Abstract
Background
The prognostic relevance of gastric tumor location has been reported and debated. Our study was conducted to examine the differences in clinicopathological features, prognostic factors, and overall survival (OS) between patients with proximal gastric cancer (PGC) and distal gastric cancer (DGC).
Patients and Methods
Patients with PGC or DGC were identified from the China National Cancer Center Gastric Cancer Database (NCCGCDB) during 1997–2017. Survival analysis was performed via Kaplan-Meier estimates and Cox proportional hazards models.
Results
We reviewed 16,119 cases of gastric cancer patients, including 6,479 of PGC and 9,640 of DGC. PGC patients presented as older patients (61.5 versus 56.4 years, P<0.001) and more males (82.9% versus 68.2%, P<0.001). Compared with DGC, PGC was more likely to be in later pT stage (pT3 and pT4, 65.0% versus 52.8%, P<0.001) and lymph node metastasis (54.8% versus 50.9%, P<0.001). In univariate analysis, PGC patients had a worse survival outcome in stage I (Hazard ratio [HR] = 2.04, 95% CI: 1.42-2.94) but a better prognosis in stage IV (HR = 0.85, 95% CI: 0.73-0.98) when compared to DGC patients. However, multivariate analysis demonstrated that PGC was not an independent predictor for poor survival (HR = 1.07, 95% CI: 1.00-1.14). Results from multivariate analysis also revealed that pT4, lymph node metastasis, distant metastasis, no gastrectomy, and Borrmann IV were independent predictors associated with poor survival for both PGC and DGC patients. Additional prognostic factors for PGC patients included underweight (BMI < 18.5) (HR = 1.29, 95% CI: 1.06-1.58), linitis plastica (HR = 2.13, 95% CI: 1.25-3.65), and overweight (23 ≤ BMI <27.5) (HR = 0.80, 95% CI: 0.71-0.90). During the 20-year study period, the 5-year OS increased significantly for both PGC and DGC, with the increase rate of 91.7% and 67.7%, respectively.
Conclusion
In China, PGC significantly differed from DGC in clinicopathological characteristics and prognostic factors. However, there was no significant relationship between survival outcome and gastric tumor location.
1. Introduction
Gastric cancer (GC) is the third leading cause of cancer-related mortality and the fifth most common cancer globally [1]. Many population-based studies have reported that the incidence of distal gastric cancer (DGC) has gradually declined, while proximal gastric cancer (PGC) has increased obviously during the last decades [2–9].
Researches have indicated that PGC differed from DGC in clinicopathological characteristics [10–13]. For example, one previous study [11] found that PGC patients were more likely to be in an advanced tumor stage and have larger tumor size as compared to DGC. Yu et al. [13] showed that PGC was more common than DGC in males. Moreover, there was no clear agreement on the link between tumor location and overall survival (OS) of GC. Some studies [11, 13–17] reported a worse prognosis in patients with PGC compared to DGC, while others [10, 12, 18] have shown no relationship between prognosis and gastric tumor location. Katsuhiko et al. [19] even demonstrated that PGC patients had a longer survival time than DGC after chemotherapy. The inconsistent findings from these previous studies could be partially due to the small sample size, with the population records ranging from 270 to 3,193.
Given the suggested but undecided differences in clinicopathological characteristics and prognosis between PGC and DGC, the aim of our study was to compare the clinicopathological features, prognostic factors, and survival outcomes between PGC and DGC based on the China National Cancer Center Gastric Cancer Database (NCCGCDB) in order to determine whether PGC conveys worse prognosis and provides evidence for the development of guiding strategies for GC patients with different tumor locations.
2. Materials and Methods
2.1. Patient Population
All the study data were abstracted from the NCCGCDB. The NCCGCDB was a clinical gastric cancer database based on a huge retrospective cohort, which was sourced from China National Cancer Center, a single but large-volume institution, and included more than 19,000 patients from all around China from 1997 to 2018. PGC was defined as tumors with the epicenter located in cardia (C16.0) or fundus (C16.1), whereas DGC was defined as lesions of the body (C16.2), antrum (C16.3), or pylorus (C16.4). Changing trends in clinicopathological characteristics and OS of total GC, PGC, and DGC were analyzed in four consecutive time periods: from 1997 to 2002 (period 1), from 2002 to 2007 (period 2), from 2007 to 2012 (period 3), and from 2012 to 2017 (period 4). The geographical locations of these gastric cancer patients can be found in Figure 1.
Figure 1.
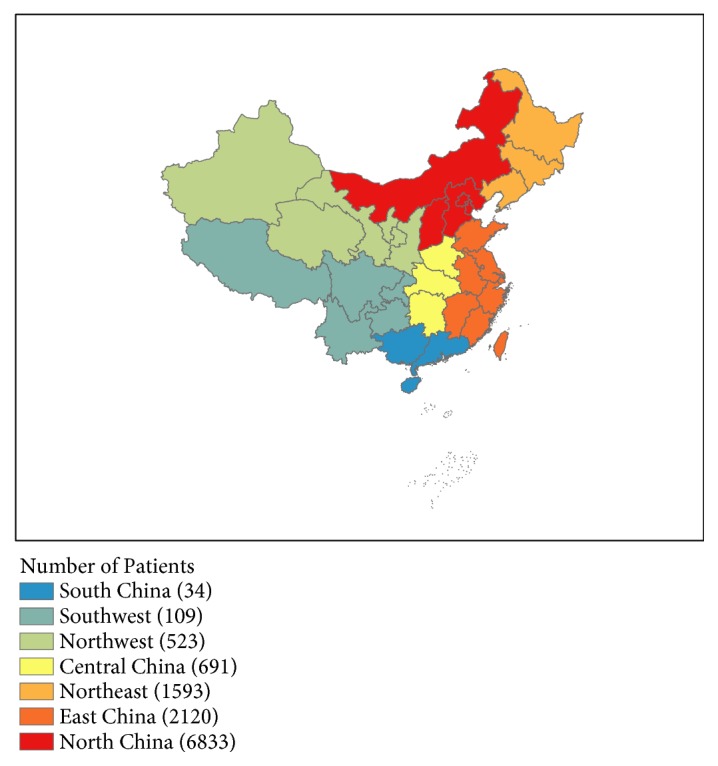
The geographical locations of PGC and DGC patients of NCCGCDB, 1997–2017.
2.2. Statistical Analyses
Categorical variables were compared using the Chi-squared test and continuous variables were analyzed by Student's t-test. OS and progression-free survival (PFS) curves were plotted for PGC and DGC groups, respectively, using the Kaplan-Meier method and compared statistically using the log-rank test. Hazard ratios (HRs) and 95% confidence intervals (CIs) were used to estimate the risk of death by employing the multivariate Cox proportional hazards models with adjustment for alcohol consumption, BMI, H. pylori infection, pT stage, pN stage, pM stage, Lauren classification, gastrectomy, surgical margin, HER2 score, linitis plastica, Borrmann classification, and gross classification. The covariates included in the final models were selected by the stepwise selection method, with a significant level for adding variables of 0.05 and a significant level for removing variables of 0.10. A two-sided P value less than 0.05 was considered as statistically significant. All the statistical analyses were performed using SAS software v9.4 (SAS Institute, Inc., Cary, NC).
3. Results
3.1. Clinicopathological Characteristics
In this study, 16,119 patients were included. The clinicopathological features of 9,640 patients (59.8%) with DGC and 6,479 patients (41.2%) with PGC were compared (Table 1), with an incidence of DGC:PGC = 1.49:1. Among our study population, a higher tumor incidence was found in DGC. There were significant differences in the distribution of age, gender, smoking, alcohol consumption, BMI, H. pylori infection, pTNM stage, Lauren classification, surgical margin, HER2 score, linitis plastica, and Borrmann classification between DGC and PGC patients. PGC was more likely to occur in older patients (61.5 versus 56.4 years, P<0.001). Both groups were predominantly males and PGC has a greater proportion of males than DGC (82.9% versus 68.2%, P<0.001). Relatively higher percentages of smokers (51.7% versus 33.9%, P<0.001), alcohol drinkers (41.7% versus 29.0%, P<0.001), and overweight/obesity (BMI≥23) (56.6% versus 51.2%, P<0.001) were shown in PGC patients as compared to DGC patients.
Table 1.
Clinicopathological characteristics by tumor location.
| Total GC | PGC | DGC | P Value | |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| Age at diagnosis (years) | ||||
| Mean (SD) | 58.5 (11.4) | 61.5 (10.0) | 56.4 (11.9) | <0.001 |
| Younger (≤35) | 590 (3.7) | 86 (1.3) | 504 (5.2) | |
| Middle-aged (36-65) | 10,842 (67.3) | 4,026 (62.1) | 6,816 (70.7) | |
| Older (≥66) | 4,685 (29.1) | 2,366 (36.5) | 2,319 (24.1) | <0.001 |
| Gender | ||||
| Male | 4,171 (25.9) | 5,374 (82.9) | 6,574 (68.2) | |
| Female | 11,948 (74.1) | 1,105 (17.1) | 3,066 (31.8) | <0.001 |
| Smoking status | ||||
| Never smokers | 9,289 (57.6) | 3,065 (47.3) | 6,224 (64.6) | |
| Smokers | 6,621 (41.1) | 3,352 (51.7) | 3,269 (33.9) | <0.001 |
| Current smokers | 4,634 (28.8) | 2,210 (34.1) | 2,424 (25.2) | |
| Ex-smokers | 1,987 (12.3) | 1,142 (17.6) | 845 (8.8) | <0.001 |
| Alcohol consumption | ||||
| Never drinkers | 10,398 (64.5) | 3,716 (57.4) | 6,682 (69.3) | |
| Drinkers | 5,496 (34.1) | 2,699 (41.7) | 2,797 (29.0) | <0.001 |
| Current drinkers | 4,752 (29.5) | 2,416 (37.3) | 2,336 (24.2) | |
| Ex-drinkers | 744 (4.6) | 283 (4.4) | 461 (4.8) | <0.001 |
| BMI (kg/m2) | ||||
| <18.5 | 1,066 (6.6) | 380 (5.9) | 686 (7.1) | |
| 18.5-22.9 | 6,097 (37.8) | 2,301 (35.5) | 3,796 (39.4) | |
| 23-27.4 | 6,576 (40.8) | 2,760 (42.6) | 3,816 (39.6) | |
| ≥27.5 | 2,028 (12.6) | 909 (14.0) | 1,119 (11.6) | <0.001 |
| H. pylori | ||||
| No | 1,247 (7.7) | 437 (6.7) | 625 (6.5) | |
| Yes | 956 (5.9) | 331 (5.1) | 810 (8.4) | |
| Unknown | 13,916 (86.3) | 5,711 (88.2) | 8,205 (85.1) | <0.001 |
| Pathologic T stage | ||||
| T0+Tis | 58 (0.4) | 14 (0.2) | 44 (0.5) | |
| T1 | 2,491 (15.5) | 596 (9.2) | 1,895 (19.7) | |
| T2 | 1,376 (8.5) | 441 (6.8) | 935 (9.7) | |
| T3 | 3,019 (18.7) | 1,640 (25.3) | 1,379 (14.3) | |
| T4 | 6,288 (39.0) | 2,573 (39.7) | 3,715 (38.5) | |
| TX | 2,887 (17.9) | 1,215 (18.8) | 1,672 (17.3) | <0.001 |
| Pathologic N stage | ||||
| N0 | 4,538 (28.2) | 1,623 (25.1) | 2,915 (30.2) | |
| N1 | 2,281 (14.1) | 983 (15.2) | 1,298 (13.5) | |
| N2 | 2,417 (15.0) | 1,081 (16.7) | 1,336 (13.9) | |
| N3 | 3,759 (23.3) | 1,489 (23.0) | 2,270 (23.6) | |
| NX | 3,124 (19.4) | 1,303 (20.1) | 1,821 (18.9) | <0.001 |
| Pathologic M stage | ||||
| M0 | 13,629 (84.6) | 5,555 (85.7) | 8,074 (83.8) | |
| M1 | 1,883 (11.7) | 651 (10.1) | 1,232 (12.8) | <0.001 |
| pTNM | ||||
| 0 | 52 (0.3) | 13 (0.2) | 39 (0.4) | |
| I | 2,989 (18.5) | 825 (12.7) | 2,164 (22.5) | |
| II | 2,112 (13.1) | 929 (14.3) | 1,183 (12.3) | |
| III | 7,354 (45.6) | 3,272 (50.5) | 4,082 (42.3) | |
| IV | 1,883 (11.7) | 651 (10.1) | 1,232 (12.8) | <0.001 |
| Lauren classification | ||||
| Intestinal | 2,390 (14.8) | 1,215 (18.8) | 1,175 (12.2) | |
| Diffuse | 2,202 (13.7) | 555 (8.6) | 1,647 (17.1) | |
| Mixed | 1,486 (9.2) | 592 (9.1) | 894 (9.3) | |
| Unknown | 10,041 (62.3) | 4,117 (63.5) | 5,924 (61.5) | <0.001 |
| Type of gastrectomy | ||||
| Gastrectomy | 13,190 (81.8) | 5,260 (81.2) | 7,930 (82.3) | |
| No surgery | 2,929 (18.2) | 1,219 (18.8) | 1,710 (17.7) | 0.068 |
| Surgical Margin | ||||
| Negative | 12,457 (77.3) | 4,950 (76.4) | 7,507 (77.9) | |
| Positive on the proximal margin | 183 (1.1) | 91 (1.4) | 92 (1.0) | |
| Positive on the distal margin | 197 (1.2) | 74 (1.1) | 123 (1.3) | |
| Positive on the proximal and distal margin | 46 (0.3) | 12 (0.2) | 34 (0.4) | 0.002 |
| HER2 score | ||||
| 0 (-) | 2,850 (17.7) | 1,037 (16.0) | 1,813 (18.8) | |
| 1 (+) | 2,620 (16.3) | 971 (15.0) | 1,649 (17.1) | |
| 2 (++) | 1,082 (6.7) | 466 (7.2) | 616 (6.4) | |
| 3 (+++) | 522 (3.2) | 275 (4.2) | 247 (2.6) | |
| Unknown | 9,045 (56.1) | 3,730 (57.6) | 5,315 (55.1) | <0.001 |
| Linitis plastica | ||||
| No | 15,670 (97.2) | 6,286 (97.0) | 9,384 (97.3) | |
| Yes | 110 (0.7) | 31 (0.5) | 79 (0.8) | 0.024 |
| Borrmann classification | ||||
| Borrmann I | 1,160 (7.2) | 714 (11.0) | 446 (4.6) | |
| Borrmann II | 4,605 (28.6) | 1,926 (29.7) | 2,679 (27.8) | |
| Borrmann III | 3,843 (23.8) | 1,574 (24.3) | 2,269 (23.5) | |
| Borrmann IV | 981 (6.1) | 347 (5.4) | 634 (6.6) | |
| Unknown | 1,807 (11.2) | 696 (10.7) | 1,111 (11.5) | <0.001 |
GC, gastric cancer; PGC, proximal gastric cancer; DGC, distal gastric cancer; SD, standard deviation.
As for tumors, PGC patients were more likely to be in later pT stage (pT3 and pT4, 65.0% versus 52.8%, P<0.001), lymph node metastasis (54.8% versus 50.9%, P<0.001), intestinal type (18.8% versus 12.2%, P<0.001), local advanced GC (76.2% versus 65.9%, P<0.001), and Borrmann I (11.0% versus 4.6%, P<0.001). The percentages of ever received surgical treatment (81.2% versus 82.3%, P=0.068) were similar between the two groups. DGC patients were more common in diffuse type (17.1% versus 8.6%, P<0.001), early stage GC (21.5% versus 11.3%, P<0.001), and distant metastasis (12.8% versus 10.1%, P<0.001).
Changing trends of clinicopathological features in GC patients were analyzed. The proportion of pT1 tumors increased gradually with time, from 9.5% in period 1 to 22.0% in period 4, whereas the proportion of pT4 had declined from 66.0% in period 1 to 28.1% in period 4. The proportion of patients with pN0 increased from 24.5% in period 1 to 33.5% in period 4, whereas patients with pN3 were gradually decreased from 26.3% in period 1 to 21.5% in period 4. The proportion of pM1 remained relatively stable (from 11.2% to 10.7%) during the past 20 years. In pTNM stage, a significant increase was observed in stages I and II (from 12.0% and 3.8% in period 1 to 24.9% and 17.6% in period 4, resp.), while the proportion of stage III had declined from 63.0% in period 1 to 37.2% in period 4.
3.2. Prognostic Factors of Survival in Univariate and Multivariate Analyses
As shown in Table 2, univariate analyses of survival revealed significantly different survival based on the following parameters: overweight/obesity (BMI ≥ 23), H. pylori infection, advanced pT, pN, pM, and pTNM stage, Lauren classification, no gastrectomy, surgical margin, linitis plastic, and Borrmann IV for both PGC and DGC groups. For patients with PGC, additional parameters including middle and older age (HR = 0.47, 95% CI: 0.33-0.65; HR = 0.51, 95% CI: 0.37-0.72, resp.) and HER2 score of 1(+) and 2(++) (HR = 0.70, 95% CI: 0.57-0.86; HR = 0.69, 95% CI: 0.53-0.89, resp.), while smoking (HR = 0.85, 95% CI: 0.78-0.93) and alcohol drinking (HR = 0.85, 95% CI: 0.77-0.94) were additional prognostic factors for DGC patients.
Table 2.
Univariate survival analysis by tumor location.
| Prognostic Factors | PGC group (N=4,716) | DGC group (N=7,228) | PGC versus DGC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | ||||
| Lower | Upper | Lower | Upper | Lower | Upper | |||||||
| Age (years) | ||||||||||||
| Younger (≤35) | 1.00 | 1.00 | 2.20 | 1.50 | 3.22 | <0.001 | ||||||
| Middle-aged (36-65) | 0.47 | 0.33 | 0.65 | <0.001 | 0.94 | 0.77 | 1.14 | 0.52 | 1.12 | 1.03 | 1.21 | 0.008 |
| Older (≥66) | 0.51 | 0.37 | 0.72 | <0.001 | 1.01 | 0.82 | 1.24 | 0.92 | 1.14 | 1.01 | 1.28 | 0.028 |
| Gender | ||||||||||||
| Male | 1.00 | 1.00 | 1.16 | 1.08 | 1.25 | <0.001 | ||||||
| Female | 1.04 | 0.91 | 1.19 | 0.55 | 1.07 | 0.98 | 1.17 | 0.12 | 1.13 | 0.98 | 1.30 | 0.097 |
| Smoking status | ||||||||||||
| Never smoker | 1.00 | 1.00 | 1.08 | 0.99 | 1.18 | 0.074 | ||||||
| Smokers | 1.00 | 0.91 | 1.10 | 0.98 | 0.85 | 0.78 | 0.93 | <0.001 | 1.27 | 1.15 | 1.41 | <0.001 |
| Current smokers | 1.03 | 0.92 | 1.14 | 0.63 | 0.89 | 0.81 | 0.99 | 0.023 | 1.25 | 1.11 | 1.40 | <0.001 |
| Ex-smokers | 0.94 | 0.82 | 1.09 | 0.41 | 0.72 | 0.60 | 0.86 | <0.001 | 1.39 | 1.13 | 1.71 | 0.002 |
| Alcohol consumption | ||||||||||||
| Never drinkers | 1.00 | 1.00 | 1.11 | 1.03 | 1.21 | 0.009 | ||||||
| Drinkers | 0.95 | 0.86 | 1.05 | 0.35 | 0.85 | 0.77 | 0.94 | 0.001 | 1.24 | 1.11 | 1.39 | <0.001 |
| Current drinkers | 0.97 | 0.87 | 1.07 | 0.55 | 0.90 | 0.82 | 1.00 | 0.048 | 1.19 | 1.06 | 1.34 | 0.004 |
| Ex-drinkers | 0.82 | 0.62 | 1.07 | 0.14 | 0.58 | 0.45 | 0.74 | <0.001 | 1.55 | 1.08 | 2.23 | 0.017 |
| BMI (kg/m2) | ||||||||||||
| <18.5 | 1.24 | 1.02 | 1.51 | 0.033 | 1.12 | 0.96 | 1.32 | 0.16 | 1.33 | 1.06 | 1.68 | 0.016 |
| 18.5-22.9 | 1.00 | 1.00 | 1.21 | 1.09 | 1.34 | <0.001 | ||||||
| 23-27.4 | 0.73 | 0.65 | 0.82 | <0.001 | 0.82 | 0.75 | 0.91 | <0.001 | 1.07 | 0.96 | 1.19 | 0.22 |
| ≥27.5 | 0.79 | 0.67 | 0.92 | 0.0024 | 0.73 | 0.63 | 0.85 | <0.001 | 1.30 | 1.08 | 1.57 | 0.006 |
| H. pylori | ||||||||||||
| No | 1.00 | 1.00 | 1.55 | 1.09 | 2.21 | 0.016 | ||||||
| Yes | 1.45 | 1.01 | 2.08 | 0.044 | 1.71 | 1.25 | 2.33 | 0.001 | 1.31 | 0.95 | 1.81 | 0.095 |
| Unknown | 2.36 | 1.80 | 3.09 | <0.001 | 3.31 | 2.60 | 4.21 | <0.001 | 1.10 | 1.03 | 1.18 | 0.005 |
| Pathologic T stage | ||||||||||||
| T0+Tis | 3.28 | 0.79 | 13.58 | 0.10 | 2.58 | 0.94 | 7.08 | 0.066 | 2.46 | 0.45 | 13.53 | 0.30 |
| T1 | 1.00 | 1.00 | 2.07 | 1.39 | 3.10 | <0.001 | ||||||
| T2 | 1.58 | 1.02 | 2.45 | 0.041 | 3.26 | 2.38 | 4.46 | <0.001 | 0.96 | 0.67 | 1.38 | 0.84 |
| T3 | 3.86 | 2.77 | 5.38 | <0.001 | 8.32 | 6.39 | 10.83 | <0.001 | 0.92 | 0.80 | 1.06 | 0.26 |
| T4 | 6.69 | 4.83 | 9.28 | <0.001 | 12.61 | 9.81 | 16.21 | <0.001 | 1.05 | 0.95 | 1.15 | 0.37 |
| TX | 11.12 | 7.99 | 15.48 | <0.001 | 27.37 | 21.21 | 35.31 | <0.001 | 0.81 | 0.72 | 0.92 | <0.001 |
| Pathologic N stage | ||||||||||||
| N0 | 1.00 | 1.00 | 1.65 | 1.35 | 2.02 | <0.001 | ||||||
| N1 | 1.91 | 1.56 | 2.36 | <0.001 | 2.39 | 1.96 | 2.90 | <0.001 | 1.31 | 1.07 | 1.60 | <0.001 |
| N2 | 2.84 | 2.34 | 3.44 | <0.001 | 3.89 | 3.26 | 4.65 | <0.001 | 1.20 | 1.01 | 1.41 | 0.034 |
| N3 | 5.00 | 4.20 | 5.95 | <0.001 | 8.42 | 7.21 | 9.83 | <0.001 | 0.97 | 0.86 | 1.08 | 0.56 |
| NX | 6.56 | 5.51 | 7.81 | <0.001 | 12.60 | 10.79 | 14.73 | <0.001 | 0.85 | 0.76 | 0.96 | 0.007 |
| Pathologic M stage | ||||||||||||
| M0 | 1.00 | 1.00 | 1.28 | 1.18 | 1.38 | <0.001 | ||||||
| M1 | 4.96 | 4.33 | 5.68 | <0.001 | 7.45 | 6.73 | 8.25 | <0.001 | 0.85 | 0.73 | 0.98 | 0.027 |
| pTNM | ||||||||||||
| 0 | 4.06 | 0.99 | 16.71 | 0.052 | 2.25 | 0.71 | 7.14 | 0.17 | 3.16 | 0.53 | 19.05 | 0.21 |
| I | 1.00 | 1.00 | 2.04 | 1.42 | 2.94 | <0.001 | ||||||
| II | 2.26 | 1.63 | 3.15 | <0.001 | 4.43 | 3.35 | 5.86 | <0.001 | 1.03 | 0.81 | 1.29 | 0.84 |
| III | 6.39 | 4.79 | 8.52 | <0.001 | 12.28 | 9.64 | 15.64 | <0.001 | 1.03 | 0.94 | 1.12 | 0.58 |
| IV | 23.33 | 17.19 | 31.68 | <0.001 | 54.77 | 42.63 | 70.38 | <0.001 | 0.85 | 0.73 | 0.98 | 0.027 |
| Lauren classification | ||||||||||||
| Intestinal | 1.00 | 1.00 | 1.68 | 1.31 | 2.15 | <0.001 | ||||||
| Diffuse | 1.93 | 1.54 | 2.42 | <0.001 | 2.09 | 1.66 | 2.62 | <0.001 | 1.54 | 1.25 | 1.90 | <0.001 |
| Mixed | 1.54 | 1.22 | 1.94 | 0.0003 | 1.37 | 1.04 | 1.81 | 0.024 | 1.88 | 1.45 | 2.46 | <0.001 |
| Unknown | 2.76 | 2.35 | 3.24 | <0.001 | 4.45 | 3.64 | 5.44 | <0.001 | 1.05 | 0.97 | 1.13 | 0.22 |
| Stage | ||||||||||||
| Early stage | 1.00 | 1.00 | 2.09 | 1.31 | 3.32 | 0.002 | ||||||
| LAGC | 5.29 | 3.65 | 7.65 | <0.001 | 9.18 | 7.24 | 13.04 | <0.001 | 1.09 | 1.01 | 1.19 | 0.032 |
| Distant | 25.33 | 17.25 | 37.20 | <0.001 | 60.25 | 44.52 | 81.52 | <0.001 | 0.85 | 0.73 | 0.98 | 0.027 |
| Type of gastrectomy | ||||||||||||
| Gastrectomy | 1.00 | 1.00 | 1.24 | 1.15 | 1.34 | <0.001 | ||||||
| No surgery | 3.01 | 2.71 | 3.35 | <0.001 | 4.47 | 4.08 | 4.88 | <0.001 | 0.84 | 0.74 | 0.94 | 0.002 |
| Surgical Margin | ||||||||||||
| Negative | 1.00 | 1.00 | 1.27 | 1.17 | 1.38 | <0.001 | ||||||
| Positive on the proximal margin | 1.96 | 1.34 | 2.87 | 0.0006 | 2.98 | 2.07 | 4.28 | <0.001 | 0.81 | 0.48 | 1.37 | 0.43 |
| Positive on the distal margin | 2.49 | 1.63 | 3.79 | <0.001 | 2.56 | 1.85 | 3.53 | <0.001 | 1.25 | 0.74 | 2.12 | 0.40 |
| Positive on the proximal and distal margin | 1.08 | 0.27 | 4.34 | 0.91 | 2.57 | 1.33 | 4.95 | 0.005 | 0.60 | 0.13 | 2.80 | 0.51 |
| HER2 score | ||||||||||||
| 0 (-) | 1.00 | 1.00 | 1.45 | 1.22 | 1.73 | <0.001 | ||||||
| 1 (+) | 0.70 | 0.57 | 0.86 | 0.001 | 0.85 | 0.72 | 1.02 | 0.073 | 1.19 | 0.98 | 1.46 | 0.083 |
| 2 (++) | 0.69 | 0.53 | 0.89 | 0.005 | 0.66 | 0.51 | 0.85 | 0.002 | 1.54 | 1.11 | 2.12 | 0.01 |
| 3 (+++) | 0.94 | 0.71 | 1.26 | 0.68 | 0.99 | 0.72 | 1.38 | 0.96 | 1.38 | 0.93 | 2.07 | 0.11 |
| Unknown | 1.72 | 1.49 | 1.98 | <0.001 | 2.44 | 2.15 | 2.77 | <0.001 | 1.03 | 0.95 | 1.11 | 0.49 |
| Linitis plastica | ||||||||||||
| No | 1.00 | 1.00 | 1.15 | 1.08 | 1.23 | <0.001 | ||||||
| Yes | 2.44 | 1.44 | 4.12 | 0.001 | 2.37 | 1.63 | 3.44 | <0.001 | 1.22 | 0.64 | 2.33 | 0.54 |
| Borrmann classification | ||||||||||||
| Borrmann I | 1.00 | 1.00 | 1.03 | 0.78 | 1.35 | 0.85 | ||||||
| Borrmann II | 1.03 | 0.85 | 1.24 | 0.79 | 0.93 | 0.73 | 1.17 | 0.52 | 1.14 | 1.00 | 1.30 | 0.045 |
| Borrmann III | 1.14 | 0.94 | 1.38 | 0.20 | 1.13 | 0.90 | 1.43 | 0.30 | 1.03 | 0.90 | 1.17 | 0.71 |
| Borrmann IV | 2.16 | 1.70 | 2.75 | <0.001 | 2.11 | 1.63 | 2.73 | <0.001 | 1.05 | 0.84 | 1.31 | 0.67 |
| Unknown | 2.44 | 1.99 | 3.01 | <0.001 | 3.09 | 2.44 | 3.93 | <0.001 | 0.81 | 0.69 | 0.94 | 0.007 |
| Location | ||||||||||||
| PGC | 1.00 | |||||||||||
| DGC | 0.87 | 0.82 | 0.93 | <0.001 | ||||||||
The univariate analysis found a survival benefit in patients with DGC (HR = 0.87, 95% CI: 0.82-0.93). After stratification by pTNM stage, further comparison between the two groups showed that, compared to patients with DGC, PGC patients had a worse survival outcome in stage I (HR = 2.04, 95% CI: 1.42-2.94) but a better prognosis in stage IV (HR = 0.85, 95% CI: 0.73-0.98). There was no significant survival difference in stages II and III (P=0.84 and 0.58, resp.). However, the multivariate analysis demonstrated that PGC was not an independent predictor for poor survival (HR = 1.07, 95% CI: 1.00-1.14).
When appropriate significant factors were taken into consideration, multivariate analysis (Table 3) revealed that pT4, lymph node metastasis, distant metastasis, no gastrectomy, and Borrmann IV were independent predictors for poor prognosis in both PGC and DGC patients. Additional factors associated with increased mortality in PGC patients included underweight (BMI < 18.5) (HR = 1.29, 95% CI: 1.06-1.58) and linitis plastica (HR = 2.13, 95% CI: 1.25-3.65). Overweight (23 ≤BMI < 27.5) was a prognostic factor associated with favorable survival outcomes only for PGC (HR = 0.80, 95% CI: 0.71-0.90). In DGC group, additional factors for poor prognosis were H. pylori infection (HR = 1.52, 95% CI: 1.11-2.07), diffuse subtype (HR = 1.32, 95% CI: 1.04-1.67), and positive on proximal or distal margin (HR = 1.67, 95% CI: 1.16-2.41; HR = 1.57, 95% CI: 1.13-2.17, resp.). Alcohol drinkers, including current drinkers and ex-drinkers, showed better survival for DGC (HR = 0.90, 95% CI: 0.81-0.99, HR = 0.72, 95% CI: 0.56-0.93, resp.).
Table 3.
Multivariate survival analysis by tumor location.
| Prognostic Factors |
Total (n=11,944) | PGC group (n=4,716) | DGC group (n=7,228) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | ||||
| Lower | Upper | Lower | Upper | Lower | Upper | |||||||
| Alcohol consumption | ||||||||||||
| Never drinkers | 1.00 | 1.00 | 1.00 | |||||||||
| Current drinkers | 0.94 | 0.87 | 1.01 | 0.08 | 0.98 | 0.88 | 1.09 | 0.69 | 0.90 | 0.81 | 0.99 | 0.034 |
| Ex-drinkers | 0.74 | 0.61 | 0.89 | 0.002 | 0.81 | 0.62 | 1.07 | 0.14 | 0.72 | 0.56 | 0.93 | 0.012 |
| BMI (kg/m2) | ||||||||||||
| <18.5 | 1.07 | 0.95 | 1.22 | 0.26 | 1.29 | 1.06 | 1.58 | 0.011 | 0.96 | 0.82 | 1.13 | 0.61 |
| 18.5-22.9 | 1.00 | 1.00 | 1.00 | |||||||||
| 23-27.4 | 0.89 | 0.83 | 0.96 | 0.002 | 0.80 | 0.71 | 0.90 | <0.001 | 0.96 | 0.88 | 1.06 | 0.44 |
| ≥27.5 | 0.91 | 0.82 | 1.01 | 0.09 | 0.94 | 0.80 | 1.09 | 0.41 | 0.87 | 0.75 | 1.01 | 0.07 |
| H. pylori | ||||||||||||
| No | 1.00 | 1.00 | 1.00 | |||||||||
| Yes | 1.43 | 1.13 | 1.81 | 0.003 | 1.35 | 0.94 | 1.94 | 0.11 | 1.52 | 1.11 | 2.07 | 0.009 |
| Unknown | 1.73 | 1.44 | 2.08 | <0.001 | 1.62 | 1.23 | 2.13 | <0.001 | 1.82 | 1.43 | 2.33 | <0.001 |
| Pathologic T stage | ||||||||||||
| T0+Tis | 1.66 | 0.72 | 3.85 | 0.23 | 2.36 | 0.56 | 9.94 | 0.24 | 1.47 | 0.52 | 4.17 | 0.46 |
| T1 | 1.00 | 1.00 | 1.00 | |||||||||
| T2 | 1.53 | 1.05 | 2.24 | 0.028 | 0.82 | 0.41 | 1.62 | 0.57 | 1.89 | 1.19 | 3.00 | 0.007 |
| T3 | 2.65 | 1.86 | 3.76 | <0.001 | 1.47 | 0.79 | 2.73 | 0.22 | 3.20 | 2.08 | 4.91 | <0.001 |
| T4 | 3.28 | 2.32 | 4.64 | <0.001 | 1.99 | 1.08 | 3.69 | 0.028 | 3.77 | 2.47 | 5.75 | <0.001 |
| TX | 2.97 | 2.02 | 4.36 | <0.001 | 1.46 | 0.76 | 2.81 | 0.26 | 3.88 | 2.42 | 6.23 | <0.001 |
| Pathologic N stage | ||||||||||||
| N0 | 1.00 | 1.00 | 1.00 | |||||||||
| N1 | 1.36 | 1.17 | 1.59 | <0.001 | 1.36 | 1.09 | 1.69 | 0.007 | 1.37 | 1.10 | 1.69 | 0.0041 |
| N2 | 1.99 | 1.73 | 2.30 | <0.001 | 1,98 | 1.61 | 2.44 | <0.001 | 2.00 | 1.65 | 2.44 | <0.001 |
| N3 | 3.44 | 3.01 | 3.92 | <0.001 | 3.29 | 2.70 | 4.01 | <0.001 | 3.58 | 2.99 | 4.28 | <0.001 |
| NX | 1.83 | 1.41 | 2.38 | <0.001 | 1.60 | 1.04 | 2.48 | 0.034 | 1.91 | 1.36 | 2.67 | <0.001 |
| Pathologic M stage | ||||||||||||
| M0 | 1.00 | 1.00 | 1.00 | |||||||||
| M1 | 3.05 | 2.59 | 3.59 | <0.001 | 3.65 | 2.75 | 4.86 | <0.001 | 2.84 | 2.32 | 3.48 | <0.001 |
| Lauren classification | ||||||||||||
| Intestinal | 1.00 | 1.00 | 1.00 | |||||||||
| Diffuse | 1.22 | 1.03 | 1.43 | 0.018 | 1.21 | 0.95 | 1.55 | 0.12 | 1.32 | 1.04 | 1.67 | 0.024 |
| Mixed | 0.99 | 0.83 | 1.19 | 0.94 | 1.06 | 0.83 | 1.35 | 0.65 | 0.96 | 0.73 | 1.27 | 0.78 |
| Unknown | 1.30 | 1.11 | 1.52 | <0.001 | 1.20 | 0.97 | 1.49 | 0.095 | 1.44 | 1.14 | 1.82 | 0.003 |
| Type of gastrectomy | ||||||||||||
| Gastrectomy | 1.00 | 1.00 | 1.00 | |||||||||
| No surgery | 1.43 | 1.22 | 1.67 | <0.001 | 1.48 | 1.15 | 1.90 | 0.003 | 1.44 | 1.17 | 1.76 | <0.001 |
| Surgical Margin | ||||||||||||
| Negative | 1.00 | 1.00 | 1.00 | |||||||||
| Positive on the proximal margin | 1.49 | 1.14 | 1.94 | 0.004 | 1.24 | 0.84 | 1.83 | 0.29 | 1.67 | 1.16 | 2.41 | 0.006 |
| Positive on the distal margin | 1.54 | 1.19 | 1.99 | 0.001 | 1.53 | 1.00 | 2.34 | 0.051 | 1.57 | 1.13 | 2.17 | 0.007 |
| Positive on the proximal and distal margin | 0.95 | 0.52 | 1.72 | 0.85 | 0.70 | 0.17 | 2.82 | 0.62 | 1.10 | 0.57 | 2.14 | 0.77 |
| HER2 score | ||||||||||||
| 0 (-) | 1.00 | 1.00 | 1.00 | |||||||||
| 1 (+) | 0.97 | 0.85 | 1.10 | 0.62 | 0.82 | 0.67 | 1.01 | 0.06 | 1.08 | 0.91 | 1.29 | 0.37 |
| 2 (++) | 0.92 | 0.76 | 1.11 | 0.39 | 0.80 | 0.61 | 1.04 | 0.09 | 1.02 | 0.78 | 1.33 | 0.88 |
| 3 (+++) | 1.16 | 0.93 | 1.45 | 0.19 | 1.13 | 0.84 | 1.52 | 0.43 | 1.15 | 0.82 | 1.61 | 0.42 |
| Unknown | 1.33 | 1.17 | 1.50 | <0.001 | 1.19 | 0.98 | 1.45 | 0.07 | 1.44 | 1.23 | 1.70 | <0.001 |
| Linitis plastica | ||||||||||||
| No | 1.00 | 1.00 | 1.00 | |||||||||
| Yes | 1.37 | 1.01 | 1.86 | 0.045 | 2.13 | 1.25 | 3.65 | 0.006 | 1.18 | 0.81 | 1.73 | 0.38 |
| Borrmann classification | ||||||||||||
| Borrmann I | 1.00 | 1.00 | 1.00 | |||||||||
| Borrmann II | 0.92 | 0.80 | 1.07 | 0.30 | 0.87 | 0.72 | 1.06 | 0.17 | 0.99 | 0.78 | 1.25 | 0.93 |
| Borrmann III | 0.98 | 0.84 | 1.14 | 0.75 | 0.90 | 0.74 | 1.10 | 0.29 | 1.06 | 0.83 | 1.35 | 0.62 |
| Borrmann IV | 1.44 | 1.21 | 1.71 | <0.001 | 1.44 | 1.12 | 1.85 | 0.005 | 1.48 | 1.14 | 1.93 | 0.003 |
| Unknown | 1.12 | 0.95 | 1.32 | 0.17 | 1.05 | 0.84 | 1.32 | 0.68 | 1.19 | 0.93 | 1.52 | 0.17 |
| Site | ||||||||||||
| PGC | 1.00 | |||||||||||
| DGC | 0.94 | 0.88 | 1.00 | 0.058 | ||||||||
3.3. Changing Trends of OS and PFS for Patients with PGC and DGC
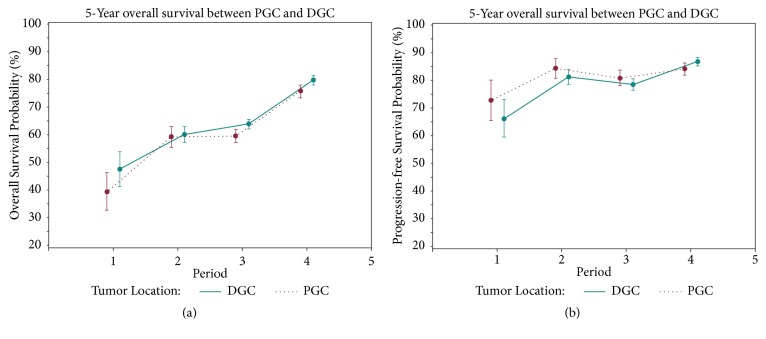
The changing trends of 5-year OS and PFS for PGC and DGC patients were shown in Figure 2(a). The total 5-year OS for GC, PGC, and DGC was 66.5% (95% CI: 65.5%-67.4%), 63.9% (95% CI: 62.4%-65.5%), and 68.1% (95% CI: 66.9%-69.3%), respectively. For total GC, 5-year OS increased from 44.1% (95% CI: 39.4%-48.7%) in period 1 to 78.4% (95% CI: 77.0%-79.7%) in period 4. The 5-year OS of PGC and DGC rose from 39.6% (95% CI: 32.8%-46.4%) to 75.9% (95% CI: 73.6%-78.1%) and from 47.7% (95% CI: 41.4%-54.0%) to 80.0% (95% CI: 78.3%-81.7%) during the 20-year study period, respectively.
Figure 2.
(a) The changing trends of 5-year OS of PGC and DGC from period 1 to period 4. (b) The changing trends of PFS of PGC and DGC from period 1 to period 4.
There was also an increase in PFS of PGC and DGC groups during the 20 years (Figure 2(b)). The total PFS for GC, PGC, and DGC was 82.0% (95% CI: 81.1%-82.9%), 82.3% (95% CI: 80.9%-83.8%), and 81.9% (95% CI: 80.7%-83.0%), respectively. The PFS of PGC and DGC in period 1 was 72.9% (95% CI: 65.5%-80.3%) and 66.2% (95% CI: 59.4%-73.0%), respectively, while the PFS of PGC and DGC in period 4 was 84.2% (95% CI: 82.1%-86.3%) and 86.9% (95% CI: 85.3%-88.4%), respectively.
4. Discussion
In this study, the clinicopathological characteristics of PGC patients presented differently with DGC patients. Although two groups were predominantly males, PGC had a greater proportion of males than DGC. This was similar to some previous reports [13, 20, 21]. Yu et al. [13] reported that the gender ratio (M:F) in PGC was up to 5:1. This may be due to poor diet and unhealthy habits in men, such as smoking or alcohol consumption [22].
In addition, our study demonstrated that PGC presented to be more frequent in older patients as compared to DGC, which was similar to two published Chinese reports [13, 21]. In contrast, Park et al. from Korea [12] had shown that PGC patients were more likely to be younger. Two European studies, however, had reported no association between age and tumor location [10, 17]. These differences may be partly attributed to the genetic distinction from populations of different countries.
A primary finding of our study was that PGC was not independently associated with overall mortality, although it has long been thought to confer worse prognosis [11, 13–17]. In the univariate analysis stratified by stage, PGC patients with stage I had worse survival when compared with DGC patients, while there was no statistical survival difference between the two groups with stages II-III. However, PGC patients with stage IV had better survival than DGC. Therefore, the variations of prognosis between PGC and DGC may be related to various stage distributions existing in different studies. The reason for survival differences between PGC and DGC by stage has stayed unclear to date, and we speculate that those in tumor biology between PGC and DGC play a role.
Interestingly, the multivariate analyses reported that BMI was an independent prognostic factor for PGC patients but not for DGC patients. Moreover, a higher BMI was associated with survival benefits, while a lower BMI was associated with higher mortality, which has not been described previously. Our study also identified that no gastrectomy was an adverse independent predictor for both PGC and DGC patients, suggesting that surgery was necessary to improve survival outcomes for resected GC. Today, systematic D2 lymphadenectomy with the goal of complete (R0) resection is a generally recognized as standard surgical procedure for gastric cancer.
Our study found that 5-year survival increased significantly during the 20 years for total GC, PGC, and DGC, with an increase of 34.3%, 36.3%, and 32.3%, respectively. This was in a concord with the changing trends of increased stages I and II, as well as the decreased lymph node. Relative survival improved steadily over time for gastric cancer, suggesting an improvement in the quality of clinical services for gastric cancer patients, such as improved access to primary healthcare, greater availability of diagnostic facilities, and improved effectiveness of the multimodal treatment [23]. In China, cancer screening and early detection programs (including cancers of the esophagus, stomach, etc.) have expanded to 31 provinces until 2015 [24]. The emerging surgical procedures like endoscopic resection and laparoscopic surgery, as well as standardized procedures, also had played an important role in the prognosis of GC [25–27]. In addition, recent studies showed that the use of individually multimodal therapies had led to an improvement in the 5-year survival rate [27–29].
One limitation of this study was that it was just conducted in a single institution, so the results might not represent the whole Chinese population. However, the volume of PGC and DGC patients was large and the source of patients usually came from the area of Northern and Eastern China, which might serve as a reference for a large population-based study.
In conclusion, PGC significantly differed from DGC in clinicopathological characteristics and prognosis factors. However, there was no significant relationship between survival outcome and gastric tumor location.
Acknowledgments
This study was funded in part by the National Key R&D Program of China (Grant no. 2017YFC0908300).
Contributor Information
Dongbin Zhao, Email: dbzhao2003@sina.com.
Yingtai Chen, Email: yingtai.chen@hotmail.com.
Data Availability
The data used to support the findings of this study are included within the article in Tables 1, 2, and 3.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Authors' Contributions
Lulu Zhao and Huang Huang contributed equally to this work. All authors made substantial contributions to the intellectual content of this paper.
References
- 1.Ferlay J., Soerjomataram I., Dikshit R., et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Blot W. J., Devesa S. S., Kneller R. W., Fraumeni J. F., Jr. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. The Journal of the American Medical Association. 1991;265(10):1287–1289. doi: 10.1001/jama.265.10.1287. [DOI] [PubMed] [Google Scholar]
- 3.Blaser M. J., Saito D. Trends in reported adenocarcinomas of the oesophagus and gastric cardia in Japan. European Journal of Gastroenterology & Hepatology. 2002;14(2):107–113. doi: 10.1097/00042737-200202000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Lee H.-J., Yang H.-K., Ahn Y.-O. Gastric cancer in Korea. Gastric Cancer. 2002;5(3):177–182. doi: 10.1007/s101200200031. [DOI] [PubMed] [Google Scholar]
- 5.Brown L. M., Devesa S. S. Epidemiologic trends in esophageal and gastric cancer in the United States. Surgical Oncology Clinics of North America. 2002;11(2):235–256. doi: 10.1016/S1055-3207(02)00002-9. [DOI] [PubMed] [Google Scholar]
- 6.Kusano C., Gotoda T., Khor C. J., et al. Changing trends in the proportion of adenocarcinoma of the esophagogastric junction in a large tertiary referral center in Japan. Journal of Gastroenterology and Hepatology. 2008;23(11):1662–1665. doi: 10.1111/j.1440-1746.2008.05572.x. [DOI] [PubMed] [Google Scholar]
- 7.Hasegawa S., Yoshikawa T. Adenocarcinoma of the esophagogastric junction: Incidence, characteristics, and treatment strategies. Gastric Cancer. 2010;13(2):63–73. doi: 10.1007/s10120-010-0555-2. [DOI] [PubMed] [Google Scholar]
- 8.Ahn H. S., Lee H.-J., Yoo M.-W., et al. Changes in clinicopathological features and survival after gastrectomy for gastric cancer over a 20-year period. British Journal of Surgery. 2011;98(2):255–260. doi: 10.1002/bjs.7310. [DOI] [PubMed] [Google Scholar]
- 9.Devesa S. S., Blot W. J., Fraumeni J. F., Jr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83(10):2049–2053. doi: 10.1002/(SICI)1097-0142(19981115)83:10<2049::AID-CNCR1>3.3.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 10.Costa L. B. D., Toneto M. G., Moreira L. F. Do proximal and distal gastric tumours behave differently? Brazilian Archives of Digestive Surgery. 2016;29(4):232–235. doi: 10.1590/0102-6720201600040005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maehara Y., Moriguchi S., Kakeji Y., et al. Prognostic factors in adenocarcinoma in the upper one-third of the stomach. PrSurg Gynecol Obstet. 1991;173:223–226. [PubMed] [Google Scholar]
- 12.Park J. C., Lee Y. C., Kim J.-H., et al. Clinicopathological features and prognostic factors of proximal gastric carcinoma in a population with high helicobacter pylori prevalence: A single-center, large-volume study in Korea. Annals of Surgical Oncology. 2010;17(3):829–837. doi: 10.1245/s10434-009-0785-x. [DOI] [PubMed] [Google Scholar]
- 13.Yu X., Hu F., Li C., Yao Q., Zhang H., Xue Y. Clinicopathologic characteristics and prognosis of proximal and distal gastric cancer. OncoTargets and Therapy. 2018;11:1037–1044. doi: 10.2147/OTT.S157378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu K., Zhang W., Chen X., et al. Comparison on Clinicopathological Features and Prognosis between Esophagogastric Junctional Adenocarcinoma (Siewert II/III Types) and Distal Gastric Adenocarcinoma: Retrospective Cohort Study, a Single Institution, High Volume Experience in China. Medicine (United States) 2015;94(34):p. e1386. doi: 10.1097/MD.0000000000001386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pacelli F., Papa V., Caprino P., Sgadari A., Bossola M., Doglietto G. B. Proximal compared with distal gastric cancer: Multivariate analysis of prognostic factors. The American Surgeon. 2001;67(7):697–703. [PubMed] [Google Scholar]
- 16.Pinto-de-Sousa J., David L., Seixas M., Pimenta A. Clinicopathologic profiles and prognosis of gastric carcinomas from the cardia, fundus/body and antrum. Digestive Surgery. 2001;18(2):102–110. doi: 10.1159/000050109. [DOI] [PubMed] [Google Scholar]
- 17.Piso P., Werner U., Lang H., Mirena P., Klempnauer J. Proximal versus distal gastric carcinoma - What are the differences? Annals of Surgical Oncology. 2000;7(7):520–525. doi: 10.1007/s10434-000-0520-0. [DOI] [PubMed] [Google Scholar]
- 18.Siewert J. R., Böttcher K., Stein H. J., Roder J. D., Busch R. Problem of proximal third gastric carcinoma. World Journal of Surgery. 1995;19(4):523–531. doi: 10.1007/BF00294713. [DOI] [PubMed] [Google Scholar]
- 19.Higuchi K., Koizumi W., Tanabe S., Saigenji K., Ajani J. A. Chemotherapy is more active against proximal than distal gastric carcinoma. Oncology. 2004;66(4):269–274. doi: 10.1159/000078326. [DOI] [PubMed] [Google Scholar]
- 20.Norouzinia M., Asadzadeh H., Shalmani H. M., Dulaimi D. A., Zali M. R. Clinical and histological indicators of proximal and distal gastric cancer in eight provinces of Iran. Asian Pacific Journal of Cancer Prevention. 2012;13(11):5677–5679. doi: 10.7314/APJCP.2012.13.11.5677. [DOI] [PubMed] [Google Scholar]
- 21.Huang Q., Fang C., Shi J., et al. Differences in clinicopathology of early gastric carcinoma between proximal and distal location in 438 chinese patients. Scientific Reports. 2015;5(1) doi: 10.1038/srep13439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalish R. J., Clancy P. E., Orringer M. B., Appelman H. D. Clinical, Epidemiologic and Morphologic Comparison Between Adenocarcinomas Arising in Barrett's Esophageal Mucosa and in the Gastric Cardia. Gastroenterology. 1984;86(3):461–467. doi: 10.1016/S0016-5085(84)80016-5. [DOI] [PubMed] [Google Scholar]
- 23.Zeng H., Chen W., Zheng R., et al. Changing cancer survival in China during 2003–15: a pooled analysis of 17 population-based cancer registries. The Lancet Global Health. 2018;6(5):e555–e567. doi: 10.1016/S2214-109X(18)30127-X. [DOI] [PubMed] [Google Scholar]
- 24.Zou X.-N. Epidemic trend, screening, and early detection and treatment of cancer in Chinese population. Cancer Biology & Medicine. 2017;14(1):50–59. doi: 10.20892/j.issn.2095-3941.2016.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim Y.-W., Baik Y. H., Yun Y. H., et al. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: Results of a prospective randomized clinical trial. Annals of Surgery. 2008;248(5):721–727. doi: 10.1097/SLA.0b013e318185e62e. [DOI] [PubMed] [Google Scholar]
- 26.Stotland P. K., Chia S., Cyriac J., Hagen J. A., Klein L. V. Safe implementation of laparoscopic gastrectomy in a community-based general surgery practice. Surgical Endoscopy. 2009;23(2):356–362. doi: 10.1007/s00464-008-9941-9. [DOI] [PubMed] [Google Scholar]
- 27.Chon S., Berlth F., Plum P. S., et al. Gastric cancer treatment in the world: Germany. Translational Gastroenterology and Hepatology. 2017;2(5):53–53. doi: 10.21037/tgh.2017.05.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song Z., Wu Y., Yang J., Yang D., Fang X. Progress in the treatment of advanced gastric cancer. Tumor Biology. 2017;39(7) doi: 10.1177/1010428317714626.101042831771462 [DOI] [PubMed] [Google Scholar]
- 29.Giampieri R., Del Prete M., Cantini L., et al. Optimal management of resected gastric cancer. Cancer Management and Research. 2018;10:1605–1618. doi: 10.2147/CMAR.S151552. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article in Tables 1, 2, and 3.