Abstract
It has been claimed that Nigella sativa seeds (NSS), also known as black cumin, have antidiabetic and lipid‐lowering properties. Our pilot study investigated the effects of powdered NSS on insulin secretion and lipid profile in healthy male volunteers. We conducted a double‐blind, randomized, placebo‐controlled 4‐week trial in 30 subjects, receiving NSS powder (1 g/day) or placebo orally (15 subjects/group). Insulin secretion as determined by the hyperglycaemic clamp technique, insulin sensitivity as well as cholesterol and triglycerides serum concentrations, were measured before and after treatment. NSS powder administration was clinically well tolerated. It did not modify fasting glycaemia and insulinaemia, and was ineffective on glucose‐induced insulin secretion and insulin sensitivity. No significant changes on serum lipids were observed after treatment in any treatment groups, nor between the two treatment groups. However, in the treated group only, there was a significant correlation between total cholesterol change after treatment and its baseline level (r = −0.71, P = 0.006, n = 13), and between low‐density lipoprotein (LDL) cholesterol change after treatment and its baseline level (r = −0.74, P = 0.004, n = 13). No such correlations were found for high‐density lipoprotein (HDL) cholesterol, and for triglycerides. These results do not confirm any NSS effect on glucose regulation; however, they suggest that NSS powder may be of interest in lowering lipid concentrations in hyperlipidaemic subjects.
Keywords: cholesterol, insulin, lipids, Nigella sativa, randomized clinical trial
What is already known about this subject
NSS have been widely used around the world as a spice and as a traditional medicine. They are especially reported to display antidiabetic properties in some pharmacopeias. Experimental results suggest that NSS could enhance insulin secretion and/or insulin sensitivity, and reduce lipid levels.
What this study adds
This study is the first clinical trial specifically designed to investigate the effect of NSS on insulin secretion in humans (primary outcome).
We could not show any effect on this metabolic parameter, or on insulin sensitivity.
Data on lipid profile suggest that NSS could have a lowering effect on total cholesterol and LDL cholesterol, which remains to be assessed in hyperlipidaemic subjects.
1. INTRODUCTION
There is increasing interest in finding new and safe alternatives to drugs for treating chronic disorders, such as type 2 diabetes and dyslipidaemia, which are both important risk factors for cardiovascular complications. Nigella sativa L. is a herbaceous annual plant of the Ranunculaceae family, cultivated in several Mediterranean and Asian countries. Its seeds are commonly used as a spice (black cumin) or as a traditional medicine against a variety of diseases, including diabetes.1 Experimentally, Nigella sativa seeds (NSS) have been reported to have a large panel of effects, including antidiabetic and lipid‐lowering effects.2 In clinical studies, antidiabetic properties and/or therapeutic effects on metabolic syndrome have been observed.3, 4, 5, 6, 7, 8 Potential mechanisms for the antidiabetic properties of NSS could be supported by an improvement of insulin sensitivity previously demonstrated in rats.9 Indeed, the effects of NSS include an increase in the peroxisome proliferator‐activated receptor gamma (PPAR‐γ) activity,10 and liver gluconeogenesis inhibition via adenosine monophosphate‐activated protein kinase (AMPK) activation.11 An inhibition of the electrogenic intestinal glucose absorption has also been observed.12 Lipid‐lowering properties and effects on metabolic syndrome could be mediated by activation of PPAR‐γ,10 increased uptake of low‐density lipoprotein (LDL) cholesterol by upregulation of hepatic LDL receptors,13 de novo suppression of cholesterol synthesis,13 and prevention of lipid peroxidation.14
In addition, we have previously shown that a NSS extract could increase the glucose‐induced insulin release from isolated pancreatic islets in a concentration‐dependent manner.15 More recently, it has been shown that a NSS extract and one of its major components, thymoquinone, were able to enhance glucose‐stimulated insulin secretion from pancreatic beta‐cells under normal conditions as well as under hyperglycaemia; this action was associated with the ability of thymoquinone to regulate carbohydrate‐to‐lipid flux via downregulation of acetyl‐CoA carboxylase and malonyl‐CoA.16 However, to date, there are no data concerning NSS effects on insulin secretion capability in humans, using the hyperglycaemic clamp technique which is the state‐of‐the‐art method.
Therefore, the primary aim of the present study was to investigate the effects of oral administration of powdered NSS on insulin secretion in healthy male subjects using the hyperglycaemic clamp technique; the secondary objectives were to investigate the effects of NSS on insulin sensitivity and lipid profile in the same population.
2. METHODS
The study was designed as a pilot 4‐week, double‐blind, randomized, placebo‐controlled parallel trial. Thirty healthy male volunteers were enrolled, with the following characteristics (mean [min‐max]): age 23 years [19–34], body mass index (BMI) 22.5 kg m−2 [19.4–24.7], fasting glycaemia 4.5 mmol L−1 [3.9–5.9], fasting insulinaemia 4.0 μU ml−1 [1.5–6.5], total cholesterol 4.4 mmol L−1 [2.0–6.3], LDL cholesterol 2.6 mmol L−1 [0.6–4.5], triglycerides 0.9 mmol L−1 [0.4–2.0]. This study was approved by the Ethics Committee “Comité de Protection des Personnes Sud‐Méditerranée IV” and conducted in accordance with the Helsinki Declaration and the International Council of Harmonisation (ICH) guideline for Good Clinical Practice. All subjects gave written informed consent to participate. They received 1 g/day of NSS powder or placebo (15 subjects per group), orally for 4 weeks. NSS were purchased from Alliance Senteur Company (Aubagne, France, batch number 424674). The main aromatic components of Nigella sativa were identified by gas chromatography–mass spectrometry from a dichloromethane seed extract; the major compound of pharmacological interest, thymoquinone, represented 50% of the extract. No damascenine was detected, which shows the absence of contamination by seeds of toxic species (Nigella damascena). The dose was chosen according to traditional pharmacopoeia and previous clinical studies. Parameters of glycaemic regulation and lipid profile were determined before and after the 4‐week treatment period.
Glucose‐induced insulin secretion and insulin sensitivity were investigated using the hyperglycaemic clamp technique, in which blood glucose was clamped at 10 mmol L−1 for 120 minutes, according to DeFronzo et al.17 The detailed protocol and calculated indexes are described elsewhere.18 Total and LDL cholesterol and triglycerides were measured. Results are expressed as means with 95% confidence interval (95% CI) in the text, and as means ± SEM in the figures. The sample size was determined based on the literature concerning the assessment of insulin secretion in humans using the hyperglycaemic clamp technique.18 Within each group, comparisons between post‐treatment and baseline values were performed using the paired t‐test. Post‐treatment means were compared between groups using analysis of covariance (ANCOVA) with baseline data as a covariate, after logarithmic transformation when appropriate. Correlations between lipid changes after treatment and their baseline level were performed using the Pearson test. Differences between the corresponding linear regression slopes were measured by the interaction between treatment and baseline lipid level on the lipid change after treatment in a two‐way analysis of variance (ANOVA). Systat 10.0 software for Windows from SPSS (Chicago, IL, USA) was used for statistical analysis. The level of significance was set at P < 0.05.
3. RESULTS
No differences were observed between the two treatment groups based on age, BMI or fasting plasma glucose, serum insulin, total cholesterol, LDL cholesterol and triglycerides values. The administration of NSS powder did not modify fasting plasma glucose and serum insulin concentrations. It was also ineffective on glucose‐induced insulin secretion, as reflected by C‐peptide concentrations (Figure 1), and on insulin sensitivity.
Figure 1.
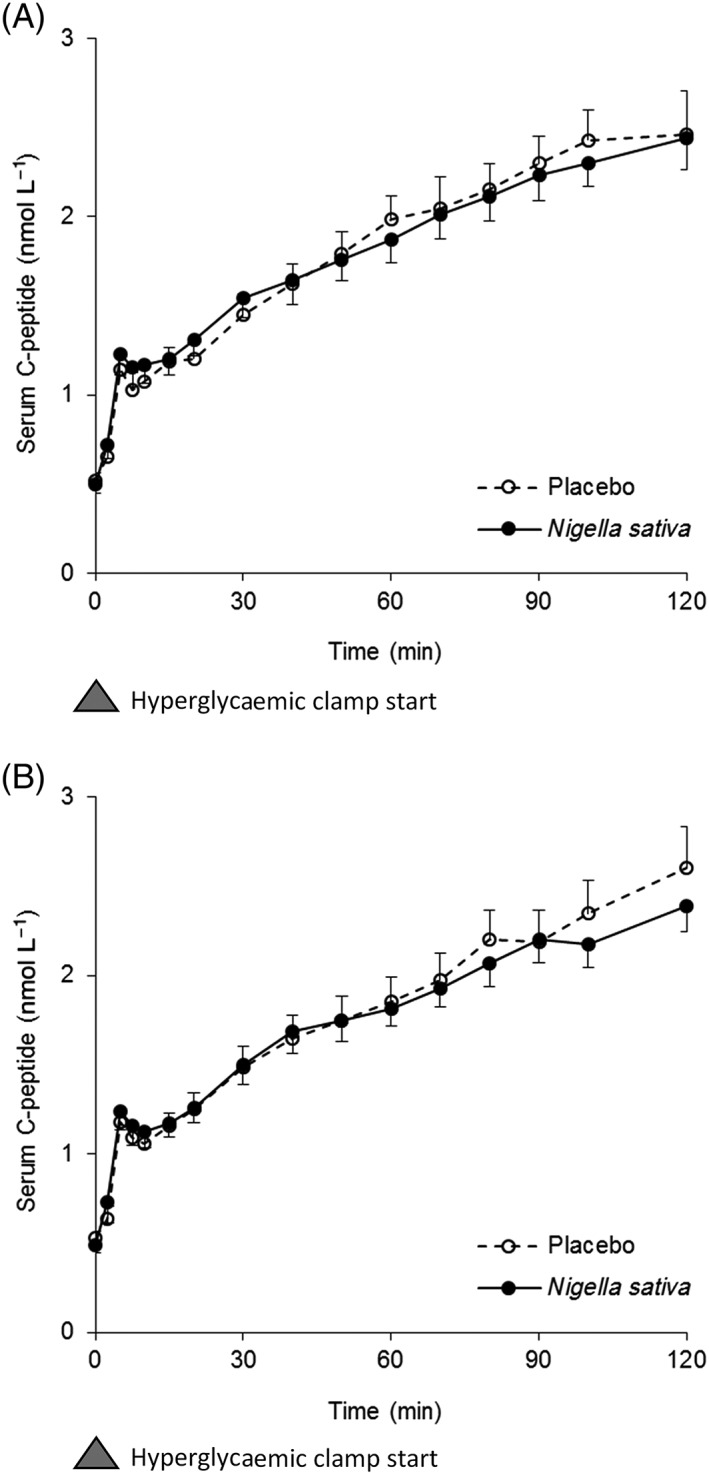
Time course of serum C‐peptide concentrations ± SEM (nmol L−1) during hyperglycaemic clamp before treatment (A), and after treatment (B)
Figure 2 shows the effect of treatment on total cholesterol. The change after treatment was −0.16 (95% CI: −0.56–0.25, P = NS, n = 13) in the NSS group and 0.08 (95% CI: −0.16–0.32, P = NS, n = 15) in the placebo group (Panel A). This difference between the two groups was non‐significant. In the NSS group, there was a significant correlation between the change in total cholesterol and its baseline level (Panel B, r = −0.71, P = 0.006, n = 13); no such correlation was found in the placebo group, and the two slopes of the corresponding linear regression lines were significantly different (P = 0.021).
Figure 2.
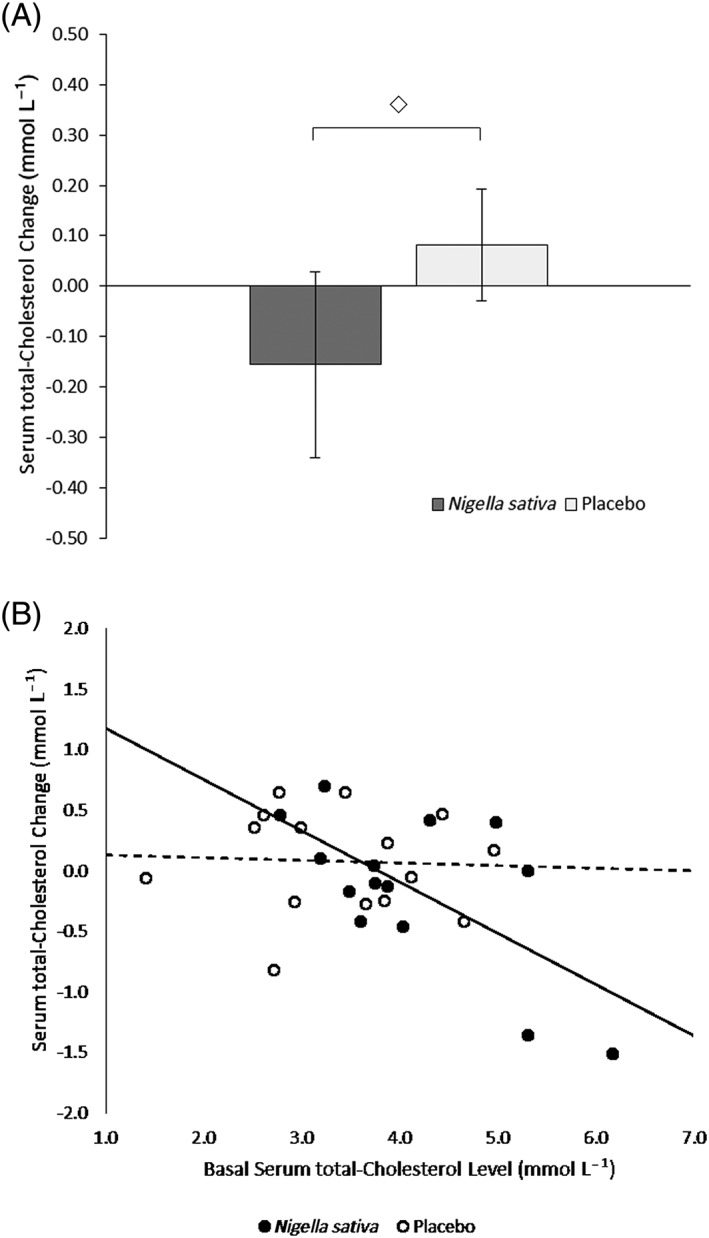
A, Serum total cholesterol changes ± SEM (mmol L−1) after 4‐week treatment (◇: Non significant). B, Correlations between serum total cholesterol change after 4‐week treatment (mmol L−1) and basal serum total cholesterol level (mmol L−1); ●: Nigella sativa, r = −0.71, P = 0.006; ○: Placebo, r = −0.05, P = NS. The slopes of the linear regression lines (full line for Nigella sativa, dashed line for placebo) were significantly different (P = 0.021)
Figure 3 shows the effect of treatment on LDL cholesterol. The change after treatment was −0.11 (95% CI: −0.40–0.18, P = NS, n = 13) in the treated group and 0.10 (95% CI: −0.09–0.29, P = NS, n = 15) for placebo (Panel A). This difference between the two groups was non‐significant. In the NSS group, there was a significant correlation as well, between the change in LDL cholesterol and its baseline level (Panel B, r = −0.74, P = 0.004, n = 13); no such correlation was found in the placebo group, and there was a trend towards statistical significance between the two slopes of the corresponding linear regression lines (P = 0.067). No correlations were found for high‐density lipoprotein (HDL) cholesterol, and for triglycerides.
Figure 3.
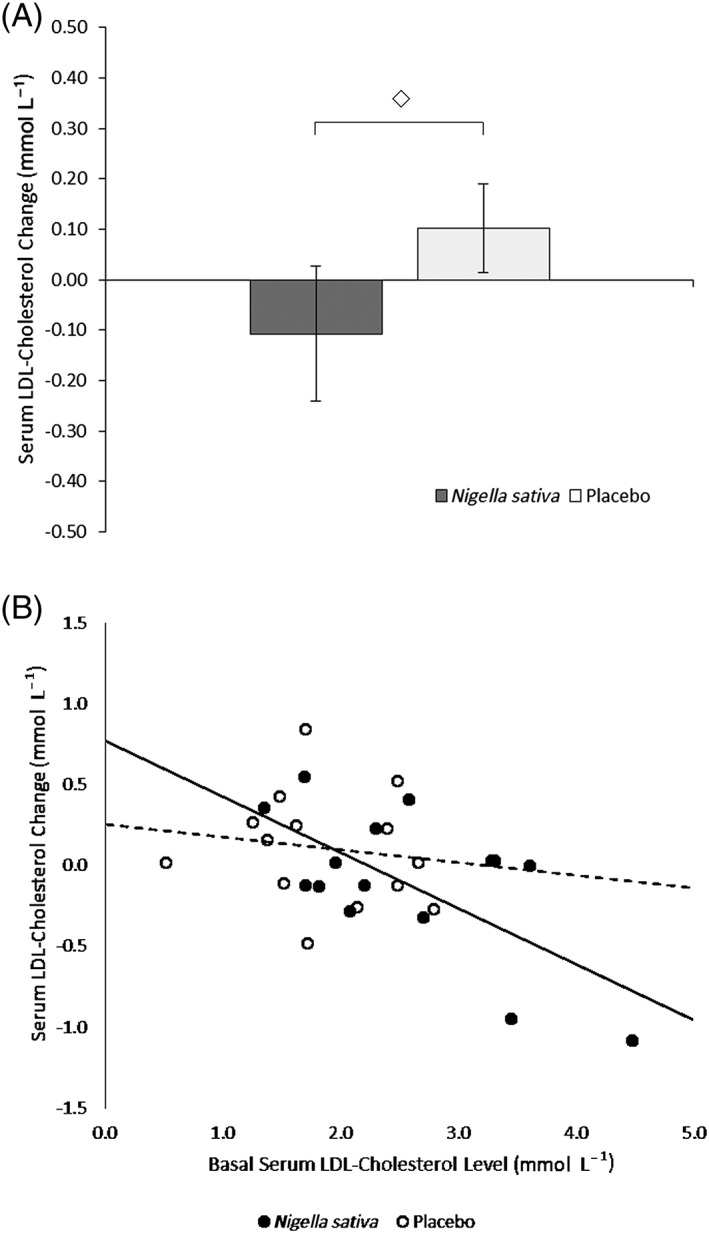
A, Serum LDL cholesterol changes ± SEM (mmol L−1) after 4‐week treatment (◇: Non significant). B, Correlations between serum LDL cholesterol change after 4‐week treatment (mmol L−1) and basal serum LDL cholesterol level (mmol L−1); ●: Nigella sativa, r = −0.74, P = 0.004; ○: Placebo, r = −0.17, P = NS. There was a trend towards statistical significance (P = 0.067) between the slopes of the linear regression lines of the Nigella sativa group (full line) and the placebo group (dashed line)
No serious adverse events were observed during the trial. The counts of platelets, leucocytes and monocytes were significantly higher at the end of the treatment period in the Nigella group as compared to placebo; however, the counts remained within normal values.
4. DISCUSSION
The present study was a pilot investigation specifically designed to evaluate the effect of a repeated oral administration of NSS powder on insulin secretion in healthy volunteers (primary outcome). The “healthy volunteers” model makes it possible to study clinical pharmacological effects without interference with any pathological condition or associated treatment. To the best of our knowledge, this is the first study to explore the effects of NSS on glucose‐induced insulin secretion in humans. The hyperglycaemic clamp technique is considered to be the gold standard for assessing beta‐cell function; it also provides an accurate estimation of insulin sensitivity.19
The 4‐week NSS treatment was clearly ineffective in modifying insulin secretion in healthy subjects, in contrast with previous experimental studies showing an enhanced glucose‐stimulated insulin secretion from rat‐isolated Langerhans islets in the presence of NSS extracts,15 or from clonal beta‐cells exposed to NSS extracts or thymoquinone.16 Nor was any effect on insulin sensitivity observed. These results concerning the glucose regulation parameters are in agreement with previous results obtained from clinical studies showing no effect on fasting glycaemia.3, 4, 20 However, a beneficial effect of NSS as an adjuvant therapy to oral hypoglycaemic agents has been shown at 2 g/day for 3 months in patients with type 2 diabetes.21
We did not observe any significant effect of NSS on mean serum lipid changes. However, significant negative correlations between baseline lipid levels and lipid changes after treatment suggest that NSS might be beneficial to hyperlipidaemic patients. Considering the low number of subjects in our study, we cannot exclude that the observed effect may be due to sampling fluctuations or regression to the mean. Conversely, this observation may result from a real pharmacological effect, in accordance with a previous clinical trial indicating that NSS may improve type 2 diabetes associated dyslipidaemia,22 and with a recent meta‐analysis of randomized controlled trials investigating the effects of Nigella sativa on plasma lipid concentrations.8
The treatment with NSS powder was well tolerated, despite a slight non‐clinically significant haematological effect.
5. CONCLUSION
As discussed above, the effect of NSS on lipids remains to be confirmed because of the small number of subjects enrolled in this study. Another limitation is the relatively low level of exposure of subjects to NSS in terms of daily dose and treatment duration (1 g/day for 1 month). Without prior data on the effects of NSS on insulin secretion in human, the dose was chosen from traditional pharmacopoeia data describing Nigella sativa use in diabetes. However, we cannot exclude that this dose was too low to be able to detect any existing insulin secreting and sensitizing effect of NSS.
In conclusion, our study in healthy volunteers does not confirm any Nigella sativa effect on insulin secretion and sensitivity; however, our data are in agreement with a potential beneficial effect of NSS on lipid concentrations in hyperlipidaemic subjects.
COMPETING INTERESTS
There are no competing interests to declare.
CONTRIBUTORS
All the authors made the following substantial contributions to the study and gave final approval of the version to be published after careful critical revision. S.P. analysis and interpretation of data, drafting the manuscript. F.G. (investigator): design of the study, acquisition and interpretation of data, drafting the manuscript. A.C. (investigator) and J.‐P.G. (investigator): data acquisition. A.‐M.B.: design of the study. Y.P., M.L., S.L., M.F. and I.G.: data acquisition. P.P. (principal investigator): conception and design of the study, data acquisition, interpretation of data, drafting the manuscript. H.C.: conception and design of the study, data acquisition, analysis and interpretation of data, drafting the manuscript.
ACKNOWLEDGEMENTS
This study was sponsored by the University Hospital of Montpellier (UF7889) with the financial support of the French Ministry of Health (PHRC régional 2006). This study was successfully carried out thanks to the expert scientific, medical, technical and logistic assistance of J.L. Allaz, B. Bories‐Azeau, M.A. Champiat, A. Dupuy, O. Gaget, V. Marco, A. Massebiau, A.M. Puech‐Cathala and I. Roch‐Torreilles.
Pelegrin S, Galtier F, Chalançon A, et al. Effects of Nigella sativa seeds (black cumin) on insulin secretion and lipid profile: A pilot study in healthy volunteers. Br J Clin Pharmacol. 2019;85:1607–1611. 10.1111/bcp.13922
French national clinical trial register number: ID RCB 2006‐A00452‐49
The authors confirm that the PI for this paper is Pierre Petit and that he had direct clinical responsibility for patients.
REFERENCES
- 1. Tahraoui A, El‐Hilaly J, Israili ZH, Lyoussi B. Ethnopharmacological survey of plants used in the traditional treatment of hypertension and diabetes in South‐Eastern Morocco (Errachidia province). J Ethnopharmacol. 2007;110(1):105‐117. [DOI] [PubMed] [Google Scholar]
- 2. Heshmati J, Namazi N. Effects of black seed (Nigella sativa) on metabolic parameters in diabetes mellitus: A systematic review. Complement Ther Med. 2015;23(2):275‐282. [DOI] [PubMed] [Google Scholar]
- 3. Datau EA, Surachmanto EE, Pandelaki K, Langi JA. Efficacy of Nigella sativa on serum free testosterone and metabolic disturbances in central obese male. Acta Med Indones. 2010;42(3):130‐134. [PubMed] [Google Scholar]
- 4. Sabzghabaee AM, Dianatkhah M, Sarrafzadegan N, Asgary S, Ghannadi A. Clinical evaluation of Nigella sativa seeds for the treatment of hyperlipidemia: A randomized, placebo controlled clinical trial. Med Arch. 2012;66(3):198‐200. [DOI] [PubMed] [Google Scholar]
- 5. Farzaneh E, Nia FR, Mehrtash M, Mirmoeini FS, Jalilvand M. The effects of 8‐week Nigella sativa supplementation and aerobic training on lipid profile and VO(2) max in sedentary overweight females. Int J Prev Med. 2014;5(2):210‐216. [PMC free article] [PubMed] [Google Scholar]
- 6. Ibrahim RM, Hamdan NS, Mahmud R, et al. A randomised controlled trial on hypolipidemic effects of Nigella sativa seeds powder in menopausal women. J Transl Med. 2014;12(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kaatabi H, Bamosa AO, Badar A, et al. Nigella sativa improves glycemic control and ameliorates oxidative stress in patients with type 2 diabetes mellitus: Placebo controlled participant blinded clinical trial. PLoS ONE. 2015;10(2):e0113486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sahebkar A, Beccuti G, Simental‐Mendia LE, Nobili V, Bo S. Nigella sativa (black seed) effects on plasma lipid concentrations in humans: A systematic review and meta‐analysis of randomized placebo‐controlled trials. Pharmacol Res. 2016;106:37‐50. [DOI] [PubMed] [Google Scholar]
- 9. Le PM, Benhaddou‐Andaloussi A, Elimadi A, Settaf A, Cherrah Y, Haddad PS. The petroleum ether extract of Nigella sativa exerts lipid‐lowering and insulin‐sensitizing actions in the rat. J Ethnopharmacol. 2004;94(2–3):251‐259. [DOI] [PubMed] [Google Scholar]
- 10. Benhaddou‐Andaloussi A, Martineau LC, Vallerand D, et al. Multiple molecular targets underlie the antidiabetic effect of Nigella sativa seed extract in skeletal muscle, adipocyte and liver cells. Diabetes Obes Metab. 2010;12(2):148‐157. [DOI] [PubMed] [Google Scholar]
- 11. Benhaddou‐Andaloussi A, Martineau L, Vuong T, et al. The in vivo antidiabetic activity of Nigella sativa is mediated through activation of the AMPK pathway and increased muscle Glut4 content. Evid Based Complement Alternat Med. 2011;2011:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meddah B, Ducroc R, El Abbes Faouzi M, et al. Nigella sativa inhibits intestinal glucose absorption and improves glucose tolerance in rats. J Ethnopharmacol. 2009;121(3):419‐424. [DOI] [PubMed] [Google Scholar]
- 13. Al‐Naqeep G, Ismail M, Allaudin Z. Regulation of low‐density lipoprotein receptor and 3‐hydroxy‐3‐methylglutaryl coenzyme A reductase gene expression by thymoquinone‐rich fraction and thymoquinone in HepG2 cells. J Nutrigenet Nutrigenomics. 2009;2(4–5):163‐172. [DOI] [PubMed] [Google Scholar]
- 14. Meral I, Yener Z, Kahraman T, Mert N. Effect of Nigella sativa on glucose concentration, lipid peroxidation, anti‐oxidant defence system and liver damage in experimentally‐induced diabetic rabbits. J Veterinary Med Ser A. 2001;48(10):593‐599. [DOI] [PubMed] [Google Scholar]
- 15. Rchid H, Chevassus H, Nmila R, et al. Nigella sativa seed extracts enhance glucose‐induced insulin release from rat‐isolated Langerhans islets. Fundam Clin Pharmacol. 2004;18(5):525‐529. [DOI] [PubMed] [Google Scholar]
- 16. Gray JP, Burgos DZ, Yuan T, et al. Thymoquinone, a bioactive component of Nigella sativa, normalizes insulin secretion from pancreatic beta‐cells under glucose overload via regulation of malonyl‐CoA. Am J Physiol Endocrinol Metab. 2016;310(6):E394‐E404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. DeFronzo R, Tobin J, Andres R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214‐E223. [DOI] [PubMed] [Google Scholar]
- 18. Farret A, Chevassus H, Roux B, Petit P, Galtier F. Direct rosiglitazone‐induced modifications in insulin secretion, action and clearance: A single‐dose hyperglycaemic clamp study. Diabetologia. 2007;50(7):1384‐1387. [DOI] [PubMed] [Google Scholar]
- 19. Mitrakou A, Vuorinen‐Markkola H, Raptis G, et al. Simultaneous assessment of insulin secretion and insulin sensitivity using a hyperglycemia clamp. J Clin Endocrinol Metab. 1992;75(2):379‐382. [DOI] [PubMed] [Google Scholar]
- 20. Qidwai W, Hamza HB, Qureshi R, Gilani A. Effectiveness, safety, and tolerability of powdered Nigella sativa (kalonji) seed in capsules on serum lipid levels, blood sugar, blood pressure, and body weight in adults: Results of a randomized, double‐blind controlled trial. J Altern Complement Med. 2009;15(6):639‐644. [DOI] [PubMed] [Google Scholar]
- 21. Bamosa AO, Kaatabi H, Lebdaa FM, Elq AM, Al‐Sultanb A. Effect of Nigella sativa seeds on the glycemic control of patients with type 2 diabetes mellitus. Indian J Physiol Pharmacol. 2010;54(4):344‐354. [PubMed] [Google Scholar]
- 22. Kaatabi H, Bamosa AO, Lebda FM, Al Elq AH, Al‐Sultan AI. Favorable impact of Nigella sativa seeds on lipid profile in type 2 diabetic patients. J Fam Community Med. 2012;19(3):155‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]


