Abstract
Background
Radiofrequency (RF) ablation is a well‐established approach to treat premature ventricular contractions (PVC) and is associated with good outcomes.
Aim
The present study sought to analyze the acute efficacy and 1‐year outcomes of PVC ablation using RF technology with an approach based on automated pace‐mapping and contact force (CF) information.
Methods
Sixty‐one consecutive patients (52.4% males, age 45.9 ± 12.5) underwent catheter ablation for symptomatic monomorphic PVC. All procedures were guided by a 3‐dimensional mapping system; site of ablation was selected based on PASO™ aided pace‐mapping; RF was started on the selected location when stable catheter position with >10 g of CF were obtained.
The procedure was defined as acutely effective if the PVC was eliminated and it did not recur during within 30 minutes. Long‐term efficacy was defined as a decrease by more than 95% at 1 year of the initial PVC burden at ECG Holter monitoring.
Results
The PVC ablation was performed in the right ventricular outflow tract in 37 patients (60.7%), left ventricle in 15 patients (24.6%), coronary cusps in 6 patients (9.8%), right ventricle in 3 patients (4.9%); PVC ablation was acutely successful in 59 of patients (96.7%). At 1‐year efficacy was obtained in 57 patients (93.4%). No major complications occurred. Mean procedural and fluoroscopy time were 94.5 ± 20.9 and 4.3 ± 2.5 minutes respectively.
Conclusion
Premature ventricular contraction RF ablation mainly guided by PASO™ and CF showed high success rate in both acute and 1‐year follow‐up (96.7% and 93.4% respectively). The best efficacy cut‐off for RF ablation of PVCs has been identified in presence of both PASO™ ≥95% and CF >10 g.
Keywords: catheter ablation, contact force, pace mapping, PVC, ventricular premature complexes
1. INTRODUCTION
Premature ventricular contractions (PVC) are very common arrhythmias in clinical practice. Many patients with PVC experience a symptomatic progression of the disease with occurrence of other symptoms apart from palpitations such as dyspnoea, atypical precordial pain, dizziness, pre‐syncope, or syncope.
Nowadays, radiofrequency (RF) ablation is a well‐established approach to treat PVC and is associated with favorable outcomes and long‐term results.1 Pace mapping of ventricular tachycardia (VT) is performed by comparing the QRS morphology during overdrive pacing to that of the clinical tachycardia. Pace‐mapping during sinus rhythm is an important tool during the ablation for monomorphic PVC.2, 3
Radiofrequency has historically been guided by two‐dimensional mapping and in recent years by three‐dimensional mapping, with satisfactory safety and efficacy.4 Recently, catheter contact force (CF) and PASO™ automated pace‐mapping module have been developed to guide PVCs ablation. The use of computerized algorithms for the comparison of paced and clinical PVC complexes might significantly improve the precision of pace‐mapping.2, 3, 5, 6, 7, 8 Data on literature investigating this approach for PVC ablation is limited.
This study sought to analyse the acute efficacy and 1‐year outcome of PVC ablation using RF technology with an approach guided by PASO™ module and contact force.
2. METHODS
2.1. Patient characteristics
Sixty‐one consecutive patients (52.4% males, age 45.9 ± 12.5) underwent catheter ablation for symptomatic PVCs from February 2015 to March 2017. All patients were refractory to medical therapy by antiarrhythmic drugs, except one who stopped the treatment because of severe bradycardia. Eight patients (13.1%) had coronary artery disease; four patients (6.5%) had an implantable cardioverter‐defibrillator (ICD). Fourty‐seven patients (77.0 %) had a normal left ventricular (LV) function (>50%). In six patients (9.8%), LVEF was slightly reduced (41%‐50%), in six patients (9.8%) moderately reduced (31%‐40%), and in two patients (3.3%) severely reduced (<30%). Before the procedure, 47 patients (77%) presented with palpitations; 18 (29.5%) suffered from presyncope, and 4 (6.5%) from syncope. No patient underwent previous cardiac resuscitation. Patient's clinical characteristics are shown in Table 1.
Table 1.
Baseline characteristics of the study population
| Age (y) | 45.9 ± 12.5 |
| Male gender | 32 (52.4%) |
| Hypertension | 14 (22.9%) |
| Diabetes mellitus | 2 (3.2%) |
| Coronary artery disease | 8 (13.1%) |
| Previous ICD implantation | 4 (6.5%) |
| LVEF | 59.2 ± 5.1% |
| Palpitations | 47 (77%) |
| Pre‐syncope | 18 (29.5%) |
| Syncope | 7 (11.4%) |
| Dyspnea | 4 (6.5%) |
| Medical therapy resistance | 26 (87) |
Abbreviations: ICD, implantable‐cardioverter defibrillator; LVEF, left ventricular ejection fraction.
PVC ablation was considered in presence of the following inclusion criteria: (a) highly symptomatic monomorphic PVC and/or a related decrease of LVEF, according to EHRA/HRS expert consensus on catheter ablation of ventricular arrhythmias9; (b) more than 10 thousands PVC/24 hours documented in at least 3 consecutive 24‐hour Holter recordings, with a maximal interval of 90 days between the last two; (c) absence of metabolic and electrolytic disorders or advanced systemic diseases; (d) clinical resistance to antiarrhythmic therapy. 92% of patients were symptomatic at least once a month, and in 70 % of patients, previous medical therapy was inefficient. All patients experienced failed attempts of antiarrhythmic medical therapy except one who presented severe bradycardia. Among patients who experienced a failure of antiarrhythmic therapy (n = 60), the majority were under beta‐blockers (78.6%; n = 48), then calcium channel blockers were given in 24.5% of patients (n = 15), class III antiarrhythmic drugs were administered in 13.1% (n = 8) and class IC antiarrhythmic drugs were tried in 9.8% (n = 6).
The 24 hours Holter ECG showed a burden of 19 398 ± 7208 PVC per patient per day (mean burden of PVC per patient was 18.3 ± 6.8% a day; range 7.5%‐30.6%).
Four patients (6.5%) had already underwent a radiofrequency ablation for PVC; all the remaining patients (93.4%) underwent their first PVC ablation procedure. All patients provided written informed consent before the procedure; the study protocol was approved by the institutional review board.
2.2. Electrophysiology study and radiofrequency ablation
Electrophysiology study and ablation was performed in the fasting state, under conscious sedation. All patients had discontinued anti‐arrhythmic medication at least five half‐lives before the procedure. A 3‐dimensional electromagnetic mapping system (CARTO 3, Biosense Webster Inc., Diamond Bar, CA) was used in all patients. Two diagnostic catheters were respectively placed in the coronary sinus and right ventricular apex. Patients with ECG criteria for presumptive right ventricular PVCs origin underwent electro‐anatomical mapping of the right ventricle (RV)10; activation mapping of the PVC and sinus rhythm substrate mapping were recorded in two distinct maps. Standard cut‐offs for low voltage (0.5‐1.5 mV) and scar area (<0.5 mV) definition were used. Patients with a distal to proximal activation of the coronary sinus (CS) during PVC underwent CS electroanatomical mapping after right ventricular mapping; if the CS was not involved in PVC origin, LV mapping was then performed. Patients with ECG criteria for presumptive PVC origin form the LV underwent activation mapping of the coronary cusps and, in absence of coronary cusps origin, the basal portion of the LV. A 3.5‐mm irrigated‐tip ablation catheter was used for mapping and ablation (SmartTouch™, Biosense Webster Inc., Diamond Bar, CA) via the right femoral vein or right femoral artery. Retrograde transaortic approach was attempted in all patients; anterograde approach with transseptal (SL Needle BRK‐1 St Jude Medical, St Paul, MN) puncture was applied when retrograde mapping did not allow sufficient CF. During LV chambers mapping heparin was administered in order to maintain an activated clotting time of approximately 300 seconds; no heparin was administered during RV and CS mapping.
SmartTouch™ (Biosense Webster Inc., Diamond Bar, CA) is a CF monitoring technology integrated into the Thermocool SmartTouch™ irrigated tip catheter and CARTO 3 electroanatomical mapping system. CF values are visualized in different ways on the CARTO 3 system, as a real time value, direction, and real time force graph. The quantitative CF is visualized on the CARTO 3 screen as an average or maximum value within a given time window; the direction of the force is displayed as a color‐coded arrow vector on the tip of the catheter image; a real‐time force graph is displayed in an independent window to visualize the chronological changes during the cardiac cycle.
After the assessment of PVC morphology on the 12 leads ECG, a 3D anatomical map of the ventricle was reconstructed. The following mapping approach was primarily guided by the PASO™ module (CARTO 3, Biosense Webster Inc., Diamond Bar, CA). Once identified the anatomical area of PVC source by PASO automated pacemapping, if the amount of PVCs was adequate, an electroanatomical map with early activation analysis was performed for that specific area in order to confirm the site location.
RF was started on the spots identified by PASO™ when stable catheter position with ≥10 g of CF were obtained.
2.3. Template matching: the PASO™ module
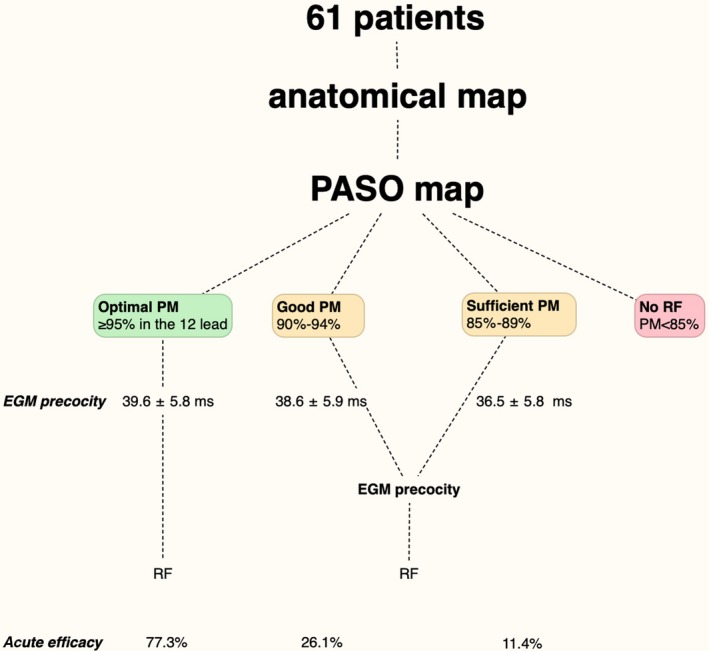
Matching between paced beats and spontaneous PVC was automatically assessed using PASO™ software module. This software compares paced electrocardiograms to a maximum of 4 different acquired arrhythmia ECGs. An average correlation match value is assessed for each of the 12 ECG leads by calculating the area from baseline to the signal for the captured interval. Pace mapping was performed at the same cycle length than the coupling interval of the PVC and using a stimulation amplitude of 1 mA greater than the diastolic threshold. We considered as optimal pace‐mapping presence of PASO™ mean correlation index ≥95% in the 12 lead, good pace‐mapping as PASO™ between 90% and 94%, sufficient pace‐mapping as PASO™ between 85% and 89%; no RF delivery was allowed in presence of PaSo <85%. In presence of PASO™ <95% RF ablation was performed on the site showing the best achievable electrograms (EGM) precocity around the region of the best Pacemapping.
Ablation was performed delivering RF energy using a power from 20 W up to 30 W, a temperature limit of 43°C and a set 17 ml/min cold saline flow. At successful RF ablation sites, energy delivery was continued for 90 seconds. When PVCs were not eliminated after 30 seconds, the energy delivery was terminated and another site was selected for remapping and ablation. The successful ablation site was defined as the elimination of PVC. After successful RF pulse, two further consolidation RF pulses were delivered for each patient.
2.4. Definitions
The procedure was defined as acutely effective if the PVC disappeared during RF and did not recur within 30 minutes after last RF delivery. Recurrence of PVC within 24 hours post ablation was defined as acute failure. A decrease >95% in PVC burden at 1‐year ECG Holter monitoring after ablation procedure, as compared to pre‐ablation ECG Holter, was defined as long‐term effective. We defined cases with discrepancy as those showing precocity higher or lower than mean ± SD in Optimal and Sufficient Categories.
2.5. Follow‐up
All patients underwent 12‐lead ECGs and 24‐hours Holter monitoring during the 24 hours after the procedure. ECG, Holter monitoring and transthoracic echocardiography were performed during the follow‐up at 1, 3, and 12 months after the ablation procedures. Antiarrhythmic drugs were discontinued after the ablation if the procedure was successful. One‐year follow‐up data were available for all patients who had undergone successful ablation.
2.6. Statistical analysis
Continuous variable are expressed as the mean ± standard deviation and compared using the T test; categorical variables are expressed as numbers and percentages and were compared using the chisquared test. Statistical significance was selected at a value of P < 0.05.
Survival tree (ST) analysis was applied to discover groupings of RF applications with homogeneous outcome, using information provided by clinical and procedural covariates. ST are nonparametric statistical procedures that, differently from traditional survival model (eg, Cox model), do not rely upon restrictive assumptions such as proportional hazards algorithms.11 The procedure is based on binary recursive partitioning of a group of subjects, aiming at the choice of optimal cut‐points for binary, ordinal or continuous covariates, which maximizes the split criterion.12, 13 The output is a decision tree, consisting of nodes and leaves, with each leaf indicating a class or a predicted outcome value. Covariates considered for the analysis were: number of PVC at preprocedure Holter recording, CF, PASO™ matching, EGM precocity. The algorithm examined all possible splits on each predictor variable and selected the best predictor and best cut point, maximizing the survival difference between nodes. ROC curve was derived and the area under the curve was estimated.
3. RESULTS
3.1. Baseline characteristics
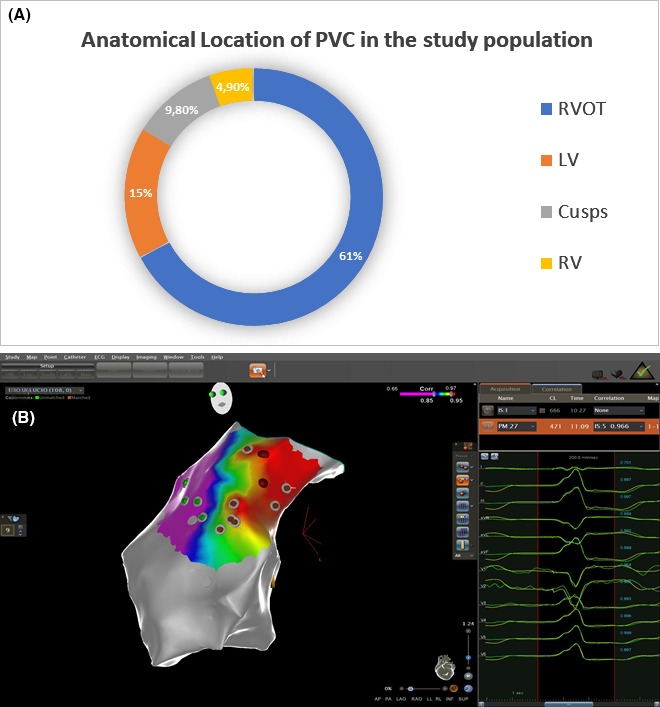
The PVC ablation was performed in the right ventricular outflow tract in 37 patients (60.7%), LV in 15 patients (24.6%), coronary cusps in 6 patients (9.8%), right ventricle in 3 patients (4.9%); location are shown in Figure 1. In six patients with previous myocardial infarction, PVC origin was located in low voltage areas (four in the anterior wall and two in the inferior wall of LV).
Figure 1.
Panel A: Anatomical distribution of PVCs in the study population. Panel B: An example of a 3D Carto anatomical mapping of RVOT showing postero‐septal PVC origin. This RVOT map concerns a 32 y old female with a burden of 29 450 monomorphic PVCs per day. PASO™ correlation was 97%, – electrograms precocity 43 ms, Contact force 14 mg. After 27 s of radiofrequency delivery PVCs disappeared. LV: left ventricle; PVC: premature ventricular contraction; RVOT: right ventricle out flow tract; RV: right ventricle; Cusps: Aortic cusps
Mean procedural and fluoroscopy time were 94.5 ± 20.9 and 4.3 ± 2.5 minutes respectively. Transeptal approach was used in eight patients to access the LV. PASO map was reconstructed with 12 ± 5 pacing points. During RF delivery, CF was 18.3 ± 6.4 g (median 17.5 g) and temperature was 39.7 ± 2.0 °C. The duration of RF applications was 335.4 ± 130.4 seconds per patient; 4.9 ± 2.0 RF pulses were delivered for each patient. Procedural data are shown in Table 2.
Table 2.
Procedural data
| Procedural time (min) | 116.0 ± 37.5 |
| Fluoroscopic time (min) | 4.4 ± 2.5 |
| RF application (s) | 335.4 ± 130.4 |
| Mean Contact force (g) | 18.3 ± 6.4 |
| Ablation temperature (°C) | 39.7 ± 2.0 |
3.2. Acute success
PVC ablation was acutely successful in 59 of patients (96.7%). In two cases the procedure was acutely ineffective. In a 37‐years‐old female with a PVC focus arising from a right para‐Hissian site, PASO™ showed a correlation of 0.95 and the activation map showed an early activity of −42 ms. After a first successful radiofrequency delivery (20 W for 60 seconds), the PVC recurred after 15 minutes and the procedure was stopped because of the proximity to the conduction system. However, the PVC burden markedly decreased from 25.9% until to 11.8% the day following the procedure and to 9.2% at 1‐year follow‐up. In a patient with PVC exit from LV summit PVC suppression was not achieved after RF delivery (20 W for 60 seconds); PASO™ showed a correlation of 0.96 and the activation map showed an early activity of −32 ms. No further RF applications due to proximity (2 mm) of the distal tip of the ablation catheter to the left main coronary artery at coronary angiography.
High power setting was required to obtain acute procedural success in three cases. One was a 39‐year‐female, with a previous failed procedure, with PVC arising from the region of septal tricuspid annulus; power setting was increased to 40 W. Two cases with PVC originating from low voltage antero‐septal scar area CF was >10 g but, to achieve acute success, power was increased up to 50 W.
No major procedure‐related complications occurred in the study population; three (4.9%) patients showed minor vascular complications not requiring surgery.
3.3. Predictors of acute success
PASO™ matching before RF delivery was 94.4 ± 2.9%. PASO was 96.8 ± 1.1% in Optimal pace‐mapping sites, 93.0 ± 1.1% in good pace‐mapping, and 91.8 ± 4.6% in sufficient pace‐mapping sites (P < 0.001). EGM precocity was 39.6 ± 5.8 ms in the optimal pace‐mapping sites, 38.6 ± 5.9 ms in the good pace‐mapping sites, and 36.5 ± 5.8 ms in the sufficient pace‐mapping sites (P = 0.57) (Figure 2). Contact force was 17.7 ± 5.6 g in the optimal pace‐mapping sites, 16.6 ± 5.2 g in the good pace‐mapping sites, and 19.4 ± 7.1 g in the sufficient pace‐mapping sites (P = 0.34). RF was effective in 77.3% in sites with optimal pace‐mapping; was 26.1% in sites with good pace‐mapping efficacy, and it was 11.4% in sites with sufficient pace‐mapping. In the effective ablation sites, the PASO™ matching was higher compared to ineffective sites (96.0 ± 2.8% vs 93.3 ± 2.5%, P < 0.01). Relationship between PaSo and efficacy, stratified for first RF application and later RF applications is shown in Figure S1. EGM precocity at the activation map was 38.9 ± 6.0 ms; it was higher in effective than in ineffective sites (41.3 ± 5.9 ms vs 37.2 ± 5.5 ms, P = 0.003). Relationship between EGM precocity and efficacy, stratified for first RF application and later RF applications is shown in Figure S2.
Figure 2.
Pacemapping results, electrogram precocity, and acute efficacy in the study population. EGM precocity was 39.6 ± 5.8 ms in the optimal pace‐mapping sites, 38.6 ± 5.9 ms in the good pace‐mapping sites, and 36.5 ± 5.8 ms in the sufficient pace‐mapping sites. EGM precocity: Electrogram precocity
Ten ineffective RF pulses were delivered in sites showing Optimal pacemapping (PM), 65 in Good PM sites, and 31 in Sufficient PM sites. After successful RF pulse, two further consolidation RF pulses were delivered for each patient. Patients with ineffective RF pulses in optimal PM sites received further RF pulses in surrounding areas that resulted in PVC elimination. Those effective RF pulses showed slightly higher PASO™, without reaching statistical significance, as compared to ineffective ones.
We found one case with discrepancy in the Optimal PASO™ category: one RF pulse showed a precocity of 29 ms, despite a PASO™ of 97%; the RF pulse was successful. In the Sufficient PASO™ category we found two discrepancies: one showing a precocity of 44 ms, despite a PASO™ of 89%; the RF pulse was unsuccessful. One RF showed a precocity of 29 ms, despite a PASO™ of 87%, the RF pulse was unsuccessful. Relationship between PaSo and EGM precocity is shown in Figure S3.
Based on ST analysis, a PASO™ >94 % was chosen as the best predictor of RF application efficacy.
3.4. Follow‐up
At 1‐year follow‐up 57 of patients (93.4%) showed absence of significant PVC burden at Holter monitoring. In one patient with PVC origin from left posterior papillary muscle mean PVC burden before the procedure was 11.8%. PASO™ showed a correlation of 0.97 and the activation map showed an early activity of −31 ms; ablation procedure was acutely effective and the Holter monitoring 24 hours later showed a PVC burden of 0.01%. However, the PVC recurred 5 days later, showing a different morphology (Figure 3); PVC burden was 8.2% at 1‐year follow‐up.
Figure 3.
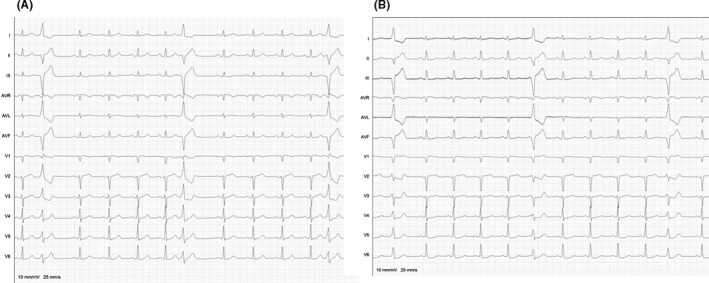
Panel A: ECG of 63 years old male with premature ventricular contraction originating from left posterior papillary muscle. Acute success in this patient was obtained with power set at 50 W. PASO™ showed a correlation of 0.97 –and electrograms precocity was 31 ms. Panel B: PVC recurred few days later with a different morphology
The other patient with PVC recurrence was affected by chronic ischemic cardiomyopathy; despite a sufficient PASO™, PVC recurrence with a similar morphology was recorded at 1‐month Holter monitoring.
4. DISCUSSION
The main findings of the present study are: (a) monomorphic PVCs ablation mainly guided by PASO™ and CF showed high success rate in both acute and 1‐year follow‐up (96.7% and 93.4%, respectively); (b) PASO™ ≥95% was associated with acute PVC elimination in 77% of cases.
4.1. Added value of pace mapping and contact force
Catheter ablation of ventricular ectopy is traditionally based both on pace‐mapping and activation mapping.14 Recent technological improvements in 3‐D mapping allowed a higher precision and better spatial resolution of activation maps14; in addition, while the results of pace‐mapping were subjected to visual interpretation by the operator, activation mapping provided more objective information. However, an approach exclusively based on activation mapping might be ineffective in absence of frequent spontaneous PVC during the electrophysiological study, which makes the acquisition of the map rather challenging. Automatic pace‐mapping method implemented in the PASO™ module, provides an objective and a quantitative assessment of the template matching. Quantitative comparison between clinical ectopy and paced morphology using mathematical waveform comparison metrics was initially described in 2003.6 Reports indicated that with pace mapping, the closer the pacing site from the tachycardia site of origin, the greater the similarity of the QRS morphology between the tachycardia and paced morphology.6
In a study by Kurosaki et al,3 the template matching was shown to be a better discriminator than subjective scoring by experienced electrophysiologists. A template matching correlation of ≥90% predicted a successful ablation site with a sensitivity of 90%. In our study, both the acute and the 12 months follow‐up success rates were high, with short fluoroscopy time and low rate minor complications.
Published detailed data on follow‐up after ablation of PVC is limited, in particular using the technologies and algorithms available nowadays. Acute success in published series ranges between 77% and 97%,2, 7, 8, 15 while long‐term success ranges between 77% and 83%.2, 7, 15 Real time CF monitoring was proven to increase acute and long‐term success for AF ablation.16 Low CF values during ablation in atrial fibrillation have been associated with higher recurrence rates.17 Mizuno et al18 showed that during ventricular substrate mapping a CF >8 g is a predictor of good contact with the tissue and is mandatory to correctly record low voltage components of ventricular electrograms. In the present study an average CF of 18.3 g was used during the PVC ablation. The CF information may be useful to prevent complications because of excessive pressure on a thin myocardium, such as in the right ventricle, and also to create a more effective and durable lesion.
In presence of an optimal or good pace‐mapping and persistence of the PVC after ablation, careful attention should be payed to CF monitoring during RF delivery; achievement of stable CF during ablation without PVC disappearance should prompt the suspect of deep origin of the PVCs that in some cases might be treated with high power RF settings (Figure 4).
Figure 4.
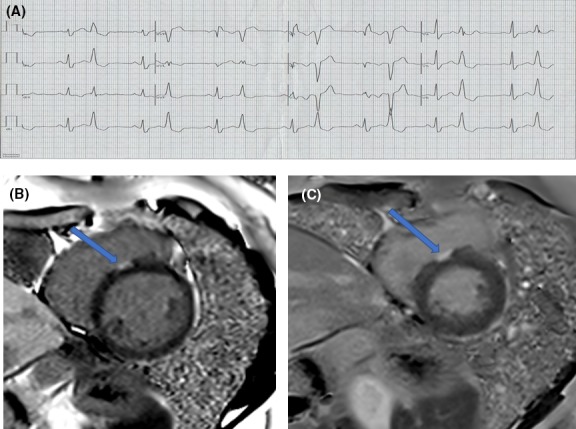
Panel A: Twelve‐leads ECG of a 39‐years‐old female highly symptomatic for frequent premature ventricular complex leading to reduce left ventricular ejection function. A first attempt to ablate PVC failed. Patient was referred to our centre for a second procedure. Panel B: Short axis LGE images of CMR. Before the second ablation a CMR was performed to evaluate left ventricular function. The left ventricular ejection fraction was 43%. A small zone of Late Gadolinum Enhancement was detected in the septal region of the tricuspid annulus (blue arrow). The max depth of the lesion in the septum was 5 mm. Panel C: Cardiac magnetic resonance was repeated 6 mo after the second successful PVC ablation. The left ventricular ejection fraction improved to 57%. The area of Late Gadolinum Enhancement (blue arrow) was larger and deeper (7 mm)
In our study, the RF ablation was guided by CF sensing targeting minimum values of 10 g; although no complications occurred, no statistical inference can be done because of the small group of patients with PVC recurrence (n = 2) and the lack of a control group.
4.2. Limitations
The most important limitation of this study is related to its retrospective design. In addition, being a single tertiary center study, results should not be generalized. Other important limitations are the relatively low number of patients enrolled in the study because of the stringent inclusion criteria and the lack of a control group. PASO™ was used as the main guide for RF ablation; in presence of PASO™ >95%, EGM precocity was not considered in the decisional step.
5. CONCLUSIONS
Premature ventricular contraction RF ablation mainly guided by PASO™ and CF showed high success rate in both acute and 1‐year follow‐up (96.7% and 93.4%, respectively) with a very low rate (4.9%) of minor complications. The best efficacy cut‐off for RF ablation of PVCs has been identified in presence of both PASO™ ≥95% and CF >10 g.
CONFLICT OF INTEREST
LC receives fees for teaching purposes from St Jude Abbott. PB receives speakers fees from Biotronik, Medtronic. GBC receives compensation for teaching purposes and proctoring from AF solutions, Medtronic. CdA receives compensation for teaching purposes and proctoring from AF solutions, Medtronic, member steering committee ETNA‐AF‐Europe Daiichi Sankyo Europe and research grants on behalf of the centre from Biotronik, Medtronic, St Jude Medical Abbot, Livanova, Boston Scientific.
Supporting information
Capulzini L, Vergara P, Mugnai G, et al. Acute and one year outcome of premature ventricular contraction ablation guided by contact force and automated pacemapping software. J Arrhythmia. 2019;35:542–549. 10.1002/joa3.12194
Lucio Capulzini and Pasquale Vergara equally contributed to the study as shared first authors.
Gianbattista Chierchia and Carlo de Asmundis equally contributed to this study as last authors.
REFERENCES
- 1. Joshi S, Wilber DJ. Ablation of idiopathic right ventricular outflow tract tachycardia: current perspectives. J Cardiovasc Electrophysiol. 2005;16(Suppl. 1):S52–549. [DOI] [PubMed] [Google Scholar]
- 2. Luker J, Sultan A, Servatius H, Berner I, Hoffmann BA, Willems S, et al. Automated template matching correlates with earliest activation during mapping of idiopathic premature ventricular contractions. IJC Heart & Vessels. 2014;4:25–9. [Google Scholar]
- 3. Kurosaki K, Nogami A, Sakamaki M, Kowase S, Sugiyasu A, Oginosawa Y, et al. Auto‐ mated template matching to pinpoint the origin of right ventricular outflow tract tachycardia. Pacing Clin Electrophysiol. 2009;32(Suppl. 1):S47–51. [DOI] [PubMed] [Google Scholar]
- 4. Yamada T, Murakami Y, Yoshida N, Okada T, Toyama J, Yoshida Y, et al. Efficacy of electroanatomic mapping in the catheter ablation of premature ventricular contractions originating from the right ventricular outflow tract. J Interv Card Electrophysiol. 2007;19:187–94. [DOI] [PubMed] [Google Scholar]
- 5. Azegami K, Wilber DJ, Arruda M, Lin AC, Denman RA. Spatial resolution of pacemapping and activation mapping in patients with idiopathic right ventricular outflow tract tachycardia. J Cardiovasc Electrophysiol. 2005;16:823–9. [DOI] [PubMed] [Google Scholar]
- 6. Gerstenfeld EP, Dixit S, Callans DJ, Rajawat Y, Rho R, Marchlinski FE. Quantitative comparison of spontaneous and paced 12‐lead electrocardiogram during right ventricular outflow tract ventricular tachycardia. J Am Coll Cardiol. 2003;41:2046–53. [DOI] [PubMed] [Google Scholar]
- 7. Darrieux FCC, Scanavacca MI, Hachul DT, Melo SL, D’Ávilla AB, Gruppi CJ, et al. Radio‐ frequency catheter ablation of premature ventricular contractions originating in the right ventricular outflow tract. Arq Bras Cardiol. 2007;88:265–72. [DOI] [PubMed] [Google Scholar]
- 8. Krittayaphong R, Sriratanasathavorn C, Bhuripanyo K, Raungratanaamporn O, Soongsawang J, Khaosaard B, et al. One‐year outcome after radiofrequency catheter ablation of symptomatic ventricular arrhythmia from right ventricular outflow tract. Am J Cardiol. 2002;89:1269–74. [DOI] [PubMed] [Google Scholar]
- 9. Aliot EM, Stevenson WG, Almendral‐Garrote JM, Bogun F, Calkins CH, Delacretaz E, et al. Rhythm Association (EHRA); Registered Branch of the European Society of Cardiology (ESC); Heart Rhythm Society (HRS); American College of Cardiology (ACC); American Heart Association (AHA). EHRA/HRS Expert Consensus on Catheter Ablation of Ventricular Arrhythmias: developed in a partnership with the European Heart Rhythm Association (EHRA), a Registered Branch of the European Society of Cardiology (ESC), and the Heart Rhythm Society (HRS). Heart Rhythm. 2009;6:886–933. [DOI] [PubMed] [Google Scholar]
- 10. Trevisi N, Silberbauer J, Radinovic A, Bavila R, Sala S, Vergara P, et al. New diagnostic criteria for identifying left‐sided ventricular ectopy using non‐contact mapping and virtual unipolar electrogram analysis. Europace. 2015;17:108–16. [DOI] [PubMed] [Google Scholar]
- 11. Breiman L, Friedman JH, Olshen RA, Stone CI. Classification and regression trees. Belmont, CA: Wadsworth; 1984. [Google Scholar]
- 12. Zhou Y, McArdle JJ. Rationale and applications of survival tree and survival ensemble methods. Psychometrika. 2015;80:811–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vergara P, Tzou WS, Tung R, Brombin C, Nonis A, Vaseghi M, et al. Predictive score for identifying survival and recurrence risk profiles in patients undergoing ventricular tachycardia ablation. Circ Arrhythm Electrophysiol. 2018;11(12):e006730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bogun F, Taj M, Ting M, Kim HM, Reich S, Good E, et al. Spatial resolution of pace mapping of idiopathic ventricular tachycardia/ectopy originating in the right ventricular outflow tract. Heart Rhythm. 2008;5:339–44. [DOI] [PubMed] [Google Scholar]
- 15. Fichtner S, Senges J, Hochadel M, Tilz R, Willems S, Eckardt L, et al. Safety and efficacy in ablation of premature ventricular contraction: data from the German ablation registry. Clin Res Cardiol. 2017;106:49–57. [DOI] [PubMed] [Google Scholar]
- 16. Reddy VY, Shah D, Kautzner J, Schmidt B, Saoudi N, Herrera C, et al. The relationship between contact force and clinical out‐ come during radiofrequency catheter ablation of atrial fibrillation in the Toccata study. Heart Rhythm. 2012;9:1789–95. [DOI] [PubMed] [Google Scholar]
- 17. Marjijon E, Fazaa S, Narayanan K, Guy‐Moyat B, Bouzeman A, Providencia R, et al. Real‐time contact force sensing for pulmonary vein isolation in the setting of paroxysmal atrial fibrillation: procedural and 1‐year results. J Cardiovasc Electrophysiol. 2013;25:130–7. [DOI] [PubMed] [Google Scholar]
- 18. Mizuno H, Vergara P, Maccabelli G, Trevisi N, Eng SC, Brombin C, et al. Contact force monitoring for cardiac mapping in patients with ventricular tachycardia. J Cardiovasc Electrophysiol. 2013;24:519–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


