh-2014-0072. [DOI] [PubMed] [Google Scholar]
- 8.Kim K.-H., Kabir E., Jahan S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017;575:525–535. doi: 10.1016/j.scitotenv.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Vakonaki E., Androutsopoulos V.P., Liesivuori J., Tsatsakis A.M., Spandidos D.A. Pesticides and oncogenic modulation. Toxicology. 2013;307:42–45. doi: 10.1016/j.tox.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Qaqish B.M., Al-Dalahmah O., Al-Motassem Y., Battah A., Ismail S.S. Occupational exposure to pesticides and occurrence of the chromosomal translocation t(14;18) among farmers in Jordan. Toxicol. Rep. 2016;3:225–229. doi: 10.1016/j.toxrep.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guyton K.Z., Loomis D., Grosse Y., El Ghissassi F., Benbrahim-Tallaa L., Guha N., Scoccianti C., Mattock H., Straif K. Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. Lancet Oncol. 2015;16:490–491. doi: 10.1016/S1470-2045(15)70134-8. [DOI] [PubMed] [Google Scholar]
- 12.Gangemi S., Gofita E., Costa C., Teodoro M., Briguglio G., Nikitovic D., Tzanakakis G., Tsatsakis A.M., Wilks M.F., Spandidos D.A., Fenga C. Occupational and environmental exposure to pesticides and cytokine pathways in chronic diseases (Review) Int. J. Mol. Med. 2016;38:1012–1020. doi: 10.3892/ijmm.2016.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mamane A., Baldi I., Tessier J.-F., Raherison C., Bouvier G. Occupational exposure to pesticides and respiratory health. Eur. Respir. Rev. 2015;24:306–319. doi: 10.1183/16000617.00006014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parrón T., Requena M., Hernández A.F., Alarcón R. Environmental exposure to pesticides and cancer risk in multiple human organ systems. Toxicol. Lett. 2014;230:157–165. doi: 10.1016/j.toxlet.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Yu Y., Yang A., Zhang J., Hu S. Maternal exposure to the mixture of organophosphorus pesticides induces reproductive dysfunction in the offspring. Environ. Toxicol. 2013;28:507–515. doi: 10.1002/tox.20741. [DOI] [PubMed] [Google Scholar]
- 16.Rapisarda V., Ledda C., Matera S., Fago L., Arrabito G., Falzone L., Marconi A., Libra M., Loreto C. Absence of t(14;18) chromosome translocation in agricultural workers after short-term exposure to pesticides. Mol. Med. Rep. 2017;15:3379–3382. doi: 10.3892/mmr.2017.6385. [DOI] [PubMed] [Google Scholar]
- 17.Semren T.Ž., Žunec S., Pizent A. Oxidative stress in triazine pesticide toxicity: a review of the main biomarker findings. Arh. Hig. Rada Toksikol. 2018;69:109–125. doi: 10.2478/aiht-2018-69-3118. [DOI] [PubMed] [Google Scholar]
- 18.Wang X., Martínez M.-A., Dai M., Chen D., Ares I., Romero A., Castellano V., Martínez M., Rodríguez J.L., Martínez-Larrañaga M.-R., Anadón A., Yuan Z. Permethrin-induced oxidative stress and toxicity and metabolism. Int. Rev. Environ. Resour. Econ. 2016;149:86–104. doi: 10.1016/j.envres.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Jabłońska-Trypuć A., Wołejko E., Wydro U., Butarewicz A. The impact of pesticides on oxidative stress level in human organism and their activity as an endocrine disruptor. J. Environ. Sci. Health B. 2017;52:483–494. doi: 10.1080/03601234.2017.1303322. [DOI] [PubMed] [Google Scholar]
- 20.Wafa T., Nadia K., Amel N., Ikbal C., Insaf T., Asma K., Hedi M.A., Mohamed H. Oxidative stress, hematological and biochemical alterations in farmers exposed to pesticides. J. Environ. Sci. Health B. 2013;48:1058–1069. doi: 10.1080/03601234.2013.824285. [DOI] [PubMed] [Google Scholar]
- 21.Roede J.R., Uppal K., Park Y., Tran V., Jones D.P. Transcriptome–metabolome wide association study (TMWAS) of maneb and paraquat neurotoxicity reveals network level interactions in toxicologic mechanism. Toxicol. Rep. 2014;1:435–444. doi: 10.1016/j.toxrep.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Şekeroğlu Z.A., Aydın A., Yedier S.K., Şekeroğlu V. Cytogenetic alterations induced by flupyradifurone, a new butenolide insecticide, in human lymphocytes. Toxicol. Ind. Health. 2018;34:737–743. doi: 10.1177/0748233718788989. [DOI] [PubMed] [Google Scholar]
- 23.Helali I., Ferchichi S., Maaouia A., Aouni M., Harizi H. Modulation of macrophage functionality induced in vitro by chlorpyrifos and carbendazim pesticides. J. Immunotoxicol. 2016;13(5):745–750. doi: 10.1080/1547691X.2016.1181124. [DOI] [PubMed] [Google Scholar]
- 24.Presti M., Mazzon E., Basile M., Petralia M., Bramanti A., Colletti G., Bramanti P., Nicoletti F., Fagone P. Overexpression of macrophage migration inhibitory factor and functionally‑related genes, D‑DT, CD74, CD44, CXCR2 and CXCR4, in glioblastoma. Oncol. Lett. 2018;16(3):2881–2886. doi: 10.3892/ol.2018.8990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mangano K., Mazzon E., Basile M.S., Di Marco R., Bramanti P., Mammana S., Petralia M.C., Fagone P., Nicoletti F. Pathogenic role for macrophage migration inhibitory factor in glioblastoma and its targeting with specific inhibitors as novel tailored therapeutic approach. Oncotarget. 2018;9(25):17951–17970. doi: 10.18632/oncotarget.24885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mammana S., Fagone P., Cavalli E., Basile M., Petralia M., Nicoletti F., Bramanti P., Mazzon E. The role of macrophages in neuroinflammatory and neurodegenerative pathways of Alzheimer’s disease, amyotrophic lateral sclerosis, and multiple sclerosis: pathogenetic cellular effectors and potential therapeutic targets. Int. J. Mol. Sci. 2018;19:831. doi: 10.3390/ijms19030831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fenga C., Gangemi S., Catania S., De Luca A., Costa C. IL-17 and IL-22 serum levels in greenhouse workers exposed to pesticides. Inflamm. Res. 2014;63(11):895–897. doi: 10.1007/s00011-014-0769-6. [DOI] [PubMed] [Google Scholar]
- 28.Costa C., Rapisarda V., Catania S., Di Nola C., Ledda C., Fenga C. Cytokine patterns in greenhouse workers occupationally exposed to α-cypermethrin: an observational study. Environ. Toxicol. Pharmacol. 2013;36:796–800. doi: 10.1016/j.etap.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Gangemi S., Miozzi E., Teodoro M., Briguglio G., De Luca A., Alibrando C., Polito I., Libra M. Occupational exposure to pesticides as a possible risk factor for the development of chronic diseases in humans. Mol. Med. Rep. 2016;14:4475–4488. doi: 10.3892/mmr.2016.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hou L., Zhang X., Wang D., Baccarelli A. Environmental chemical exposures and human epigenetics. Int. J. Epidemiol. 2012;41:79–105. doi: 10.1093/ije/dyr154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ritz B.R., Paul K.C., Bronstein J.M. Of pesticides and men: a California story of genes and environment in Parkinson’s disease. Curr. Environ. Health Rep. 2016;3:40–52. doi: 10.1007/s40572-016-0083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koutros S., Andreotti G., Berndt S.I., Hughes Barry K., Lubin J.H., Hoppin J.A., Kamel F., Sandler D.P., Burdette L.A., Yuenger J., Yeager M., Alavanja M.C.R., Freeman L.E.B. Xenobiotic-metabolizing gene variants, pesticide use, and the risk of prostate cancer. Pharmacogenet. Genomics. 2011;21:615–623. doi: 10.1097/FPC.0b013e3283493a57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Falzone Lupo, Rosa Crimi, Anfuso Salemi, Rapisarda Libra. Candido, identification of novel MicroRNAs and their diagnostic and prognostic significance in oral cancer. Cancers (Basel) 2019;11:610. doi: 10.3390/cancers11050610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sisto R., Capone P., Cerini L., Sanjust F., Paci E., Pigini D., Gatto M.P., Gherardi M., Gordiani A., L’Episcopo N., Tranfo G., Chiarella P. Circulating microRNAs as potential biomarkers of occupational exposure to low dose organic solvents. Toxicol. Rep. 2019;6:126–135. doi: 10.1016/j.toxrep.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Falzone L., Romano G., Salemi R., Bucolo C., Tomasello B., Lupo G., Anfuso C., Spandidos D., Libra M., Candido S. Prognostic significance of deregulated microRNAs in uveal melanomas. Mol. Med. Rep. 2019;19(4):2599–2610. doi: 10.3892/mmr.2019.9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Battaglia R., Palini S., Vento M.E., La Ferlita A., Lo Faro M.J., Caroppo E., Borzì P., Falzone L., Barbagallo D., Ragusa M., Scalia M., D’Amato G., Scollo P., Musumeci P., Purrello M., Gravotta E., Di Pietro C. Identification of extracellular vesicles and characterization of miRNA expression profiles in human blastocoel fluid. Sci. Rep. 2019;9:84. doi: 10.1038/s41598-018-36452-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basile M.S., Fagone P., Mangano K., Mammana S., Magro G., Salvatorelli L., Li Destri G., La Greca G., Nicoletti F., Puleo S., Pesce A. KCNMA1 expression is downregulated in colorectal cancer via epigenetic mechanisms. Cancers (Basel) 2019;11:245. doi: 10.3390/cancers11020245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Falzone L., Scola L., Zanghì A., Biondi A., Di Cataldo A., Libra M., Candido S. Integrated analysis of colorectal cancer microRNA datasets: identification of microRNAs associated with tumor development. Aging (Albany. NY) 2018;10:1000–1014. doi: 10.18632/aging.101444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCubrey J.A., Fitzgerald T.L., Yang L.V., Lertpiriyapong K., Steelman L.S., Abrams S.L., Montalto G., Cervello M., Neri L.M., Cocco L., Martelli A.M., Laidler P., Dulińska-Litewka J., Rakus D., Gizak A., Nicoletti F., Falzone L., Candido S., Libra M. Roles of GSK-3 and microRNAs on epithelial mesenchymal transition and cancer stem cells. Oncotarget. 2017;8 doi: 10.18632/oncotarget.13991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Falzone L., Candido S., Salemi R., Basile M.S., Scalisi A., McCubrey J.A., Torino F., Signorelli S.S., Montella M., Libra M. Computational identification of microRNAs associated to both epithelial to mesenchymal transition and NGAL/MMP-9 pathways in bladder cancer. Oncotarget. 2016;7 doi: 10.18632/oncotarget.11805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vrijens K., Bollati V., Nawrot T.S. MicroRNAs as potential signatures of environmental exposure or effect: a systematic review. Environ. Health Perspect. 2015;123:399–411. doi: 10.1289/ehp.1408459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hafsi S., Candido S., Maestro R., Falzone L., Soua Z., Bonavida B., Spandidos D.A., Libra M. Correlation between the overexpression of Yin Yang 1 and the expression levels of miRNAs in Burkitt’s lymphoma: a computational study. Oncol. Lett. 2016;11:1021–1025. doi: 10.3892/ol.2015.4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Q., Shao W., Zhang C., Xu C., Wang Q., Liu H., Sun H., Jiang Z., Gu A. Organochloride pesticides modulated gut microbiota and influenced bile acid metabolism in mice. Environ. Pollut. 2017;226:268–276. doi: 10.1016/j.envpol.2017.03.068. [DOI] [PubMed] [Google Scholar]
- 44.Vivarelli S., Salemi R., Candido S., Falzone L., Santagati M., Stefani S., Torino F., Banna G., Tonini G., Libra M. Gut microbiota and cancer: from pathogenesis to therapy. Cancers (Basel) 2019;11:38. doi: 10.3390/cancers11010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Banna G.L., Torino F., Marletta F., Santagati M., Salemi R., Cannarozzo E., Falzone L., Ferraù F., Libra M. Lactobacillus rhamnosus GG: an overview to explore the rationale of its use in cancer. Front. Pharmacol. 2017;8 doi: 10.3389/fphar.2017.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roy Sarkar S., Banerjee S. Gut microbiota in neurodegenerative disorders. J. Neuroimmunol. 2019;328:98–104. doi: 10.1016/j.jneuroim.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 47.Liang Y., Zhan J., Liu D., Luo M., Han J., Liu X., Liu C., Cheng Z., Zhou Z., Wang P. Organophosphorus pesticide chlorpyrifos intake promotes obesity and insulin resistance through impacting gut and gut microbiota. Microbiome. 2019;7:19. doi: 10.1186/s40168-019-0635-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kapka-Skrzypczak L., Cyranka M., Skrzypczak M., Kruszewski M. Biomonitoring and biomarkers of organophosphate pesticides exposure – state of the art. Ann. Agric. Environ. Med. 2011;18:294–303. [PubMed] [Google Scholar]
- 49.Tawfik Khattab A.M., Zayed A.A., Ahmed A.I., AbdelAal A.G., Mekdad A.A. The role of PON1 and CYP2D6 genes in susceptibility to organophosphorus chronic intoxication in Egyptian patients. Neurotoxicology. 2016;53:102–107. doi: 10.1016/j.neuro.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 50.Hollman A., Tchounwou P., Huang H.-C. The association between gene-environment interactions and diseases involving the human GST superfamily with SNP variants. Int. J. Environ. Res. Public Health. 2016;13:379. doi: 10.3390/ijerph13040379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sunay S.Z., Kayaaltı Z., Bayrak T., Söylemezoğlu T. Effect of paraoxonase 1 192 Q/R polymorphism on paraoxonase and acetylcholinesterase enzyme activities in a Turkish population exposed to organophosphate. Toxicol. Ind. Health. 2015;31:1061–1068. doi: 10.1177/0748233713487246. [DOI] [PubMed] [Google Scholar]
- 52.Kaur G., Jain A.K., Singh S. CYP/PON genetic variations as determinant of organophosphate pesticides toxicity. J. Genet. 2017;96:187–201. doi: 10.1007/s12041-017-0741-7. http://www.ncbi.nlm.nih.gov/pubmed/28360405 [DOI] [PubMed] [Google Scholar]
- 53.Godoy F.R., Costa E.O.A., da Silva Reis A.A., Batista M.P., de Melo A.V., Gonçalves M.W., Cruz A.S., de Araújo Melo C.O., Minasi L.B., Ribeiro C.L., da Cruz A.D., de Melo e Silva D. Do GSTT1 and GSTM1 polymorphisms influence intoxication events in individuals occupationally exposed to pesticides? Environ. Sci. Pollut. Res. 2014;21:3706–3712. doi: 10.1007/s11356-013-2349-7. [DOI] [PubMed] [Google Scholar]
- 54.Gómez-Martín A., Altakroni B., Lozano-Paniagua D., Margison G.P., de Vocht F., Povey A.C., Hernández A.F. Increased N7-methyldeoxyguanosine DNA adducts after occupational exposure to pesticides and influence of genetic polymorphisms of paraoxonase-1 and glutathione S -transferase M1 and T1. Environ. Mol. Mutagen. 2015;56:437–445. doi: 10.1002/em.21929. [DOI] [PubMed] [Google Scholar]
- 55.Costa C., Gangemi S., Giambo F., Rapisarda V., Caccamo D., Fenga C. Oxidative stress biomarkers and paraoxonase 1 polymorphism frequency in farmers occupationally exposed to pesticides. Mol. Med. Rep. 2015;12:6353–6357. doi: 10.3892/mmr.2015.4196. [DOI] [PubMed] [Google Scholar]
- 56.Costa C., Miozzi E., Teodoro M., Fenga C. Influence of genetic polymorphism on pesticide-induced oxidative stress. Curr. Opin. Toxicol. 2019;13:1–7. [Google Scholar]
- 57.Saad-Hussein A., Noshy M., Taha M., El-Shorbagy H., Shahy E., Abdel-Shafy E.A. GSTP1 and XRCC1 polymorphisms and DNA damage in agricultural workers exposed to pesticides. Mutat. Res. Toxicol. Environ. Mutagen. 2017;819:20–25. doi: 10.1016/j.mrgentox.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 58.Elfaki I., Mir R., Almutairi F.M., Duhier F.M.A. Cytochrome P450: polymorphisms and roles in cancer, diabetes and atherosclerosis. Asian Pac. J. Cancer Prev. 2018;19:2057–2070. doi: 10.22034/APJCP.2018.19.8.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Docea A.O., Vassilopoulou L., Fragou D., Arsene A.L., Fenga C., Kovatsi L., Petrakis D., Rakitskii V.N., Nosyrev A.E., Izotov B.N., Golokhvast K.S., Zakharenko A.M., Vakis A., Tsitsimpikou C., Drakoulis N. CYP polymorphisms and pathological conditions related to chronic exposure to organochlorine pesticides. Toxicol. Rep. 2017;4:335–341. doi: 10.1016/j.toxrep.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ellison C.A., Abou El-Ella S.S., Tawfik M., Lein P.J., Olson J.R. Allele and genotype frequencies of CYP2B6 and CYP2C19 polymorphisms in egyptian agricultural workers. J. Toxicol. Environ. Health Part A. 2012;75:232–241. doi: 10.1080/15287394.2012.641201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Costa C., Silvari V., Melchini A., Catania S., Heffron J.J., Trovato A., De Pasquale R. Genotoxicity of imidacloprid in relation to metabolic activation and composition of the commercial product. Mutat. Res. Toxicol. Environ. Mutagen. 2009;672:40–44. doi: 10.1016/j.mrgentox.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 62.Ginsberg G., Neafsey P., Hattis D., Guyton K.Z., Johns D.O., Sonawane B. Genetic polymorphism in paraoxonase 1 (PON1): population distribution of PON1 activity. J. Toxicol. Environ. Health B Crit. Rev. 2009;12:473–507. doi: 10.1080/10937400903158409. [DOI] [PubMed] [Google Scholar]
- 63.Costa L.G., Cole T.B., Furlong C.E. Polymorphisms of paraoxonase (PON1) and their significance in clinical toxicology of organophosphates. J. Toxicol. Clin. Toxicol. 2003;41:37–45. doi: 10.1081/clt-120018269. http://www.ncbi.nlm.nih.gov/pubmed/12645966 [DOI] [PubMed] [Google Scholar]
- 64.Rohr P., da Silva J., Erdtmann B., Saffi J., Guecheva T.N., Antônio Pêgas Henriques J., Kvitko K. BER gene polymorphisms (OGG1 Ser326Cys and XRCC1 Arg194Trp) and modulation of DNA damage due to pesticides exposure. Environ. Mol. Mutagen. 2011;52:20–27. doi: 10.1002/em.20562. [DOI] [PubMed] [Google Scholar]
- 65.Declerck K., Remy S., Wohlfahrt-Veje C., Main K.M., Van Camp G., Schoeters G., Vanden Berghe W., Andersen H.R. Interaction between prenatal pesticide exposure and a common polymorphism in the PON1 gene on DNA methylation in genes associated with cardio-metabolic disease risk—an exploratory study. Clin. Epigenetics. 2017;9:35. doi: 10.1186/s13148-017-0336-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sánchez S., Vera B., Montagna C., Magnarelli G. Characterization of placental cholinesterases and activity induction associated to environmental organophosphate exposure. Toxicol. Rep. 2015;2:437–442. doi: 10.1016/j.toxrep.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Manthripragada A.D., Costello S., Cockburn M.G., Bronstein J.M., Ritz B. Paraoxonase 1, agricultural organophosphate exposure, and Parkinson disease. Epidemiology. 2010;21:87–94. doi: 10.1097/EDE.0b013e3181c15ec6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee P.-C., Rhodes S.L., Sinsheimer J.S., Bronstein J., Ritz B. Functional paraoxonase 1 variants modify the risk of Parkinson’s disease due to organophosphate exposure. Environ. Int. 2013;56:42–47. doi: 10.1016/j.envint.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Paul K.C., Sinsheimer J.S., Cockburn M., Bronstein J.M., Bordelon Y., Ritz B. Organophosphate pesticides and PON1 L55M in Parkinson’s disease progression. Environ. Int. 2017;107:75–81. doi: 10.1016/j.envint.2017.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Androutsopoulos V.P., Kanavouras K., Tsatsakis A.M. Role of paraoxonase 1 (PON1) in organophosphate metabolism: implications in neurodegenerative diseases. Toxicol. Appl. Pharmacol. 2011;256:418–424. doi: 10.1016/j.taap.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 71.Ginsberg G., Smolenski S., Hattis D., Guyton K.Z., Johns D.O., Sonawane B. Genetic polymorphism in glutathione transferases (GST): population distribution of GSTM1, T1, and P1 conjugating activity. J. Toxicol. Environ. Health Part B. 2009;12:389–439. doi: 10.1080/10937400903158375. [DOI] [PubMed] [Google Scholar]
- 72.Sharma E., Mustafa M., Pathak R., Guleria K., Ahmed R.S., Vaid N.B., Banerjee B.D. A case control study of gene environmental interaction in fetal growth restriction with special reference to organochlorine pesticides. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012;161:163–169. doi: 10.1016/j.ejogrb.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 73.Pathak R., Suke S.G., Ahmed T., Ahmed R.S., Tripathi A., Guleria K., Sharma C., Makhijani S., Banerjee B. Organochlorine pesticide residue levels and oxidative stress in preterm delivery cases. Hum. Exp. Toxicol. 2010;29:351–358. doi: 10.1177/0748233710363334. [DOI] [PubMed] [Google Scholar]
- 74.Da Silva F.R., Da Silva J., Allgayer Mda C., Simon C.F., Dias J.F., dos Santos C.E.I., Salvador M., Branco C., Schneider N.B., Kahl V., Rohr P., Kvitko K. Genotoxic biomonitoring of tobacco farmers: biomarkers of exposure, of early biological effects and of susceptibility. J. Hazard. Mater. 2012;225–226:81–90. doi: 10.1016/j.jhazmat.2012.04.074. [DOI] [PubMed] [Google Scholar]
- 75.Kahl V.F.S., da Silva F.R., Alves Jda S., da Silva G.F., Picinini J., Dhillon V.S., Fenech M., de Souza M.R., Dias J.F., de Souza C.T., Salvador M., Branco Cdos S., Thiesen F.V., Simon D., da Silva J. Role of PON1, SOD2, OGG1, XRCC1, and XRCC4 polymorphisms on modulation of DNA damage in workers occupationally exposed to pesticides. Ecotoxicol. Environ. Saf. 2018;159:164–171. doi: 10.1016/j.ecoenv.2018.04.052. [DOI] [PubMed] [Google Scholar]
- 76.Siddarth M., Datta S.K., Mustafa M., Ahmed R.S., Banerjee B.D., Kalra O.P., Tripathi A.K. Increased level of organochlorine pesticides in chronic kidney disease patients of unknown etiology: Role of GSTM1/GSTT1 polymorphism. Chemosphere. 2014;96:174–179. doi: 10.1016/j.chemosphere.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 77.Sharma T., Jain S., Verma A., Sharma N., Gupta S., Arora V.K., Dev Banerjee B. Gene environment interaction in urinary bladder cancer with special reference to organochlorine pesticide: a case control study. Cancer Biomark. 2013;13:243–251. doi: 10.3233/CBM-130346. [DOI] [PubMed] [Google Scholar]
- 78.de S. Pinhel M.A., Sado C.L., dos S. Longo G., Gregorio M.L., Amorim G.S., da S. Florim G.M., Mazeti C.M., Martins D.P., de N. Oliveira F., Nakazone M.A., Tognola W.A., Souza D.R.S. Nullity of GSTT1/GSTM1 related to pesticides is associated with Parkinson’s disease. Arq. Neuropsiquiatr. 2013;71:527–532. doi: 10.1590/0004-282X20130076. [DOI] [PubMed] [Google Scholar]
- 79.Fortes C., Mastroeni S., Bottà G., Boffetta P., Antonelli G., Venanzetti F. Glutathione S-transferase M1 null genotype, household pesticides exposure and cutaneous melanoma. Melanoma Res. 2016;26:625–630. doi: 10.1097/CMR.0000000000000295. [DOI] [PubMed] [Google Scholar]
- 80.Bhat M.A., Gandhi G. Association of GSTT1 and GSTM1 gene polymorphisms with coronary artery disease in North Indian Punjabi population: a case-control study. Postgrad. Med. J. 2016;92:701–706. doi: 10.1136/postgradmedj-2015-133836. [DOI] [PubMed] [Google Scholar]
- 81.Kahl V.F.S., Simon D., de Souza M.R., da Rosa V.H., Nicolau C., Da Silva F.R., Kvitko K., Peres A., Dorneles G.P., de Souza C.T., Dias J.F., Da Silva J. Base excision repair (OGG1 and XRCC1) and metabolism (PON1) gene polymorphisms act on modulation of DNA damage and immune parameters in tobacco farmers. Mutat. Res. Toxicol. Environ. Mutagen. 2018;836:9–18. doi: 10.1016/j.mrgentox.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 82.Mabley J.G., Pacher P., Deb A., Wallace R., Elder R.H., Szabó C. Potential role for 8-oxoguanine DNA glycosylase in regulating inflammation. FASEB J. 2005;19:290–292. doi: 10.1096/fj.04-2278fje. [DOI] [PubMed] [Google Scholar]
- 83.Marsin S., Vidal A.E., Sossou M., Murcia J.M., Le Page F., Boiteux S., de Murcia G., Radicella J.P. Role of XRCC1 in the coordination and stimulation of oxidative DNA damage repair initiated by the DNA glycosylase hOGG1. J. Biol. Chem. 2003;278:44068–44074. doi: 10.1074/jbc.M306160200. [DOI] [PubMed] [Google Scholar]
- 84.Rohr P., da Silva J., Erdtmann B., Henriques J.A.P., Kvitko K. The BER pathway genes and PON1 polymorphism: influence on DNA damage in agriculture exposed workers. Theoria. 2006;15:69–77. [Google Scholar]
- 85.de M. Adad L.M., de Andrade H.H.R., Kvitko K., Lehmann M., de C.M. Cavalcante A.A., Dihl R.R. Occupational exposure of workers to pesticides: toxicogenetics and susceptibility gene polymorphisms. Genet. Mol. Biol. 2015;38:308–315. doi: 10.1590/S1415-475738320140336. [DOI] [PMC free article] [PubMed] [Google Scholar]