Abstract
Aims
We aimed to investigate the impact of various genetic polymorphisms affecting thiopurine metabolism pathways and toxicity in paediatric acute lymphoblastic leukaemia patients for the first time in Korea.
Methods
From May 2006 to September 2016, 139 paediatric acute lymphoblastic leukaemia patients treated with combination chemotherapy including 6‐mercaptopurine were included in the study. One hundred and twenty‐three variants in 43 genes, including TMPT and NUDT15, were screened using targeted genotyping, such as a MassARRAY system, direct sequencing and polymerase chain reaction‐restriction fragment length polymorphism methods. Among the polymorphisms screened, 103 polymorphisms of 43 genes were included for further analyses.
Results
The genetic polymorphisms in the ABCC4, AHCY, ATIC, FAM8A6P, GART, GNG2, GSTA1, MTHFD1, MTHFR, NUDT15, PACSIN2, TYMS and XDH genes, and an intronic polymorphism between HIVEP2 and AIG1, and TPMT genotype were associated with thiopurine metabolism (P < 0.05). Genetic polymorphisms in the ABCC4, ADK, ATIC, GART, GMPS, GSTP1, IMPDH1, ITPA, KCNMA1, MOCOS, MTRR, NUDT15, SLC19A1, SLC28A3, SLC29A1, SLCO1B1, TYMP and XDH genes were associated with thiopurine‐related toxicities; neutropenia, hepatotoxicity and treatment interruption (P < 0.05).
Conclusions
Findings of this study may provide basic knowledge for personalized medicine for thiopurinxe treatment in paediatric acute lymphoblastic leukaemia patients.
Keywords: acute leukaemia, genome‐wide association study, paediatric, polymorphisms, thiopurine
What is already known about this subject
Thiopurine‐related toxicity is frequently observed in Asian populations, with more importance of the NUDT15 genotype rather than the TPMT genotype.
There is a lack of study focused on the genetic polymorphisms and metabolites, which can provide information on toxicity in Korean patients.
What this study adds
This is the first study focused on the various genetic polymorphisms in association with thiopurine metabolism in Korean paediatric leukaemia patients.
Among the 103 SNPs analysed, 32 SNPs in 24 genes (including NUDT15) other than TPMT genotype were associated with either or both thiopurine metabolism and any adverse effects.
1. INTRODUCTION
Mercaptopurine (6‐MP), the thiopurine, is used in combination chemotherapy as a myelosuppressive maintenance therapy and is required for more than 2 years in paediatric patients with acute lymphoblastic leukaemia (ALL) to achieve a long‐term cure.1, 2, 3 However, adverse effects of 6‐MP, including myelotoxicity which can lead to life‐threatening infection, and hepatotoxicity often lead to decreased thiopurine dosage or treatment interruption.2 The pharmacokinetic and pharmacogenetic characteristics of thiopurine drugs are complicated (Figure S1), with high intra‐ and inter‐individual variability.4, 5
Thiopurine S‐methyltransferase (TPMT) is one of the well‐known key enzymes involved in the metabolism of thiopurine drugs and its activity is largely influenced by polymorphisms of the TPMT gene.6 6‐MP is converted into active or inactive metabolites, such as 6‐thioguanine nucleotides (6‐TGN) and 6‐methylmercaptopurine (6‐MeMPN) by TPMT intracellularly.7 Patients with inherited TPMT deficiency influenced by the polymorphism in TPMT gene present with higher 6‐TGN level and have an increased risk of myelotoxicity and/or with higher 6‐MeMPN level and have an increased risk of hepatotoxicity, respectively.8 Despite a lower frequency of TPMT variant alleles in Asians, the incidence of thiopurine‐induced leukopenia is higher in Asians than in individuals of European descent.9 Studies have found an association between several genetic polymorphisms and toxicity in paediatric ALL patients, but the association varies among ethnic groups.5, 10 For example, thiopurine‐related toxicity is frequently observed in Asian populations, with importance of the Nudix hydrolase 15 (NUDT15) genotype rather than the thiopurine methyltransferase (TPMT) genotype.11
It has been suggested that pharmacogenomics should be incorporated as a dosage‐calibrating tool in the treatment of paediatric ALL in order to predict and minimize the occurrence of serious toxicity in these patients.5 However, to the best of our knowledge, there is a lack of pharmacogenetic studies focused on the various genetic polymorphisms in association with thiopurine metabolism and toxicity in Korean paediatric ALL patients. Therefore, the aims of this study were to explore the genetic polymorphisms of genes involved in the thiopurine and folate metabolism pathways and their relationships with thiopurine metabolism and toxicity in Korean paediatric ALL patients. These findings may serve as a basis for thiopurine therapy in paediatric ALL patients.
2. METHODS
2.1. Study population and definition
This retrospective study included paediatric ALL patients who were treated with 6‐MP at the Paediatric Department of Samsung Medical Center (Seoul, Korea) from May 2006 to September 2016. Clinical information including 6‐MP dosage (mg/body surface area/day), duration of 6‐MP treatment, and adverse effects related to treatment were investigated. The treatment protocol has been described in detail previously.12 Patients who were over 10 years of age or under 12 months or who had initial white blood cell (WBC) count of 50 × 109 L−1 or more were considered as high‐risk ALL patients (the others were standard risk ALL patients) according to the risk stratification criteria of the National Cancer Institute.13 Laboratory data including 6‐TGN and 6‐MeMPN levels, WBC count, neutrophil count, aspartate aminotransferase (AST), alanine aminotransferase (ALT) and bilirubin were collected.
We defined the terms used to describe the adverse effects of thiopurine treatment as follows: leukopenia, WBC count <1.5 × 109 L−1; neutropenia, absolute neutrophil count <0.5 × 109 L−1; hepatotoxicity, ALT or AST enzyme activity >3 times the upper limit of the reference range and bilirubin >1.5 times the upper limit of the reference range after starting 6‐MP and during a stable disease state; and treatment interruption, any period in which 6‐MP therapy was discontinued due to an adverse drug reaction. Treatment was interrupted when patients showed significant hematopoietic toxicity (absolute neutrophil count below 0.5 × 109 L−1 or platelet count below 50 × 109 L−1) or serious infectious events.12 This study was approved by the Institutional Review Board of Samsung Medical Center (IRB file No. SMC 2012‐04‐106‐014).
2.2. Measurement of thiopurine metabolites
Measurements of RBC levels of thiopurine metabolites (6‐TGN and 6‐MeMPN) were performed using a liquid chromatography–tandem mass spectrometry as described previously.4, 14, 15 The lower limit of quantification (LOQ) was 0.1 μmol L−1 for 6‐TGN and 0.5 μmol L−1 for 6‐MeMPN.4, 15 The details of the measurement method have been described previously.15 For each individual patient, the ratio of 6‐MeMPN to 6‐TGN and the ratio of the 6‐TGN to 6‐MP dose was calculated to analyse the patient's pharmacological phenotype.16
2.3. SNP selection and targeted genotyping
Genetic polymorphisms associated with thiopurine metabolism were reviewed through a literature review and public databases, such as the PubMed and PharmGKB databases (http://www.pharmgkb.org/pathway, Figure S1) using search keywords in various combinations of the following words: ‘leukaemia/leukemia’, ‘thiopurine’ and ‘polymorphism’.17 A total of 123 genetic polymorphisms of 43 genes including the TPMT gene and the NUDT15 gene were included for genetic analyses.
Single nucleotide polymorphisms (SNPs) were genotyped using the MassARRAY® system with SpectroTYPER™ software (Sequenom, Inc., CA, USA).4 The TPMT genotype and NUDT15 genotype of each patient were determined by direct sequencing analysis as described previously.4, 18 The TYMS polymorphism (28‐bp repeat) was analysed using polymerase chain reaction as described previously.4, 19 Among 121 SNPs in 43 genes, after excluding 18 SNPs of 13 genes with a call rate < 90%, a minor allele frequency (MAF) < 0.01, or a Hardy–Weinberg equilibrium P‐value <0.001, 103 autosomal genetic polymorphisms in 43 genes and the TPMT genotype were included for statistical analysis (Table S1). The variant of c.416G>A in the NUDT15 gene (rs147390019, also reported as the *4 allele11) was excluded in the final analysis because of its low allele frequency in this study (<0.01).
2.4. Statistical analysis
Quantitative non‐normally distributed variables are expressed as medians and interquartile ranges (IQR). Categorical variables are presented as frequencies and percentages. Nonparametric methods were used for non‐normally distributed data. The association between mean dose of 6‐MP and metabolites (6‐TGN and 6‐MeMPN) was analysed using the generalized estimating equations. Linear regression analysis was performed under genotype, dominant and recessive genetic models in order to evaluate the association of thiopurine metabolites and ratios with each polymorphism with a frequency of more than 10% in this study population.20 The P‐value was corrected with the false‐discovery rate (FDR) for candidate polymorphisms according to the linear step‐up procedure.21 Fisher's exact test was performed under genotype, dominant and recessive genetic models in order to evaluate the association of adverse effects of thiopurine treatment.20 The FDR correction was used to adjust the P‐values for multiple comparisons. Statistical analyses were performed using R 3.1.0 (R Foundation for Statistical Computing, Vienna, Austria) and SAS version 9.3 (SAS Institute, Cary, NC). P‐values less than 0.05 were considered to be significant.
2.5. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY.22
3. RESULTS
3.1. Demographic characteristics
A total of 139 patients (64 boys and 75 girls) treated with 6‐MP (median dose, 30.1 mg m−2 per day) were included in this study. The median (range) duration of treatment with 6‐MP to measure thiopurine metabolites used for analysis was 23.7 months (1.4–91.3 months). The follow‐up period for these patients ranged from 1.5–10.7 years (median 5.9 years). The length of treatment duration ranged from 1.4 to 92.9 months (median 25.6 months). During the follow‐up period, 1–14 times (median 7 times) of thiopurine metabolites measurement were performed for each individual patient. Among the 139 patients, six had a TPMT variant (two patients heterozygous for *3C, two heterozygous for *6, one heterozygous for the *32 allele, and one heterozygous for the variant of uncertain significance, c.532T>C23). The length of treatment interruptions (median, IQR) in the first year of maintenance therapy was 20.0 (7.0–32.0) days. Patient characteristics are summarized in Table 1.
Table 1.
Patient characteristics (139 paediatric patients with acute lymphoblastic leukaemia)
| Sex, n (%) | |
| Male | 64 (46) |
| Female | 75 (54) |
| Age, median (IQR), years | 5.1 (3.7–10.6) |
| Follow‐up period, median (IQR), years | 5.9 (3.6–7.9) |
| Body weight, median (IQR), kg | 19.4 (15.2–37.5) |
| Body surface area, median (IQR), m2 | 0.79 (0.66–1.19) |
| Diagnosis | |
| B‐lymphoblastic leukaemia, n (%) | 131 (94) |
| T‐lymphoblastic leukaemia, n (%) | 8 (6) |
| Risk group | |
| Standard risk, n (%) | 86 (62) |
| High risk, n (%) | 53 (38) |
| Number of thiopurine metabolite measurements per patient, median (range) | 7 (1–14) |
| 6‐MP daily dose, median (IQR), mg m−2 per day | 30.1 (22.2–38.9) |
| 6‐TGN level, median (IQR), pmol per 8 × 108 RBCs | 388.5 (301.1–555.2) |
| 6‐MeMPN level, median (IQR), pmol per 8 × 108 RBCs | 5089.0 (2064.2–9363.7) |
| 6‐MeMPN/6‐TGN ratio, median (IQR) | 13.9 (5.2–23.5) |
| 6‐TGN/6‐MP dose ratio, median (IQR) | 12.8 (9.3–18.1) |
| TPMT genotype, n (%) | |
| *1/*1 | 133 (96) |
| *1/*3C | 2 (1) |
| *1/*6 | 2 (1) |
| *1/*32 | 1 (1) |
| *1/? (c.532T>C) (novel allele) | 1 (1) |
| Toxicity, n (%)a | |
| Leukopenia (WBC < 1.5 x 109 L−1) | 38 (27) |
| Neutropenia (ANC < 0.5 x 109 L−1) | 40 (29) |
| Hepatotoxicity | 64 (46) |
| Treatment interruption, n (%) | 114 (82) |
Abbreviations: ANC, absolute neutrophil count; IQR, interquartile range; WBC, white blood cell.
Any toxicity experienced during the follow‐up period.
3.2. Association between 6‐MP dosage and thiopurine metabolites
The levels of thiopurine metabolites were significantly associated with 6‐MP dosage: 6‐TGN, ρ = 0.2085 (95% CI 0.1502–0.2667, Z = 7.01, P < 0.0001) and 6‐MeMPN, ρ = 0.7687 (95% CI 0.6107–0.9268, Z = 9.53, P < 0.0001, Figure 1).
Figure 1.
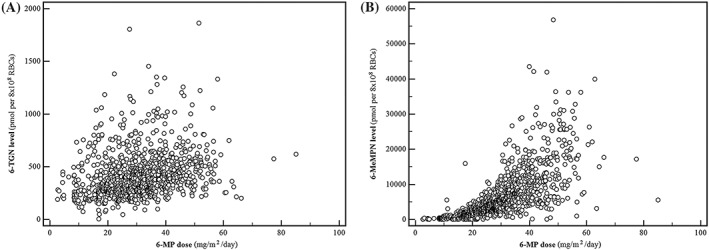
Correlation between the 6‐TGN measurements and 6‐MP dose (A) and 6‐MeMPN measurements and 6‐MP dose (B)
3.3. Genetic polymorphisms associated with thiopurine metabolites and 6‐TGN level/6‐MP dose ratio
Table 2 shows the SNPs associated with thiopurine metabolites. Different genetic polymorphisms were associated with 6‐TGN and 6‐MeMPN levels. Among 139 patients, 36 (25.9%) patients had one or more variant alleles of NUDT15. The allele frequencies were 86.0% for NUDT15*1, and 4.3, 7.6, 1.4 and 0.7% for *2, *3, *5 and *6, respectively. The TPMT variants had significantly lower 6‐MeMPN levels and 6‐MeMPN/6‐TGN ratios. Figure 2A shows the 6‐MeMPN/6‐TGN ratios among patients with different TPMT and NUDT15 genotypes. Patients who had NUDT15 variants showed variable 6‐MeMPN/6‐TGN ratios.
Table 2.
Predictors of log‐transformed thiopurine metabolites (mean ± standard deviation)
| Variables | rsID | Maj | Min | Genotype | Dominant | Recessive | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maj hom | Het | Min hom | P | FDR P | Maj hom + het | Min hom | P | FDR P | Maj hom | Het + min hom | P | FDR P | ||||
| 6‐TGN | ||||||||||||||||
| ATIC | rs16853834 | C | T | 2.56 ± 0.20 | 2.65 ± 0.22 | 2.59 ± 0.12 | 0.0903 | 0.9914 | 2.60 ± 0.21 | 2.59 ± 0.12 | 0.9149 | 0.9553 | 2.56 ± 0.20 | 2.64 ± 0.20 | 0.0415 | 0.9077 |
| GART | rs2070388 | G | C | 2.57 ± 0.20 | 2.69 ± 0.19 | 2.51 ± 0.35 | 0.0090 | 0.3135 | 2.60 ± 0.20 | 2.51 ± 0.35 | 0.2263 | 0.9553 | 2.57 ± 0.20 | 2.65 ± 0.23 | 0.0351 | 0.9077 |
| HIVEP2‐AIG1 a | rs200148 | G | A | 2.60 ± 0.20 | 2.41 ± 0.35 | 0.0194 | 0.5052 | |||||||||
| XDH | rs2295475 | G | A | 2.57 ± 0.25 | 2.59 ± 0.18 | 2.71 ± 0.19 | 0.0634 | 0.9914 | 2.58 ± 0.21 | 2.71 ± 0.19 | 0.0235 | 0.9553 | 2.57 ± 0.25 | 2.62 ± 0.18 | 0.2216 | 0.9077 |
| NUDT15 | rs116855232 | C | T | 2.64 ± 0.19 | 2.51 ± 0.15 | 1.95 ± 0.27 | <.0001 | <.0001 | 2.61 ± 0.19 | 1.95 ± 0.27 | <.0001 | <.0001 | 2.64 ± 0.19 | 2.46 ± 0.24 | <.0001 | 0.0024 |
| NUDT15 | rs186364861 | G | A | 2.62 ± 0.19 | 2.39 ± 0.28 | <.0001 | 0.0040 | |||||||||
| 6‐MeMPN | ||||||||||||||||
| ATIC | rs3821353 | T | G | 3.48 ± 0.67 | 3.76 ± 0.46 | 3.52 ± 0.56 | 0.0438 | 0.9027 | 3.62 ± 0.59 | 3.52 ± 0.56 | 0.3589 | 0.9666 | 3.48 ± 0.67 | 3.65 ± 0.52 | 0.1125 | 0.7348 |
| ATIC | rs16853834 | C | T | 3.53 ± 0.57 | 3.74 ± 0.52 | 3.77 ± 0.46 | 0.0779 | 0.9027 | 3.62 ± 0.56 | 3.77 ± 0.46 | 0.3735 | 0.9666 | 3.53 ± 0.57 | 3.74 ± 0.51 | 0.0240 | 0.7348 |
| FAM8A6P | rs1040637 | G | A | 3.64 ± 0.55 | 3.32 ± 0.73 | 3.42 ± 0.54 | 0.1131 | 0.9027 | 3.6 ± 0.57 | 3.42 ± 0.54 | 0.5977 | 0.9978 | 3.64 ± 0.55 | 3.34 ± 0.69 | 0.0383 | 0.7348 |
| MTHFD1 | rs2236225 | G | A | 3.51 ± 0.62 | 3.71 ± 0.47 | 3.98 ± 0.39 | 0.0775 | 0.9027 | 3.59 ± 0.57 | 3.98 ± 0.39 | 0.2443 | 0.9666 | 3.51 ± 0.62 | 3.73 ± 0.46 | 0.0338 | 0.7348 |
| MTHFR | rs1801133 | C | T | 3.58 ± 0.60 | 3.55 ± 0.59 | 3.88 ± 0.33 | 0.1226 | 0.9027 | 3.56 ± 0.59 | 3.88 ± 0.33 | 0.0418 | 0.9666 | 3.58 ± 0.60 | 3.60 ± 0.57 | 0.8647 | 0.9763 |
| NUDT15 | rs147390019 | G | A | 3.62 ± 0.55 | 2.97 ± 0.94 | 0.0239 | 0.9027 | |||||||||
| NUDT15 | rs186364861 | G | A | 3.62 ± 0.58 | 3.42 ± 0.43 | 0.2224 | 0.9027 | |||||||||
| TPMT | *1 | Other | 3.62 ± 0.55 | 3.05 ± 0.69 | 0.0156 | 0.9027 | ||||||||||
| 6‐MeMPN/6‐TGN ratio | ||||||||||||||||
| ABCC4 | rs2274407 | C | A | 0.98 ± 0.58 | 1.08 ± 0.49 | −0.09 ± 0.00 | 0.0932 | 0.9573 | 1.01 ± 0.55 | −0.09 ± 0.00 | 0.0481 | 0.9388 | 0.98 ± 0.58 | 1.05 ± 0.51 | 0.5103 | 0.9371 |
| ATIC | rs3821353 | T | G | 0.88 ± 0.72 | 1.18 ± 0.43 | 0.92 ± 0.57 | 0.0329 | 0.9573 | 1.03 ± 0.61 | 0.92 ± 0.57 | 0.3294 | 0.9388 | 0.88 ± 0.72 | 1.06 ± 0.51 | 0.1006 | 0.9371 |
| FAM8A6P | rs1040637 | G | A | 1.04 ± 0.55 | 0.74 ± 0.78 | 0.75 ± 0.77 | 0.1339 | 0.9573 | 1.01 ± 0.58 | 0.75 ± 0.77 | 0.4579 | 0.9550 | 1.04 ± 0.55 | 0.74 ± 0.75 | 0.0446 | 0.9371 |
| GNG2 | rs12886319 | T | C | 1.14 ± 0.50 | 0.89 ± 0.65 | 1.10 ± 0.43 | 0.0507 | 0.9573 | 0.99 ± 0.61 | 1.10 ± 0.43 | 0.4289 | 0.9550 | 1.14 ± 0.50 | 0.93 ± 0.61 | 0.0481 | 0.9371 |
| MTHFD1 | rs2236225 | G | A | 0.91 ± 0.66 | 1.10 ± 0.41 | 1.41 ± 0.28 | 0.0905 | 0.9573 | 0.98 ± 0.58 | 1.41 ± 0.28 | 0.2080 | 0.9388 | 0.91 ± 0.66 | 1.12 ± 0.41 | 0.0457 | 0.9371 |
| NUDT15 | rs147390019 | G | A | 1.02 ± 0.56 | 0.37 ± 1.17 | 0.0269 | 0.9573 | |||||||||
| NUDT15 | rs186364861 | G | A | 1.00 ± 0.60 | 1.03 ± 0.39 | 0.0269 | 0.9864 | |||||||||
| TPMT | *1 | Other | 1.03 ± 0.57 | 0.39 ± 0.50 | 0.0085 | 0.8871 | ||||||||||
Abbreviations: FDR, false‐discovery rate; Het, heterozygote; Maj: major allele; Maj hom, major allele homozygote; Min hom, minor allele homozygote; Min, minor allele.
P‐values < 0.05 calculated by linear regression analysis are shown in bold.
Intronic SNP between theAIG1 and HIVEP2 gene.
Figure 2.
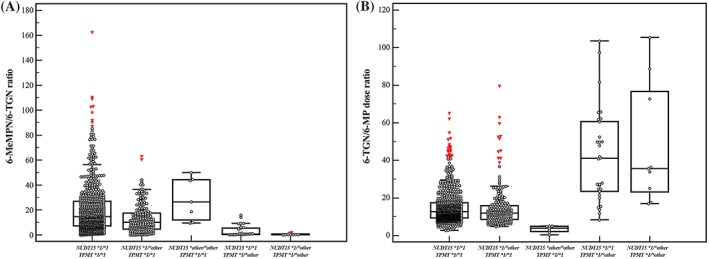
The 6‐TGN/6‐MP dose ratio among patients with TPMT genotypes and NUDT15 variants: The 6‐TGN/6‐MP dose ratio (A) at all metabolite measurements and (B) at the metabolite measurement used to study the association with genetic polymorphisms
Table 3 shows the genetic polymorphisms associated with the 6‐TGN/6‐MP dose ratio. Among the genetic polymorphisms, rs2274407 in the ABCC4 gene, rs819146 in the AHCY gene, rs2070388 in the GART gene, rs2413739 in the PACSIN2 gene, rs34743033 in the TYMS gene, and rs116855232 in the NUDT15 gene were significantly related to the 6‐TGN/6‐MP dose ratio (P < 0.05). Univariate analysis did not show statistical significance for genetic polymorphisms after FDR correction, except for NUDT15 rs116855232. Figure 2B shows the 6‐TGN/6‐MP dose ratios among patients with different TPMT and NUDT15 genotypes. The TPMT variants were associated with a higher 6‐TGN/6‐MP dose ratio while NUDT15 variants were associated with a lower 6‐TGN/6‐MP dose ratio (P < 0.05).
Table 3.
Predictors of log‐transformed 6‐TGN/6‐MP dose ratio (mean ± standard deviation)
| Genes | rsID | Maj | Min | Total number of patients | Genotype | Dominant | Recessive | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maj hom | Het | Min hom | Maj hom | Het | Min hom | P | FDR P | Maj hom + het | Min hom | P | FDR P | Maj hom | Het + min hom | P | FDR P | ||||
| ABCC4 | rs2274407 | C | A | 89 | 42 | 1 | 1.14 ± 0.28 | 1.12 ± 0.21 | 1.79 | 0.0406 | 0.8253 | 1.13 ± 0.26 | 1.79 | 0.0124 | 0.5312 | 1.14 ± 0.28 | 1.14 ± 0.23 | 0.9277 | 0.9873 |
| AHCY | rs819146 | T | G | 78 | 39 | 9 | 1.18 ± 0.26 | 1.06 ± 0.27 | 1.06 ± 0.23 | 0.0484 | 0.8253 | 1.14 ± 0.27 | 1.06 ± 0.23 | 0.3666 | 0.9941 | 1.18 ± 0.26 | 1.06 ± 0.26 | 0.0137 | 0.6891 |
| GART | rs2070388 | G | C | 93 | 35 | 9 | 1.11 ± 0.23 | 1.24 ± 0.31 | 1.05 ± 0.32 | 0.0369 | 0.8253 | 1.15 ± 0.26 | 1.05 ± 0.32 | 0.2739 | 0.9941 | 1.11 ± 0.23 | 1.20 ± 0.32 | 0.0878 | 0.7726 |
| NUDT15 | rs116855232 | C | T | 109 | 27 | 3 | 1.15 ± 0.24 | 1.14 ± 0.30 | 0.55 ± 0.24 | 0.0003 | 0.0335 | 1.15 ± 0.25 | 0.55 ± 0.24 | <.0001 | 0.0052 | 1.15 ± 0.24 | 1.08 ± 0.34 | 0.1959 | 0.8077 |
| PACSIN2 | rs2413739 | C | T | 116 | 13 | 1.12 ± 0.26 | 1.28 ± 0.32 | 0.0386 | 0.8253 | ||||||||||
| TPMT | *1 | Other | 133 | 6 | 1.12 ± 0.25 | 1.47 ± 0.37 | 0.0016 | 0.0847 | |||||||||||
| TYMS | rs34743033 | ≥3 R | 2 R | 91 | 45 | 3 | 1.18 ± 0.28 | 1.07 ± 0.21 | 1.07 ± 0.09 | 0.0556 | 0.8253 | 1.14 ± 0.27 | 1.07 ± 0.09 | 0.6575 | 0.9941 | 1.18 ± 0.28 | 1.07 ± 0.20 | 0.0160 | 0.6891 |
Abbreviations: FDR, false‐discovery rate; Het, heterozygote; Hom, homozygote; Maj, major allele; Min, minor allele; R, repeats.
P‐values < 0.05 calculated by linear regression analysis are shown in bold.
3.4. Genetic polymorphisms associated with toxicity
Table 4 shows the genetic polymorphisms associated with neutropenia, hepatotoxicity and treatment interruption. The results of this study are summarized in Table 5 and Table S2 with findings from previous studies performed in paediatric ALL patients or in patients with other diseases, in vitro studies or theoretical studies.
Table 4.
Influence of genetic polymorphisms on leukopenia, neutropenia, hepatotoxicity, and treatment interruption (number and %)
| Total number of patients | Genotype | Dominant | Recessive | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Category | Adverse effect | Gene | rsID | Maj | Min | Maj hom | Het | Min hom | Maj hom | Het | Min hom | P | FDR P | Maj hom + het | Min hom | P | FDR P | Maj hom | Het + min hom | P | FDR P |
| Significant | Leukopenia | NUDT15 | rs116855232 | C | T | 109 | 27 | 3 | 2 (2) | 3 (11) | 2 (67) | <0.0001 | 0.0013 | 5 (4) | 2 (67) | 0.0064 | 0.5512 | 2 (2) | 5 (17) | 0.0053 | 0.4565 |
| Possible trend toward significancea | Treatment interruption | GART | rs2070388 | G | C | 93 | 35 | 9 | 83 (89) | 20 (57) | 9 (100) | 0.0522 | 0.7622 | 103 (81) | 9 (100) | 0.2112 | 1 | 83 (89) | 29 (66) | 0.0017 | 0.0717 |
| MOCOS | rs3744900 | G | A | 114 | 23 | 1 | 99 (87) | 13 (57) | 1 (100) | 0.0027 | 0.2839 | 112 (82) | 1 (100) | 1 | 1 | 99 (87) | 14 (58) | 0.0025 | 0.0717 | ||
| NUDT15 | rs116855232 | C | T | 109 | 27 | 3 | 84 (77) | 27 (100) | 3 (100) | 0.0056 | 0.2931 | 111 (82) | 3 (100) | 1 | 1 | 84 (77) | 30 (100) | 0.0022 | 0.0717 | ||
| Others | Leukopenia | ABCC4 | rs2274405 | C | T | 42 | 65 | 29 | 2 (5) | 1 (2) | 4 (14) | 0.1481 | 0.8886 | 3 (3) | 4 (14) | 0.0373 | 0.7674 | 2 (5) | 5 (5) | 1 | 1 |
| ABCC4 | rs2274406 | C | T | 51 | 56 | 30 | 3 (6) | 4 (13) | 0.2858 | 0.8886 | 3 (3) | 4 (13) | 0.0410 | 0.7674 | 3 (9) | 4 (5) | 0.7113 | 1 | |||
| SLC29A1 | rs9394992 | C | T | 86 | 49 | 4 | 7 (8) | 0.0419 | 0.8886 | 7 (5) | 1 | 1 | 7 (8) | 0.0440 | 0.9770 | ||||||
| Neutropenia | NUDT15 | rs116855232 | C | T | 109 | 27 | 3 | 4 (4) | 1 (4) | 2 (67) | 0.0063 | 0.6501 | 5 (4) | 2 (67) | 0.0064 | 0.5512 | 4 (4) | 3 (10) | 0.1714 | 1 | |
| ABCC4 | rs4773866 | C | T | 124 | 13 | 2 | 5 (4) | 1 (8) | 1 (50) | 0.0242 | 1 | 6 (4) | 1 (50) | 0.0985 | 1 | 5 (4) | 2 (13) | 0.1662 | 1 | ||
| SLC28A3 | rs7867504 | C | T | 52 | 68 | 18 | 7 (10) | 0.3167 | 1 | 7 (6) | 0.5942 | 1 | 7 (8) | 0.0448 | 1 | ||||||
| Hepatotoxicity | MOCOS | rs3744900 | G | A | 114 | 23 | 1 | 47 (41) | 16 (70) | 1 (100) | 0.0067 | 0.3501 | 63 (46) | 1 (100) | 0.4638 | 1 | 47 (41) | 17 (71) | 0.0123 | 0.5291 | |
| MTRR | rs1801394 | A | G | 77 | 51 | 8 | 44 (57) | 15 (29) | 3 (38) | 0.0067 | 0.3501 | 59 (46) | 3 (38) | 0.7273 | 1 | 44 (57) | 18 (31) | 0.0030 | 0.2585 | ||
| SLCO1B1 | rs11045879 | T | C | 47 | 62 | 25 | 16 (34) | 28 (45) | 16 (64) | 0.0166 | 0.5754 | 44 (40) | 16 (64) | 0.0442 | 1 | 16 (34) | 44 (51) | 0.0720 | 0.8654 | ||
| IMPDH1 | rs2278294 | A | G | 36 | 74 | 28 | 22 (61) | 32 (43) | 10 (36) | 0.0370 | 0.7396 | 54 (49) | 10 (36) | 0.2886 | 1 | 22 (61) | 42 (41) | 0.0518 | 0.8654 | ||
| ADK | rs10824095 | C | T | 58 | 63 | 12 | 19 (33) | 34 (54) | 6 (50) | 0.0427 | 0.7396 | 53 (44) | 6 (50) | 0.7651 | 1 | 19 (33) | 40 (53) | 0.0223 | 0.6405 | ||
| XDH | rs494852 | G | A | 101 | 27 | 2 | 43 (43) | 16 (59) | 2 (100) | 0.0375 | 0.7396 | 59 (46) | 2 (100) | 0.2182 | 1 | 43 (43) | 18 (62) | 0.0906 | 0.8654 | ||
| TYMP | rs470119 | C | T | 82 | 48 | 8 | 36 (44) | 21 (44) | 7 (88) | 0.1327 | 0.8625 | 57 (44) | 7 (88) | 0.0247 | 1 | 36 (44) | 28 (50) | 0.4927 | 1 | ||
| Treatment interruption | ATIC | rs3821353 | T | G | 45 | 46 | 40 | 34 (76) | 37 (80) | 38 (95) | 0.0178 | 0.4351 | 71 (78) | 38 (95) | 0.0207 | 0.8912 | 34 (76) | 75 (87) | 0.1380 | 0.9974 | |
| ATIC | rs4673993 | T | C | 83 | 44 | 8 | 63 (76) | 39 (89) | 8 (100) | 0.0251 | 0.4351 | 102 (80) | 8 (100) | 0.3507 | 1 | 63 (76) | 47 (90) | 0.0413 | 0.8875 | ||
| GMPS | rs61750368 | T | C | 102 | 31 | 5 | 87 (85) | 24 (77) | 2 (40) | 0.0202 | 0.4351 | 111 (84) | 2 (40) | 0.0414 | 0.9917 | 87 (85) | 26 (72) | 0.1285 | 0.9974 | ||
| KCNMA1 | rs12765834 | C | T | 118 | 20 | 0 | 93 (79) | 20 (100) | 0.0242 | 0.4351 | |||||||||||
| ABCC4 | rs1678387 | C | T | 86 | 46 | 7 | 72 (84) | 39 (85) | 3 (43) | 0.1141 | 0.8681 | 111 (84) | 3 (43) | 0.0200 | 0.8912 | 72 (84) | 42 (79) | 0.5052 | 1 | ||
| SLC19A1 | rs1051266 | G | A | 41 | 66 | 26 | 33 (81) | 58 (88) | 17 (65) | 0.2264 | 0.8753 | 91 (85) | 17 (65) | 0.0461 | 0.9917 | 33 (81) | 75 (82) | 1 | 1 | ||
Abbreviations: FDR, false‐discovery rate; Het, heterozygote; Hom, homozygote; Maj, major allele; Min, minor allele.
Polymorphisms were arranged in a sequence starting from the lowest P‐value and were corrected using the false‐discovery rate of genotype mode analysis for each adverse outcome.
P‐values < 0.05 are shown in bold.
Possible trend toward significance with P < 0.10 corrected with a false‐discovery rate.
Table 5.
Identified SNPs in this study in comparison with previous studies
| Gene | rsID | Maj | Min | MAF in this study | Related drug pathway | Results of this study (identified associations) | Results of previous studies in paediatric ALL patients | Comments (studies in other diseases) | Comparable with previous study? |
|---|---|---|---|---|---|---|---|---|---|
| GART | rs2070388 | G | C | 0.193 | Folate cycle | GG genotype: ↑treatment interruption?a | Overexpression of GART gene was reported in association with late relapse in children with ALL.24 Polymorphisms in the GART gene showed increased sensitivity to 6‐TGN and 6‐mercaptopurine but the significance disappeared after applying a Bonferroni correction.25 | Glycinamide ribonucleotide transformylase (GART) is an important trifunctional enzymes involved in purine synthesis.26 This gene is involved in the methotrexate pathway.17 This variant has been studied in paediatric IBD patients and showed no significant association between this variant and thiopurine metabolite concentrations or any adverse outcomes including leukopenia, neutropenia, hepatotoxicity, gastrointestinal intolerance, alopecia, arthralgia, rash, and hair loss in patients treated with azathioprine.4 | Unknown |
| MOCOS | rs3744900 | G | A | 0.091 | Thiopurine metabolism | GG genotype: ↑treatment interruption?a | –b | Molybdenum cofactor sulfurase (MOCOS) is a protein‐coding gene which sulfurates the molybdenum cofactor in XDH and AOX1, key enzymes involved in the degradation of thiopurines.27 It was observed in 2 individual patients with IBD who had intolerance to thiopurines in a cohort of 100 patients.27 Studies performed in kidney transplant recipients receiving azathioprine showed no association between this polymorphism and azathioprine dose requirements.28 | Unknown |
| NUDT15 | rs116855232 | C | T | 0.119 | Thiopurine metabolism |
T allele: ↑leukopenia CC genotype (wild): Treatment interruption?a |
Well established in Asian populations | Comparable | |
| NUDT15 | rs186364861 | G | A | 0.014 | Thiopurine metabolism | GG genotype (wild): ↑6‐TGN levels compared to GA genotype | Well established in Asian populations | Comparable | |
| TPMT | *1 | Other | 0.022 | Thiopurine metabolism | Variant allele: ↑6‐TGN/6‐MP dose ratio | Well established in various populations | Comparable | ||
Abbreviations: ALL, acute lymphoblastic leukaemia; AOX1, aldehyde oxidase 1; IBD, inflammatory bowel disease; MAF, minor allele frequency; Maj, major allele; Min, minor allele; XDH; xanthine dehydrogenase.
Possible trend toward significance with P < 0.10 corrected with a false‐discovery rate.
This polymorphism is rarely investigated in paediatric ALL patients.
4. DISCUSSION
Among the genetic polymorphisms analysed, 32 SNPs in 24 genes (including rs116855232 and rs186364861 of the NUDT15 gene) other than TPMT genotype were identified as having an association with either or both the levels of thiopurine metabolites and any adverse effects. Among the 32 SNPs, results of 16 SNPs were comparable with previous studies, while the other 16 SNPs were needed to validate through further confirmatory clinical or biochemical studies (Table S2). Among these polymorphisms, four SNPs (rs2070388 in the GART gene, rs3744900 in the MOCOS gene, rs116855232 and rs186364861 in the NUDT15 gene) and TPMT genotype were statistically significant in this study (Table 5).
In this study, patients with TPMT variant alleles had significantly higher 6‐TGN/6‐MP dose ratios (Figure 2) which confirmed previous findings on the importance of TPMT in patients who are treated with thiopurines.17, 29 TPMT genotyping before administering thiopurines is recommended to determine the proper initial dosing and consideration of an alternate agent or extreme dose reduction of azathioprine for patients with low or deficient TPMT activity by clinical guidelines (start at 30–70% of target dose for patients with intermediate enzyme activity).8, 29 In this study only 4.3% of patients (6/139) had TPMT variant alleles. This finding was comparable to previous studies performed in Asian populations (variant allele frequency 2.3–5.0%)6 which was lower than for individuals of European descent.8, 29 Meanwhile, in this study, the frequency of NUDT15 variant alleles was 14.0% which was comparable to a previous study in the Korean population.18 NUDT15, another important gene with a similar pathway as TPMT, was first identified as a candidate gene by a genome‐wide association study (GWAS) of inflammatory bowel disease patients9 and in children with ALL.30 NUDT15 was associated with thiopurine‐related hematopoietic toxicity and has been confirmed in several other studies to be associated with thiopurine tolerance and/or thiopurine‐related toxicity in leukaemia patients.11, 12, 31, 32, 33 In this study, two SNPs of NUDT15 (rs116855232 and rs186364861) were identified in association with thiopurine metabolites and therapeutic interruption which confirmed and reflected previous findings on its association with thiopurine‐related hematopoietic toxicity (Tables 2, 3, 4). Because the frequency of the variant alleles range from 0.2–17.2% in different ethnic populations and the variant alleles are more common in East Asian populations, standard guidelines including thiopurine dosage adjustment on the basis of NUDT15 genotyping is needed for safe thiopurine therapy.18 In this study, patients with NUDT15 variants showed significantly lower 6‐TGN/6‐MP dose ratios (Figure 2). It has been suggested that NUDT15 may prevent incorporation of 6‐TGN into DNA and loss of function variants in the NUDT15 gene cause thiopurine‐induced cytotoxicity.11 Another study identified that DNA‐6‐TGN accumulated more efficiently in vivo with an increasing number of variant alleles of NUDT15 and suggested that DNA‐6‐TGN is a more relevant metabolite rather than 6‐TGN in RBCs because 6‐TGN levels were significantly lower in patients with NUDT15 deficiency, likely because of toxicity‐related 6‐MP dose reduction.2 In this study, clinical implementation of NUDT15 genotype‐guided 6‐MP dose individualization was performed by measuring levels of RBC 6‐TGN. Future studies are needed to clarify the relationship between NUDT15 and metabolites including DNA‐6‐TGN and 6‐TGN in RBCs.
Because the folate cycle is important in treatments using antimetabolite drugs including analogues of purines (thiopurines such as 6‐MP, 6‐thioguanine and azathiopurine), pyrimidines (fluoropyrimidines such as 5‐fluorouracil, tegafur and capecitabine), and antifolates (methotrexate, pemetrexed and raltitrexed) and co‐medication is widely used in order to improve clinical outcomes in paediatric ALL patients, future studies assessing information about co‐medication in association with various genetic polymorphisms are needed.17
Most polymorphisms identified in this study were not statistically significant after FDR correction; however, rs2070388 in the GART gene and rs3744900 in the MOCOS gene showed a possible trend toward an association (corrected P = 0.07) with treatment interruption (Tables 2, 3, 4, 5 and S2). In previous research on possible trans expression associations with SNPs, rs200148 in the region between HIVEP2 and AIG1 showed the association with reduced TPMT activity and increased sensitivity to 6‐TGN and 6‐MP.34 This SNP was associated with expression of the thiopurine pathway genes ADA (reduced expression) and GART (increased expression).25, 34, 35 The protein encoded by the GART gene (glycinamide ribonucleotide transformylase) is a trifunctional polypeptide (phosphoribosylglycinamide formyltransferase, phosphoribosylglycinamide synthetase and phosphoribosylaminoimidazole synthetase activity) required for de novo purine biosynthesis in association with folate metabolism and has long been considered a potential target for the development of anti‐neoplastic therapeutics.26 In the present study, rs200148 in the region between HIVEP2 and AIG1 was associated with decreased 6‐TGN levels, in contrast to the findings of previous studies.25, 34, 35 The complex mechanisms of these SNPs and their clinical impacts should be elucidated through future studies. The polymorphism rs3744900 in the MOCOS gene has rarely been investigated in paediatric ALL patients. Molybdenum cofactor sulfurase is encoded by the MOCOS gene, which sulfurates the molybdenum cofactor in xanthine dehydrogenase and aldehyde oxidase 1, key enzymes involved in the degradation of thiopurines.28 Two patients with inflammatory bowel disease were reported to exhibit intolerance to thiopurines.27 However, in kidney transplant recipients receiving azathioprine, there was no association between this polymorphism and azathioprine dose requirements.28 Although the mechanisms of these SNPs have not been fully explained by previously published studies, these genetic polymorphisms in candidate genes with potential effects on the pharmacokinetics of thiopurine metabolism should be considered and validated with further studies.10
Although the relatively small number of patients included in this study might be a limitation, those SNPs have not previously been examined to determine if they are associated with thiopurine‐related toxicity in Korean paediatric ALL patients. Dosing compliance might be another limitation that could affect thiopurine‐related toxicity.
The value of the present study lies primarily in the emphasis placed on the genetic variants in the Korean paediatric ALL patient population which has not been assessed previously. Validation of the results presented here requires confirmatory clinical or biochemical studies, especially because functional data are not yet available for some genetic polymorphisms. Considering that the frequency of genetic polymorphisms is different among different populations and pharmacokinetics in association with pharmacogenetic differences among different populations, future studies in different ethnic populations are needed to confirm the relevance of our data.
In conclusion, this is the first study to explore various genetic polymorphisms related to thiopurine treatment in Korean paediatric ALL patients. This study provides basic knowledge for personalized thiopurine treatment in paediatric ALL patients. Further studies to enable more comprehensive pharmacogenetic‐based thiopurine dose adjustments across diverse populations are needed.
COMPETING INTERESTS
There are no competing interests to declare.
CONTRIBUTORS
R.C. conceptualized and designed the study, collected data, carried out the initial analyses, drafted the initial manuscript, and reviewed and revised the manuscript. J.W.L., Y. M. and E.S.Y. designed the data collection instruments and collected data, and reviewed and revised the manuscript. I.S. and M‐J.K. carried out the initial analyses, and reviewed and revised the manuscript. H.I.W., H.H.K. and S‐Y.L. conceptualized and designed the study, coordinated and supervised data collection, and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work. The authors would like to express their gratitude to the patients and their families for their participation in the study.
Supporting information
TABLE S1 List of the 103 genetic polymorphisms in 43 genes other than NUDT15 and TPMT genotypes analysed in this study
TABLE S2 Identified SNPs other than NUDT15 and TPMT genotypes in this study compared with previous studies (before correction with the false‐discovery rate correction)
FIGURE S1 Genes involved in the transport, metabolism, or actions of (A) thiopurine and (B) the folate cycle (from PharmGKB, http://www.pharmgkb.org/pathway). Parts A and B adapted with permission from PharmGKB and Stanford University (A, https://www.pharmgkb.org/pathway/PA2040; B, https://www.pharmgkb.org/pathway/PA165291575). Copyright PharmGKB.
ACKNOWLEDGEMENTS
This study was supported by a grant from the Korean Foundation for Cancer Research (KFCR‐2017‐D‐1).
Choi R, Sohn I, Kim M‐J, et al. Pathway genes and metabolites in thiopurine therapy in Korean children with acute lymphoblastic leukaemia. Br J Clin Pharmacol. 2019;85:1585–1597. 10.1111/bcp.13943
Principal Investigator: The authors confirm that the PI for clinical data reused in this paper is Hong Hoe Koo and that he had direct clinical responsibility for patients.
Contributor Information
Hong Hoe Koo, Email: hhkoo@skku.edu.
Soo‐Youn Lee, Email: sy117.lee@samsung.com.
REFERENCES
- 1. Schmiegelow K, Nielsen SN, Frandsen TL, Nersting J. Mercaptopurine/methotrexate maintenance therapy of childhood acute lymphoblastic leukemia: clinical facts and fiction. J Pediatr Hematol Oncol. 2014;36(7):503‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moriyama T, Nishii R, Lin TN, et al. The effects of inherited NUDT15 polymorphisms on thiopurine active metabolites in Japanese children with acute lymphoblastic leukemia. Pharmacogenet Genomics. 2017;27(6):236‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhatia S, Landier W, Hageman L, et al. 6MP adherence in a multiracial cohort of children with acute lymphoblastic leukemia: a Children's oncology group study. Blood. 2014;124(15):2345‐2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee MN, Kang B, Choi SY, et al. Impact of genetic polymorphisms on 6‐thioguanine nucleotide levels and toxicity in pediatric patients with IBD treated with azathioprine. Inflamm Bowel Dis. 2015;21(12):2897‐2908. [DOI] [PubMed] [Google Scholar]
- 5. Rudin S, Marable M, Huang RS. The promise of pharmacogenomics in reducing toxicity during acute lymphoblastic leukemia maintenance treatment. Genomics Proteomics Bioinformatics. 2017;15(2):82‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim HY, Lee SH, Lee MN, et al. Complete sequence‐based screening of TPMT variants in the Korean population. Pharmacogenet Genomics. 2015;25(3):143‐146. [DOI] [PubMed] [Google Scholar]
- 7. Adam de Beaumais T, Fakhoury M, Medard Y, et al. Determinants of mercaptopurine toxicity in paediatric acute lymphoblastic leukemia maintenance therapy. Br J Clin Pharmacol. 2011;71(4):575‐584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Relling MV, Gardner EE, Sandborn WJ, et al. Clinical pharmacogenetics implementation consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing: 2013 update. Clin Pharmacol Ther. 2013;93(4):324‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang SK, Hong M, Baek J, et al. A common missense variant in NUDT15 confers susceptibility to thiopurine‐induced leukopenia. Nat Genet. 2014;46(9):1017‐1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maxwell RR, Cole PD. Pharmacogenetic predictors of treatment‐related toxicity among children with acute lymphoblastic leukemia. Curr Hematol Malig Rep. 2017;12(3):176‐186. [DOI] [PubMed] [Google Scholar]
- 11. Moriyama T, Nishii R, Perez‐Andreu V, et al. NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat Genet. 2016;48(4):367‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yi ES, Choi YB, Choi R, et al. NUDT15 variants cause hematopoietic toxicity with low 6‐TGN levels in children with acute lymphoblastic leukemia. Cancer Res Treat. 2018;50(3):872‐882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smith M, Arthur D, Camitta B, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol off J am Soc Clin Oncol. 1996;14(1):18‐24. [DOI] [PubMed] [Google Scholar]
- 14. Dervieux T, Meyer G, Barham R, et al. Liquid chromatography‐tandem mass spectrometry analysis of erythrocyte thiopurine nucleotides and effect of thiopurine methyltransferase gene variants on these metabolites in patients receiving azathioprine/6‐mercaptopurine therapy. Clin Chem. 2005;51(11):2074‐2084. [DOI] [PubMed] [Google Scholar]
- 15. Yoo IY, Lee K, Ji OJ, Woo HI, Lee SY. Evaluation of stability of thiopurine metabolites using a validated LC‐MS/MS method. Ann Lab Med. 2018;38(3):255‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stocco G, Cuzzoni E, De Iudicibus S, et al. Deletion of glutathione‐s‐transferase M1 reduces azathioprine metabolite concentrations in young patients with inflammatory bowel disease. J Clin Gastroenterol. 2014;48(1):43‐51. [DOI] [PubMed] [Google Scholar]
- 17. Whirl‐Carrillo M, McDonagh EM, Hebert JM, et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther. 2012;92(4):414‐417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim HT, Choi R, Won HH, et al. NUDT15 genotype distributions in the Korean population. Pharmacogenet Genomics. 2017;27(5):197‐200. [DOI] [PubMed] [Google Scholar]
- 19. Huang MY, Fang WY, Lee SC, Cheng TL, Wang JY, Lin SR. ERCC2 2251A>C genetic polymorphism was highly correlated with early relapse in high‐risk stage II and stage III colorectal cancer patients: a preliminary study. BMC Cancer. 2008;8(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lewis CM. Genetic association studies: design, analysis and interpretation. Brief Bioinform. 2002;3(2):146‐153. [DOI] [PubMed] [Google Scholar]
- 21. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc B. 1995;57(1):289‐300. [Google Scholar]
- 22. Harding SD, Sharman JL, Faccenda E, et al. The IUPHAR/BPS guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res. 2018;46(D1):D1091‐D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee MN, Woo HI, Lee YM, et al. Successful azathioprine treatment with metabolite monitoring in a pediatric inflammatory bowel disease patient homozygous for TPMT*3C . Yonsei Med J. 2013;54(6)1545‐1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hogan LE, Meyer JA, Yang J, et al. Integrated genomic analysis of relapsed childhood acute lymphoblastic leukemia reveals therapeutic strategies. Blood. 2011;118(19):5218‐5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fridley BL, Jenkins GD, Batzler A, et al. Multivariate models to detect genomic signatures for a class of drugs: application to thiopurines pharmacogenomics. Pharmacogenomics J. 2012;12(2):105‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Connelly, DeMartino JK, Boger DL, et al. Biological and structural evaluation of 10R‐ and 10S‐methylthio‐DDACTHF reveals a new role for sulfur in inhibition of glycinamide ribonucleotide transformylase. Biochemistry. 2013;52(30):5133‐5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Coelho T, Andreoletti G, Ashton JJ, et al. Genes implicated in thiopurine‐induced toxicity: comparing TPMT enzyme activity with clinical phenotype and exome data in a paediatric IBD cohort. Sci Rep. 2016;6(1):34658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kurzawski M, Dziewanowski K, Safranow K, Drozdzik M. Polymorphism of genes involved in purine metabolism (XDH, AOX1, MOCOS) in kidney transplant recipients receiving azathioprine. Ther Drug Monit. 2012;34(3):266‐274. [DOI] [PubMed] [Google Scholar]
- 29. Kim S, Yun YM, Chae HJ, et al. Clinical pharmacogenetic testing and application: laboratory medicine clinical practice guidelines. Ann Lab Med. 2017;37(2):180‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang JJ, Landier W, Yang W, et al. Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia. J Clin Oncol off J am Soc Clin Oncol. 2015;33(11):1235‐1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tanaka Y, Kato M, Hasegawa D, et al. Susceptibility to 6‐MP toxicity conferred by a NUDT15 variant in Japanese children with acute lymphoblastic leukaemia. Br J Haematol. 2015;171(1):109‐115. [DOI] [PubMed] [Google Scholar]
- 32. Chiengthong K, Ittiwut C, Muensri S, et al. NUDT15 c.415C>T increases risk of 6‐mercaptopurine induced myelosuppression during maintenance therapy in children with acute lymphoblastic leukemia. Haematologica. 2016;101(1):e24‐e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liang DC, Yang CP, Liu HC, et al. NUDT15 gene polymorphism related to mercaptopurine intolerance in Taiwan Chinese children with acute lymphoblastic leukemia. Pharmacogenomics J. 2016;16(6):536‐539. [DOI] [PubMed] [Google Scholar]
- 34. Matimba A, Li F, Livshits A, et al. Thiopurine pharmacogenomics: association of SNPs with clinical response and functional validation of candidate genes. Pharmacogenomics. 2014;15(4):433‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang L, Weinshilboum R. Thiopurine S‐methyltransferase pharmacogenetics: insights, challenges and future directions. Oncogene. 2006;25(11):1629‐1638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 List of the 103 genetic polymorphisms in 43 genes other than NUDT15 and TPMT genotypes analysed in this study
TABLE S2 Identified SNPs other than NUDT15 and TPMT genotypes in this study compared with previous studies (before correction with the false‐discovery rate correction)
FIGURE S1 Genes involved in the transport, metabolism, or actions of (A) thiopurine and (B) the folate cycle (from PharmGKB, http://www.pharmgkb.org/pathway). Parts A and B adapted with permission from PharmGKB and Stanford University (A, https://www.pharmgkb.org/pathway/PA2040; B, https://www.pharmgkb.org/pathway/PA165291575). Copyright PharmGKB.


