Abstract
Background
Apixaban, a non‐vitamin K oral anticoagulant (NOAC), was approved in Japan in 2012 for the prevention of thromboembolic events in patients with nonvalvular atrial fibrillation (NVAF). However, the safety and effectiveness of apixaban in clinical practice have not yet been elucidated thoroughly among Japanese NVAF patients.
Methods
A postmarketing surveillance study was conducted to determine the safety and effectiveness of apixaban. Patients were followed‐up for 104 weeks. Outcome events included adverse drug reactions (ADRs), hemorrhages, and thromboembolic events (ischemic stroke, systemic embolism [SE], and transient ischemic attack [TIA]).
Results
Among 6306 NVAF patients in the safety analysis set (age, 74.5 ± 10.1 years; women, 41.1%; and CHADS 2 score, 2.0 ± 1.4), 3600 patients (57.1%) received the standard dose (5 mg twice daily) and 2694 (42.7%) received a reduced dose (2.5 mg twice daily) of apixaban. ADRs occurred in 604 patients (9.58%), with the most common being epistaxis (0.86%), subcutaneous hemorrhage (0.67%), and hematuria (0.57%). Incidence rate of any hemorrhages and major hemorrhage was 5.52% per year and 2.36% per year, respectively. Incidence rate of ischemic stroke/SE/TIA was 1.00% per year among 6286 patients in the effectiveness analysis set. Among three subgroups (3106 apixaban initiators, 2038 patients switched from warfarin, and 1118 patients switched from other NOACs), incidence rates of major hemorrhage (P = 0.221 for trend) and ischemic stroke/SE/TIA (P = 0.686 for trend) were comparable.
Conclusions
No new safety signals of apixaban were identified in Japanese NVAF patients. Safety and effectiveness of apixaban were consistent with those in the ARISTOTLE study.
Keywords: apixaban, atrial fibrillation, postmarketing surveillance, safety
1. INTRODUCTION
Atrial fibrillation (AF) is the most common cardiac arrhythmia, with an estimated prevalence of 0.56%‐1.6% in Asian countries (China, Japan, Korea, and Singapore).1, 2 AF is an independent risk factor for stroke (a nearly five‐fold increase3) and is associated with stroke death (a nearly three‐fold increase4), making the growing prevalence of AF a major healthcare concern. For decades, warfarin has been used for prevention of ischemic stroke in patients with AF. However, several new non‐vitamin K oral anticoagulants (NOACs) are now available in many countries to alleviate the unmet need left by traditional anticoagulants, and are recommended for use in the latest guidelines in the United States,5 Europe,6 and Japan.7
The randomized controlled trial ARISTOTLE showed that apixaban, an oral direct factor Xa inhibitor, was effective in preventing stroke and systemic embolism (SE) in patients with nonvalvular AF (NVAF).8, 9 Apixaban received regulatory approval in Japan in 2012 for the prevention of ischemic stroke and SE in patients with NVAF. To date, the real‐world safety and effectiveness of apixaban in patients with NVAF have been assessed in retrospective claims database studies both outside of Japan10, 11, 12, 13 and in Japan.14 However, data are still limited in Japanese NVAF patients.
After introduction of NOACs into clinical practice, the proportion of NVAF patients now given NOACs has been increasing worldwide.15, 16 In a certain proportion of these patients, warfarin was switched to NOACs and NOACs were switched to other NOACs.16 However, reasons for the switch, as well as safety and effectiveness of the switch have not been elucidated thoroughly yet in clinical practice.
As required for a new medicine approved by the Ministry of Health, Labour and Welfare, safety and effectiveness information for the medicine should be provided in the setting of routine practice. Consequently, we conducted a postmarketing surveillance (PMS) study, STroke prevention ANticoagulant Drug Apixaban Real‐world Data (STANDARD, ClinicalTrials.gov ID: NCT02007655), to assess the safety and effectiveness of apixaban in Japanese NVAF patients in routine clinical practice. Herein, we report baseline clinical characteristics and incidences of outcome events among all the study patients, as well as among the patient groups based on prior status of anticoagulation therapy.
2. METHODS
2.1. Study design, study period, and sample size setting
This PMS study was conducted at 811 sites across Japan from September 2013 (start of registration) until August 2016 (end of follow‐up). On the basis of the incidence rate of major bleeding observed in the ARISTOTLE study,8 a target sample size of 1000 and 1600 was set for patients receiving apixaban 2.5 and 5 mg twice daily (BID), respectively. These sample size settings aimed to extract background risk factors having a risk ratio of ≥2.5 by using the chi‐squared test. Taking into account the proportion of patients meeting the dose reduction criteria in daily clinical settings, it was anticipated that by the time 1000 patients receiving apixaban 2.5 mg BID were registered, 4495 patients receiving 5 mg BID would have been registered. Accordingly, the final sample size was set to 5500 including at least 1000 patients receiving apixaban 2.5 mg BID.
Registration was possible before the start of treatment with apixaban or within 14 days after the start of treatment. Apixaban doses were selected at the discretion of each treating physician. The follow‐up period was 104 weeks for each patient. Data collection was performed at baseline and at 12, 52, and 104 weeks of treatment or at discontinuation of apixaban using case report forms (CRFs) from the participating sites.
This PMS study was conducted as a condition of approval for the use of apixaban in Japanese NVAF patients, and in accordance with the Declaration of Helsinki and the Good Post‐marketing Study Practice (GPSP) ordinance of Japan. Approval of the institutional review board of each participating institution and written informed consent were not required because this study was conducted as a regulatory and legal requirement in accordance with the GPSP guidelines.
2.2. Assessments and outcomes
Assessment included baseline characteristics, ie, age, gender, body weight, serum creatinine value, past history, comorbidities, past anticoagulant treatment, CHADS2 scores,17 CHA2DS2‐VASc scores,18 HAS‐BLED scores,19 and apixaban dosing conditions (dosing period and dosage). Creatinine clearance (CrCl) was estimated using the Cockcroft‐Gault equation.20
Outcome events for safety analysis included adverse drug reactions (ADRs) and hemorrhages. All patients were evaluated for safety during apixaban use and for 28 days after the last study drug dose to confirm the safety profile of apixaban under routine, daily practice or to obtain information on any previously unsuspected ADRs. ADRs were events with a possible causal relationship with apixaban. Hemorrhage included all the major and nonmajor hemorrhagic events. Major hemorrhages were defined as per the criteria of the International Society of Thrombosis and Haemostasis21 with modification in the number of blood transfusion units and included (a) fatal hemorrhage; (b) hemorrhage with a decrease in hemoglobin levels of 2 g/dL or more; (c) hemorrhage requiring blood transfusion of four units or more; and (d) hemorrhage in a critical region or organ, including intracranial, intraspinal, intraocular, pericardial, intra‐articular, and retroperitoneal hemorrhages, and intramuscular hemorrhage with compartment syndrome. Outcome events for effectiveness analysis included thromboembolic events, such as ischemic stroke, SE, and transient ischemic attack (TIA).
2.3. Statistical analysis
Data are expressed as mean ± standard deviation (SD), median, or percentage. The incidence (%) of ADRs was tabulated. The incidence rates (% per year) of major hemorrhage, any hemorrhage, and effectiveness outcomes were also calculated, and cumulative incidence was analyzed using a Kaplan‐Meier curve. In the three subgroups categorized by prior anticoagulation status, differences in continuous variables were analyzed using analysis of variance, differences in categorical variables were analyzed using the chi‐squared test, and incidence rates were tested by the log‐rank test. P < 0.05 were considered statistically significant. Statistical analyses were performed with sas software, version 9.2 (sas Institute Inc., Cary, NC, USA).
3. RESULTS
Of 6455 patients enrolled from September 2013 to August 2014, four patients were excluded from the registration owing to duplicate registration (n = 1), absence of study drug initiation (n = 2), and registration from an institution not participating in this registry (n = 1); CRFs were not obtained from 79 patients (Figure 1). Additionally, 66 patients were not included in the analysis; therefore, 6306 patients constituted the study group for safety analysis. Of these, 20 patients who were given apixaban for treatment of diseases other than AF were excluded, and the remaining 6286 patients thus constituted the study group for the effectiveness analysis (Figure 1). Four patients had a CrCl value of <15 mL/min, a contraindication of apixaban treatment, but were included in the present analyses.
Figure 1.

Patient disposition. AF, atrial fibrillation; CRF, case report form
3.1. Baseline clinical characteristics
Table 1 shows the baseline clinical characteristics of the 6306 patients in the safety analysis set. Of these, 34.6% of patients were ≥80 years of age, 52.0% weighed ≤60 kg, and 3.5% had serum creatinine values of ≥1.5 mg/dL. Moderate‐to‐severe renal dysfunction (CrCl ≤ 50 mL/min) was observed in 31.4% of patients. Standard dose apixaban, ie, 5 mg BID, was given to 57.1% of patients, and reduced dose, ie, 2.5 mg BID, was given to 42.7% of patients. The remaining patients (0.2%) received other doses: 5 mg once daily in five patients, 2.5 mg once daily in six patients, and 1.25 mg BID in one patient. Of 2694 patients receiving 2.5 mg BID apixaban, 1682 patients (62.4%) met ≥2 dose reduction criteria (age ≥80 years, body weight ≤60 kg, and serum creatinine ≥1.5 mg/dL),22 but 941 patients (34.9%) met <2 dose reduction criteria. Information of the dose reduction criteria was not obtained in 71 patients (2.6%). Mean risk scores were 2.0 for CHADS2, 3.4 for CHA2DS2‐VASc, and 1.7 for HAS‐BLED (Figure S1).
Table 1.
Baseline demographics and characteristics of 6306 patients in the safety analysis set
| Characteristics | Overall n = 6306 |
|---|---|
| Women | 2592 (41.1) |
| Age (y) | 74.5 ± 10.1 (median 76.0) |
| <70 | 1715 (27.2) |
| ≥70 to <75 | 1189 (18.9) |
| ≥75 to <80 | 1220 (19.3) |
| ≥80 to <85 | 1231 (19.5) |
| ≥85 to <90 | 729 (11.6) |
| ≥90 | 222 (3.5) |
| Weight (kg) (n = 5896) | 59.6 ± 12.6 (median 59.0) |
| ≤60 | 3282 (52.0) |
| >60 | 2614 (41.5) |
| Unknown | 410 (6.5) |
| Serum creatinine (mg/dL) (n = 5820) | 0.90 ± 0.37 |
| <1.5 | 5597 (88.8) |
| ≥1.5 | 223 (3.5) |
| Unknown | 486 (7.7) |
| Creatinine clearance (mL/min) (n = 5552) | 62.2 ± 26.8 |
| <15 | 4 (0.1) |
| ≥15 to <30 | 382 (6.1) |
| ≥30 to ≤50 | 1590 (25.2) |
| >50 to ≤80 | 2426 (38.5) |
| >80 | 1150 (18.2) |
| Unknown | 754 (12.0) |
| Past history | |
| Clinically important hemorrhage | 221 (3.5) |
| Congenital or acquired hemorrhagic disease | 102 (1.6) |
| Gastrointestinal ulcer | 147 (2.3) |
| Ischemic stroke and transient ischemic attack | 1101 (17.5) |
| Comorbidity | |
| Hypertension | 3855 (61.1) |
| Heart failure | 1921 (30.5) |
| Diabetes mellitus | 1121 (17.8) |
| Renal disorder | 930 (14.7) |
| Liver disorder | 771 (12.2) |
| Apixaban dosage | |
| 5 mg twice daily | 3600 (57.1) |
| 2.5 mg twice daily | 2694 (42.7) |
| Othersa | 12 (0.2) |
| Antiplatelet drug useb | 1184 (18.8) |
Note: Values are mean ± standard deviation or number (%) of patients.
See text for details.
Antiplatelet drugs that were used during the apixaban dosing period.
Of 6306 patients included in the safety analysis set, 3106 patients (49.3%) started apixaban as their initial anticoagulation treatment for NVAF. In 2038 patients (32.3%), warfarin was switched to apixaban, and in 1118 patients (17.7%), other NOACs (dabigatran in 613 and rivaroxaban in 508 patients; three patients had received both dabigatran and rivaroxaban) were switched to apixaban. Among those patients who switched, nine patients had received both warfarin and NOAC. Other anticoagulants including heparin were switched to apixaban in 53 patients.
Of 2038 patients in whom warfarin was switched to apixaban, the most common reason (37.3%) for switching was poor control of INR values (poor INR control, 36.4%; INR control and adverse events, 0.9%; Table 2). Of 760 patients with poor INR control, 69.5% had INR values of <2.0 at the time of switching as recommended in the Japanese package insert.22 However, 12.4% of patients had INR values of ≥2.0. Reasons for switching from other NOACs are also shown in Table 2.
Table 2.
Reasons for switching from other anticoagulants to apixaban
| Reasons for switching from warfarin to apixaban (n = 2038) | |
| Poor INR control | 36.4% |
| INR control and adverse events | 0.9% |
| Physician's decision | 25.0% |
| Patient's preference | 10.3% |
| Dietary restrictions | 7.3% |
| Others | 10.6% |
| Unknown | 9.4% |
| INR values at the time of switching from warfarin (n = 760) | |
| <1.6 | 61.2% |
| 1.6 to <2.0 | 8.3% |
| 2.0 to <2.6 | 2.9% |
| 2.6 to <3.0 | 2.4% |
| ≥3.0 | 7.1% |
| Unknown | 18.1% |
| Reasons for switching from other NOACs (n = 776) | |
| Physician's decision | 33.1% |
| Efficacy | 15.3% |
| Safety concerns | 5.9% |
| Blood test results | 5.3% |
| Adverse events | 23.3% |
| Gastrointestinal symptoms | 10.0% |
| Bleeding | 6.1% |
| Patient characteristics | 19.8% |
| Renal function | 12.9% |
| Age | 4.9% |
| Patient's preference | 15.7% |
| Others | 8.1% |
Abbreviations: INR, international normalized ratio; NOACs, non‐vitamin K oral anticoagulants.
Baseline clinical characteristics of the three subgroups are shown in Table 3. Patients in whom warfarin had been switched to apixaban were older, had lower body weight and CrCl values, and had higher thromboembolic and bleeding risk scores compared with the remaining two subgroups.
Table 3.
Baseline demographics and characteristics by subgroup (safety analysis set)
| Characteristics | Apixaban initiators (n = 3106) | From warfarin (n = 2038) | From other NOACs (n = 1118) | P value for trend |
|---|---|---|---|---|
| Women | 1278 (41.1) | 844 (41.4) | 450 (40.3) | 0.811 |
| Age (y) | 73.6 ± 10.8 | 76.0 ± 9.0 | 74.3 ± 9.6 | <0.001 |
| Weight (kg) | 60.0 ± 12.6 (n = 2923) | 58.8 ± 12.6 (n = 1898) | 59.8 ± 12.9 (n = 1032) | 0.004 |
| Serum creatinine (mg/dL) | 0.87 ± 0.43 (n = 2914) | 0.93 ± 0.29 (n = 1855) | 0.93 ± 0.30 (n = 1007) | <0.001 |
| Creatinine clearance (mL/min) | 65.4 ± 27.0 (n = 2796) | 58.0 ± 26.0 (n = 1760) | 60.4 ± 26.0 (n = 953) | <0.001 |
| CHADS2 score | 1.9 ± 1.3 | 2.3 ± 1.4 | 2.1 ± 1.4 | <0.001 |
| CHA2DS2‐VASc score | 3.2 ± 1.7 | 3.7 ± 1.7 | 3.5 ± 1.7 | <0.001 |
| HAS‐BLED score | 1.4 ± 1.0 | 2.1 ± 1.1 | 1.7 ± 1.0 | <0.001 |
| Antiplatelet use | 534 (17.2) | 442 (21.7) | 200 (17.9) | <0.001 |
Note: Values are mean ± standard deviation or number of patients (%).
Abbreviation: NOACs, non‐vitamin K oral anticoagulants.
3.2. Outcome events
Of 6306 patients included in the safety analysis set, follow‐up was discontinued in 2381 patients (37.8%) before completion of the 104‐week follow‐up; before 52 weeks in 1807 patients and between 52 and 104 weeks in 574 patients. Reasons for discontinuation of follow‐up were adverse events in 585 patients, transfer to other hospitals/clinics in 837, and discontinuation of visit in 381, death in 119, poor adherence in 108, surgery/invasive procedures in 82, discontinuation of apixaban owing to medication cost in 58, and others.
Major hemorrhage occurred in 210 patients (2.36% per year) during a mean follow‐up period of 17.4 months (Table 4, Figure 2). Intracranial hemorrhage occurred in 67 patients (0.75% per year). Incidence rates of major hemorrhage by baseline HAS‐BLED scores are shown in Figure 3. Common (≥0.5%) ADRs were epistaxis, subcutaneous hemorrhage, and hematuria (Table S1). Of 6286 patients included in the effectiveness analysis set, ischemic stroke, SE, and TIA occurred in 89 patients (1.00% per year; Table 5, Figure 2). Of 79 episodes of ischemic stroke, 57% were of cardioembolic origin. Incidence rates of ischemic stroke/SE/TIA by baseline risk scores are shown in Figure 3. No outcome events were observed in four patients (79‐89 years old; three women; and apixaban 2.5 mg BID in three and 5 mg BID in one) who had baseline CrCl values of <15 mL/min.
Table 4.
Incidence rate of hemorrhagic events
| Overall (n = 6306) | Apixaban initiators (n = 3106) | From warfarin (n = 2038) | From other NOACs (n = 1118) | P value for trenda | |
|---|---|---|---|---|---|
| Any hemorrhage | 480 (5.52) | 204 (5.03) | 179 (5.88) | 94 (5.98) | 0.127 |
| Major hemorrhage | 210 (2.36) | 86 (2.07) | 82 (2.63) | 40 (2.49) | 0.221 |
| Intracranial | 67 (0.75) | 31 (0.74) | 22 (0.70) | 13 (0.80) | 0.939 |
| Cerebral | 44 (0.49) | 21 (0.50) | 18 (0.57) | 5 (0.31) | 0.446 |
| Subarachnoid | 7 (0.08) | 2 (0.05) | 1 (0.03) | 4 (0.25) | NA |
| Chronic subdural | 9 (0.10) | 7 (0.17) | 1 (0.03) | 1 (0.06) | NA |
| Others | 8 (0.09) | 1 (0.02) | 2 (0.06) | 4 (0.25) | NA |
| Gastrointestinal | 90 (1.01) | 34 (0.82) | 37 (1.18) | 17 (1.05) | 0.232 |
Note: Number of patients (% per year).
Abbreviations: NA, not assessed due to lower event rates; NOACs, non‐vitamin K oral anticoagulants.
Comparison among three subgroups.
Figure 2.
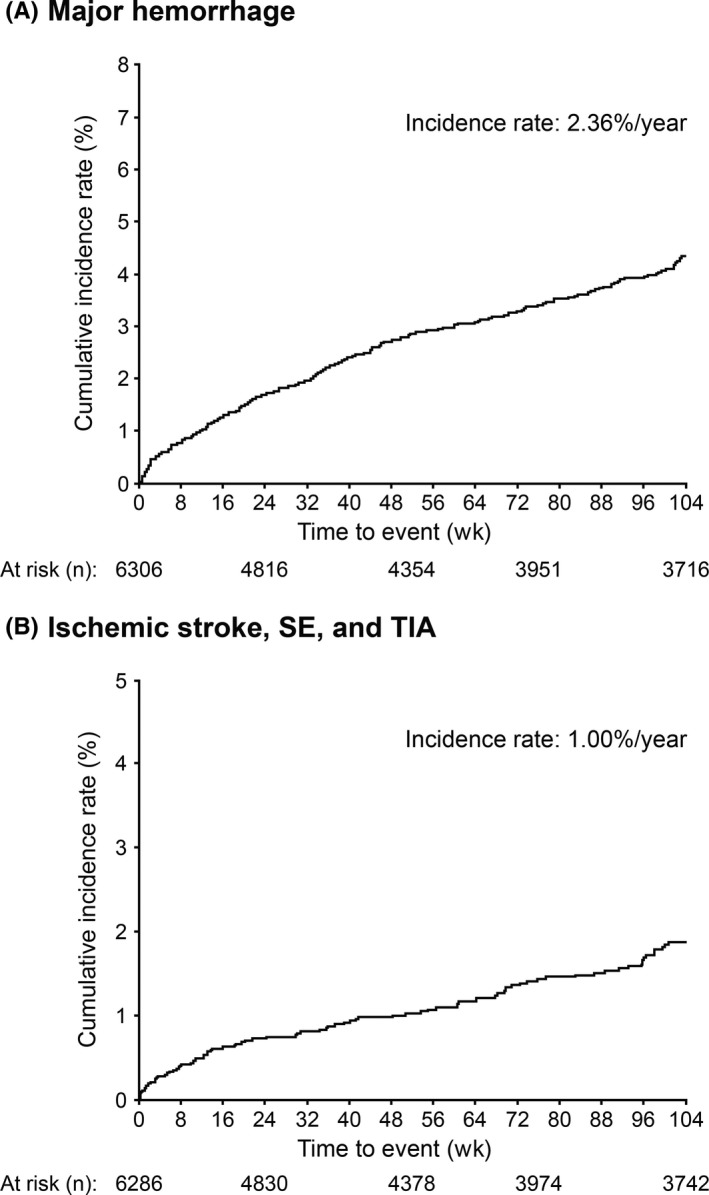
Cumulative incidence rate of (A) major hemorrhage and (B) ischemic stroke, SE, and TIA. SE, systemic embolism; TIA, transient ischemic attack
Figure 3.
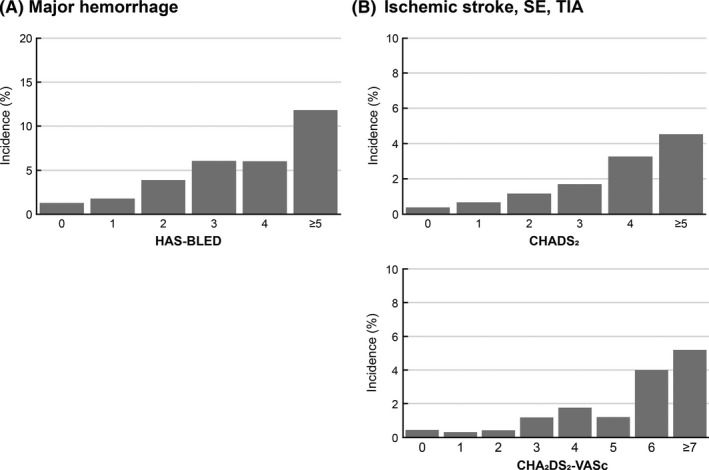
Incidence of (A) major hemorrhage by baseline HAS‐BLED score and (B) ischemic stroke, SE, and TIA by baseline CHADS 2 score and CHA 2 DS 2‐VASc score. SE, systemic embolism; TIA, transient ischemic attack
Table 5.
Incidence rate of thromboembolic events
| Overall (n = 6286) | Apixaban initiators (n = 3093) | From warfarin (n = 2034) | From other NOACs (n = 1118) | P value for trenda | |
|---|---|---|---|---|---|
| Ischemic stroke/SE/TIA | 89 (1.00) | 42 (1.01) | 33 (1.05) | 13 (0.80) | 0.686 |
| Ischemic stroke | 79 (0.88) | 36 (0.87) | 30 (0.96) | 12 (0.74) | 0.717 |
| Atherothrombotic | 8 (0.09) | 6 (0.14) | 2 (0.06) | 0 (0) | NA |
| Cardioembolic | 45 (0.50) | 19 (0.46) | 17 (0.54) | 8 (0.49) | 0.836 |
| Lacunar | 7 (0.08) | 5 (0.12) | 0 (0) | 2 (0.12) | NA |
| Cryptogenic | 9 (0.10) | 4 (0.10) | 4 (0.13) | 1 (0.06) | NA |
| Unknown | 6 (0.07) | 2 (0.05) | 3 (0.10) | 1 (0.06) | NA |
| Others | 5 (0.06) | 1 (0.02) | 4 (0.13) | 0 (0) | NA |
| SE | 3 (0.03) | 2 (0.05) | 0 (0) | 1 (0.06) | NA |
| TIA | 8 (0.09) | 4 (0.10) | 4 (0.13) | 0 (0) | NA |
Note: Number of patients (% per year).
Abbreviations: NA, not assessed due to lower event rates; NOACs, non‐vitamin K oral anticoagulants; SE, systemic embolism; TIA, transient ischemic attack.
Comparison among three subgroups.
As shown in Tables 4 and 5, incidence rates of outcome events did not differ significantly among the three subgroups divided by prior status of anticoagulation therapy.
4. DISCUSSION
This PMS study included more than 6000 patients with NVAF and identified the following major findings. First, 42.7% of the patients received 2.5 mg BID apixaban. Second, incidence rates of major hemorrhage and thromboembolic events were 2.36% per year and 1.00% per year, respectively. No new safety signals were identified. Third, some clinical characteristics differed among the three subgroups based on the status of anticoagulation therapy prior to apixaban treatment, but incidence rates of hemorrhagic and thromboembolic events did not differ among the three subgroups.
4.1. Comparison with the ARISTOTLE study
A comparison with the apixaban group of the ARISTOTLE study8 showed that patients in the present study were at a higher hemorrhagic risk associated with their higher age (76.0 years [median] vs 70 years [median]), lower body weight (59.0 kg [median] vs 82 kg [median]), and reduced renal function (CrCl >80 mL/min, 18.2% vs 41.2%). Nevertheless, the incidence rates of major hemorrhage (present study, 2.36% per year vs ARISTOTLE, 2.13% per year), ischemic stroke (0.88% per year vs 0.97% per year), and SE (0.03% per year vs 0.09% per year) were comparable between the two studies. Similarly, a comparison with the ARISTOTLE subgroup analysis in an East Asian population (median age, 69 years; median body weight, 66 kg; CrCl >80 mL/min, 25.7%)23 also revealed that the incidence rates of major hemorrhage (present study, 2.36% per year vs ARISTOTLE subgroup analysis, 2.02% per year), any hemorrhages (5.52% per year vs 20.47% per year), and ischemic stroke (0.88% per year vs 2.22% per year) were comparable or lower in the present study. Consequently, the safety and effectiveness profiles of apixaban observed in our study were consistent with those reported in the ARISTOTLE study.8, 23
4.2. Patient characteristics
Age (mean, 74.5 years) and thromboembolic risk (mean CHADS2 score, 2.0 and CHA2DS2‐VASc score, 3.4) of the present study population were similar to those in the Fushimi AF Registry (74.2 years, 2.09, and 3.43, respectively),24 and a Japanese claims database study (77.0 years, 2.2 and 3.4, respectively).25 This finding indicates that the patients included in the present study were at a similar risk of thromboembolism compared with the general Japanese population with NVAF.
The hemorrhagic risk of apixaban, dabigatran, and rivaroxaban, compared with warfarin, was assessed in a retrospective claims database study in Japanese NVAF patients using a propensity‐matched analysis.14 Before the propensity matching, patients receiving apixaban were older (mean age, 76.7 vs 72.3‐74.4 years) and had reduced renal function (comorbid renal dysfunction, 4.9% vs 2.4%‐3.3%) compared with those receiving dabigatran or rivaroxaban.14 These findings were consistent with the present study in comparison with the PMS studies of rivaroxaban26 and dabigatran27 in terms of higher mean age (74.5 vs 73.126 and 70.8 years27) and reduced CrCl (62.2 vs 67.726 and 72.8 mL/min27) in patients receiving apixaban.
In the present study, 42.7% of the patients received 2.5 mg BID apixaban. This frequency was similar to that in Japanese registration studies (40.4%28 and 44%16), but lower than that in a Japanese claims database study (60.6%25).
4.3. Switch from other anticoagulants
Patients who switched from warfarin to apixaban were older, and had lower body weight and CrCl values compared with the other two subgroups. Moreover, thromboembolic and bleeding risk scores were higher in this subgroup. Nevertheless, incidence rates of outcome events did not differ significantly among all the subgroups.
In our study, poor INR control was the most common reason for switching from warfarin to apixaban, with the risk score of stroke or hemorrhage being higher in patients who switched from warfarin compared with apixaban initiators (Tables 2 and 3). Indeed, results from a nationwide database study indicated that Japanese NVAF patients who continued to receive warfarin had both poor or inadequate INR control and higher stroke risk compared with those who switched from warfarin to NOACs.29
Approximately 70% of patients who switched from warfarin to apixaban due to uncontrolled INR started apixaban at an INR value of <2.0 as recommended in the Japanese package insert of apixaban.22 Importantly, the incidence rate of hemorrhagic events did not increase in patients switching from warfarin to apixaban (Table 4). Thus, it remains crucial for physicians to follow this INR prerequisite in patients who are to switch from warfarin to apixaban. With regard to switching from other NOACs, a number of reasons were reported for doing so in the present study (Table 2). Medication switching from an index NOAC occurs in one in five patients with NVAF in the United States and is therefore not uncommon30; however, the reasons behind switching have not been well studied, and practical clinical guidelines for switching between NOACs are lacking. Our findings, therefore, provide some insight into the real‐world experience in switching between NOACs in Japan.
4.4. Limitations
This study had some limitations. First, the single‐cohort design prevented comparisons with warfarin or other NOACs. Second, 37.8% of the patients discontinued apixaban treatment or were lost to follow during the 104‐week follow‐up period. Discontinuation of periodical visits and transfer to other hospitals/clinics accounted for 51% of these patients for whom the follow‐up was discontinued. This rate seemed a little higher, but the clinical status of these patients at the discontinuation of apixaban was determined, yielding a mean follow‐up period of 17.4 months. This result was similar to that of a previous Japanese PMS of another NOAC, which was designed to follow the patients for 104 weeks, but yielded a mean follow‐up period of approximately 15 months.31 The shorter follow‐up period than designed would have underestimated event rates in the present study as well as in that PMS study.31 Third, as a result of the inherent limitations of a noninterventional design, this study may not have generated unbiased relative risk estimates or absolute incidence rates. Fourth, since some of the reasons for switching from other anticoagulants to apixaban are subjective, the possibility of selection bias and confounding bias cannot be ruled out. Fifth, possible misclassifications of events cannot be ruled out since events were assessed by treating physicians and were not confirmed by an independent adjudication committee. Sixth, adherence of prescribing apixaban was not evaluated in the present study. Seventh, upon comparison of patient background and incidence rates, safety and effectiveness among the three subgroups categorized by prior anticoagulation therapy, power was not considered for the P value calculation. Finally, changes in apixaban doses were not considered in the course of treatment, and event rates were determined using the baseline apixaban doses. Approximately 900 patients did not meet ≥2 dose reduction criteria but received 2.5 mg BID apixaban, so‐called underdose. The association of apixaban doses and dose reduction criteria with outcome events seems clinically relevant, but is not reported herein. This will be reported separately.
5. CONCLUSIONS
No new safety signals of apixaban were identified in Japanese NVAF patients. The safety and effectiveness profile for apixaban was consistent with that in the ARISTOTLE study. There was no notable difference in safety or effectiveness event rates among apixaban initiators, patients switched from warfarin, and those switched from other NOACs.
CONFLICT OF INTEREST
HI has received remuneration from Bristol‐Myers Squibb, Nippon Boehringer Ingelheim, Bayer Healthcare, and Daiichi Sankyo. MY has received remuneration from Bristol‐Myers Squibb, Nippon Boehringer Ingelheim, Bayer Healthcare, and Daiichi Sankyo. MU, TY, and AK are employees of Bristol‐Myers Squibb. HH is an employee of Pfizer Japan. This study is registered as ClinicalTrials.gov ID: NCT02007655.
Supporting information
ACKNOWLEDGEMENTS
The authors would like to thank all the medical institutions and physicians who participated in this surveillance for their cooperation.
Inoue H, Umeyama M, Yamada T, Hashimoto H, Komoto A, Yasaka M. Safety and effectiveness of apixaban in Japanese patients with nonvalvular atrial fibrillation in clinical practice: A regulatory postmarketing surveillance, the STANDARD study. J Arrhythmia. 2019;35:506–514. 10.1002/joa3.12184
Funding Information
This study was funded and conducted by Bristol‐Myers Squibb K.K. and Pfizer Japan Inc. Medical writing services were provided by Mami Hirano, MS, of Cactus Communications and funded by Bristol‐Myers Squibb K.K.
REFERENCES
- 1. Ma C. Current antithrombotic treatment in East Asia: some perspectives on anticoagulation and antiplatelet therapy. Thromb Haemost. 2012;107(6):1014–18. [DOI] [PubMed] [Google Scholar]
- 2. Inoue H, Fujiki A, Origasa H, Ogawa S, Okumura K, Kubota I, et al. Prevalence of atrial fibrillation in the general population of Japan: an analysis based on periodic health examination. Int J Cardiol. 2009;137(2):102–7. [DOI] [PubMed] [Google Scholar]
- 3. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke. 1991;22(8):983–8. [DOI] [PubMed] [Google Scholar]
- 4. Ohsawa M, Okayama A, Okamura T, Itai K, Nakamura M, Tanno K, et al. Mortality risk attributable to atrial fibrillation in middle‐aged and elderly people in the Japanese general population: nineteen‐year follow‐up in NIPPON DATA80. Circ J. 2007;71(6):814–19. [DOI] [PubMed] [Google Scholar]
- 5. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1–76. [DOI] [PubMed] [Google Scholar]
- 6. Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non‐vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39(16):1330–93. [DOI] [PubMed] [Google Scholar]
- 7. JCS Joint Working Group . Guidelines for pharmacotherapy of atrial fibrillation (JCS 2013). Circ J. 2014;78(8):1997–2021. [DOI] [PubMed] [Google Scholar]
- 8. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–92. [DOI] [PubMed] [Google Scholar]
- 9. Ogawa S, Shinohara Y, Kanmuri K. Safety and efficacy of the oral direct factor xa inhibitor apixaban in Japanese patients with non‐valvular atrial fibrillation. The ARISTOTLE‐J study. Circ J. 2011;75(8):1852–514. [DOI] [PubMed] [Google Scholar]
- 10. Coleman CI, Antz M, Bowrin K, Evers T, Simard EP, Bonnemeier H, et al. Real‐world evidence of stroke prevention in patients with nonvalvular atrial fibrillation in the United States: the REVISIT‐US study. Curr Med Res Opin. 2016;32(12):2047–53. [DOI] [PubMed] [Google Scholar]
- 11. Deitelzweig S, Luo X, Gupta K, Trocio J, Mardekian J, Curtice T, et al. Comparison of effectiveness and safety of treatment with apixaban vs. other oral anticoagulants among elderly nonvalvular atrial fibrillation patients. Curr Med Res Opin. 2017; 33(10): 1745–54. [DOI] [PubMed] [Google Scholar]
- 12. Hohnloser SH, Basic E, Nabauer M. Comparative risk of major bleeding with new oral anticoagulants (NOACs) and phenprocoumon in patients with atrial fibrillation: a post‐marketing surveillance study. Clin Res Cardiol. 2017;106(8):618–28. [DOI] [PubMed] [Google Scholar]
- 13. Chan YH, See LC, Tu HT, Yeh YH, Chang SH, Wu LS, et al. Efficacy and safety of apixaban, dabigatran, rivaroxaban, and warfarin in Asians with nonvalvular atrial fibrillation. J Am Heart Assoc. 2018;7(8):e008150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kohsaka S, Murata T, Izumi N, Katada J, Wang F, Terayama Y. Bleeding risk of apixaban, dabigatran, and low‐dose rivaroxaban compared with warfarin in Japanese patients with non‐valvular atrial fibrillation: a propensity matched analysis of administrative claims data. Curr Med Res Opin. 2017;33(11):1955–63. [DOI] [PubMed] [Google Scholar]
- 15. Camm AJ, Accetta G, Ambrosio G, Atar D, Bassand JP, Berge E, et al. Evolving antithrombotic treatment patterns for patients with newly diagnosed atrial fibrillation. Heart. 2017;103(2):307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yamashita Y, Uozumi R, Hamatani Y, Esato M, Chun YH, Tsuji H, et al. Current status and outcomes of direct oral anticoagulant use in real‐world atrial fibrillation patients. Circ J. 2017;81(9):1278–85. [DOI] [PubMed] [Google Scholar]
- 17. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285(22):2864–70. [DOI] [PubMed] [Google Scholar]
- 18. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on atrial fibrillation. Chest. 2010;137(2):263–72. [DOI] [PubMed] [Google Scholar]
- 19. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093–100. [DOI] [PubMed] [Google Scholar]
- 20. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. [DOI] [PubMed] [Google Scholar]
- 21. Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost. 2005;3(4):692–4. [DOI] [PubMed] [Google Scholar]
- 22. Pharmaceuticals and Medical Devices Agency [internet]. Eliquis® tablets Japanese package insert version 8 [updated Apr 2017]. http://www.info.pmda.go.jp/go/pack/3339004F1029_1_12/. Accessed October 23, 2018.
- 23. Goto S, Zhu J, Liu L, Oh BH, Wojdyla DM, Aylward P, et al. Efficacy and safety of apixaban compared with warfarin for stroke prevention in patients with atrial fibrillation from East Asia: a subanalysis of the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. Am Heart J. 2014;168(3):303–514. [DOI] [PubMed] [Google Scholar]
- 24. Akao M, Chun YH, Wada H, Esato M, Hashimoto T, Abe M, et al. Current status of clinical background of patients with atrial fibrillation in a community‐based survey: the Fushimi AF Registry. J Cardiol. 2013;61(4):260–6. [DOI] [PubMed] [Google Scholar]
- 25. Kohsaka S, Katada J, Saito K, Terayama Y. Safety and effectiveness of apixaban in comparison to warfarin in patients with nonvalvular atrial fibrillation: a propensity‐matched analysis from Japanese administrative claims data. Curr Med Res Opin. 2018;34(9):1627–34. [DOI] [PubMed] [Google Scholar]
- 26. Ogawa S, Minematsu K, Ikeda T, Kitazono T, Nakagawara J, Miyamoto S, et al. Design and baseline characteristics of the Xarelto Post‐Authorization Safety & effectiveness Study in Japanese patients with atrial fibrillation (XAPASS). J Arrhythm. 2018;34(2):167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Inoue H, Uchiyama S, Atarashi H, Okumura K, Koretsune Y, Yasaka M, et al. Post‐marketing surveillance on the long‐term use of dabigatran in Japanese patients with nonvalvular atrial fibrillation: preliminary report of the J‐dabigatran surveillance. J Arrhythm. 2016;32(2):145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Okumura Y, Yokoyama K, Matsumoto N, Tachibana E, Kuronuma K, Oiwa K, et al. Current use of direct oral anticoagulants for atrial fibrillation in Japan: findings from the SAKURA AF Registry. J Arrhythm. 2017;33(4):289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hirano T, Kaneko H, Mishina S, Wang F, Morita S. Suboptimal anticoagulant management in Japanese patients with nonvalvular atrial fibrillation receiving warfarin for stroke prevention. J Stroke Cerebrovasc Dis. 2017;26(10):2102–10. [DOI] [PubMed] [Google Scholar]
- 30. Manzoor BS, Walton SM, Sharp LK, Galanter WL, Lee TA, Nutescu EA. High number of newly initiated direct oral anticoagulant users switch to alternate anticoagulant therapy. J Thromb Thrombolysis. 2017;44(4):435–41. [DOI] [PubMed] [Google Scholar]
- 31. Inoue H, Uchiyama S, Atarashi H, Okumura K, Koretsune Y, Yasaka M, et al. Effectiveness and safety of long‐term dabigatran among patients with non‐valvular atrial fibrillation in clinical practice: J‐Dabigatran Surveillance. J Cardiol. 2019. 10.1016/j.jjcc.2018.12.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


