Summary
The significance of intracellular Ap4A levels over immune activity of dendritic cells (DCs) has been studied in Nudt2fl/fl/CD11c-cre mice. The transgenic mice have been generated by crossing floxed NUDT2 gene mice with DC marker CD11c recombinase (cre) mice. The DCs derived from these mice have higher levels of Ap4A (≈30-fold) compared with those derived from Nudt2+/+ mice. Interestingly, the elevated Ap4A in DCs has led them to possess higher motility and lower directional variability. In addition, the DCs are able to enhance immune protection indicated by the higher cross-presentation of antigen and priming of CD8+ OT-I T cells. Overall, the study denotes prominent impact of Ap4A over the functionality of DCs. The Nudt2fl/fl/CD11c-cre mice could serve as a useful tool to study the influence of Ap4A in the critical immune mechanisms of DCs.
Subject Areas: Molecular Mechanism of Behavior, Immunology, Immune Response, Model Organism
Graphical Abstract
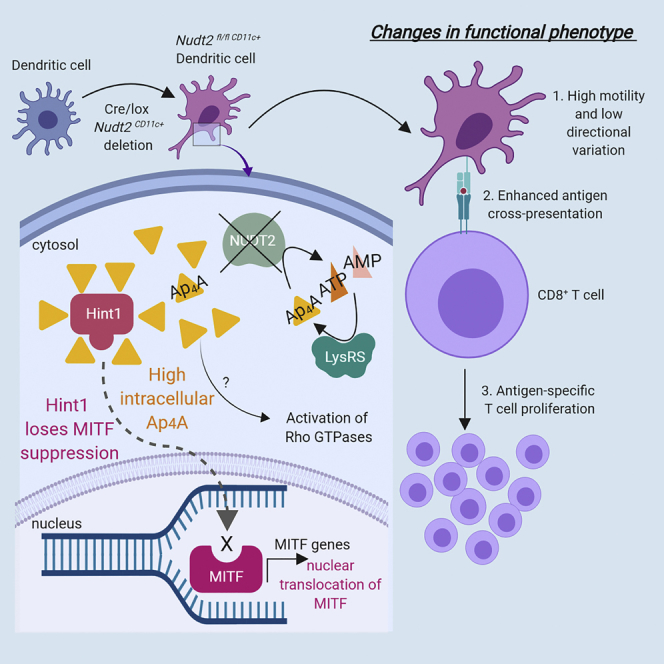
Highlights
-
•
DCs of Nudt2fl/fl/CD11c-cre mice exhibit low directional variability and high motility
-
•
DCs elevate proliferation of OVA-specific T cell receptor transgenic CD8+ T cells
-
•
The escalation of Ap4A levels in DCs could enhance their immune protective activity
-
•
Mice can serve as useful functional tool to study the role of Ap4A in various cells
Molecular Mechanism of Behavior; Immunology; Immune Response; Model Organism
Introduction
Transfer ribonucleic acid (tRNA) synthetases play an important role in the central dogma of molecular biology. The specific function of tRNA synthetases is to conjugate tRNAs with the cognate amino acid for correct translation of polypeptides from mRNA. Progressively, highly conserved and non-canonical activities of tRNA synthetases that are unique for each amino acid-charging tRNA synthetase have been discovered. Lysyl-tRNA synthetase (LysRS), a tRNA that charges lysine onto lysine-tRNA for use in ribosome for translation, have also been serving an evolutionarily conserved, non-canonical enzymatic activity to produce diadenosine tetraphosphate (Ap4A), a small signaling molecule composed of two adenosine moieties joined through a 5′-5′ linkage by a chain of four phosphates. This non-canonical pathway in LysRS is triggered by the phosphorylation of LysRS on serine 207 (P-s207 LysRS) via p38 mitogen-activated protein kinase activity. Phosphorylation leads to the dissociation of P-s207 LysRS from multi-synthetase complex (MSC) and promotes the production of Ap4A (Ofir-Birin et al., 2013). In turn, the synthesis of Ap4A is regulated by the housekeeping protein Ap4A hydrolase (Ap4AHy) that converts Ap4A back into its original building blocks (one molecule of ATP and one molecule of AMP) thereby creating a regulatory feedback to maintain intracellular Ap4A levels (Vollmayer et al., 2003).
Ap4A synthesis activity by LysRS can directly control specific response programming in immune-specialized cells (Nechushtan et al., 2009). Our group has previously demonstrated that non-canonical LysRS activity can drive increased intracellular Ap4A and control USF2 transcriptional activity, which up-regulates transforming growth factor-β2 in FcepsilonRI-activated mast cells (Lee and Razin, 2005). Ap4A can enhance phorbol myristate acetate (PMA)-stimulated reactive oxygen species production in lymphocytes (Schepers et al., 2010) and has been implicated in key immunological responses (Carracedo et al., 2013, Castany et al., 2011, Chang et al., 2014, Louie et al., 1988). Another pathway that is driven by the increase of intracellular Ap4A is the activation of microphthalmia-associated transcription factor (MITF), a master regulator in melanocyte development (Levy et al., 2006). Hint1, a co-suppressor of MITF, is also inactivated by high intracellular concentration of Ap4A and dissociates from Hint1. Released MITF can translocate to the nucleus and initiate transcription of downstream genes (Lee et al., 2004a).
Ap4A is also implicated in the control of antigen presentation. Low levels of Ap4A are observed in patients with Chediak-Higashi syndrome (Kim et al., 1985), where delayed major histocompatibility complex (MHC) class II-restricted antigen presentation increases pathogen load (Martin-Fernández et al., 2005). The expression of MHC class II is a defining feature of antigen-presenting cells (APCs). Among APCs, dendritic cells (DCs) possess vastly efficient antigen cross-presentation response over all other known immune cell types. DCs possess high motility to bring sampled antigens to naive T cells located in lymph nodes to stimulate adaptive immune response. Hence DCs act as connecting linkers between innate and acquired immunity. Immature DCs, although present in blood, are more prevalent in pathogen-prone peripheral tissues. Mature DCs travel toward lymph nodes for the antigen presentation to T and B cells, thus activating acquired immune response (Clark et al., 2000). Harnessing the ability of DC as a professional APC to enhance antigen-specific T cell immune response with high precision is particularly useful for the development of more effective cancer immunotherapy vaccines (Zamarin and Postow, 2015).
DCs adopt a dynamic behavior by migrating to lymph nodes for naive T or B cell priming and maintaining acquired immune activity (Germain et al., 2012). Hence migratory ability of DC is directly correlated with its ability to stimulate immune response through antigen presentation to naive T and B cells in remotely accessible regions of lymph nodes awaiting stimulation by DCs.
We reasoned that if a highly conserved function of APCs such as antigen presentation can be precisely impaired by low levels of intracellular Ap4A, APCs may functionally benefit from the increase of intracellular Ap4A concentration. We hypothesized that Ap4A is able to enhance functional capacities of APCs by improving either mobility or antigen presentation, or both.
The NUDT2 gene encodes Ap4A hydrolase, a member of the nudix-type family of enzymes that hydrolyze a wide range of pyrophosphates. CD11c+ is preferentially expressed in murine DCs. Ap4A hydrolase allele was floxed and crossed with a CD11c+ promoter-specific Cre mice to generate the deletion of NUDT2 gene in CD11c+ cells (Nudt2fl/fl/CD11c-cre mice). The present study is aimed at investigating the immune modulatory effect of Ap4A in DCs from Nudt2fl/fl/CD11c-cre mice. We report the influence of Ap4A levels over DCs' viability, motility, and expression of immune activation markers.
Results
Selective Knockout of Ap4A Hydrolase in Dendritic Cells of Nudt2fl/fl/CD11c+ Mice
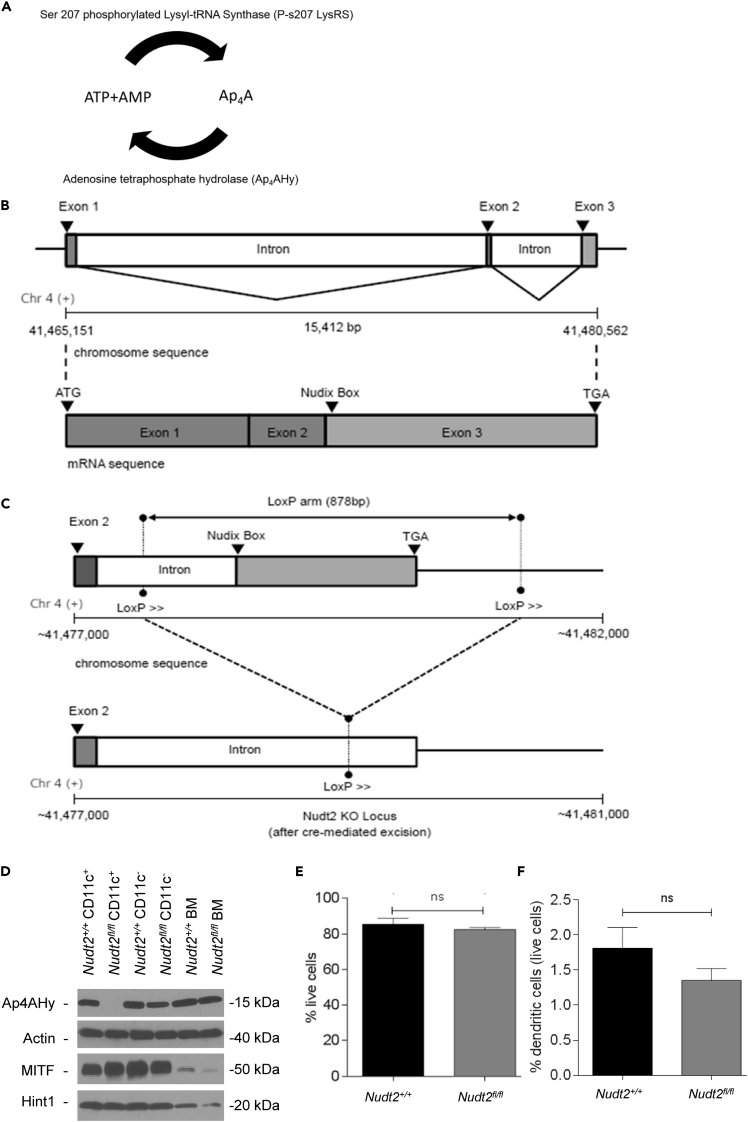
The intracellular level of Ap4A is regulated by two enzymes: lysyl tRNA synthetase (LysRS) and Ap4A hydrolase (Ap4AHy; Figure 1A). To investigate the function of Ap4A in DCs, we have utilized a floxed Ap4A hydrolase allele mouse (Nudt2fl/fl; Figure 1B), which we crossed with CD11c+ transgenic mice thereby targeting deletion of Ap4A hydrolase to cells that expresses the transmembrane surface protein CD11c, i.e., DCs (Figure 1C). The western blot of splenic DC extracts of the mice showed neither any detectable expression of Ap4AHy nor any disruption in the expression of MITF and Hint1 in Nudt2fl/fl/CD11c+ cells when compared with Nudt2+/+ CD11c+ cells, confirming successful knockout of Ap4A hydrolase in DCs of Nudt2fl/fl/CD11c+ mice (Figure 1D).
Figure 1.
Generation of Ap4A Hydrolase Knockout Mice and Viability of Dendritic Cells
(A) The phosphorylated form of Lysyl-tRNA transferase (phosphoSerine 207 LysRS) synthesizes Ap4A from ATP and AMP, and is in turn broken down into same by Ap4A hydrolase (Ap4AHy).
(B) The gene locus and mRNA structure of NUDT2. The full open reading frame for Ap4AHy (15,412 bp) consists of three exons interspersed with two introns. The Nudix box is nothing but the enzymatically functional site for Ap4AHy and is located at exon 3.
(C) The deletion of Ap4AHy by gene floxing and deletion strategy. The two loxP sites were generated on either side of the gene encompassing the full sequence of exon 3 and a portion of immediate intron upstream to it.
(D–F) (D) Western blot showing expression levels of Ap4AHy, MITF, and Hint1 in splenic dendritic cells of Nudt2+/+ and Nudt2fl/fl/CD11c+ (Ap4A hydrolase knockout) mice (represents four independent experiments). Immature bone marrow-derived cells (BM). (E) and (F) Viability percentage of isolated BMDCs and splenic DCs. Results (mean ± SEM) represent two independent experiments. The significant difference of test in comparision to control. nsp> 0.05 (Student's t test).
Based on the increased cellular permeability and binding ability with free amines retained in dead cells than live cells, saturation of cells with amines conjugated to florescent APC-Cy7 probe were measured by using flow cytometry to determine the live and dead cell population percentages. The study has revealed a slight decrease in viability of bone marrow DCs (BMDCs) as well as splenic DCs in case of Nudt2fl/fl/CD11c+ mice when compared with Nudt2+/+ mice and is found to be statistically insignificant (Figures 1E and 1F).
Characterization of Ap4AHy Nudt2fl/fl/CD11c+ DCs
Higher Accumulation of Ap4A Is Observed in Nudt2fl/fl/CD11c+DCs
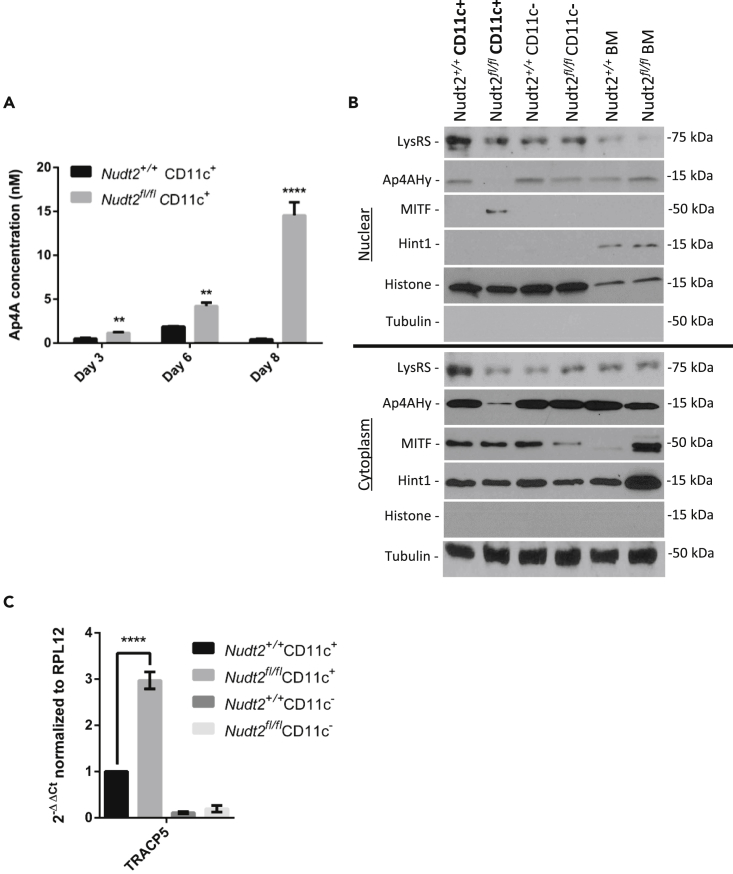
The intracellular Ap4A level of BMDCs generated from Nudt2fl/fl/CD11c+ and Nudt2+/+ mice was examined using a diadenosine nucleotide assay. Compared with Nudt2+/+ CD11c+ mice, the BMDCs of Nudt2fl/fl/CD11c+ mice contained prominently higher levels of Ap4A on their third and sixth days of culture (2.3- and 3.2-folds, respectively), whereas the levels reached peak state on eighth day of culture (approximately 32-fold; Figure 2A).
Figure 2.
Detailed Functional Characterization of Nudt2fl/fl/CD11c-cre DC
(A) Intracellular concentrations of Ap4A in Nudt2+/+ and Nudt2fl/fl/CD11c-cre BMDC were generated from bone marrow cultured with granulocyte-macrophage colony-stimulating factor and examined on days 3, 6, and 8, using a luciferase assay.
(B and C) (B) Western blot of LysRS, Ap4AHy, MITF, and Hint1 in Nudt2+/+ and Nudt2fl/fl/CD11c-cre CD11c+ splenic cells. Immature bone marrow-derived cells (BM) (C). Real-time PCR result of MITF-specific gene (TRACP5) expression in BMDCs. The results (mean ± SEM) are representative of four independent experiments and the significant difference of test in comparison to control. **p < 0.01; ****p < 0.0001 (Mann-Whitney test for multiple comparison).
Another correlative activation from the increase in intracellular Ap4A concentration is the initiation of LysRS-Ap4A-Hint1-MITF pathway. The MITF nuclear localization was examined in splenic DCs and was detected prominently (without concomitant increase in expression) in splenic Nudt2fl/fl/CD11c+ cells, but not in Nudt2+/+ CD11c+ cells (Figure 2B). Furthermore, Hint1, a suppressor of MITF, is not present in the nucleus of matured DCs and monocytes and is only present in unmatured BM cells. Hint1 translocation also posits that MITF suppression is released from increased Ap4A concentration within Nudt2fl/fl/CD11c+ splenic cells.
To validate the translocation of MITF in to nucleus, which leads to gene transcription, MITF-specific gene tartrate-resistant acid phosphatase 5 (TRACP5) was examined for expression (Luchin et al., 2010). TRACP5 is strongly expressed in both Nudt2+/+ and Nudt2fl/fl CD11c+ cells and is upregulated by 2.97-fold in Nudt2fl/fl/CD11c+ cells compared with Nudt2+/+ CD11c+ cells (Figure 2C).
To review the association of MITF with LysRS, which occurs during activation of MITF, a pull-down of MITF using LysRS antibody via co-immunoprecipitation experiment was performed on BMDCs. MITF is co-immunoprecipitated using LysRS antibody pull-down in both Nudt2+/+ and Nudt2fl/fl DCs, indicating the that binding of LysRS to MITF occurs at baseline level in DCs and the association is increased in Nudt2fl/fl DCs (Figure S1).
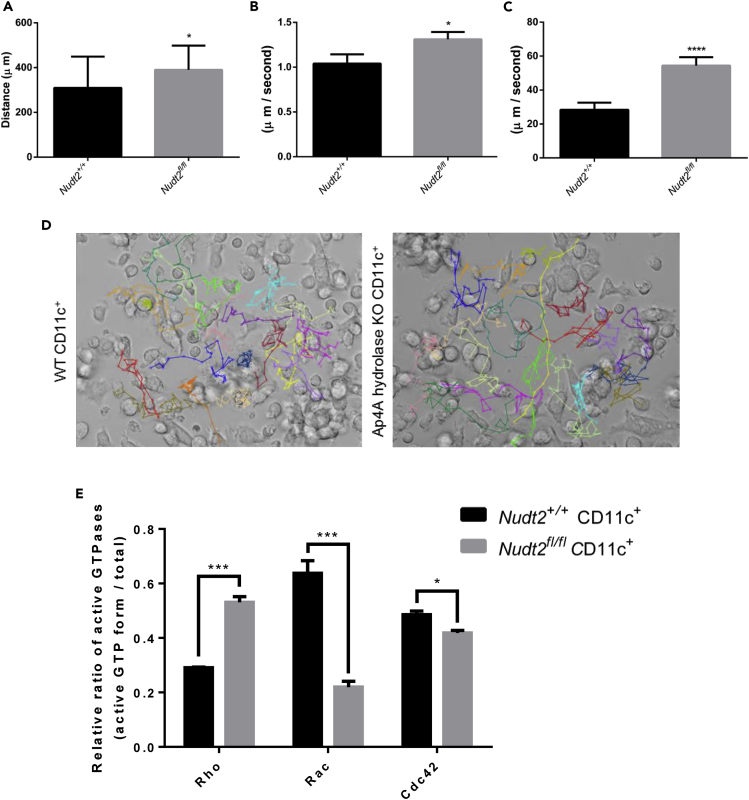
Nudt2fl/fl/CD11c+ BMDCs Possess Greater Motility with Lower Directional Variation
The Nudt2fl/fl/CD11c+ BMDCs have shown vigorous motility as indicated by higher values of distance, speed and displacement compared with Nudt2+/+ BMDCs (Figures 3A–3D). Less directional variability indicates enhanced organization of cytoskeletal dynamics, and such organization generally requires the activation of small GTPases, such as Rho GTPases. The Rho GTPases play a crucial role in cellular migration, of which Rho, Rac, and Cdc42 are widely studied proteins and are highly conserved among eukaryotes (Ridley, 2015). To examine a possible relationship between intracellular Ap4A increase in DCs and small GTPase activation, active Rac1, cdc42, and RhoA levels were measured (GTP-bound form). The results show a significant decrease in active Rac1 and cdc42, and an increase in RhoA activation levels in case of BMDCs of Nudt2fl/fl/CD11c+ mice compared with that of Nudt2+/+ CD11c+ mice (Figure 3E).
Figure 3.
Comparative Motility of DCs from Nudt2fl/fl/CD11c+ and Nudt2+/+ Mice
(A) Live imaging microscopy of granulocyte-macrophage colony-stimulating factor-treated DCs (8 days). The post-treated DCs on observational Petri dish were chosen randomly (n = 20) and traced minute wise (time lap ≈30 min) to find the change in cell movement and the distance moved.
(B) The speed of cellular movement and the ratio between the distance moved and difference of two time frames.
(C) Cellular displacement.
(D) A still frame taken from the video created for live cell imaging of Nudt2+/+ and Nudt2fl/fl/CD11c+ DCs.
(E) Small GTPase activation state in Nudt2+/+ and Nudt2fl/fl/CD11c+ DCs (n = 2). Results (mean ± SEM) represent two independent experiments. The significant difference of test in comparision to control. *p < 0.05, ***p < 0.001, and ****p < 0.0001 (Student's t test or Mann-Whitney test for multiple comparison).
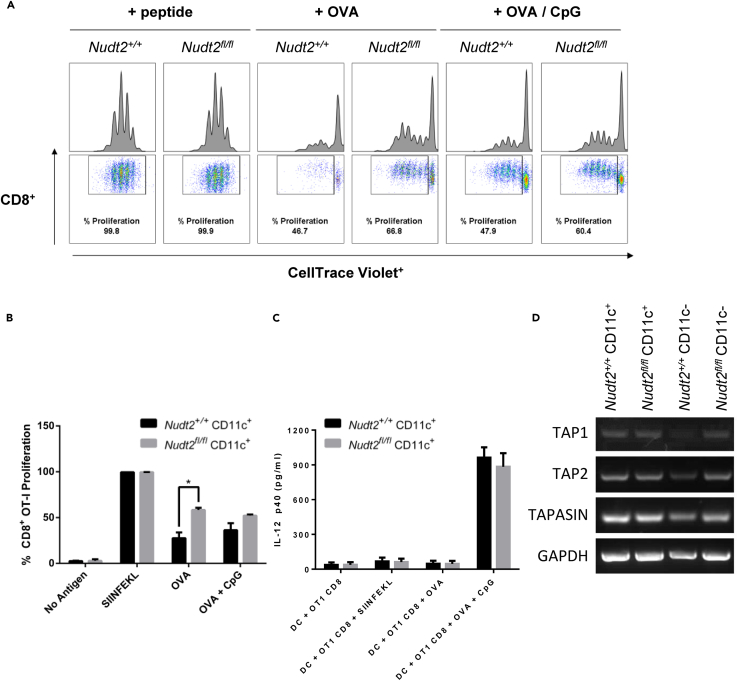
Nudt2fl/fl/CD11c+ DCs Possess Higher Antigen-Presenting and CD8+ T Cell-Priming Potential
The possibility of NUDT2 gene directly controlling fundamental immune activity of DCs was also investigated. It is equally important to know whether Nudt2 is important for DC maturation because its status directly impacts the immune function as APCs. Although DCs are highly specialized immune cells that specialize as APCs, they are initially phagocytic before maturation. A change in phagocytic potential would indicate aberrant DC maturation signaling compared with Nudt2+/+ DCs. To measure the phagocytic potential, uptake of fluorescein isothiocyanate (FITC) dextran by DCs was measured. The phagocytic potential of DCs of Nudt2fl/fl/CD11c+ mice was found to be unchanged when compared with that of Nudt2+/+ DCs as the uptake of FITC dextran DCs from Nudt2fl/fl/CD11c + mice was identical to Nudt2+/+ DCs both at 4°C and 37°C (Figure S2). Therefore, these DCs do not differ in phagocytic capacity, a defining attribute in DC maturation status, indicating that DC maturation status is identical. To determine if antigen presentation functionality is affected, the capacity of DCs to trigger proliferation in antigen-specific T cells that requires DCs to present highly specific antigens to these T cells to trigger activation and proliferation was also investigated. The cultures of BMDCs from either the Nudt2fl/fl/CD11c+ or the Nudt2+/+/CD11c+ mice incubated with OT-1 T cell were co-incubated with positive control SIINFEKL peptides, and have given a similar extent of stimulation of proliferation of CD8+ T cells, indicating an overall lack of involvement in the activation of T cells by DCs, which does not require antigen cross-presentation (Figures 4A and 4B). Remarkably, when cultured with DCs co-incubated either with whole ovalbumin (OVA) or OVA and cytosine-phosphodiester linked guanine ligodeoxynucleotides (CpG ODN), there was an average of 20% and 15% higher stimulus in the proliferation of CD8+ T cell population, respectively, by the BMDCs from Nudt2fl/fl/CD11c+ mice compared with the Nudt2+/+ BMDCs. Nudt2 gene is therefore involved in the control of antigen cross-presentation machinery in DCs.
Figure 4.
Nudt2fl/fl/CD11c+ DCs' Potential in Influencing OT-I CD8+ T Cells Antigen Cross-Priming
(A) The percentage of OT-I CD8+ T cells that have proliferated following co-culture for 3 days either with Nudt2fl/fl/CD11c+ BMDCs or Nudt2+/+ CD11c+ BMDCs (1:10) incubated with SIINFEKL peptide, OVA and CpG ODN. The harvested cells were analyzed by flow cytometry to quantify CD8+ CellTrace Violet+ population for proliferation.
(B) Graph represents the percentage of OT-I CD8+ T cells proliferated when cultured with Nudt2+/+ or Nudt2fl/fl/CD11c+ DCs in response to different antigenic preparations.
(C) IL-12 p40 production by DCs during the above co-culture conditions.
(D) mRNA levels for TAP1, TAP2, and TAPASIN in Nudt2+/+ and Nudt2fl/fl/CD11c-cre in splenic DCs measured by RT-PCR. Results (mean ± SEM) represent three independent experiments and the significant difference of test in comparision to control. *p < 0.05 (Student's t test or Mann-Whitney test for multiple comparison).
To determine the involvement of interleukin (IL)-12 p40, which is a DC-specialized T cell activation cytokine that controls enhancement of antigen cross-presentation by Nudt2fl/fl/CD11c+ BMDCs, the cytokine levels were measured by ELISA technique. The results revealed a lack of involvement of IL-12 p40 in enhancing the priming of CD8+ T cells by Nudt2fl/fl/CD11c+ BMDCs as there was no observable difference in the levels of IL-12 p40 between Nudt2fl/fl/CD11c+ BMDCs and Nudt2+/+ BMDCs (Figure 4C).
Antigen cross-presentation can also be skewed by the change in expression of TAP proteins required for assembly of short peptides onto MHC-I molecules. The mRNA levels of TAP1, TAP2, and TAPASIN were measured in Nudt2fl/fl/CD11c+ DCs as well as in Nudt2+/+ DCs. The results have shown a lack of difference in TAP1, TAP2, or TAPASIN between Nudt2fl/fl/CD11c-cre or Nudt2+/+ DCs indicating lack of their involvement (Figure 4D). Taken together, Nudt2 gene is involved in controlling antigen cross-presentation machinery that directly signals T cell proliferation.
Discussion
This study shows that intracellular Ap4A can be upregulated in immune cells of a specific lineage (DCs) within an in vivo model. The DCs from these mice are lacking Ap4A hydrolase, and a consequent rise in intracellular Ap4A amounts is observed, revealing an obvious substantial regulation of Ap4A concentrations in DCs by Ap4A hydrolase through the NUDT2 gene. The Nudt2fl/fl/CD11c+ mice did not exhibit any observable physiological differences to wild-type, leading to the inference that they harbor similar small population of CD11c+ cells as are normally present. In addition, their viability remained almost similar.
DCs play a pivotal role in adaptive immunity and tolerance. Efficient migration for accurate positioning to capture antigens from invading pathogens and capacity to process the foreign peptides into recognizable MHC-I-bound complexes through antigen-cross presentation are two immutable purposes of DCs. These functions are therefore critical for immune protection. Most of the immature and highly phagocytic DCs begin their voyage from the bone marrow where they are generated, followed by entry into the blood and sequential movement into peripheral lymphoid tissues (PLTs) and non-lymphoid tissues (NLTs). The DCs possess advanced migratory skills, and their main function in NLTs is the carriage and presentation of antigenic components into and within secondary lymphoid organs (Alvarez et al., 2008). Once primed by the phagocytosis of foreign antigens the enrouted Ag-bearing DCs in lymph nodes begin to build up an immune-stimulatory phenotype, exhibiting increased expression of MHC complexes as well as upregulation of the co-stimulatory molecules and cytokines required for effective T cell priming. The non-retained DCs enter into the blood stream (blood-borne DCs) and deliver the antigenic components to the spleen (Mullins et al., 2003) and also to PLTs (Cavanagh et al., 2005).
The relationship between Nudt2 and DC is unknown, but its association with immune function has been speculated. A recent RNA sequencing analysis of Nudt2 knockout myelogenous leukemia cells revealed that the majority of the target genes are linked to immune-specific processes such as interferon-associated inflammatory responses, MHC-II antigen presentation, allograft rejection, and B cell development (Marriott et al., 2016). The current study focused on the influence of Ap4A over immune activity of DCs from Nudt2fl/fl/CD11c+ mice and has confirmed that Nudt2 gene has influence over MHC-II antigen presentation.
Ap4A regulates MITF activation through the release of Hint1 suppression and consequent nuclear localization of MITF. The nuclear localization of MITF, as a result of increase of Ap4A, is a hallmark of the LysRS-Ap4A-Hint1-MITF pathway (Carmi-Levy et al., 2008, Carmi-Levy et al., 2011, Huete et al., 2011, Lee and Razin, 2005, Lee et al., 2004b, Tshori et al., 2013). The LysRS-Ap4A-Hint1-MITF pathway has been described in mast cells (Lee et al., 2004a), but it was not clear whether the same pathway is active in DCs.
The LysRS-Ap4A-Hint1-MITF pathway was examined in DCs in this study, and it was revealed that the increase in MITF nuclear localization, but not HINT-1, in response to the elevated Ap4A levels is due to the knockout of NUDT2 gene. The binding of MITF to LysRS using co-immunoprecipitation pull down of MITF using LysRS antibody establishes the existence of LysRS-Ap4A-Hint1-MITF pathway in DCs and further confirms that Hint1 suppression is lifted so that LysRS can bind active MITF.
The BMDCs from Nudt2fl/fl/CD11c-cre mice cultured from undifferentiated monocytes did not show any morphological or phenotypic differences to Nudt2+/+ DCs. Interestingly, the DCs of Nudt2fl/fl/CD11c+ mice exhibited a unique pattern of cellular motility with higher velocity and lower directional variability. This suggests that the majority population of DCs of Nudt2fl/fl/CD11c+ mice is having uniform directional imprint that may indicate effective migration toward the immune reaction zones. Furthermore, we found such behavior was correlated to the variation in their expression of small GTPases, i.e., cdc42, Rac1, and RhoA. Our findings provide evidence that reduced activation of Rac1 in DCs leads to increased directional motility, consistent with the earlier studies over fibroblasts that had shown their enhancement of directional motility by the inactivation of the same GTPases (Hanna and El-Sibai, 2013). Among small GTPases that are important in cell motility, Rho GTP is critical for inducing actomyosin contraction and inhibiting actin filament disassembly for cell polarity and directional migration (Kimura et al., 1998, Maekawa et al., 1999). Upregulation of active Rho is observed in CD11c+ DC derived from Nudt2fl/fl mice. Pharmacologic Rho effector blockades (e.g., ROCK inhibitors) can profoundly decrease DC migration capability. Hence increased bioavailability of RhoGTP can correlate with the increased mobility observed in CD11c+ DC derived from Nudt2fl/fl mice. Increase in Rho activity also causes decreased availability of Rac (Ohta et al., 2006), and this is likewise also observed in CD11c+ DC derived from Nudt2fl/fl mice.
The defining feature of DCs is their ability to take up antigen in the periphery, through their dendrites; to withdraw those dendrites; and then to migrate to lymph nodes. Upon regaining their former shapes in lymph node, DCs will present antigenic peptides to CD4+ and CD8+ T cells. Thus DCs may be regarded as the “shape-shifters” of the immune system. Cross-presentation is the intracellular degradation of extrinsic antigen and its presentation by MHC-I. This important DC function activates CD8+ T cells during an immunological response to intracellular pathogens (Murphy and McLennan, 2004, Murphy et al., 2000) and is a highly energy-dependent process and requires movement of the cells into finding suitable T cells. Though identical levels of phagocytic potential, Class-II MHC display and expression of co-stimulatory molecules were seen in DCs of Nudt2fl/fl/CD11c+ mice, there was an enhancement in their immune functionality. These DCs have increased immune activity in terms of antigen processing and cross-presentation in priming the proliferation of CD8+ T cells. In vitro antigen presentation assay using OT-1 CD8+ T cells is well suited to validate this result as an antigen-specific response. The activation of CD8+ T cells of OT-1 mice is exclusive for antigen presentation of OVA through DC cross-presentation machinery, and the subsequent trigger of proliferation in OT-1 CD8+ T cells is a highly specific response against cross-presented MHC-I antigen on APCs. CD11c+ DC derived from Nudt2fl/fl mice are capable of driving the proliferation of antigen-specific CD8+ T cells without the need for CpG ODN, a potent immune agonist (Ramírez-Pineda et al., 2004). However, this observation was found not associated with the expected alteration in the elements of MHC-I machinery, reflecting the possible positive influence of directional motility making effective contact to the T cells and also the rise in the duration of functioning in antigen presentation process. There is direct evidence in support of one of our assumption. Other groups have also reported that overexpression of Rho can directly increase the ability of DC to present OVA peptide to specific T cells (Shurin et al., 2005). Our studies indicate that the increased CD8+ T cell cross-priming potential of DC from Nudt2fl/fl/CD11c+ mice compared with Nudt2+/+ is more likely the result of RhoA activation and RAC1 repression. This is in agreement with the earlier studies (Hanna and El-Sibai, 2013, Shurin et al., 2005, Wu et al., 2009).
Another important feature of DCs is their capacity to steer the immune response into clinically beneficial Th1-type immune response by the production of IL-12 (Muller-Berghaus et al., 2005). Without stimulation, IL-12 production is not observed to be different than Nudt2+/+ for CD11c+ DC derived from Nudt2fl/fl mice, indicating that the resultant DCs are not activated immune response without stimuli. Presence of CpG ODN increased IL-12 production but by an identical amount to Nudt2+/+ mice, suggesting that the CD11c+ DC derived from Nudt2fl/fl mice are neither functionally impaired or overactivated compared with Nudt2+/+ DC counterparts.
In conclusion, increased Ap4A concentration in DC of Nudt2fl/fl/CD11c+ mice led to the localization of MITF into the nucleus during restive conditions compared with Nudt2+/+ DC. These Ap4A enriched DCs have prominently exhibited alteration in small GTPases rising their motility and antigen presenting potentiality. This study documents an in vivo model that can modulate the intracellular Ap4A concentration by knocking out Ap4A hydrolase and enhancing antigen cross-presentation by DC. Fundamentally, enhanced antigen cross-presentation in a controlled manner will benefit a wide variety of APC-associated functions such as earlier pathogen recognition by the immune system and better loading and presentation of tumor antigens to cytotoxic CD8+ T cells in response to cancer. The highly specific control of intracellular Ap4A concentration through manipulation of NUDT2 gene makes this mechanism an attractive pharmacological target to enhance antigen presentation while keeping the complex repertoire of transcriptional activation of APCs as intact as possible.
Limitations of the Study
The currently studied Nudt2fl/fl/CD11c+ mice model is an excellent in vivo system to understand the consequences of increased intracellular Ap4A. It is important and yet to be determined whether the circulatory DCs in Nudt2fl/fl/CD11c+ mice exhibit elevation in antigen presentation to T cells. Also, the identification of key cytokines responsible for DC-specialized T cell activation would become significant. In addition, this study necessitates the elucidation of Ap4A-binding proteins that are associated with cellular migration in future.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
The present study was supported in part by grants from the National University of Singapore to D.M.K.; Israel Science Foundation, 115/2013, and Hebrew-University-National Research Foundation of Singapore HUJ-CREATE (R182-005-172-281) to E.R. Graphical abstract was created with bio-render.com.
Author Contributions
S.L.S., N.Q.T., A.S.Y.F., K.H.L., E.G.L.K., and A.L. performed the experimental work. Y.L.C. produced the Nudt2fl/fl/CD11c-cre mice and C.M.Y. designed the experiments. S.L.S. and L.B.P. analyzed the data and drafted the manuscript. D.M.K. and E.R., the principal investigators, conceived the study design and wrote the grant, and with H.N corrected the manuscript. The authors thank Paul Hutchinson and Teoh Guo Hui of the NUS immunology programme flow cytometry facility for their help and advice on the flow cytometry assays used in this study.
Declaration of Interests
The authors declare no competing interests.
Published: June 28, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.05.045.
Contributor Information
Hovav Nechushtan, Email: hovavnech@hadassah.org.il.
Ehud Razin, Email: ehudr@ekmd.huji.ac.il.
David Michael Kemeny, Email: mickdm@nus.edu.sg.
Supplemental Information
References
- Alvarez D., Vollmann E.H., von Andrian U.H. Mechanisms and consequences of dendritic cell migration. Immunity. 2008;29:325–342. doi: 10.1016/j.immuni.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]; Alvarez, D., Vollmann, E.H., and von Andrian, U.H.. (2008). Mechanisms and consequences of dendritic cell migration. Immunity 29, 325-342. [DOI] [PMC free article] [PubMed]
- Carmi-Levy I., Yannay-Cohen N., Kay G., Razin E., Nechushtan H. Diadenosine tetraphosphate hydrolase is part of the transcriptional regulation network in immunologically activated mast cells. Mol. Cell. Biol. 2008;28:5777–5784. doi: 10.1128/MCB.00106-08. [DOI] [PMC free article] [PubMed] [Google Scholar]; Carmi-Levy, I., Yannay-Cohen, N., Kay, G., Razin, E., and Nechushtan, H.. (2008). Diadenosine tetraphosphate hydrolase is part of the transcriptional regulation network in immunologically activated mast cells. Mol. Cell. Biol. 28, 5777-5784. [DOI] [PMC free article] [PubMed]
- Carmi-Levy I., Motzik A., Ofir-Birin Y., Yagil Z., Yang C.M., Kemeny D.M., Han J.M., Kim S., Kay G., Nechushtan H. Importin beta plays an essential role in the regulation of the LysRS-Ap(4)A pathway in immunologically activated mast cells. Mol. Cell. Biol. 2011;31:2111–2121. doi: 10.1128/MCB.01159-10. [DOI] [PMC free article] [PubMed] [Google Scholar]; Carmi-Levy, I., Motzik, A., Ofir-Birin, Y., Yagil, Z., Yang, C.M., Kemeny, D.M., Han, J.M., Kim, S., Kay, G., Nechushtan, H., et al. (2011). Importin beta plays an essential role in the regulation of the LysRS-Ap(4)A pathway in immunologically activated mast cells. Mol. Cell. Biol. 31, 2111-2121. [DOI] [PMC free article] [PubMed]
- Carracedo G., Guzman-Aranguez A., Loma P., Pintor J. Diadenosine polyphosphates release by human corneal epithelium. Exp. Eye Res. 2013;113:156–161. doi: 10.1016/j.exer.2013.05.022. [DOI] [PubMed] [Google Scholar]; Carracedo, G., Guzman-Aranguez, A., Loma, P., and Pintor, J.. (2013). Diadenosine polyphosphates release by human corneal epithelium. Exp. Eye Res. 113, 156-161. [DOI] [PubMed]
- Castany M., Jordi I., Catala J., Gual A., Morales M., Gasull X., Pintor J. Glaucoma patients present increased levels of diadenosine tetraphosphate, Ap4A, in the aqueous humour. Exp. Eye Res. 2011;92:221–226. doi: 10.1016/j.exer.2010.12.004. [DOI] [PubMed] [Google Scholar]; Castany, M., Jordi, I., Catala, J., Gual, A., Morales, M., Gasull, X., and Pintor, J. (2011). Glaucoma patients present increased levels of diadenosine tetraphosphate, Ap4A, in the aqueous humour. Exp. Eye Res. 92, 221-226. [DOI] [PubMed]
- Cavanagh L.L., Bonasio R., Mazo I.B., Halin C., Cheng G., van der Velden A.W.M., Cariappa A., Chase C., Russell P., Starnbach M.N. Activation of bone marrow–resident memory T cells by circulating, antigen-bearing dendritic cells. Nat. Immunol. 2005;6:1029. doi: 10.1038/ni1249. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cavanagh, L.L., Bonasio, R., Mazo, I.B., Halin, C., Cheng, G., van der Velden, A.W.M., Cariappa, A., Chase, C., Russell, P., Starnbach, M.N., et al. (2005). Activation of bone marrow-resident memory T cells by circulating, antigen-bearing dendritic cells. Nat. Immunol. 6, 1029. [DOI] [PMC free article] [PubMed]
- Chang H., Yanachkov I.B., Dix E.J., Yanachkova M., Li Y., Barnard M.R., Wright G.E., Michelson A.D., Frelinger A.L. Antiplatelet activity, P2Y1 and P2Y12 inhibition, and metabolism in plasma of stereoisomers of diadenosine 5′,5‴-P1,P4-dithio-P2,P3-chloromethylenetetraphosphate. PLoS One. 2014;9:e94780. doi: 10.1371/journal.pone.0094780. [DOI] [PMC free article] [PubMed] [Google Scholar]; Chang, H., Yanachkov, I.B., Dix, E.J., Yanachkova, M., Li, Y., Barnard, M.R., Wright, G.E., Michelson, A.D., and Frelinger, A.L.. (2014). Antiplatelet activity, P2Y1 and P2Y12 inhibition, and metabolism in plasma of stereoisomers of diadenosine 5′,5‴-P1,P4-dithio-P2,P3-chloromethylenetetraphosphate. PLoS One 9, e94780. [DOI] [PMC free article] [PubMed]
- Clark G.J., Angel N., Kato M., López J.A., MacDonald K., Vuckovic S., Hart D.N. The role of dendritic cells in the innate immune system. Microbes Infect. 2000;2:257–272. doi: 10.1016/s1286-4579(00)00302-6. [DOI] [PubMed] [Google Scholar]; Clark, G.J., Angel, N., Kato, M., Lopez, J.A., MacDonald, K., Vuckovic, S., and Hart, D.N.. (2000). The role of dendritic cells in the innate immune system. Microbes Infect. 2, 257-272. [DOI] [PubMed]
- Germain R.N., Robey E.A., Cahalan M.D. A decade of imaging cellular motility and interaction dynamics in the immune system. Science. 2012;336:1676–1681. doi: 10.1126/science.1221063. [DOI] [PMC free article] [PubMed] [Google Scholar]; Germain, R.N., Robey, E.A., and Cahalan, M.D.. (2012). A decade of imaging cellular motility and interaction dynamics in the immune system. Science 336, 1676-1681. [DOI] [PMC free article] [PubMed]
- Hanna S., El-Sibai M. Signaling networks of Rho GTPases in cell motility. Cell. Signal. 2013;25:1955–1961. doi: 10.1016/j.cellsig.2013.04.009. [DOI] [PubMed] [Google Scholar]; Hanna, S., and El-Sibai, M.. (2013). Signaling networks of Rho GTPases in cell motility. Cell. Signal. 25, 1955-1961. [DOI] [PubMed]
- Huete F., Guzman-Aranguez A., Ortin J., Hoyle C.H.V., Pintor J. Effects of diadenosine tetraphosphate on FGF9-induced chloride flux changes in achondroplastic chondrocytes. Purinergic Signal. 2011;7:243–249. doi: 10.1007/s11302-011-9234-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; Huete, F., Guzman-Aranguez, A., Ortin, J., Hoyle, C.H.V., and Pintor, J.. (2011). Effects of diadenosine tetraphosphate on FGF9-induced chloride flux changes in achondroplastic chondrocytes. Purinergic Signal. 7, 243-249. [DOI] [PMC free article] [PubMed]
- Kim B.K., Chao F.C., Leavitt R., Fauci A.S., Meyers K.M., Zamecnik P.C. Diadenosine 5’,5’’’-p1,p4-tetraphosphate deficiency in blood platelets of the Chediak-Higashi syndrome. Blood. 1985;66:735–737. [PubMed] [Google Scholar]; Kim, B.K., Chao, F.C., Leavitt, R., Fauci, A.S., Meyers, K.M., and Zamecnik, P.C.. (1985). Diadenosine 5’,5’’’-p1,p4-tetraphosphate deficiency in blood platelets of the Chediak-Higashi syndrome. Blood 66, 735-737. [PubMed]
- Kimura K., Fukata Y., Matsuoka Y., Bennett V., Matsuura Y., Okawa K., Iwamatsu A., Kaibuchi K. Regulation of the association of adducin with actin filaments by rho-associated kinase (Rho-kinase) and myosin phosphatase. J. Biol. Chem. 1998;273:5542–5548. doi: 10.1074/jbc.273.10.5542. [DOI] [PubMed] [Google Scholar]; Kimura, K., Fukata, Y., Matsuoka, Y., Bennett, V., Matsuura, Y., Okawa, K., Iwamatsu, A., and Kaibuchi, K.. (1998). Regulation of the association of adducin with actin filaments by rho-associated kinase (Rho-kinase) and myosin phosphatase. J. Biol. Chem. 273, 5542-5548. [DOI] [PubMed]
- Lee Y.-N., Razin E. Nonconventional involvement of LysRS in the molecular mechanism of USF2 transcriptional activity in FcɛRI-activated mast cells. Mol. Cell. Biol. 2005;25:8904–8912. doi: 10.1128/MCB.25.20.8904-8912.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lee, Y.-N., and Razin, E.. (2005). Nonconventional involvement of LysRS in the molecular mechanism of USF2 transcriptional activity in FcɛRI-activated mast cells. Mol. Cell. Biol. 25, 8904-8912. [DOI] [PMC free article] [PubMed]
- Lee Y.-N., Nechushtan H., Figov N., Razin E. The function of lysyl-tRNA synthetase and Ap4A as signaling regulators of MITF activity in FcepsilonRI-activated mast cells. Immunity. 2004;20:145–151. doi: 10.1016/s1074-7613(04)00020-2. [DOI] [PubMed] [Google Scholar]; Lee, Y.-N., Nechushtan, H., Figov, N., and Razin, E.. (2004a). The function of lysyl-tRNA synthetase and Ap4A as signaling regulators of MITF activity in FcepsilonRI-activated mast cells. Immunity 20, 145-151. [DOI] [PubMed]
- Lee Y.-N., Nechushtan H., Figov N., Razin E. The function of Lysyl-tRNA synthetase and Ap4A as signaling regulators of MITF activity in FcɛRI-activated mast cells. Immunity. 2004;20:145–151. doi: 10.1016/s1074-7613(04)00020-2. [DOI] [PubMed] [Google Scholar]; Lee, Y.-N., Nechushtan, H., Figov, N., and Razin, E.. (2004b). The function of Lysyl-tRNA synthetase and Ap4A as signaling regulators of MITF activity in FcɛRI-activated mast cells. Immunity 20, 145-151. [DOI] [PubMed]
- Levy C., Lee Y.-N., Nechushtan H., Schueler-Furman O., Sonnenblick A., Hacohen S., Razin E. Identifying a common molecular mechanism for inhibition of MITF and STAT3 by PIAS3. Blood. 2006;107:2839–2845. doi: 10.1182/blood-2005-08-3325. [DOI] [PubMed] [Google Scholar]; Levy, C., Lee, Y.-N., Nechushtan, H., Schueler-Furman, O., Sonnenblick, A., Hacohen, S., and Razin, E.. (2006). Identifying a common molecular mechanism for inhibition of MITF and STAT3 by PIAS3. Blood 107, 2839-2845. [DOI] [PubMed]
- Louie S., Kim B.K., Zamecnik P. Diadenosine 5’,5’’’-p1,p4 -tetraphosphate, a potential antithrombotic agent. Thromb. Res. 1988;49:557–565. doi: 10.1016/0049-3848(88)90253-8. [DOI] [PubMed] [Google Scholar]; Louie, S., Kim, B.K., and Zamecnik, P.. (1988). Diadenosine 5’,5’’’-p1,p4 -tetraphosphate, a potential antithrombotic agent. Thromb. Res. 49, 557-565. [DOI] [PubMed]
- Luchin A., Purdom G., Murphy K., Clark M.-Y., Angel N., Cassady A.I., Hume D.A., Ostrowski M.C. The microphthalmia transcription factor regulates expression of the tartrate-resistant acid phosphatase gene during terminal differentiation of osteoclasts. J. Bone Miner. Res. 2010;15:451–460. doi: 10.1359/jbmr.2000.15.3.451. [DOI] [PubMed] [Google Scholar]; Luchin, A., Purdom, G., Murphy, K., Clark, M.-Y., Angel, N., Cassady, A.I., Hume, D.A., and Ostrowski, M.C.. (2010). The microphthalmia transcription factor regulates expression of the tartrate-resistant acid phosphatase gene during terminal differentiation of osteoclasts. J. Bone Miner. Res. 15, 451-460. [DOI] [PubMed]
- Maekawa M., Ishizaki T., Boku S., Watanabe N., Fujita A., Iwamatsu A., Obinata T., Ohashi K., Mizuno K., Narumiya S. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285:895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]; Maekawa, M., Ishizaki, T., Boku, S., Watanabe, N., Fujita, A., Iwamatsu, A., Obinata, T., Ohashi, K., Mizuno, K., and Narumiya, S. (1999). Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science 285, 895-898. [DOI] [PubMed]
- Marriott A.S., Vasieva O., Fang Y., Copeland N.A., McLennan A.G., Jones N.J. NUDT2 disruption elevates diadenosine tetraphosphate (Ap4A) and down-regulates immune response and cancer promotion genes. PLoS One. 2016;11:e0154674. doi: 10.1371/journal.pone.0154674. [DOI] [PMC free article] [PubMed] [Google Scholar]; Marriott, A.S., Vasieva, O., Fang, Y., Copeland, N.A., McLennan, A.G., and Jones, N.J.. (2016). NUDT2 disruption elevates diadenosine tetraphosphate (Ap4A) and down-regulates immune response and cancer promotion genes. PLoS One 11, e0154674. [DOI] [PMC free article] [PubMed]
- Martin-Fernández J.M., Cabanillas J.A., Rivero-Carmena M., Lacasa E., Pardo J., Anel A., Ramirez-Duque P.R., Merino F., Rodriguez-Gallego C., Regueiro J.R. Herpesvirus saimiri-transformed CD8+ T cells as a tool to study Chediak-Higashi syndrome cytolytic lymphocytes. J. Leukoc. Biol. 2005;77:661–668. doi: 10.1189/jlb.0904500. [DOI] [PubMed] [Google Scholar]; Martin-Fernandez, J.M., Cabanillas, J.A., Rivero-Carmena, M., Lacasa, E., Pardo, J., Anel, A., Ramirez-Duque, P.R., Merino, F., Rodriguez-Gallego, C., and Regueiro, J.R.. (2005). Herpesvirus saimiri-transformed CD8+ T cells as a tool to study Chediak-Higashi syndrome cytolytic lymphocytes. J. Leukoc. Biol. 77, 661-668. [DOI] [PubMed]
- Muller-Berghaus J., Olson W.C., Moulton R.A., Knapp W.T., Schadendorf D., Storkus W.J. IL-12 production by human monocyte-derived dendritic cells. J. Immunother. 2005;28:306–313. doi: 10.1097/01.cji.0000163594.74533.10. [DOI] [PubMed] [Google Scholar]; Muller-Berghaus, J., Olson, W.C., Moulton, R.A., Knapp, W.T., Schadendorf, D., and Storkus, W.J.. (2005). IL-12 production by human monocyte-derived dendritic cells. J. Immunother. 28, 306-313. [DOI] [PubMed]
- Mullins D.W., Sheasley S.L., Ream R.M., Bullock T.N.J., Fu Y.-X., Engelhard V.H. Route of immunization with peptide-pulsed dendritic cells controls the distribution of memory and effector T cells in lymphoid tissues and determines the pattern of regional tumor control. J. Exp. Med. 2003;198:1023–1034. doi: 10.1084/jem.20021348. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mullins, D.W., Sheasley, S.L., Ream, R.M., Bullock, T.N.J., Fu, Y.-X., and Engelhard, V.H.. (2003). Route of immunization with peptide-pulsed dendritic cells controls the distribution of memory and effector T cells in lymphoid tissues and determines the pattern of regional tumor control. J. Exp. Med. 198, 1023-1034. [DOI] [PMC free article] [PubMed]
- Murphy G.A., McLennan A.G. Synthesis of dinucleoside tetraphosphates in transfected cells by a firefly luciferase reporter gene. Cell. Mol. Life Sci. 2004;61:497–501. doi: 10.1007/s00018-003-3420-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; Murphy, G.A., and McLennan, A.G.. (2004). Synthesis of dinucleoside tetraphosphates in transfected cells by a firefly luciferase reporter gene. Cell. Mol. Life Sci. 61, 497-501. [DOI] [PMC free article] [PubMed]
- Murphy G.A., Halliday D., McLennan A.G. The Fhit tumor suppressor protein regulates the intracellular concentration of diadenosine triphosphate but not diadenosine tetraphosphate. Cancer Res. 2000;60:2342–2344. [PubMed] [Google Scholar]; Murphy, G.A., Halliday, D., and McLennan, A.G.. (2000). The Fhit tumor suppressor protein regulates the intracellular concentration of diadenosine triphosphate but not diadenosine tetraphosphate. Cancer Res. 60, 2342-2344. [PubMed]
- Nechushtan H., Kim S., Kay G., Razin E. Chapter 1: the physiological role of lysyl tRNA synthetase in the immune system. Adv. Immunol. 2009;103:1–27. doi: 10.1016/S0065-2776(09)03001-6. [DOI] [PubMed] [Google Scholar]; Nechushtan, H., Kim, S., Kay, G., and Razin, E.. (2009). Chapter 1: the physiological role of lysyl tRNA synthetase in the immune system. Adv. Immunol. 103:1-27. [DOI] [PubMed]
- Ofir-Birin Y., Fang P., Bennett S., Zhang H.-M., Wang J., Rachmin I., Shapiro R., Song J., Dagan A., Pozo J. Structural switch of lysyl-tRNA synthetase between translation and transcription. Mol. Cell. 2013;49:30–42. doi: 10.1016/j.molcel.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ofir-Birin, Y., Fang, P., Bennett, S., Zhang, H.-M., Wang, J., Rachmin, I., Shapiro, R., Song, J., Dagan, A., Pozo, J., et al. (2013). Structural switch of lysyl-tRNA synthetase between translation and transcription. Mol. Cell 49, 30-42. [DOI] [PMC free article] [PubMed]
- Ohta Y., Hartwig J.H., Stossel T.P. FilGAP, a Rho- and ROCK-regulated GAP for Rac binds filamin A to control actin remodelling. Nat. Cell Biol. 2006;8:803–814. doi: 10.1038/ncb1437. [DOI] [PubMed] [Google Scholar]; Ohta Y., Hartwig J.H. and Stossel T.P., (2006). FilGAP, a Rho- and ROCK-regulated GAP for Rac binds filamin A to control actin remodelling, Nat. Cell Biol. 8, 803-814. [DOI] [PubMed]
- Ramírez-Pineda J.R., Fröhlich A., Berberich C., Moll H. Dendritic cells (DC) activated by CpG DNA ex vivo are potent inducers of host resistance to an intracellular pathogen that is independent of IL-12 derived from the immunizing DC. J. Immunol. 2004;172:6281–6289. doi: 10.4049/jimmunol.172.10.6281. [DOI] [PubMed] [Google Scholar]; Ramirez-Pineda, J.R., Frohlich, A., Berberich, C., and Moll, H.. (2004). Dendritic cells (DC) activated by CpG DNA ex vivo are potent inducers of host resistance to an intracellular pathogen that is independent of IL-12 derived from the immunizing DC. J. Immunol. 172, 6281-6289. [DOI] [PubMed]
- Ridley A.J. Rho GTPase signalling in cell migration. Curr. Opin. Cell Biol. 2015;36:103–112. doi: 10.1016/j.ceb.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ridley, A.J.. (2015). Rho GTPase signalling in cell migration. Curr. Opin. Cell Biol. 36, 103-112. [DOI] [PMC free article] [PubMed]
- Schepers E., Glorieux G., Jankowski V., Dhondt A., Jankowski J., Vanholder R. Dinucleoside polyphosphates: newly detected uraemic compounds with an impact on leucocyte oxidative burst. Nephrol. Dial. Transplant. 2010;25:2636–2644. doi: 10.1093/ndt/gfq080. [DOI] [PubMed] [Google Scholar]; Schepers, E., Glorieux, G., Jankowski, V., Dhondt, A., Jankowski, J., and Vanholder, R.. (2010). Dinucleoside polyphosphates: newly detected uraemic compounds with an impact on leucocyte oxidative burst. Nephrol. Dial. Transplant. 25, 2636-2644. [DOI] [PubMed]
- Shurin G.V., Tourkova I.L., Chatta G.S., Schmidt G., Wei S., Djeu J.Y., Shurin M.R. Small rho GTPases regulate antigen presentation in dendritic cells. J. Immunol. 2005;174:3394–3400. doi: 10.4049/jimmunol.174.6.3394. [DOI] [PubMed] [Google Scholar]; Shurin, G.V., Tourkova, I.L., Chatta, G.S., Schmidt, G., Wei, S., Djeu, J.Y., and Shurin, M.R.. (2005). Small rho GTPases regulate antigen presentation in dendritic cells. J. Immunol. 174, 3394-3400. [DOI] [PubMed]
- Tshori S., Razin E., Nechushtan H. Amino-acyl tRNA synthetases generate dinucleotide polyphosphates as second messengers: functional implications. Top. Curr. Chem. 2013;344:189–206. doi: 10.1007/128_2013_426. [DOI] [PubMed] [Google Scholar]; Tshori, S., Razin, E., and Nechushtan, H.. (2013). Amino-acyl tRNA synthetases generate dinucleotide polyphosphates as second messengers: functional implications. Top. Curr. Chem. 344, 189-206. [DOI] [PubMed]
- Vollmayer P., Clair T., Goding J.W., Sano K., Servos J., Zimmermann H. Hydrolysis of diadenosine polyphosphates by nucleotide pyrophosphatases/phosphodiesterases. Eur. J. Biochem. 2003;270:2971–2978. doi: 10.1046/j.1432-1033.2003.03674.x. [DOI] [PubMed] [Google Scholar]; Vollmayer, P., Clair, T., Goding, J.W., Sano, K., Servos, J., and Zimmermann, H.. (2003). Hydrolysis of diadenosine polyphosphates by nucleotide pyrophosphatases/phosphodiesterases. Eur. J. Biochem. 270, 2971-2978. [DOI] [PubMed]
- Wu Y.I., Frey D., Lungu O.I., Jaehrig A., Schlichting I., Kuhlman B., Hahn K.M. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature. 2009;461:104–108. doi: 10.1038/nature08241. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wu, Y.I., Frey, D., Lungu, O.I., Jaehrig, A., Schlichting, I., Kuhlman, B., and Hahn, K.M.. (2009). A genetically encoded photoactivatable Rac controls the motility of living cells. Nature 461, 104-108. [DOI] [PMC free article] [PubMed]
- Zamarin D., Postow M.A. Immune checkpoint modulation: rational design of combination strategies. Pharmacol. Ther. 2015;150:23–32. doi: 10.1016/j.pharmthera.2015.01.003. [DOI] [PubMed] [Google Scholar]; Zamarin, D., and Postow, M.A.. (2015). Immune checkpoint modulation: rational design of combination strategies. Pharmacol. Ther. 150, 23-32. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.