Abstract
Aims
With a prevalence of 16%, diabetes mellitus (DM) is one of the most frequent non‐communicable comorbidities of tuberculosis (TB). DM is a major risk factor for adverse TB outcomes and may require personalized TB drug dosing regimens. However, information on the inclusion of DM in TB drug trials is lacking. We aimed to assess the percentage of recent TB drug efficacy trials that included DM patients.
Methods
A systematic review was performed and reported according to PRISMA guidelines. PubMed, Science Direct, and ClinicalTrials.gov databases were systematically searched for TB drug trials published between 1 January 2012 and 12 September 2017. Primary outcome was the percentage of TB drug trials performed around the world that included DM patients.
Results
Out of the included 41 TB drug trials, 12 (29.3%) reported DM comorbidity among the study participants. Nine trials (21.9%) excluded all patients with DM comorbidity, ten (24.4%) excluded only insulin‐dependent or uncontrolled DM, and 10 (24.4%) did not mention whether DM was included or excluded. Of the 12 trials that included DM comorbidity, the majority did not report the diagnostic criteria for DM and none reported outcomes in the DM subpopulation. Inclusion of DM was higher in drug‐resistant‐TB trials (67%, P = .003, vs drug‐susceptible) and trials performed in Asia (60%, P = .006, vs Africa).
Conclusions
Fewer than 1/3 recent TB drug trials reported the inclusion of DM. To better reflect real‐world DM prevalence and differential TB drug effectiveness, inclusion of DM patients requires increased attention for future TB drug trials.
Keywords: diabetes, drug trials, review, tuberculosis
What is already known about this subject
Globally, around 16% of tuberculosis (TB) patients suffer from comorbid diabetes mellitus
Diabetes is a risk factor for TB, altered pharmacokinetics and can thus impact pharmacological TB treatment outcomes
In recent years, multiple TB drug trials have been performed, yet a systematic overview of the inclusion of diabetes comorbidity and potential differential outcomes within these trials is lacking.
What this study adds
This systematic review provides an overview of diabetes inclusion in recent TB drug trials performed around the world
Of the 41 studies included, <1/3 TB drug trials reported the inclusion of patients with diabetes
A total of 12 studies (29%) reported the inclusion of patients with diabetes, yet the vast majority of TB drug trials did not report the diagnostic criteria for diabetes
None of the studies reported differential outcomes for the TB–diabetes overlap subpopulation, warranting increased attention on the design and analyses of future TB drug trials
1. INTRODUCTION
The dual burden of tuberculosis (TB) and diabetes mellitus (DM) is a major global public health problem.1 In 2017, the World Health Organization reported 10 million cases of TB and 1.3 million TB‐related deaths.2 Approximately 415 million people worldwide live with DM and another 318 million people have impaired glucose tolerance—a marker for future diabetes.3 By 2040, these numbers are likely to grow to 642 million and 481 million, respectively.4
The global burden of TB‐DM overlap is high, with a prevalence of 16% globally, 17% in Asia, 7% in Africa, 24% in North America, 23% in Oceania, 11% in South America, and 6% in Europe.1 The International Diabetes Federation (IDF) estimates that 46% of diabetes cases worldwide (around 175 million) are not diagnosed, with the highest proportions concentrated in Africa (62%) and southeast Asia (54%), coinciding with the greatest TB burden. Globally, 84% of all people with undiagnosed diabetes live in low‐income and middle‐income countries where the management of these people is rarely optimal.5 DM could severely threaten TB control and may become most profound in resource‐poor areas where TB thrives.6
A systematic review and meta‐analysis of studies published between 1980–2010 reported that DM is associated with 69% higher risk of death and increased risk of TB relapse than TB patients without DM.7 Since 2010, several large cohort studies reported unfavourable effects of DM on TB outcomes. DM was associated with more severe clinical manifestations of TB such as higher frequency of cavities on chest X‐ray and higher hospitalization rates.8, 9, 10 Patients with DM were more likely to have up to 2 times higher TB reactivation, recurrence, and relapse.8, 9, 10, 11 TB‐DM patients were more likely to have delayed sputum conversion and higher probability of treatment failure.8, 9, 12 A recent systematic review showed that glycaemic control has a favourable effect on TB treatment outcomes and, conversely, uncontrolled DM or poor glycaemic control (i.e. HbA1c > 7%) was associated with delayed sputum conversion.13, 14
Early screening for TB‐DM comorbidity can help clinicians to act promptly, thereby resulting in improved TB treatment outcomes.15 Notably, given the profound impact of DM comorbidity on TB treatment outcomes and the call for intensified precision drug therapy, this comorbidity should receive higher priority in prospective randomized clinical TB drug efficacy trials. However, an overview of current data on TB‐DM comorbidity in recent TB drugs trials is lacking. This overview may help to raise awareness on the inclusion of DM comorbidity and could benefit the design of future TB drug trials. We therefore aimed to systematically review the inclusion of DM comorbidity in recent TB drug efficacy trials, with specific emphasis on differential outcomes of TB‐DM overlap patients.
2. METHODS
2.1. Study design
A systematic review was performed and reported according to the Preferred Reporting Items for Systematic review and Meta‐Analyses (PRISMA) statement (Supporting information Appendix S1). The review was registered at PROSPERO (registration number: 71203) and is available online on https://www.crd.york.ac.uk/prospero/display_record.php? RecordID = 71203.
2.2. Information sources and search strategy
In this review, the PubMed, Science Direct and ClinicalTrials.gov databases were systematically searched (in September 2017) for TB drug trials published between 1 January 2012 and 12 September 2017 using combinations of the keywords “tuberculosis”, AND “drug” AND “trial”. Full search criteria can be found in Supporting information Appendix S2.
2.3. Inclusion criteria
The following eligibility criteria were applied for studies to be considered for inclusion: (i) published in peer‐reviewed journals; (ii) clinical trials or interventional studies of TB drug efficacy in TB confirmed (i.e. sputum smear or culture positive) patients that have been completed and published; and (iii) in English16 and reflecting an original study. All criteria were required to be met for inclusion.
2.4. Exclusion criteria
Exclusion criteria were: (i) studies only assessing risk factors, biomarkers (and not drugs) in the TB trials; (ii) reviews, comments, conference abstracts, case reports or editorials; and (iii) study designs other than clinical trials.
2.5. Study selection
Study screening based on title and abstract and selection based on full‐text assessment was first performed by one researcher (N.L.) and checked by a second researcher (M.Z.). Any discrepancies were solved by consensus and/or consultation of a third researcher if needed.
2.6. Data extraction and data items
Data extracted included the studies' first author, the year of publication, study design, study sample size, number and percentage of comorbid DM patients, diagnostic criteria for DM, type of TB population, drug(s) studied, and country where the trial was performed. Again, data extraction was first performed by one researcher (N.L.) and subsequently checked by a second researcher (M.Z.). Any discrepancies were solved by consensus and/or consultation of a third researcher if needed.
2.7. Study measures and outcomes
The primary outcome of interest was the percentage of TB drug trials performed around the world that included DM patients. Additionally, results were assessed per continent. Exploratory, more descriptive outcomes included differential outcomes of TB‐DM patients (if reported). Chi‐square tests were performed to assess potential statistical differences in inclusion (yes/no) of DM comorbidity across subgroups (e.g. type of TB and continent were trials were performed). A P‐value <.05 was considered statistically significant.
2.8. Assessment of reporting bias
To assess potential reporting bias, we searched for study protocols of each study to check recruitment criteria of DM patients in the eligible trials against reported population characteristics. If unclear, we contacted the study authors to get more information about the DM criteria and reported outcomes.
3. RESULTS
3.1. Study selection
After duplicates were removed, a total of 1177 records were screened based on abstract and title. We identified 54 potentially eligible full‐text papers of which 13 studies were excluded after detailed review (10 studies were noninterventional, and 3 assessed a diagnostic tool or the pharmacokinetics of TB drugs and not the efficacy of the TB drug itself). A flow diagram is presented in Figure 1 and study characteristics of the final selection of 41 trials are presented in Table 1. Eligibility decisions for in‐ and excluded studies have been provided in Supporting information Appendix S3. Of note, compared to our initial study protocol we did not apply the full‐text being available as an inclusion criterion, given that we did not restrict ourselves to online available full‐texts only but also contacted study authors to retrieve full‐texts.
Figure 1.
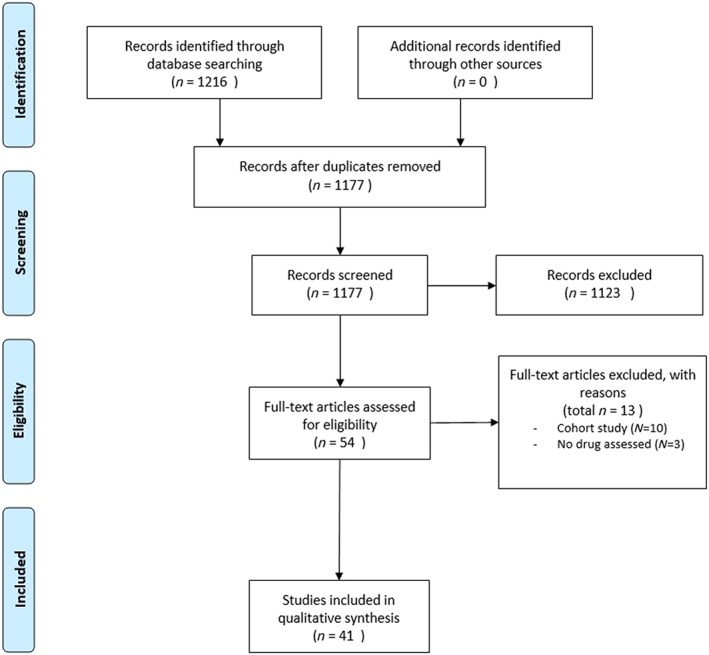
PRISMA flow diagram
Table 1.
Study characteristics of randomized clinical trials of anti‐tuberculosis (TB) drugs (n = 41)
| Author ref | Year | Type of study | Sample size | DM patients (n, %) | Diagnostic criteria for DM | Type of TB | Drugs | Country |
|---|---|---|---|---|---|---|---|---|
| Diacon et al.17 | 2012 | Phase 2 RCT | 85 | DM insulin dependent were excluded | Judge by the investigator | PTB | Proteomanid, pyrazinamide, bedaquiline, rifafour, moxifloxacin | South Africa |
| Diacon et al.18 | 2012 | Phase 2 RCT | 89 | DM insulin dependent were excluded | Judge by the investigator | PTB | Proteomanid, rifafour | South Africa |
| Lee et al.19 | 2012 | Phase 2 RCT | 39 | 14 (36%) | Unexplained, medical history and blood examination performed | XDR PTB | Linezolid | South Korea |
| Gler et al.20 | 2012 | Phase 2 RCT | 481 | Evidence of clinically significant metabolic, endocrine diseases were excluded | unexplained | MDR‐PTB | Delamanid | Philippines, Peru, Latvia, Estonia, China, Japan, Korea, Egypt and USA |
| Zhang et al.21 | 2013 | Phase 2 RCT | 38 | 5 (13.2%) | Unexplained, blood biochemistry performed | MDR‐TB | Delamanid | China |
| Jawahar et al.22 | 2013 | Phase 3 RCT | 416 | Excluded | PTB | Moxifloxacin, gatifloxacin | India | |
| Wang et al.23 | 2013 | Phase 2 RCT | 100% | Fasting blood glucose and oral glucose tolerance test | PTB | Retinol, vitamin D | China | |
| Jindani et al.24 | 2014 | Phase 3 RCT 3 | 827 | Unclear | PTB | Rifapentine, moxifloxacin | Zimbabwe, Botswana, Zambia, South Africa | |
| Diacon et al.25 | 2014 | Phase 2 RCT | 160 | Unclear | MDR‐PTB | Bedaquiline | Brazil, India, Philippines, Latvia, Peru, South Africa, Thailand | |
| Gillespie et al.26 | 2014 | Phase 3 RCT | 1931 | Excluded | Unexplained | PTB | Moxifloxacin | South Africa, India, Tanzania, Kenya Thailand, Malaysia, Zambia, China, Mexico |
| Nunn et al.27 | 2014 | Phase 3 RCT | 1348 | Unclear | PTB | Isoniazid, rifampicin, pyrazinamide, ethambutol, prothionamide | Africa, Asia, Latin America | |
| Luangchosiri et al.28 | 2015 | Phase 4 RCT | 55 | Unclear | PTB | Silymarin | Thailand | |
| Diacon et al.29 | 2015 | Phase 2 RCT | 105 | DM insulin dependent were excluded | Unexplained | PTB | Bedaquiline, proteomanid, pyrazinamide, clofazimine, rifafour | South Africa |
| Daley et al.30 | 2015 | Phase 3 RCT | 247 | Unclear | PTB | Vitamin D | India | |
| Dawson et al.31 | 2015 | Phase 2 RCT | 207 | Excluded | History of DM | PTB | Moxifloxacin, proteomanid, pyraziamide | South Africa, Tanzania |
| Dorman et al.32 | 2015 | Phase 2 RCT | 334 | Unclear | PTB | Rifapentine | USA, Brazil, Uganda, Canada, South Africa, Spain | |
| Heinrich et al.33 | 2015 | Phase 2 RCT | 90 | DM insulin dependent were excluded | Random blood glucose | PTB | SQ109, rifampicin | South Africa |
| Merle et al.34 | 2015 | Phase 3 RCT | 1836 | Excluded | PTB | Gatifloxacin | Benin, Guinea, Kenya, Senegal and South Africa | |
| Mily et al.35 | 2015 | Phase 2 RCT | 288 | Excluded | History of DM | PTB | Vitamin D3 | Bangladesh |
| Dawson et al.31 | 2015 | Phase 2 RCT | 153 | Evidence of clinically significant metabolic endocrine diseases, DM insulin dependent were excluded | Random blood glucose | PTB | Rifapentine | Tanzania, South Africa |
| Wu et al.36 | 2015 | Phase 4 RCT | 161 | 24 (14.9%) | Random blood glucose | PTB | Isoniazid, rifampicin, pyrazinamide, ethambuthol (FDC compared to separate formulation) | Taiwan |
| Tang et al.37 | 2015 | Phase 4 RCT | 65 | 13 (20%) | Random blood glucose | XDR‐TB | Linezolid | China |
| Tang et al.38 | 2015 | Phase 4 RCT | 105 | 21 (20%) | History of DM | MDR PTB | Clofazimin | China |
| Tukvadze et al.39 | 2015 | Phase 2 RCT | 199 | 10 (5%) | History of DM | PTB | Vitamin D | Georgia |
| Aseffa et al.40 | 2016 | Phase 4 RCT | 1000 | Excluded | Fasting blood glucose | PTB | Isoniazid, rifampicin, pyrazinamide, ethambuthol: FDC vs loose regimen | Ethiopia, Nigeria |
| Conde et al.41 | 2016 | Phase 2 RCT | 121 | HbA1c >8 g/dl were excluded | HbA1c | PTB | Rifapentine, moixifloxacin, pyrazinamide, isoniazid | Brazil |
| Furin et al.42 | 2016 | Phase 2 RCT | 75 | Excluded | Random blood glucose | PTB | Azd5847 | South Africa |
| Heemskerk et al.43 | 2016 | Phase 4 RCT | 817 | Unclear | Meningitis TB | Rifampicin, levofloxacin, isoniazid, pyrazinamide, ethambutol, streptomycin | Vietnam | |
| Kang et al.44 | 2016 | Phase 3 RCT | 151 | 5 (3.3%) | Unexplained | MDR‐TB | Levofloxacin, moxifloxacin | South Korea |
| Milstein et al.45 | 2016 | Phase 2 RCT | 180 | Uncontrolled DM HbA1c >7.5 excluded | HbA1c | PTB | Higher dose rifampin | Peru, USA, UK |
| Pym et al.46 | 2016 | Phase 2 RCT | 233 | Unclear | MDR‐PTB and XDR‐PTB | Bedaquiline | China, Estonia, Kenya, Korea, Latvia, Peru, Philippines, Russia, South Africa, Thailand, Turkey, Ukraine | |
| Chesdachai et al.47 | 2016 | Phase 2 RCT | 31 | 3 (9.7%) | History of DM | PTB | Vitamin D3 | Georgia |
| Zhang et al.48 | 2016 | Phase 4 RCT | 370 | 34 (9.2%) | Unexplained | Unspecified | Silybum marianum capsule as hepatoprotectant | China |
| Aarnoutse et al.49 | 2017 | Phase 2 RCT | 150 | Excluded | Medical History | PTB | Rifampicin | Tanzania |
| Alsultan et al.50 | 2017 | Phase 2 RCT | 60 | Excluded | Random blood glucose > 150 mg/dL excluded | PTB |
AZD‐5847 Rifafour |
South Africa |
| Boeree et al.51 | 2017 | Phase 2 RCT | 365 | Uncontrolled or DM insulin dependent were excluded | History of DM | PTB | Rifampicin, moxifloxacin, SQ109 | Tanzania, South Africa |
| Boutoun et al.52 | 2017 | Phase 2 RCT | 111 | Poorly controlled DM (HbA1c >9%) were excluded | HbA1c | MDR‐PTB | Levofloxacin | Peru, South Africa |
| Batbold et al.53 | 2017 | Phase 3 RCT | 269 | 2 (0.7%) | Random blood glucose | PTB | Imunoxel honey lozenges | Mongolia, Ukraine |
| Lee et al.54 | 2017 | Phase 2 RCT | 429 | Unclear | PTB | Linezolid, ethambutol | South Korea | |
| Sigal et al.55 | 2017 | Phase 2 RCT | 389 | Unclear | PTB | Rifapentine, isoniazid, pyrazinamide, ethambutol | USA | |
| Ganmaa et al.56 | 2017 | Phase 4 RCT | 380 | 19 (5%) | Unexplained | PTB | Vitamin D3 | Mongolia |
DM: diabetes mellitus; RCT: randomised controlled trial; PTB: pulmonary tuberculosis; MDR: multidrug resistant; XDR: extensively drug resistant, FDC: fixed drug combination.
3.2. Study characteristics
The vast majority of TB drug trials was exclusively performed in Asia (n = 15; 37%) and Africa (n = 13; 32%). North America (USA) and South America (Brazil) contributed only one trial each, while others were performed in Europe (n = 2, both in Georgia) or in multiple sites around the world (n = 9). Study size varied between 31 patients for a TB trial performed in Georgia25 and 1931 for a multicentre trial with moxifloxacin.47 Drugs mostly studied were isoniazid, rifampicin, pyrazinamide and ethambutol.
3.3. Overview of DM comorbidity in TB drug trials
Out of the included 41 trials, 12 (29.3%) reported DM comorbidity among the study participants (Figure 2).
Figure 2.
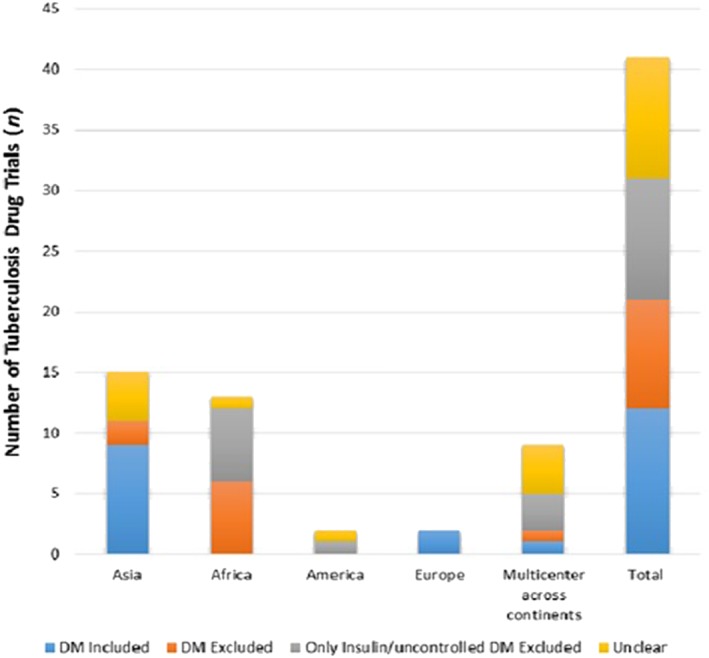
Inclusion status of diabetes mellitus (DM) in tuberculosis drug trials 2012–2017 (total n = 41)
Nine trials (21.9%) clearly excluded patients with any DM comorbidity, 10 (24.4%) excluded only insulin‐dependent or uncontrolled DM but did not report data of noninsulin dependent DM patients, and 10 (24.3%) did not mention whether DM was included or excluded. DM was included in 9 of the 15 (60%) trials performed in Asia and in both European trials (Figure 2). In 12 of the 13 (92.3%) African TB trials, patients with DM comorbidity were excluded. There was a significant difference (P = .006) between DM inclusion in Asian and African TB drug trials. Of the 12 trials that included patients with DM comorbidity regardless of severity, 5 studies did not report the diagnostic criteria for DM. Three studies used random blood glucose.36, 37, 54 One study used fasting plasma glucose and 2‐hour oral glucose tolerance test23 and 3 studies obtained DM comorbidity from patients' history.38, 39, 48 The prevalence of DM among TB patients in the 12 trials ranged from 0.7% in Mongolia and Ukraine54 to 36% in South Korea19 with overall median DM prevalence of 12.3%. Naturally, in the study that specifically focused on TB‐DM overlap, this was 100%.23 Three out of 12 trials reporting DM comorbidity showed that DM was the most common comorbidity.37, 38, 39 Of the 12 trials reporting DM comorbidity, none of the studies assessed any potential effects of DM on anti‐TB drugs outcomes. Of note, 6 out of 9 (67%) drug trials for drug‐resistant TB included DM comorbidity in their baseline characteristics, while only 4 out of the 32 (12.5%) drug‐susceptible TB trials included DM comorbidity in their baseline characteristics, and this differed significantly (P = .003).
4. DISCUSSION
Data from this systematic review indicate that <1/3 recent TB drug efficacy trials reported the inclusion of patients with DM comorbidity regardless of DM severity. If included, diagnostic criteria for DM were often unclear. Notably, inclusion of DM was relatively higher in MDR‐TB drug trials and trials performed in Asia. Although DM patients were included in some studies, no differential outcomes for DM‐TB overlap patients were reported.
Asia has high DM prevalence among TB patients; therefore, it is not surprising that most trials that included DM comorbidity were conducted in Asia, mainly China. China and India are 2 leading countries that have piloted the TB‐DM collaborative framework and have demonstrated bidirectional screening for both diseases.57, 58, 59 Although India is one of the pilot countries, most drug trials conducted in India had unclear criteria for DM comorbidity, and one Indian trial even excluded DM patients. Most trials that excluded DM comorbidity were conducted in Africa. Notably, South Africa has high prevalence of TB, and TB ranks third in diseases that causes life‐years lost,60 but none of the TB drug trials conducted in South Africa screened for DM comorbidity. For South Africa, this omission may be related to the relatively low comorbid DM rates compared with, for example, comorbid human immunodeficiency virus/acquired immune deficiency syndrome. However, the few TB drug trials conducted in America also did not assess DM comorbidity, while DM rates in these continents are relatively high.1 In particular, multidrug‐resistant TB (MDR‐TB) continues to be a public health crisis and in MDR‐TB the importance of DM comorbidity seems more widely acknowledged. Indeed, in a meta‐analysis it was shown that DM was an independent risk factor for MDR‐TB and, in most drug‐resistant TB trials, DM comorbidity was more often included.61 Regarding the effect of DM comorbidity to unfavourable treatment of TB, none of the TB trials that included DM comorbidity reported specific outcomes related to the TB‐DM subpopulation. A systematic review suggested a phase III clinical trial to ensure the safe use of new TB drugs in diabetes patients.62 Indeed, there is still a lack of sufficient data regarding pharmacokinetic and clinical data of TB drugs in DM patients, despite the continuous growth of DM patients in the future that will cause a further threat to TB control.
Some TB drug trials excluded insulin‐dependent DM patients. Insulin‐dependent diabetes will usually reflect uncontrolled DM.63 As TB patients with uncontrolled DM are more likely to fail on treatment, trials that are specifically designed to show efficacy of a new TB drug usually exclude those patients as they could compromise trial results.64
Several underlying mechanisms to understand adverse treatment outcomes of TB due to hyperglycaemia have been suggested.65, 66 One mechanism is related to an altered immunological response67, 68, 69 which is important, but difficult to account for in TB treatment decisions. Another factor that explains unfavourable treatment outcomes are the drug–drug and drug–disease interactions. A systematic review that assessed the pharmacokinetics of first‐line TB drugs showed that age, sex, malnutrition, food intake, genetic factors and comorbidities (mainly human immunodeficiency virus and diabetes) could all play a role.70
Altered pharmacokinetics of anti‐TB drugs may warrant a need for routine monitoring and modification of the regimens in patients with DM. American Thoracic Society, Center for Disease Control and Prevention, and Infectious Diseases Society of America guidelines suggest early identification of patients at increased risk of relapse such as those with DM71 as well as therapeutic drug monitoring (TDM). TDM does allow for timely, informed decisions regarding the need for dose adjustment when necessary. TDM is considered to be helpful in situations in which clinicians are confronted with drug malabsorption, drug under‐dosing, or clinically important drug–drug or drug–disease interactions, such as diabetes comorbidity.72
To our knowledge, this is the first systematic review specifically focusing on the inclusion of DM in TB drug trials. Major strengths are the search within 3 different databases, double checking of inclusion and data extraction and reporting according to the standardized PRISMA statement. Also, some limitations need to be mentioned. First, given the focus on English language manuscripts and our own restricted language knowledge, we had to exclude the few trials that were only published in a local language. These trials could potentially be informative but may often be less generalizable as the larger multicountry trials. Second, the studies included in this review used different diagnostic criteria for DM that could induce the risk of over‐ or under representation of DM patients among studies. Also, we should consider that if studies did not explicitly listed DM as an exclusion criterion, it may well be that DM patients were eligible but were not included in the trial. Third, no meta‐analysis was performed because we felt that simply combining all rates would be less informative than providing separate DM inclusion rates by region/continent. Fourth, in the PubMed search, we applied a full‐text available filter (see Supporting information Appendix S2). This could have excluded some full manuscripts that only had an abstract available in PubMed. Retrospectively, we have checked the impact of this filter. In the search without the filter, 21 additional hits (equalling 3.7% more hits) were found, although, after inspection, none were eligible. Finally, we could not assess reporting bias as clinical trials around the world can be registered in many different databases and we received little response from contacting the study authors. Therefore, comparing published trials with registered trials was not feasible.
Regarding future research and policies, it is important for TB drug trials to screen for DM comorbidity, aim for a representative, real‐life, DM percentage according to the location and appropriately diagnose DM. Alternatively, a separate multicentre trial in diabetic patients could be considered where also more emphasis can be placed on diabetes‐specific outcomes such as hypoglycaemias. Intensified research and development of TB drugs, particularly in the context of comorbidities such as DM, play a crucial role to improve TB control and contribute to reductions in TB incidence and mortality required to reach global TB targets by 2035, one of the pillars of World Health Organization's Post‐2015 Global TB Strategy.16
Including DM comorbidity in TB drug trials will allow for the study of possible DM‐TB drug–drug and drug–disease interactions that can alter the pharmacokinetics, safety and clinical effects of the TB drugs. Eventually, these findings will enable us to assess TB‐DM patients' individual need for personalize treatment options and lead to better real‐world TB‐DM outcomes and possibly lower resistance rates.
5. CONCLUSION
To conclude, current inclusion of DM comorbidity in recent TB drug efficacy trials is suboptimal compared with its increasing prevalence and significance. Considering the considerable prevalence and impact of DM comorbidity, the inclusion of patients with DM in future TB efficacy drug trials warrants increased attention and requires a joint effort of trialists, clinicians and policy makers alike.
5.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY.73
COMPETING INTERESTS
The authors declare no competing interests.
CONTRIBUTORS
The study was designed by J.B. and J.A. Data collection was done by N.L. and M.Z. The initial manuscript was drafted by J.B. and N.L. All other authors helped with data interpretation and commented on the study design and the first draft. All authors helped with completing the final manuscript. J.A. is the guarantor of the study.
Supporting information
APPENDIX S1 PRISMA checklist.
APPENDIX S2 Search protocol.
APPENDIX S3 Studies included and excluded from the systematic review during full‐text assessment.
ACKNOWLEDGEMENTS
N.L. and M.Z. were supported by a visiting fellowship grant from the University Medical Centre Groningen.
Lutfiana NC, van Boven JFM, Masoom Zubair MA, Pena MJ, Alffenaar J‐WC. Diabetes mellitus comorbidity in patients enrolled in tuberculosis drug efficacy trials around the world: A systematic review. Br J Clin Pharmacol. 2019;85:1407–1417. 10.1111/bcp.13935
REFERENCES
- 1. Workneh MH, Bjune GA, Yimer SA. Prevalence and associated factors of tuberculosis and diabetes mellitus comorbidity: A systematic review. PloS One. 2017;12(4):e0175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . Global tuberculosis report. 2018. Available at http://apps.who.int/iris/bitstream/handle/10665/274453/9789241565646‐eng.pdf?ua=1. Accessed September 2 2018.
- 3. International Diabetes Federation . IDF Diabetes atlas. 7th Edition. 2015. International Diabetes Federation, Brussels. http://www.idf.org/diabetesatlas/. Accessed June 13 2017.
- 4. Riza AL, Pearson F, Ugarte‐Gil C, et al. Clinical management of concurrent diabetes and tuberculosis and the implication for patient services. Lancet Diabetes Endocrinol. 2014;2(9):740‐753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Odone A, Houben RMGJ, White RG, Lönnroth K. The effect of diabetes and undernutrition trends on reaching 2035 global tuberculosis targets. Lancet Diabetes Endocrinol. 2014;2(9):754‐764. [DOI] [PubMed] [Google Scholar]
- 6. Deng C, Wang X, Liao Y. Current recommendations on managing tuberculosis patients with diabetes & its epidemiology. Microb Pathog. 2016;92:43‐45. [DOI] [PubMed] [Google Scholar]
- 7. Baker MA, Harries AD, Jeon CY, et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med. 2011;9(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perez‐Navarro LM, Restrepo BI, Fuentes‐Dominguez FJ, et al. The effect size of type 2 diabetes mellitus on tuberculosis drug resistance and adverse treatment outcomes. Tuberculosis. 2017;103:83‐91. [DOI] [PubMed] [Google Scholar]
- 9. Jiménez‐Corona ME, Cruz‐Hervert LP, García‐García L, et al. Association of diabetes and tuberculosis: impact on treatment and post‐treatment outcomes. Thorax. 2013;68(3):214‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reed GW, Choi H, Lee SY, et al. Impact of diabetes and smoking on mortality in tuberculosis. PLoS ONE. 2013;8(2):58‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang Q, Ma A, Bygbjerg IC, et al. Rationale and design of a randomized controlled trial of the effect of retinol and vitamin D supplementation on treatment in active pulmonary tuberculosis patients with diabetes. BMC Inf Dis. 2013;13(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mi F, Tan S, Liang L, et al. Diabetes mellitus and tuberculosis: pattern of tuberculosis, two‐month smear conversion and treatment outcomes in Guangzhou, China. Trop Med Int Health. 2013;18(2):1379‐1385. [DOI] [PubMed] [Google Scholar]
- 13. Shewade HD, Jeyashree K, Mahajan P, et al. Effect of glycemic control and type of diabetes treatment on unsuccessful TB treatment outcomes among people with TB‐diabetes: a systematic review. PLoS ONE. 2017;12(10):e0186697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chiang CY, Baik KJ, Chien ST, et al. The influence of diabetes, glycemic control, and diabetes‐related comorbidities on pulmonary tuberculosis. PloS ONE. 2015;10(3):e0121698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Health Organization . Collaborative Framework for Care and Control of Tuberculosis and Diabetes. 2011. Available at http://www.who.int/tb/publications/tb‐diabetes‐framework/en/. Accessed June 13 2017. [PubMed]
- 16. Wallis RS, Maeurer M, Mwaba P, et al. Tuberculosis—advances in development of new drugs, treatment regimens, host‐directed therapies, and biomarkers. Lancet Infect Dis. 2016;16:34‐34. [DOI] [PubMed] [Google Scholar]
- 17. Diacon AH, Dawson R, Groote‐Bidlingmaier F, et al. 14‐day bactericidal activity of PA‐824, bedaquiline, pyrazinamide, and moxifloxacin combinations: a randomised trial. Lancet. 2012;380(9846):986‐993. [DOI] [PubMed] [Google Scholar]
- 18. Diacon AH, Dawson R, du Bois J, et al. Phase II dose‐ranging trial of the early bactericidal activity of PA‐824. Antimicrob Agents Chemother. 2012;56(6):3027‐3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee MS, Lee JS, Carroll MW, et al. Linezolid for treatment of chronic extensively drug‐resistant tuberculosis. N Engl J Med. 2012;367(16):1508‐1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gler MT, Skripconoka V, Sanchez‐Garavito E, et al. Delamanid for multidrug‐resistant pulmonary tuberculosis. N Engl J Med. 2012;366(23):2151‐2160. [DOI] [PubMed] [Google Scholar]
- 21. Zhang Q, Liu Y, Tang S, Sha W, Xiao H. Clinical benefit of delamanid (OPC‐67683) in the treatment of multidrug‐resistant tuberculosis patients in China. Cell Biochem Biophys. 2013;67(3):957‐963. [DOI] [PubMed] [Google Scholar]
- 22. Jawahar MS, Banurekha VV, Paramasivan CN, et al. Randomized clinical trial of thrice‐weekly 4‐month moxifloxacin or gatifloxacin containing regimens in the treatment of new sputum positive pulmonary tuberculosis patients. PLoS ONE. 2013;8(7):e67030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang Q, Ma A, Bygbjerg IC, et al. Rationale and design of a randomized controlled trial of the effect of retinol and vitamin D supplementation on treatment in active pulmonary tuberculosis patients with diabetes. BMC Infect Dis. 2013;13(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jindani A, Harrison TS, Nunn AJ, et al. High‐dose rifapentine with moxifloxacin for pulmonary tuberculosis. N Engl J Med. 2014;371(17):1599‐1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Diacon AH, Pym A, Grobusch MP, et al. Multidrug‐resistant tuberculosis and culture conversion with bedaquiline. N Engl J Med. 2014;371:723‐732. [DOI] [PubMed] [Google Scholar]
- 26. Gillespie SH, Crook AM, McHugh TD, et al. Four‐month moxifloxacin‐based regimens for drug‐sensitive tuberculosis. N Engl J Med. 2014;371(17):1577‐1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nunn AJ, Cook SV, Burgos M, et al. Results at 30 months of a randomised trial of FDCs and separate drugs for the treatment of tuberculosis. Int J Tuberc Lung Dis. 2014;18(10):1252‐1254. [DOI] [PubMed] [Google Scholar]
- 28. Luangchosiri C, Thakkinstian A, Chitphuk S, Stitchantrakul W, Petraksa S, Sobhonslidsuk A. A double‐blinded randomized controlled trial of silymarin for the prevention of antituberculosis drug‐induced liver injury. BMC Complement Altern Med. 2015;15(1):334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Diacon AH, Dawson R, von Groote‐Bidlingmaier F, et al. Bactericidal activity of pyrazinamide and clofazimine alone and in combinations with pretomanid and bedaquiline. Am J Respir Crit Care Med. 2015;191(8):943‐953. [DOI] [PubMed] [Google Scholar]
- 30. Daley P, Jagannathan V, John KR, et al. Adjunctive vitamin D for treatment of active tuberculosis in India: a randomised, double‐blind, placebo‐controlled trial. Lancet Inf Dis. 2015;15(5):528‐534. [DOI] [PubMed] [Google Scholar]
- 31. Dawson R, Diacon AH, Everitt D, et al. Efficiency and safety of the combination of moxifloxacin, pretomanid (PA‐824), and pyrazinamide during the first 8 weeks of antituberculosis treatment: a phase 2b, open‐label, partly randomised trial in patients with drug‐susceptible or drug‐resistant pulmonary tuberculosis. Lancet. 2015;385(9979):1738‐1747. [DOI] [PubMed] [Google Scholar]
- 32. Dorman SE, Savic RM, Goldberg S, et al. Daily rifapentine for treatment of pulmonary tuberculosis. A randomized, dose‐ranging trial. Am J Respir Crit Care Med. 2015;191(3):333‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Heinrich N, Dawson R, du Bois J, et al. Early phase evaluation of SQ109 alone and in combination with rifampicin in pulmonary TB patients. J Antimicrob Chemother. 2015;70(5):1558‐1566. [DOI] [PubMed] [Google Scholar]
- 34. Merle CS, Fielding K, Sow OB, et al. A four‐month gatifloxacin‐containing regimen for treating tuberculosis. N Engl J Med. 2014;371(17):1588‐1598. [DOI] [PubMed] [Google Scholar]
- 35. Mily A, Rekha RS, Kamal SMM, et al. Significant effects of oral phenylbutyrate and vitamin d3 adjunctive therapy in pulmonary tuberculosis: a randomized controlled trial. PLoS ONE. 2015;10(9):e0138340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wu JT, Chiu CT, Wei YF, Lai YF. Comparison of the safety and efficacy of a fixed‐dose combination regimen and separate formulations for pulmonary tuberculosis treatment. Clinics (Sao Paulo). 2015;70(6):429‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tang S, Yao L, Hao X, et al. Efficacy, safety and tolerability of linezolid for the treatment of XDR‐TB: a study in China. Eur Respir J. 2015;45(1):161‐170. [DOI] [PubMed] [Google Scholar]
- 38. Tang S, Yao L, Hao X, et al. Clofazimine for the treatment of multidrug‐resistant tuberculosis: prospective, multicenter, randomized controlled study in China. Clin Infect Dis. 2015;60(9):1361‐1367. [DOI] [PubMed] [Google Scholar]
- 39. Tukvadze N, Sanikidze E, Kipiani M, et al. High‐dose vitamin D3 in adults with pulmonary tuberculosis: a double‐blind randomized controlled trial. Am J Clin Nutr. 2015;102(5):1059‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Aseffa A, Chukwu JN, Vahedi M, et al. Efficacy and safety of 'fixed dose' versus 'loose' drug regimens for treatment of pulmonary tuberculosis in two high TB‐burden African countries: a randomized controlled trial. PLoS One. 2016;11(6):e0157434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Conde MB, Mello FCQ, Duarte RS, et al. A phase 2 randomized trial of a rifapentine plus moxifloxacin‐based regimen for treatment of pulmonary tuberculosis. Plos One. 2016;11(5):e0154778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Furin JJ, Du Bois J, van Brakel E, et al. Early bactericidal activity of AZD5847 in patients with pulmonary tuberculosis. Antimicrob Agents Chemother. 2016;60(11):6591‐6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Heemskerk D, Bang ND, Mai NTH, et al. Intensified antituberculosis therapy in adults with tuberculous meningitis. N Engl J Med. 2016;374(2):124‐134. [DOI] [PubMed] [Google Scholar]
- 44. Kang YA, Shim TS, Koh WJ, et al. Choice between levofloxacin and moxifloxacin and multidrug‐resistant tuberculosis treatment outcomes. Ann Am Thorac Soc. 2016;13(3):364‐370. [DOI] [PubMed] [Google Scholar]
- 45. Milstein M, Lecca L, Peloquin C, et al. Evaluation of high‐dose rifampin in patients with new, smear‐positive tuberculosis (HIRIF): study protocol for a randomized controlled trial. BMC Infect Dis. 2016;16(1):453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pym AS, Diacon AH, Tang SJ, et al. Bedaquiline in the treatment of multidrug‐ and extensively drug‐resistant tuberculosis. Eur Respir J. 2016;47(2):564‐574. [DOI] [PubMed] [Google Scholar]
- 47. Chesdachai S, Zughaier SM, Hao L, et al. The effects of first‐line anti‐tuberculosis drugs on the actions of vitamin D in human macrophages. J Clin Transl Endocrinol. 2016;6:23‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang S, Pan H, Peng X, et al. Preventive use of a hepatoprotectant against anti‐tuberculosis drug‐induced liver injury: A randomized controlled trial. J Gastroenterol Hepatol. 2016;31(2):409‐416. [DOI] [PubMed] [Google Scholar]
- 49. Aarnoutse RE, Kibiki GS, Rither K, et al. Pharmacokinetics, tolerability, and bacteriological response of rifampin administered 600, 900, and 1200 milligrams daily in patients with pulmonary tuberculosis. Antimicrob Agents Chemother. 2017;61(11):e01054‐e01017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Alsultan A, Furin JJ, DuBois J, et al. Population pharmacokinetics of AZD‐5847 in adults with pulmonary tuberculosis. Antimicrob Agents Chemother. 2017;61(10):e01066‐e01017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Boeree MJ, Heinrich N, Aarnoutse R, et al. High‐dose rifampicin, moxifloxacin, and SQ109 for treating tuberculosis: a multi‐arm, multi‐stage randomised controlled trial. Lancet Infect Dis. 2017;17(1):39‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Boutoun TC, Phillips PPJ, Mitnick CD, et al. An optimized background regimen design to evaluate the contribution of levofloxacin to multidrug‐resistant tuberculosis treatment regimens: study protocol for a randomized controlled trial. Trials. 2017;18(1):563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Batbold U, Butov DO, Kutsyna GA, et al. Double‐blind, placebo‐controlled, 1:1 randomized Phase III clinical trial of immunoxel honey lozenges as an adjunct immunotherapy in 269 patients with pulmonary tuberculosis. Immunotherapy. 2017;9(1):13‐24. [DOI] [PubMed] [Google Scholar]
- 54. Lee JY, Kim DK, Lee JK, et al. Substitution of ethambutol with linezolid during the intensive phase of treatment of pulmonary tuberculosis: study protocol for a prospective, multicenter, randomized, open‐label, phase II trial. Trials. 2017;18(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sigal GB, Segal MR, Mathew A, et al. Biomarkers of tuberculosis severity and treatment effect: a directed screen of 70 host markers in randomized clinical trial. EBioMedicine. 2017;25:112‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ganmaa D, Munkhzul B, Fawzi W, et al. High‐dose vitamin D3 during tuberculosis treatment in mongolia. a randomized controlled trial. Am J Respir Crit Care Med. 2017;196(5):628‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wallis RS, Maeurer M, Mwaba P, et al. Screening patients with diabetes mellitus for tuberculosis in China. Trop Med Int Health. 2012;17(10):1302‐1308. [DOI] [PubMed] [Google Scholar]
- 58. India Diabetes Mellitus–Tuberculosis Study Group . Screening patients with diabetes mellitus for tuberculosis in India. Trop Med Int Health. 2013;18(5):646‐654. [DOI] [PubMed] [Google Scholar]
- 59. India Tuberculosis‐Diabetes Study Group . Screening patients with tuberculosis for diabetes mellitus in India. Trop Med Int Health. 2013;18(5):636‐645. [DOI] [PubMed] [Google Scholar]
- 60. GBD 2013 Mortality and Causes of Death Collaborators . Global, regional, and national age–sex specific all‐cause and cause‐specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Liu Q, Li W, Xue M, et al. Diabetes mellitus and the risk of multidrug resistant tuberculosis: a meta‐analysis. Sci Rep. 2017;7(1):1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hu M, Zheng C, Gao F. Use of bedaquiline and delamanid in diabetes patients: clinical and pharmacological considerations. Drug Des Devel Ther. 2016;10:3983‐3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. International Diabetes Federation . 2012. Global Guideline for Type 2 Diabetes. International Diabetes Federation, Brussels.
- 64. Yoon YS, Jung JW, Jeon EJ, et al. The effect of diabetes control status on treatment response in pulmonary tuberculosis: a prospective study. Thorax. 2017;72(3):263‐270. [DOI] [PubMed] [Google Scholar]
- 65. Critchley JA, Restrepo BI, Ronacher K, et al. Defining a research agenda to address the converging epidemics of tuberculosis and diabetes. Part 1: Epidemiology and clinical management. Chest. 2017;152(1):165‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ronacher K, van Crevel R, Critchley JA, et al. Defining a research agenda to address the converging epidemics of tuberculosis and diabetes. Part 2: Underlying biological mechanisms. Chest. 2017;152(1):174‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hodgson K, Morris J, Bridson T, Govan B, Rush C, Ketheesan N. Immunological mechanisms contributing to the double burden of diabetes and intracellular bacterial infections. Immunology. 2014;144:171‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Restrepo BI, Twahirwa M, Rahbar MH, Schlesinger LS. Phagocytosis via complement or Fc‐gamma receptors is compromised in monocytes from type 2 diabetes patients with chronic hyperglycemia. PloS ONE. 2014;9(3):e92977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. van Crevel R, Dockrell HM, TANDEM Consortium . TANDEM. Understanding diabetes and tuberculosis. Lancet Diabetes Endocrinol. 2014;2(4):270e2. [DOI] [PubMed] [Google Scholar]
- 70. Daniel BD, Ramachandran G, Swaminathan S. The challenges of pharmacokinetic variability of first‐line anti‐TB drugs. Expert Rev Clin Pharmacol. 2017;12:461‐479. [DOI] [PubMed] [Google Scholar]
- 71. Nahid P, Dorman SE, Alipanah N, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: treatment of drug‐susceptible tuberculosis. Clin Inf Dis. 2016;63(7):147‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Alffenaar JC, Tiberi S, Verbeeck RK, Heysell SK, Grobusch MP. Therapeutic drug monitoring in tuberculosis: practical application for physicians. Clin Inf Dis. 2017;64(1):104‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Harding SD, Sharman JL, Faccenda E, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acid Res. 2018;46: S1091–S1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
APPENDIX S1 PRISMA checklist.
APPENDIX S2 Search protocol.
APPENDIX S3 Studies included and excluded from the systematic review during full‐text assessment.


