Abstract
Aims
Antazoline is a first‐generation antihistaminic drug used primarily in eye drop formulations. When administered intravenously, antazoline displays antiarrhythmic properties resulting in a rapid conversion of recent‐onset atrial fibrillation (AF) to sinus rhythm (SR).
The aim of the study was to assess the influence of antazoline on atrio‐venous conduction and other electrophysiological parameters in patients undergoing AF ablation.
Methods
An experimental prospective study. Patients scheduled for the first‐time AF ablation, in SR and not on amiodarone were enrolled. Atrio‐venous conduction assessment and invasive electrophysiological study (EPS) were performed before and after intravenous administration of 250 mg of antazoline. In case of AF induction during EPS, antazoline was administered until conversion to SR or a cumulative dose of 300 mg.
Results
We enrolled 14 patients: 13 (93%) men, mean age 63.4 (59.9–66.8) years, mean CHA2DS2‐VASc score 1.6 (1.0–2.2). Antazoline was administered in a mean dose 257.1 (246.7–267.6) mg. Pulmonary vein potentials and atrial capture during pulmonary vein stimulation were present before and after the administration of antazoline. Wenckebach point and atrial conduction times did not change significantly, but atrio‐ventricular node effective refractory period improved—324.7 (275.9–373.5) ms vs 284.3 (256.2–312.4) ms, P = 0.02. Antazoline was effective in all 5 (100%) cases of AF induction during EPS. There were no serious adverse events.
Conclusion
Due to the lack of influence on atrio‐venous conduction and high clinical effectiveness, antazoline may be suitable for pharmacological cardioversion of AF occurring during AF ablation.
Keywords: antazoline, atrial fibrillation, ablation, conduction, pulmonary vein
What is already known about this subject
Antazoline is an effective and safe drug used intravenously during pharmacological cardioversion of recent‐onset atrial fibrillation (AF) in a wide variety of patients
There are no data available concerning its influence on atrio‐venous conduction and other electrophysiological parameters in patients undergoing AF ablation
What this study adds
Antazoline administered intravenously in clinically effective dose did not change atrio‐venous conduction in any of 14 patients
Antazoline did not change the Wenckebach point or atrial conduction times, but significantly lowered atrioventricular node effective refractory period
The sinus rhythm conversion rate of AF inducted during EPS was 5/5 (100%) with mean time to conversion 8.4 ± 6.2 min. (range 4–19 min.)
1. INTRODUCTION
Pulmonary vein isolation (PVI) with subsequent demonstration of the lack of electrical conduction between the left atrium and pulmonary veins are cornerstones of atrial fibrillation (AF) ablation—the most effective rhythm‐control therapy for AF patients.1, 2 An AF induction during the procedure may affect PVI assessment and generally requires electrical or pharmacological cardioversion. Antazoline is a I generation antihistaminic drug displaying antiarrhythmic properties.3 Antazoline is administered intravenously in 50–100 mg boluses until conversion of AF to sinus occurs or up to a cumulative dose of 250–300 mg.4, 5, 6, 7, 8 In a single clinical study over healthy volunteers, after the injection of 100 mg of antazoline the drug concentration in plasma declined rapidly to 10% of baseline within half an hour. The following measurements were taken (expressed in means): terminal elimination half‐life 2.29 h, volume of distribution 315 L, mean residence time 3.45 h and clearance 80.5 L/h. In 1 participant, pharmacokinetic parameters were significantly different from the rest of the study group.9
The detailed mechanism of antiarrhythmic properties of antazoline is not entirely clear but current data point to a multichannel mode of action involving sodium and potassium channels.3, 5, 10, 11, 12 Animal model studies report a significant increase of atrial and ventricular effective refractory periods (ERP) leading to a remarkable increase in atrial and ventricular postrepolarization refractoriness, an antiarrhythmic mechanism observed in amiodarone or quinidine.3, 10
In human healthy volunteers, the administration of antazoline prolonged significantly P wave, QRS and QTc duration measured in surface echocardiography. Haemodynamically, a decrease in stroke volume was noted without a significant influence on cardiac output, total peripheral resistance or blood pressure.12
In a recent study of patients scheduled for ablation of supraventricular tachycardia, antazoline was shown to influence a series of electrophysiological parameters, including the prolongation of interatrial conduction and left atrium ERP. Similarly to the previous study, there was an increase in QTc duration and no significant change in systolic or diastolic blood pressure.5
Both experimental and clinical studies indicate that biological effects exerted by antazoline are dose‐dependent.3, 5, 10, 12
In a randomized controlled trial, antazoline was highly effective in recent‐onset AF conversion to sinus rhythm (72.2% conversion rate) and median time to conversion 16 minutes.8 Its effectiveness in the cases of AF induced during electrophysiological procedures was even higher and ranged between 92% and 100% in the cases of paroxysmal AF and sinus rhythm at the beginning of pulmonary vein isolation or AF inducted during accessory pathway ablation, respectively.4, 13 Time to conversion in those studies was 20 minutes and 425 ± 365 seconds, respectively.4, 13 Despite studies into the drug's electrophysiological properties or effectiveness in the electrophysiology laboratory, there are no data on its influence on a parameter crucial for PVI—atrio‐venous conduction.
The aim of the study was to assess the influence of antazoline on atrio‐venous conduction and other electrophysiological parameters in patients undergoing pulmonary vein isolation.
2. METHODS
AntaEP was an experimental prospective study without a control group. The study protocol was in full compliance with the Declaration of Helsinki and was approved by the Local Ethics Committee at our Centre.
We enrolled patients with paroxysmal or persistent AF scheduled for the first AF ablation according to standard clinical indications.2 Eligible patients were in sinus rhythm on the day of ablation and off antiarrhythmic drugs (AADs) for at least 3 drug half‐lives. The last dose of betablocker was allowed 1 day before the scheduled procedure. Exclusion criteria were: redo procedure; planned ablation beyond PVI (documented flutters); a history of cardiosurgical procedures; AF on the day of the procedure; chronic amiodarone; acute cardio‐vascular episode during 3 months prior to ablation; heart failure NYHA class ≥2; chronic medications influencing cardiac ion channels (e.g. antipsychotics, antihistamines); and known intolerance to the study drug.
2.1. EPS and study measurements
All patients underwent balloon cryoablation with PVI as the desired outcome of the procedure.1
After a transseptal puncture, a decapolar catheter remained in the coronary sinus (CS), a quadripolar catheter was positioned in the high right atrium and a circular catheter was positioned in consecutive pulmonary veins to assess the baseline atrio‐venous conduction. During the ensuing electrophysiological study (EPS) the circular catheter remained in the left superior PV (LSPV, Figure 1).
Figure 1.
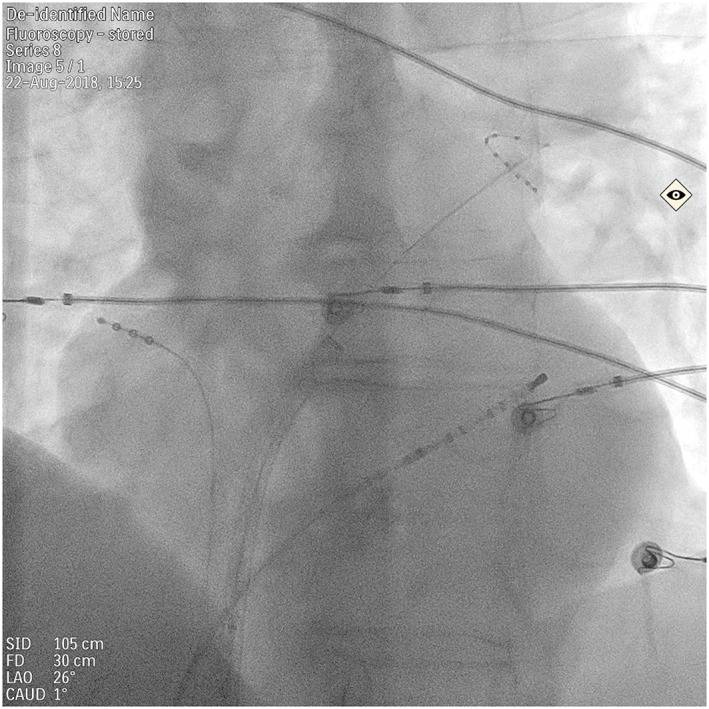
Catheter position during electrophysiological study. A decapolar catheter placed in the coronary sinus, a quadripolar catheter in the high right atrium position, a circular catheter visible in the left superior pulmonary vein
The EPS protocol consisted of atrial extrastimulus pacing (S1‐S2: 500–450 ms), atrial incremental pacing and sinus node testing. The following measurements were noted: right atrium and atrio‐ventricular node effective refractory periods (RA and AVN ERP), Wenckebach point, sinus node recovery time (SNRT) at 160/min and 130/min. The inter‐ and intra‐atrial conduction times (ms): high right atrium–proximal CS (CS 9/10), CS 9/10–LSPV, distal CS–LSPV were measured at baseline atrial pacing (500 ms) and 10 ms above the atrial ERP.
All measurements were taken once by an experienced electrophysiologist, similarly to the standard EPS, using LABSYSTEM Pro EP Recording System (Boston Scientific, Melbourne, MA, USA). We did not repeat pacing manoeuvres to minimize the risk of AF induction, which would have rendered measurements impossible.
Atrio‐venous conduction was verified in all PVs and EPS was repeated according to the same protocol 2 minutes after the last bolus of antazoline, AF conversion to sinus or potential electrical cardioversion (CV; see below). The atrio‐venous conduction in consecutive veins was assessed as the presence or absence of PV potentials and atrial capture during PV stimulation.
2.2. Administration of antazoline
If AF had been induced during EPS, antazoline was administered intravenously until conversion to sinus or cumulative dose of 300 mg.7, 8 In case of drug ineffectiveness, electrical CV was the treatment of choice. If no AF had been induced during EPS, antazoline was administered in divided doses until a cumulative dose of 250 mg. This scheme was based on our previous research.6, 7 In our opinion, beyond 300 mg the incremental effectiveness of antazoline is marginal, but the risk of adverse effects increases significantly. In the AnPAF randomized trial the maximum allowed dose of antazoline was 250 mg.8 We decided to maximize the chance of successful cardioversion in patients with AF induced during EPS by allowing the 300 mg threshold and minimize the risk of adverse effects in patients in sinus by administering antazoline up to 250 mg. Since both of those doses have been effective in previously published pragmatic studies, we did not conduct any plasma concentration measurements.6, 7, 8
2.3. Outcomes
The primary outcome of the AntaEP study was the assessment of atrio‐venous conduction before and after the infusion of antazoline in a clinically effective dose.
Secondary outcomes comprised: changes in electrophysiological properties of atrial and AV nodal tissue; effectiveness and safety of antazoline in AF conversion to sinus. Serious adverse events were defined as: systolic blood pressure <90 mmHg, chest pain/discomfort, tachycardia >180/min (including AF or flutter with rapid ventricular response), any ventricular arrhythmia.
2.4. Statistical analysis
Continuous variables were observed to approximate a normal distribution and are presented as means and standard deviation. Differences between group means were tested by paired Student t‐test. Categorical variables are reported as frequencies and percentages and compared by McNemar test. All tests were 2‐tailed and a P‐value of <.05 was considered statistically significant. All statistical calculations were performed using SAS version 9.4.
3. RESULTS
We enrolled 21 patients, but 7 were not included in the analysis due to AF present at the beginning of the procedure or AF induction during catheter manipulation or initiation of EPS. Among 14 patients included in the analysis, 13 (93%) were men. Mean age was 63.4 ± 5.9 years old (range 53–71 years), mean CHA2DS2‐VASc score 1.6 ± 1.0 (range 0–3) and mean left atrium diameter 41.6 ± 4.3 mm.
Antazoline was administered intravenously in a mean dose 257.1 ± 18.2 mg (range 250–300 mg) per patient over 5.21 ± 1.19 min. There were no serious adverse effects of the infusion.
Both PV potentials and atrial capture during PV stimulation were present in 4 veins in all patients; there was not a single case of left common pulmonary trunk in this series. Infusion of antazoline did not change PV potentials and atrial capture during PV stimulation (Figure 2) in any patient (κ = 1).
Figure 2.
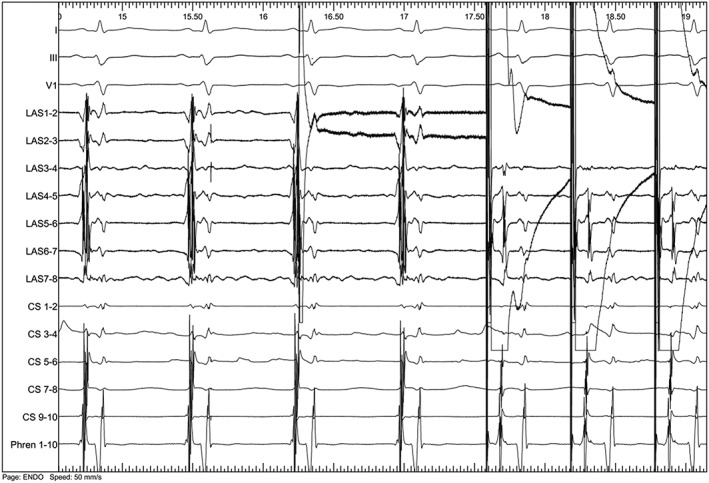
Endocardial tracing depicting pulmonary vein potentials (LAS 1/2 to 7/8, left side of the tracing) and conduction to the left atrium while pacing from the left superior pulmonary vein (right side of the tracing) after intravenous administration of 250 mg of antazoline
The electrophysiological parameters before and after the drug infusion are presented in Table 1. Table 2 summarizes atrial conduction times. AV node dual physiology was detected in 3 patients (21.4%). both before and after infusion.
Table 1.
Basic electrophysiological parameters measured before and after the administration of antazoline
| Baseline | Antazoline | P | |
|---|---|---|---|
| Right atrium ERP (ms) | 250 ± 23.9 | 259.3 ± 25.6 | .0422 |
| AV node ERP (ms) | 324.7 ± 84.5 | 284.3 ± 48.6 | .0214 |
| SNRT 160/min (s) | 1306.0 ± 159.9 | 1339.2 ± 150.5 | .0592 |
| SNRT 130/min (s) | 1230.3 ± 337.2 | 1222.3 ± 278.9 | .9643 |
| Wenckebach point (/min) | 151.6 ± 24.4 | 148.1 ± 23.6 | .1394 |
AV, atrio‐ventricular; ERP, effective refractory period; SNRT, sinus node recovery time.
Table 2.
Atrial conduction times before and after the administration of antazoline expressed in ms
| Baseline | Antazoline | P | |
|---|---|---|---|
| HRA–proximal CS (CS 9/10) | 111.4 ± 20.9 | 115.7 ± 25.5 | .1559 |
| HRA–proximal CS (CS 9/10) refa | 159.0 ± 43.3 | 179.4 ± 35.5 | .1526 |
| Proximal CS (CS 9/10)– LSPV | 4.21 ± 19.6 | 6.43 ± 26.2 | .6437 |
| Proximal CS (CS 9/10) – LSPV refa | 13.0 ± 35.6 | 1.5 ± 26.6 | .2282 |
| Distal CS (CS 1/2)– LSPV | −24.2 ± 20.1 | −27.7 ± 19.8 | .3349 |
| Distal CS (CS 1/2) ‐ LSPV refa | −33.7 ± 28.4 | −40.1 ± 18.2 | .2407 |
CS, coronary sinus; HRA, high right atrium; LSPV, left superior pulmonary vein.
measured 10 ms above the right atrium effective refractory period (RA ERP).
AF was induced during EPS in 5 (35.7%) patients. Antazoline was effective in all cases with a mean conversion time 8.4 ± 6.2 min (range 4–19 min). Intravenous 5 mg metoprolol was added to antazoline in 1 patient (7.1%).
4. DISCUSSION
The AntaEP study has shown that antazoline administration does not change atrio‐venous conduction (Figure 2). It has also confirmed its high effectiveness and rapid onset of action making antazoline a suitable choice for pharmacological cardioversion of AF during PVI. The consensus end point of AF ablation is the achievement of PVI defined as the lack of electrical conduction between the left atrium and pulmonary veins.1, 2 In practice, as in major clinical trials (CABANA, CASTLE AF), PVI is demonstrated when pacing from within the ablation line, which encircles the ipsilateral veins, captures local atrio‐venous tissue but does not propagate beyond the line and does not activate the atrium.14, 15 This can be reliably demonstrated only in sinus rhythm. Drugs influencing atrio‐venous conduction can potentially interfere with this assessment leading to a false evaluation of an AF ablation endpoint. Previous antazoline studies conducted in the electrophysiology laboratory concentrated either on its electrophysiological properties or clinical effectiveness, yet none reported any information on atrio‐venous conduction.4, 5, 13 This observation was of paramount interest in terms of PVI and the results of this study were straightforward: antazoline did not alter atrio‐venous conduction in any patient (Figure 2). Despite the clinical practice of class Ic AADs (flecainide, propafenone) administration during AF ablation there are no widely known studies describing their effect on electrophysiological parameters of human LA, atrio‐venous conduction or even clinical effectiveness in AF induced during the EP procedure. The present study fills those gaps in our knowledge in terms of antazoline while previously published retrospective studies already showed high AF conversion rates during ablation procedures.4, 13
The exact antiarrhythmic mechanism of antazoline remains unclear but recent animal studies derived significant data in this matter. In an experimental model of AF, antazoline modestly but significantly increased interatrial conduction time, a parameter not affected by flecainide. More interestingly, antazoline prolonged atrial action potential duration and increased the atrial effective refractory period. This resulted in an increase of atrial postrepolarization refractoriness, an antiarrhythmic mechanism shared by other AADs (amiodaron, quinidine).3 An experimental model of long/short QT further reinforced this theory, demonstrating the prolongation of ventricular ERP and a remarkable increase in ventricular PPR.10 The clinical effect observed in both studies was the abolition of atrial fibrillation and ventricular arrhythmia. EP studies in humans, involving supraventricular tachycardia patients and a current study over AF patients, show the drug's more or less pronounced influence on atrial and AV nodal ERP, with varied influence on inter‐ and intra‐atrial conduction times and an excellent clinical effect.5, 13
Clinical effectiveness of antazoline was investigated in 1 double‐blind randomized controlled trial and in a series of retrospective case‐controlled studies covering a wide variety of patients.4, 6, 7, 8, 13, 16, 17 In a short‐standing (<48‐hour) AF, conversion rates to sinus were about 70–80% and time to conversion ranged between 7 and 20 minutes.6, 7, 8, 16, 17 For AF induced during the EP procedure, antazoline effectiveness reached almost 100%.4, 13 Antazoline was reported to be as effective or superior to propafenone and clearly more effective than amiodaron when administered in an emergency department.7, 17
Similarly to published data, the AntaEP study has shown lack of significant influence of antazoline on Wenckebach point and SNRT.5 In our study, AV node ERP was significantly lower after the drug infusion (Table 1), which is not entirely different from the previous publication, where the absolute values of AV node ERP were markedly distinct before and after the drug infusion – 290.67 ± 20.15 ms vs 252.0 ± 22.39 ms, although the difference did not reach statistical significance.5 The reason for such discrepancy remains unclear as the enrolment was similar in both experiments. Contrary to the study by Binkowski et al., there were no significant differences in atrial conduction times before and after antazoline. Our population was older (age range 53–71 years vs 17–72 years) and scheduled for AF ablation where patients in the other study were scheduled for ablation of supraventricular arrhythmias.3, 5
The sinus conversion rate in our study was 100% (5/5) and was comparable with previous studies, where antazoline effectiveness in AF inducted during the EP procedure was as high as 90–100%.4, 13 While the time to conversion was generally short (8.4 ± 6.2 minutes), in 1 patient, it took 19 minutes and sinus returned as the operator was waiting for an anaesthesiologist to perform electrical CV. The authors of the biggest analysis comprising patients undergoing PVI and treated with antazoline (141 patients, 55 in sinus at the beginning of the procedure) assessed drug's effectiveness after 20 minutes.4
There were no serious adverse events during the study. Antazoline is known to induce a plethora of mild side effects: nausea, metallic taste, blushing, among others.4, 8, 18 The drug can also prolong QTc interval, convert AF to sustained atrial tachycardia or flutter (including 1:1 conduction), unmask underlying conduction disturbances or sick sinus syndrome, exacerbate existing heart failure, and provoke chest pain or hypotension.4, 6, 7, 8, 12, 16, 18, 19 Therefore, antazoline should be administered intravenously under continuous cardiac monitoring in the setting of emergency department, cardiological ward, cardiac intensive care unit or electrophysiology catheterization laboratory.
While eye drops containing antazoline are available across Europe, intravenous antazoline is produced and marketed in Poland. It has a Summary of Product Characteristics and is registered for pharmacological cardioversion of AF in Poland. Data on its development are limited as the drug was studied extensively around the world in 1960s and 1970s.19, 20, 21, 22 Intravenous antazoline was effective against both supraventricular and ventricular arrhythmias but failed to prevent AF recurrence while given orally.18 There have also been case studies suggesting an elevated risk of thrombocytopenia, cardiac arrest or 1:1 flutter; the latter has been confirmed in contemporary studies.23, 24, 25 Published data indicate that antazoline is available in the experimental setting in Germany, Egypt and China, while less is known about clinical practice.3, 26, 27
4.1. Limitations
This was a single‐centre study without a control group that enrolled relatively few patients. In our opinion, unequivocal results of the primary outcome and secondary results comparable to previously published data offset at least some of those limitations.
Since antazoline doses of 250–300 mg have been effective in previously published pragmatic studies we considered them the target doses and did not conduct any plasma concentration measurements. Unfortunately, this fact ruled out any possibilities to study the association between the concentration of the drug and electrophysiological parameters or assess drug metabolism. This is a major limitation of the study.
Study measurements were taken by an experienced electrophysiologist once during an EPS. This was similar to the previously published report but averaged measurements might have been more reliable than a single result. Also, pacing manoeuvres performed during the study were limited to a minimum and not repeated to minimize the risk of AF induction. Both of those facts should be considered when interpreting the results of the study.
5. CONCLUSION
Antazoline does not influence atrio‐venous conduction, has high effectiveness and rapid onset of action, therefore may be suitable for pharmacological cardioversion of atrial fibrillation induced during an atrial fibrillation ablation procedure.
COMPETING INTERESTS
There are no competing interests to declare.
CONTRIBUTORS
M.M.F.: concept/design, data collection, data analysis/interpretation and manuscript preparation. A.M.: data collection, data analysis/interpretation and critical revision ofarticle. I.K.: Statistics, critical revision of article M.K.: critical revision of article. H.S.: critical revision of article. M.P.: data analysis/interpretation and critical revision of article.
ACKNOWLEDGEMENTS
This work was supported by Institute of Cardiology research grant 2.27/IV/16.
Farkowski MM, Maciag A, Kowalik I, Konka M, Szwed H, Pytkowski M. Intravenous antazoline, a first‐generation antihistaminic drug with antiarrhythmic properties, is a suitable agent for pharmacological cardioversion of atrial fibrillation induced during pulmonary vein isolation due to the lack of influence on atrio‐venous conduction and high clinical effectiveness (AntaEP Study). Br J Clin Pharmacol. 2019;85:1552–1558. 10.1111/bcp.13940
The authors confirm that the Principal Investigator for this paper is Michal M. Farkowski and that he had direct clinical responsibility for patients.
REFERENCES
- 1. Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. 2018;20(1):e1‐e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609‐1678. [DOI] [PubMed] [Google Scholar]
- 3. Frommeyer G, Sterneberg M, Dechering DG, et al. Effective suppression of atrial fibrillation by the antihistaminic agent antazoline: first experimental insights into a novel antiarrhythmic agent. Cardiovasc Ther. 2017;35(2). 10.1111/1755-5922.12244 [DOI] [PubMed] [Google Scholar]
- 4. Balsam P, Kozluk E, Peller M, et al. Antazoline for termination of atrial fibrillation during the procedure of pulmonary veins isolation. Adv Med Sci. 2015;60(2):231‐235. [DOI] [PubMed] [Google Scholar]
- 5. Binkowski BJ, Makowski M, Kubinski P, et al. Effect of antazoline on electrophysiological properties of atrial muscle and conduction system of the heart. Cardiovasc Drugs Ther. 2018;32(2):169‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Farkowski MM, Maciag A, Zurawska M, et al. Clinical effectiveness and safety of antazoline‐based therapy in patients with stable coronary artery disease undergoing pharmacological cardioversion of short‐duration atrial fibrillation in the emergency department. Cardiovasc Ther. 2018;36:e12469. [DOI] [PubMed] [Google Scholar]
- 7. Farkowski MM, Maciag A, Zurawska M, et al. Comparative effectiveness and safety of antazoline‐based and propafenone‐based strategies for pharmacological cardioversion of short‐duration atrial fibrillation in the emergency department. Pol Arch Med Wew. 2016;126:381‐387. [DOI] [PubMed] [Google Scholar]
- 8. Maciag A, Farkowski MM, Chwyczko T, et al. Efficacy and safety of antazoline in the rapid cardioversion of paroxysmal atrial fibrillation (the AnPAF Study). Europace. 2017;19(10):1637‐1642. [DOI] [PubMed] [Google Scholar]
- 9. Giebultowicz J, Piotrowski R, Baran J, et al. Application of a novel liquid chromatography/tandem mass spectrometry method for the determination of antazoline in human plasma: result of ELEPHANT‐I [ELEctrophysiological, pharmacokinetic and hemodynamic effects of PHenazolinum (ANTazoline mesylate)] human pharmacokinetic study. J Pharm Biomed Anal. 2016;123:113‐119. [DOI] [PubMed] [Google Scholar]
- 10. Ellermann C, Sterneberg M, Kochhauser S, et al. Antiarrhythmic effect of antazoline in experimental models of acquired short‐ and long‐QT‐syndromes. Europace. 2018;20(10):1699‐1706. [DOI] [PubMed] [Google Scholar]
- 11. Lee K, Groh WJ, Blair TA, Maylie JG, Adelman JP. Imidazoline compounds inhibit KATP channels in guinea pig ventricular myocytes. Eur J Pharmacol. 1995;285(3):309‐312. [DOI] [PubMed] [Google Scholar]
- 12. Piotrowski R, Giebultowicz J, Baran J, et al. Antazoline‐insights into drug‐induced electrocardiographic and hemodynamic effects: results of the ELEPHANT II substudy. Ann Noninvasive Electrocardiol. 2017;22:e12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Piotrowski R, Krynski T, Baran J, et al. Antazoline for rapid termination of atrial fibrillation during ablation of accessory pathways. Cardiol J. 2014;21(3):299‐303. [DOI] [PubMed] [Google Scholar]
- 14. Marrouche NF, Brachmann J, Andresen D, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378(5):417‐427. [DOI] [PubMed] [Google Scholar]
- 15. Packer DL, Mark DB, Robb RA, et al. Catheter ablation versus antiarrhythmic drug therapy for atrial fibrillation (CABANA) trial: study rationale and design. Am Heart J. 2018;199:192‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Srzednicki M, Sadowski Z, Kulikowski A. Evaluation of the anti‐arrhythmia effectiveness of Phenazolinum Polfa in paroxysmal atrial fibrillation. Pol Tyg Lekarski. 1990;45:924‐927. [PubMed] [Google Scholar]
- 17. Wybraniec MT, Wrobel W, Wilkosz K, et al. Pharmacological cardioversion with antazoline in atrial fibrillation: the results of the CANT study. J Am Heart Assoc. 2018;7:e010153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reynolds EW Jr, Baird WM, Clifford ME. A clinical trial of antazoline in the treatment of arrhythmias. Am J Cardiol. 1964;14:513‐521. [DOI] [PubMed] [Google Scholar]
- 19. Leon‐Sotomayor L. A clinical evaluation of the antiarrhythmic properties of antazoline. Am J Cardiol. 1963;11:646‐653. [DOI] [PubMed] [Google Scholar]
- 20. Higazi AM, el‐Ahmdai LM, Ageeb M. The antiarrythmic action of antazoline (an experimental study). J Egypt Med Assoc. 1971;54(5):376‐384. [PubMed] [Google Scholar]
- 21. Muniz M, Bellini AJ. Treatment of paroxysmal tachycardias. our experience with antazoline. Folha Med. 1965;50:97‐118. [PubMed] [Google Scholar]
- 22. Shah SS, Vaidya CH, Doshi HV. Antazoline in the treatment of cardiac arrhythmias. Postgrad Med J. 1972;48(559):304‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Andersen M, Hansen PF, Sandoe E. Antazoline (antistina) as an antiarrhythmic agent: the risk of cardiac arrest. Acta Med Scand. 1965;177:761‐763. [PubMed] [Google Scholar]
- 24. Gassel WD, Schneider D. Acute drug allergic thrombocytopenia caused by antazoline. Blut. 1974;29(3):195‐202. [DOI] [PubMed] [Google Scholar]
- 25. Yahini JH, Nathan D, Charuzi Y, et al. Atrial flutter with 1:1 atrioventricular conduction precipitated by antazoline (antistine). Isr J Med Sci. 1966;2:329‐332. [PubMed] [Google Scholar]
- 26. Abdel‐Halim LM, Abd‐El Rahman MK, Ramadan NK, et al. Comparative study between recent methods manipulating ratio spectra and classical methods based on two‐wavelength selection for the determination of binary mixture of antazoline hydrochloride and tetryzoline hydrochloride. Spectrochim Acta A Mol Biomol Spectrosc. 2016;159:98‐105. [DOI] [PubMed] [Google Scholar]
- 27. Wang R, Chu Y, Li X, et al. Determination of antazoline hydrochloride in rat plasma and excreta by reversed‐phase ion‐pair chromatography and its application to pharmacokinetics. Biomed Chromatogr. 2013;27(12):1595‐1602. [DOI] [PubMed] [Google Scholar]


