Abstract
Behavioral flexibility provides an individual with the ability to adapt its behavior in response to environmental changes. Studies on mammals, birds, and teleosts indicate greater behavioral flexibility in females. Conversely, males appear to exhibit greater behavioral persistence. We, therefore, investigated sex differences in behavioral flexibility in 2 closely related molly species (Poecilia latipinna, P. mexicana) and their more distant relative, the guppy P. reticulata by comparing male and female individuals in a serial, visual reversal learning task. Fish were first trained in color discrimination, which was quickly learned by all females (guppies and mollies) and all molly males alike. Despite continued training over more than 72 sessions, male guppies did not learn the general test procedure and were, therefore, excluded from further testing. Once the reward contingency was reversed serially, molly males of both species performed considerably better by inhibiting their previous response and reached the learning criterion significantly faster than their respective conspecific females. Moreover, Atlantic molly males clearly outperformed all other individuals (males and females) and some of them even reached the level of 1-trial learning. Thus, the apparently universal pattern of higher female behavioral flexibility seems to be inverted in the 2 examined molly species, although the evolutionary account of this pattern remains highly speculative. These findings were complemented by the observed lower neophobia of female sailfin mollies compared with their male conspecifics. This sex difference was not observed in Atlantic mollies that were observed to be significantly less distressed in a novel situation than their consexuals. Hypothetically, sex differences in behavioral flexibility can possibly be explained in terms of the different roles that males and females play in mating competition, mate choice, and reproduction or, more generally, in complex social interactions. Each of these characteristics clearly differed between the closely related mollies and the more distantly related guppies.
Keywords: behavioural and cognitive flexibility; colour discrimination; neophobia; serial reversal learning; Poecilia mexicana; Poecilia latipinna, Poecilia reticulata
The capability to learn provides an individual with the behavioral flexibility pivotal to living in a variable environment (Dodson 1988). In this context, “behavioral flexibility” is the ability to adapt one’s behavior to better cope with changing environmental contingencies or sometimes erratic occurrence of resources (Bond et al. 2007). It requires an individual to alter or to persist no longer viable strategies rapidly and to develop new ones to attain new associations when environmental demands change (Moore and Malinowski 2009; Rayburn-Reeves et al. 2017a, 2017b). Accordingly, adaptive behavior may change to varying degrees, ranging from changes that are slightly more than reflexes or tropic responses (i.e., to reflect a change in environmental conditions, but without the engagement of cognitive processes) to behavioral modifications that are attributable to (predictable) environmental changes. Thus, behavioral flexibility echoes a modification of an individual’s cognitive state regarding perceived environmental contingencies (Brown and Tait 2015). Hence, determining an individual’s behavioral flexibility allows to indirectly examine its level of “cognitive flexibility”, which is the ability to switch attention between different tasks (e.g., in response to an alteration of rules or demands; Scott 1962). Cognitive processes underlying cognitive flexibility can be evaluated in terms of associative learning (learning to associate a specific reward/punishment with an outcome), reversal learning (incorporating new information to improve decision-making), and innovativeness or learning flexibility in solving problems or coping with challenges (Cummings 2018).
Selective pressures can cause sex differences in cognition. The association between an individual’s cognitive abilities, its behavioral flexibility, and other behavioral traits (Thornton and Lukas 2012; Lucon-Xiccato and Bisazza 2017), as well as the relevance of these properties in the light of mate choice (Řežucha and Reichard 2016; White 2017) is as complex as it is critical for understanding how cognitive differences between individuals, sex, or species arise. Other aspects such as the interaction of different genotypes or different genetic bases additionally contribute to these associations. These can be found in vertebrates across different taxa, from fish to humans. Generally, females show greater cognitive flexibility and lower persistence than males in a number of polygamous mammals and birds (e.g., Ha et al. 2011; Roelofs et al. 2017). In fish, the aspect of behavioral flexibility received particular attention through experiments on guppies in the context of several different tasks. Female guppies Poecilia reticulata displayed greater behavioral flexibility than males: compared with their male conspecifics female guppies appeared to be more innovative and interested in problem solving when given a novel foraging task involving spatial exploration (Laland and Reader 1999). Likewise, female guppies solved a learning flexibility task faster than males (Lucon-Xiccato and Bisazza 2017). In this study, individuals had to move around a transparent barrier (i.e., gain distance to the goal represented by shoaling partners; detour reaching) before successfully approaching the goal. These results appear to support previous results indicating that female guppies were faster at color discrimination reversal learning than male guppies (Lucon-Xiccato and Bisazza 2014). However, male guppies outperformed females in learning a complex spatial task (Lucon-Xiccato and Bisazza 2017) and made decisions faster (though not more correct) than females in visual color discrimination learning (Lucon-Xiccato and Bisazza 2016). Likewise, female guppies were observed to outperform males in a spatial orientation task requiring them to learn to select the correct arm of a T-maze to rejoin a group of conspecifics and in a numerical task requiring them to discriminate between 5 and 10 dots to obtain a food reward (Petrazzini et al. 2017). However, although female guppies appeared to exhibit a generally higher cognitive flexibility than males, the extent of this sex difference seemed to depend on the task to be solved. A recent study examined western mosquitofish Gambusia affinis regarding the association between independent traits of activity, exploration, anxiety, and sociability and the individual’s associative learning performance in numerical discrimination experiments (Etheredge et al. 2018). The authors concluded that despite the convergence of their learning performance, sexes differ in their cognitive-behavioral responses that could possibly be attributed to different sexual selection pressures (Etheredge et al. 2018).
The behavioral flexibility and the ability to learn more than simple associations in response to 2 stimuli can be investigated by reversal learning experiments, in which a particular response must be replaced due to changes in reward contingencies (e.g., Jones and Mishkin 1972; Rolls 2000). Accordingly, the individual is initially trained to choose a predetermined stimulus from 2 alternative options to attain a reward. On learning the association, the reward contingency is reversed and speed in acquiring the new association is interpreted an indicator of flexibility (Shettleworth 2010). The ability to perform serial reversal learning, where animals gradually improve their performance in view of repeatedly changing reward contingencies (e.g., Roth and Dicke 2005; Raine and Chittka 2012) has been frequently found in animals living in complex physical and/or social environments (e.g., Godfrey-Smith 2002; Bond et al. 2007). Visual reversal learning of colors, shapes, or contours has been studied in numerous vertebrate species across many different taxonomic groups, for instance mammals (e.g., Warren 1966; Bissonette and Powell 2012), birds (Boogert et al. 2010, Caves et al. 2018), reptiles (e.g., Leal and Powell 2012), amphibians (e.g., Liu et al. 2016), and fish (e.g., Parker et al. 2012; Fuss et al. 2014; Lucon-Xiccato and Bisazza 2014). Even invertebrates have already been successfully challenged with visual reversal tasks (e.g., Liedtke and Schneider 2014; Bublitz et al. 2017; Maharaj et al. 2018).
Inspired by the study of Lucon-Xiccato and Bisazza (2014, 2016), in which females were observed to have a greater behavioral flexibility and appeared to be more apt to adapt their responses to new situations in captive-bred pet shop guppies, we conducted this comparative study on reversal learning on 3 poeciliid species to further investigate possible sex differences. This study is one of the first comparative studies dealing with behavioral flexibility in the context of (cognitive) sex-specific differences in 3 related fish species. We aimed to investigate sex differences with regard to the ability to exploit previously gained knowledge using a simple color discrimination paradigm and, subsequently, the behavioral flexibility using serial reversal learnings of 2 closely related molly species (P. latipinna, P. mexicana) and their more distant relative, the guppy P. reticulata. We expected sex differences regarding the learning speed during the initial color discrimination task as well as their behavioral flexibility in serial reversals in all 3 species. We expected females of all 3 species to exhibit a higher behavioral flexibility compared with their conspecific males during serial reversals.
Materials and Methods
Animals and housing facilities
All species trained in the present experiments belong to the family Poeciliidae (Meffe and Snelson 1989), which are livebearers without parental care and live in mixed-sex shoals year round. The guppies P. reticulata (30 males, 15 females) were wild type mature descen-dants from wild-caught individuals from a population from Trinidad, caught by the University of Bielefeld in the 1980s. The sailfin mollies P. latipinna (15 males, 16 females) were also sexually mature descendants of wild-caught fish in 2007. The Atlantic mollies P. mexicana (16 males, 18 females) used in our experiments were sexually mature adult descendants from a population from Tampico, Mexico, caught in 1995.
All individuals were housed in groups of ∼45 individuals separated by species. They were kept in several large aquaria (80 cm × 50 cm × 40 cm) filled with aerated, filtered water (conductance: about 250 µs/cm) at 24°C ± 2°C under a 14:10 h light:dark cycle, providing constant environmental conditions (conductivity, temperature, and pH). The aquaria were equipped with gravel substrates, plants, and several hiding places. All experiments were conducted during the day. Food (JBL NovoBel flake food, thawed red mosquito larvae or brine shrimps) was obtainable 5 days per week (i.e., on 3 days only during experiments, on 2 other days ad libitum). To enhance motivation, no food was available on Saturdays and Sundays. On commencement of the experiments, experimental fish were separated in mixed-sex groups of 5–8 individuals in smaller tanks (40 cm × 40 cm × 25 cm); they were identified based on phenotypic characteristics.
Experimental setups
For experimental trials, a rectangular tank (44 cm × 33 cm × 30 cm) made of opaque white plastic walls was used to prevent unintentional cueing or potentially disturbing external influences. The floor was covered with a dark Perspex acrylic sheet to reduce stress levels. During all experiments, the tank was filled with water (T = 24°C ± 2°C) to a depth of about 10 cm. Ceiling mounted fluorescent tubes (18 W) including ultraviolet (UV) light spectrum provided an even illumination during all experiments. The experimental tank contained a plastic hole board (12.5 cm × 8 cm × 1.5 cm; a 24-well plate, Stemcell Technologies™) perforated with 24 equidistant holes (1.5 cm in diameter). It was placed horizontally along the front side. To counteract the hole-board’s buoyancy by weighing it down, each hole closest to its corners was filled with small light-colored gravel stones. In each trial, 2 holes concealing food (thawed red mosquito larvae or brine shrimps) were covered with colored plastic chips with 2 cm in diameter (weight: 0.643 g) from “Tiddleywinks”, Noris-Spiele (Georg Reulein GmbH & Co. KG, Fürth, Germany). A Plexiglas cylinder (11 cm in diameter with 1 transparent half and one half laminated with light gray self-adhesive foil; hereafter referred to as “starting cylinder”) served as a starting compartment and was placed about 25 cm ahead of the hole-board (Figure 1).
Figure 1.
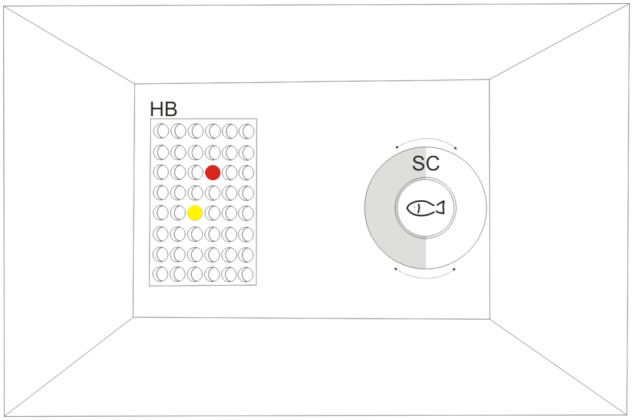
Experimental setup used for color discrimination tests. Prior to each trial, the individual was placed in the starting cylinder before it was allowed to approach and dislodge the colored plastic chips. SC, starting cylinder featuring an opaque, light grey and a transparent side; HB, hole-board with 24 equi-spaced holes placed horizontally along the front side. In each trial, 2 holes concealing food were covered with colored plastic chips (red or yellow in guppies, green and yellow in mollies).
Experimental training
Individuals were given 2 sessions per day on 3 consecutive days per week (i.e., Mondays to Wednesdays). Each training session consisted of 12 trials. The behavioral training consisted of 4 phases: 1) acclimatization, 2) pretraining and training, 3) reversal learning, and 4) controls to examine unintentional cueing.
Acclimatization
Poeciliidae naturally live in larger groups. Therefore, and to facilitate their acclimatization, 4–5 individuals were introduced simultaneously to the handling procedure and the experimental setup. Initially, they were gently shooed into a small water-filled beaker and transferred from the small tank into the experimental setup prior to experiments (Figure 1). Individuals were then placed in the starting cylinder (opaque side turned in the direction to the hole-board to block the fish’s view) for allowing them to settle in and habituate to the handling procedure for a period of 6 min. To introduce and familiarize individuals with the regular starting procedure, the starting cylinder was first turned to allow the fish to observe the hole-board and, subsequently, removed from the experimental setup after another 30 s. Then, each fish was allowed to explore the experimental setup for up to 20 min at a time and to search for food (thawed red mosquito larvae or brine shrimps) concealed in freely accessible holes without any colored chips around. When they did so, acclimatization period was finished and the pretraining phase commenced.
Pretraining
Initially, 4–5 individuals were trained simultaneously to dislodge the chips laying on the hole-board and partly covering the holes by shoving them away with their snout during pretraining. Individuals were placed in the starting cylinder (opaque side turned in the direction to the hole-board to block the fish’s view) allowing them to settle in and habituate to the handling procedure for a period of 5 min. Then, the starting cylinder was turned, allowing the individuals to observe the hole-board for 30 s before they could approach the chips. To avoid any kind of conditioning prior to the actual color discrimination training, 4 chips (blue, green, red, and yellow, for color spectra compare Appendix Figure A1) were used simultaneously in this phase. All chips (regardless of color) were placed on the hole-board such as to leave a gap, thereby concealing food only partially. The gap was reduced as trials progressed. Each pretraining session consisted of 12 trials. Once an individual swam freely throughout the experimental setup and dislodged the chips with the snout to look for food, initial color discrimination training commenced for this individual. Following the last pretraining session, the standard body length of each fish (from the tip of its snout to the end of its caudal peduncle) was recorded.
Initial color discrimination training
To test sex differences to exploit previously gained knowledge, experiments were conducted as 2-alternative forced choice experiments, that is, only 2 chips were used during training. Each training session consisted of 12 trials. During the initial color discrimination training, the reversals and during all controls, guppies were always presented with a red and a yellow chip. These colors were selected in accordance with a previous study on pet shop guppies performed by Lucon-Xiccato and Bisazza (2014) to facilitate comparability. However, all mollies clearly avoided the red chip and visited it much less frequently than the other chips during pretraining. To avoid a (sensory or aversive) bias, the red chip was thence replaced by a green one. Accordingly, during the initial color discrimination training, the reversals and during all controls, mollies were presented with a green and a yellow chip. All other training parameters remained the same. The rewarded color was counterbalanced across all individuals of a species (random group assignment). At this point, it is important to note that this study was not about recognizing colors (i.e., red as red, green as green, and yellow as yellow). An individual was supposed to simply recognize 2 different colored chips and to learn to distinguish a positive (rewarded) from a negative (unrewarded) stimulus, followed by several reversal tasks.
On commencement of the initial color discrimination training, each fish was trained individually. Before each trial, an individual was placed in the starting cylinder. To prevent the individual from observing the chips being moved or food being inserted into a particular hole, the opaque side of the cylinder was turned in the direction of the hole-board (i.e., blocking the fish’s view). Both chips (red and yellow in female guppies, green and yellow in mollies) were positioned in a predefined pseudorandom order on the hole-board. A small piece of food was hidden under a chip defined as the positive (rewarded) stimulus. To start a trial, the starting cylinder was turned, allowing the individual to observe the hole-board for 30 s before it could approach the chips. Subsequently, the starting cylinder was removed and the individual could choose and dislodge a chip to find food. Although only first choices were analyzed for every trial, the individual was allowed to correct itself in case of an incorrect choice within 30 s to promote learning (Lucon-Xiccato and Bisazza 2014). Subsequently, the fish was gently guided back into the starting cylinder using a transparent plastic rod for guidance. Learning was assumed successful as soon as a learning criterion of 75% correct responses within 12 consecutive trials was reached. This comparatively weak learning criterion was established to avoid overtraining in view of the successive serial reversal learning (Warren 1960; Lucon-Xiccato and Bisazza 2014). As soon as an individual reached the learning criterion, the next phase commenced. If an individual did not reach the learning criterion within 10 training sessions (i.e., 120 trials), it was excluded from further training.
Reversal learning
Following successful learning, a series of 4 reversals was performed to evaluate 1) behavioral flexibility and 2) potential sex-specific differences in the 3 species under investigation. The general training procedure (cf. section “Initial color discrimination training”) remained the same. Each reversal learning session consisted of 12 trials. Learning was assumed successful as soon as a learning criterion of 75% correct responses in 12 consecutive trials was reached. Each time an individual reached the learning criterion, the contingency of reinforcement was reversed between the 2 colors. This procedure was continued until 4 reversals were completed.
Controls to examine unintentional cueing
In this study, the food reward was hidden beneath the chips in the hole-board and may have been detected olfactorily by the fish. Furthermore, individuals could have relied on other external cues (e.g., cues on the chips themselves, on the hole-board, or cues given inadvertently by the experimenter). Therefore, 3 different controls (C1–C3) were performed following the reversal learning period. In C1 trials, there were 2 chips of the same, previously rewarded color with one hiding food (random choice). If individuals had used olfactory cues, they would have preferred the chip covering food. Likewise, both of the 2 colored chips (C2) or neither of them (C3) concealed food in the hole-board in additional control trials. If individuals had observed these cues for guidance, they would prefer a certain chip or a certain area of the hole-board. All 3 controls were presented at the same frequency (i.e., 12 trials each for each individual), but in random order. Ten individuals per sex and species (i.e., 30 females, 20 males; male guppies did not participate), which had performed successfully during initial training and reversals, were presented with these 3 different controls, each following the same procedure as described earlier.
Data analysis
Data analysis was only performed on successful individuals per sex and species. Those individuals, who were unsuccessful for any reason during the pretraining or initial color discrimination training, were excluded from all further statistical analyses. For every individual, first choices, the number of errors to criterion (based on the first choices per trial) and right and left choices were analyzed (and are reported as mean ± SD). To compare the number of errors to criterion made during 1) the initial color discrimination training and the 1st reversal and 2) the 1st and 4th reversal, a one-way analysis of variance (ANOVA) for repeated measures was used per sex for each species. An analysis of covariance (ANCOVA) for 2 independent samples (sex and reinforced color as factors and body length as a covariate) was used to examine 1) potential sex differences in the number of errors to criterion during the initial color discrimination training (for both molly species) and 2) potential sex differences during serial reversal learning. The latency until an individual’s first decision during the first initial training trial was recorded as a “behavioral trait” to be used as a predictor variable in the learning analysis. It hence served as a proxy of an individual’s neophobia. The shorter its latency, the higher an individuals’ motivation to explore a new situation and the lower its neophobia was considered to be. To evaluate potential sex differences, a 2-tailed Mann–Whitney U-Test (MWU) was performed for both molly species. To assess neophobia, the respective latencies of the females of all 3 species were compared using a Kruskal–Wallis test, those of the males of both molly species using a 2-tailed MWU. Binomial tests were performed to analyze the results of the controls C1–C3. In addition, the Holm–Bonferroni method was applied to correct the level of significance for multiple comparisons to Palpha ≤ 0.01.
Ethical statement
The research reported herein was performed under the guidelines established by the EU Directive 2010/63/EU for animal experiments and the current German animal protection law and had been approved by the Landesamt für Natur, Umwelt und Verbraucherschutz NRW (approval number 53.6 55-05).
Results
Initial color discrimination training
Poecilia reticulata
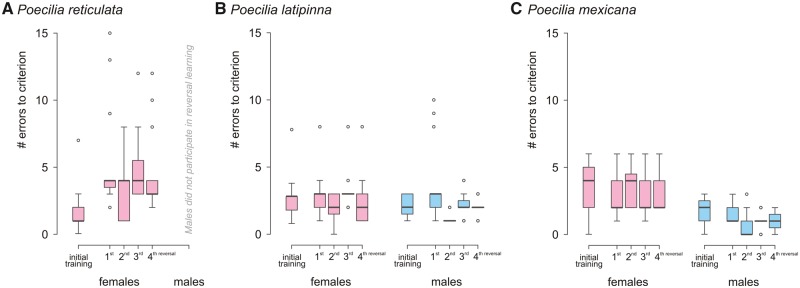
Fifteen female and 30 male guppies P. reticulata (SL: females: 25.7 ± 7.6 mm, males: 16.9 ± 3.3 mm) participated in the pretraining and/or training procedure. The initial training task was successfully learned by all 15 female guppies within 12.8 ± 2.99 trials (of a maximum of 120 trials) with an average of 1.6 ± 1.66 errors to criterion (Figure 2A). Conversely, none of the males was able to pass the pretraining phase. All males got used to the handling and starting procedure and learned to search for food in the uncovered and partly covered holes concealing food. They were pushing the chips aside inadvertently, thereby proving their physical ability to solve the task. However, they did not learn to deliberately shove the chips aside to access the hidden food as soon as the chips completely covered the holes. Therefore, males’ pretraining was terminated after 3 months (i.e., 72 pretraining sessions, equaling 864 pretraining trials).
Figure 2.
Box plots of number of errors to criterion. During the initial color discrimination and during the reversals 1–4 for (A)P. reticulata females and males (15 females and 30 males), (B)P. latipinna females and males (15 females and 15 males) and (C)P. mexicana females and males (15 females and 15 males). Median, 1st and 3rd quartile are displayed; outliers are indicated as circles. Female results are colored light pink, male results are colored light blue.
Poecilia latipinna
Fifteen female and 15 male sailfin mollies P. latipinna (SL: females: 43.2 ± 6.05 mm, males: 46.2 ± 8.78 mm) successfully finished the whole training procedure (i.e., pretraining, initial training, and reversals). A 16th female showed severe motivational deficits to the point of refusal to participate during the 2nd reversal and was therefore excluded from all following statistical analyses. The initial training task was successfully learned by all female sailfin mollies within 12.8 ± 2.99 trials with an average of 2.7 ± 1.57 errors to criterion. The task was learned by all male sailfin mollies within 12 ± 0 trials with an average of 2.2 ± 0.84 errors to criterion (Figure 2B). We detected no sex difference in color discrimination learning in sailfin mollies (ANCOVA: F1, 30 = 0.45, P = 0.508).
Poecilia mexicana
Fifteen female and 15 male Atlantic mollies P. mexicana (SL: females: 39 ± 2.44 mm; males: 42.1 ± 2.72 mm) successfully finished the training procedure. Three additional females and 1 additional male showed severe motivational deficits to the point of refusal to participate during the 1st or 2nd reversal and were therefore excluded from all following statistical analyses. The initial training task was successfully learned by all female Atlantic mollies within 16 ± 5.66 trials with an average of 3.6 ± 1.78 errors to criterion. The task was learned by all male Atlantic mollies within 12 ± 0 trials with an average of 1.8 ± 0.91 errors to criterion (Figure 2C). Male Atlantic mollies learned the initial training task significantly faster than females (ANCOVA: F1, 30 = 13.72, P < 0.001).
Latency as an indicator of neophobia
As an indicator of an individual’s neophobia, we determined the latency until its first decision to shove a chip in the first initial training trial. The shorter its latency, the higher an individual's motivation to explore a new situation and the lower its neophobia was considered to be. The guppy females’ average latency was 20.49 ± 9.88 s. Although the sailfin molly females’ average latency was 24.35 ± 15.88 s, it was on average 32.27 ± 12.18 s for their conspecific males (MWU: U = 63, z = –2.032, P2-tailed = 0.042). Hence, sailfin molly females were observed to be less neophobic than males in this experimental setup. Conversely, the Atlantic molly females’ average latency was 13.18 ± 4.47 s, whereas it was 11.68 ± 2.35 s for their conspecific males (MWU: U = 97.5, z = 0.601, P2-tailed = 0.548).
Atlantic molly females performed significantly faster than guppy or sailfin molly females (Kruskal–Wallis test: H1, 30 = 9.891, P = 0.007). Hence, Atlantic molly females were observed to be the less neophobic of all females tested in this experimental setup. Likewise, Atlantic molly males were observed to perform significantly faster than male sailfin mollies (MWU: U = 9, z = 4.272, P2-tailed < 0.001). Therefore, they were judged to be significantly less neophobic than male sailfin mollies.
Reversal learning
Poecilia reticulata
In the 1st reversal, guppy females made on average 5.3 ± 3.72 errors to reach the learning criterion. Subsequently, during serial reversal learning the number of errors continuously decreased to 3 ± 1.89 errors (Figure 2A). In the 1st reversal, female guppies made significantly more errors compared with initial training (ANOVA: F1, 15 = 12.048, P = 0.004). Although the absolute number of errors decreased, this effect was not significant (ANOVA: F1, 15 = 3.721, P = 0.075).
Poecilia latipinna
In the 1st reversal, sailfin molly females made on average 2.9 ± 1.59 errors to reach the learning criterion, whereas males made on average 3.6 ± 2.82 errors (Figure 2B). During the following reversals, the number of errors continuously decreased to 2.1 ± 0.93 errors in females, but no significant differences in the number of errors to criterion were observed neither between the initial training and the 1st reversal (ANOVA: F1, 15 = 0.049, P = 0.828), nor between the 1st and the 4th reversal (ANOVA: F1, 15 = 3.027, P = 0.104). In the 1st reversal, sailfin molly males made on average 3.6 ± 2.82 errors (Figure 2B). During the following reversals, the number of errors continuously decreased to 1.2 ± 0.40 errors but no significant differences in the number of errors to criterion were observed between the initial training and the 1st reversal in male sailfin mollies (ANOVA: F1, 15 = 3.442, P = 0.085). However, the number of errors to criterion significantly decreased until the 4th reversal (ANOVA: F1, 15 = 10.839, P = 0.005). The ANCOVA revealed no significant effects of sex (F1, 30 = 0.4, P = 0.533), body length (F1, 30 = 3.31, P = 0.079), or interaction (F1, 30 = 1.77, P = 0.076).
Poecilia mexicana
In the 1st reversal, Atlantic molly females made on average 3 ± 1.46 errors to reach the learning criterion (Figure 2C). During the following reversals, the number of errors slightly increased to 3.4 ± 1.40 errors in females, but no significant differences in the number of errors to criterion were observed neither between the initial training and the 1st reversal (ANOVA: F1, 15 = 0.860, P = 0.369), nor between the 1st and the 4th reversal (ANOVA: F1, 15 = 0.706, P = 0.415). In the 1st reversal, Atlantic molly males made on average 1.4 ± 0.62 errors (Figure 2C). During the following reversals, the number of errors decreased to 0.6 ± 0.88 errors. While no significant difference in the number of errors to criterion was observed between the initial training and the 1st reversal in male Atlantic mollies (ANOVA: F1, 15 = 1.0, P = 0.335), they significantly improved their performance until the 4th reversal and made fewer errors (ANOVA: F1, 15 = 8.895, P = 0.009). The ANCOVA revealed a significant effect of sex (F1, 30 = 46.29, P < 0.001), but no effect of body length (F1, 30 = 0.01, P = 0.921), or interaction (F1, 30 = 0.49, P = 0.619).
Controls to examine unintentional cueing
Three different controls were performed to examine unintentional cueing, which could have influenced the fish’s choice. Fifty individuals (30 females, 20 males) participated in each of the C1–C3 controls.
In C1 trials, there were 2 chips of the same, previously rewarded color with only 1 hiding food (random choice). If individuals had used olfactory cues, they would have preferred the chip covering food. However, this chip was not preferred over the chip covering no food, neither by a particular sex (2-tailed binomial test: P. reticulata females: P = 0.315; P. latipinna females: P = 0.523; P. latipinna males: P = 0.784; P. mexicana females: P = 0.073; P. mexicana males: P = 0.411) nor by any of the 3 species (2-tailed binomial test: P. reticulata: P = 0.315; P. latipinna: P = 0.478; P. mexicana: P = 0.561). While 35 individuals chose indifferently between both alternatives (18 females, 17 males), 15 individuals (12 females, 3 males) developed side biases and predominantly chose a particular side (i.e., left or right).
Furthermore, individuals could have relied on other external cues (e.g., cues on the chips themselves, on the hole-board, or cues given inadvertently by the experimenter). In this case, they would prefer a certain chip or a certain area of the hole-board. However, individuals preferred neither of the 2 chips (both concealing food) in the C2 control trials regardless of sex (2-tailed binomial test: P. reticulata females: P = 0.073; P. latipinna females: P = 0.171; P. latipinna males: P = 0.083; P. mexicana females: P = 0.648; P. mexicana males: P = 0.927) or species (2-tailed binomial test: P. latipinna: P = 0.333; P. mexicana: P = 0.651). While 42 individuals chose indifferently between both alternatives (23 females, 19 males), 8 individuals (7 females, 1 male) developed side biases and predominantly chose a particular side (i.e., left or right). Likewise, individuals preferred neither of the 2 chips (none of them concealing food) in the C3 control trials regardless of sex (2-tailed binomial test: P. reticulata females: P = 0.648; P. latipinna females: P = 0.120; P. latipinna males: P = 0.523; P. mexicana females: P = 0.784; P. mexicana males: P = 0.784) or species (2-tailed binomial test: P. latipinna: P = 0.106; P. mexicana: P = 0.081). While 38 individuals chose indifferently between both alternatives (24 females, 14 males), 12 individuals (6 females, 6 males) developed side biases and predomi-nantly chose a particular side (i.e., left or right).
In all 3 controls, we found no indication that olfactory or other unintentional cues would have guided the fish in its choosing of any chip. None of the 3 species showed 1) a significant bias for right or left or 2) significant sex differences. Nevertheless, distinct individual differences were observed regardless of sex and species.
Discussion
In this study, we investigated sex differences in behavioral flexibility by using serial learning in 3 poeciliid species. While no sex differences were observed in sailfin mollies, male Atlantic mollies learned to solve the initial color discrimination task significantly faster than their conspecific females. Surprisingly and contrasting our expectations of a reflection of the results of a previous study on guppies P. reticulata (Lucon-Xiccato and Bisazza 2014), only females solved the initial task in our study. Their conspecific males did not even succeed in pretraining (i.e., learning to deliberately push chips aside to uncover food). Moreover, mollies and female guppies were well able to respond to the changing reward contingencies in serial reversals by improving their response with experience as shown by the reduction in errors with repeated reversals. Regarding the expected sex differences in accuracy and behavioral flexibility, we observed different results for the 3 species under investigation. Compared with previous studies or other vertebrate taxa, the hitherto apparently universal pattern (i.e., females showing higher behavioral flexibility) seems to be inverted in the 2 examined molly species. Due to the guppy males’ poor performance, we unfortunately cannot deduce conclusions on sex differences in guppies.
Sex differences within the 3 species
In a previous study by Lucon-Xiccato and Bisazza (2014), guppies of both sexes were described to learn the initial training task within comparable periods of time (i.e., females: 0.86 ± 1.35, males: 0.50 ± 0.91 errors to criterion; Lucon-Xiccato and Bisazza 2014, Supplementary material). However, in our study, only guppy females were able to match these results. Although their error rate increased significantly between their initial training to the 1st reversal, they were able to improve their performance until the 4th reversal. Although all males grew accustomed to the experimental procedure, learned to search for food in the uncovered or partly covered holes containing food and, inadvertently, pushed the chips aside during pretraining, they did not learn to shove the chips aside deliberately as soon as the chips completely covered the holes despite experience from 72 pretraining sessions. Therefore, we assume a lack of association learning (i.e., linking “shoving the chip aside” with “finding food”) that does not reflect a lack of males’ physical size or strength. As long as guppy males had the opportunity to see the food, the association seemingly led to success, but as soon as the food became invisible underneath the chips, the association seemingly deteriorated.
Male sailfin mollies showed a learning progress comparable with that of guppy females during initial training and the 1st reversal, but the errors made to succeed in the following reversals (i.e., reversals 2–4) decreased considerably faster compared with female guppies. A different pattern of learning was observed in female sailfin mollies. Although they made more errors to criterion in the initial training task compared with female guppies, they made considerably less errors in the 1st reversal. However, they did not improve their performance in the 2nd reversal. Subsequently, the number of errors gradually decreased in consecutive reversals (i.e., reversals 3 and 4), albeit nonsignificantly and less pronounced compared with female guppies.
Male Atlantic mollies clearly outperformed all other individuals, both males and females, by making the fewest errors during initial training and even improving their performance during reversals. Some individuals were able to improve to the point of 1-trial learning during the 4th reversal. Conversely, female Atlantic mollies performed distinctly different compared with the other 2 species. Comparatively, they made the most errors during the initial training task. Initially, they showed a similar behavioral flexibility during the 1st reversal compared with sailfin mollies and easily outperformed the female guppies in this 1st reversal task. However, in contrast to their male conspecifics and the other 2 poeciliid species, female Atlantic mollies were apparently unable to gradually improve or maintain their performance, resulting in more errors even in the 4th reversal.
Controls clearly showed that neither olfactory cues nor unintentional cueing had influenced the fish’s choice. Accordingly, we are confident that our procedure was robust in terms of the objectionable influence tested.
Comparison to previous studies
The behavioral flexibility to adapt one’s own behavior when facing a spatial and/or visual reversal task has been demonstrated in nearly all vertebrate taxa including mammals (e.g., Sutherland 1964; Hamilton et al. 2004), birds (e.g., Range et al. 2008), amphibians and reptiles (e.g., Day et al. 2003; Jenkin and Laberge 2010), and several fish species (e.g., teleosts: Lopez et al. 1999; Hughes and Blight 2000; Colwill et al. 2005; elasmobranchs: Fuss et al. 2014). Generally, individuals facing (serial) reversals following an initial training task will first persist to use their previously successful strategy before they start testing alternatives. Accordingly, learning in a reversal task will initially proceed slower than in the preceding task(s) (Sutherland, 1964). This learning behavior was also observed in female guppies and male sailfin and Atlantic mollies examined in this study. However, there seems to be another, apparently “delayed” form of learning, as one might assume from the example of female sailfin mollies. Although individuals made more and more errors during the 1st 2 reversals, they improved substantially during the last 2 reversals. This may apply to female Atlantic mollies alike, but further reversals are required for confirmation.
There is, however, another aspect that is worth mentioning. Lucon-Xiccato and Bisazza (2014) advert to the effect of domestication, which possibly influences learning in fish (Huntingford 2004). While they examined guppies of a domesticated strain (which are frequently bred particularly with regard to certain phenotypic characteristics such as an extremely prominent body ornamentation or large, colorful caudal fins), we assessed wild-type descendants of wild-caught individuals. Nevertheless, we were able to mirror the results of guppy females, although our females made about twice as many errors (i.e., 1.6 ± 1.66 errors to criterion) compared with the domesticated ones (i.e., 0.86 ± 1.35 errors to criterion; Lucon-Xiccato and Bisazza 2014, Supplemental material). Regarding serial reversal learning, female guppies of both studies were well able to inhibit their previous response and successfully solved all tasks they were assigned to. Surprisingly, female guppies of this study made only about a third of the errors to criterion (i.e., 5.3 ± 3.72 errors) than the females of the previous study (16.69 ± 11.58 errors; Lucon-Xiccato and Bisazza 2014, Supplemental material) during the very 1st reversal task. This could be a first tentative indication that domestication in guppies facilitates learning ability but inhibits behavioral flexibility.
Cognitive traits and neophobia
Potential factors responsible for individual differences in cognitive traits (e.g., behavioral flexibility regarding learning and memory) include sex differences (e.g., Range et al. 2006; Halpern 2012) or genetic variations (Dukas 2004 for review). Moreover, distinct differences in cognitive ability (Sih and Del Giudice 2012; Griffin et al. 2015) have been assumed to be functionally associated with an individual’s personality (i.e., reliable differences between individuals in behavioral responses; Gosling 2001; Dall et al. 2004; Sih et al. 2004), which could serve as a “behavioral trait” to be used as a predictor variable in the learning analysis. Certain personality traits are frequently associated with the speed of learning across taxa (Dougherty and Guillette 2018 for review) such as activity (e.g., mice: Matzel et al. 2006; zebra finches: Brust et al. 2013), explorative behavior (e.g., guppies: Brown et al. 2018; convict cichlids: Jones and Godin 2010; black-capped chickadees: Guillette et al. 2015; mallards: Bousquet et al. 2015), boldness (e.g., guppies: Dugatkin and Alfieri 2003; Eastern water skink: Carazo et al. 2014), or neophobia (Atlantic mollies: Sommer-Trembo et al. 2016; cichlids: Hoskins 2018; three-spined sticklebacks: Keagy et al. 2019; Florida scrub-jays: Bebus et al. 2016). Generally, bold, fast exploring individuals can often be observed to learn simple tasks very quickly, but make more errors when tasks change or become more challenging (e.g., Coppens et al. 2010; Koolhaas et al. 2010). Conversely, shyer individuals were found to learn new tasks comparatively slowly, but payed more attention and therefore made fewer mistakes in response to environmental changes (e.g., Jones and Godin 2010; Sih and Del Giudice 2012). Having said that this study was not designed to determine individual differences or explicitly examine individual traits, but to determine general sex-specific differences in a population of a given species. Additionally to the differences in latencies used as a proxy of neophobia, considerable differences have been observed across all 3 species with respect to habituation to the handling and (pre-) training procedure and motivation to participate. Particularly male Atlantic mollies were characterized by a comparatively short habituation and pretraining phase. While Atlantic mollies were highly motivated almost immediately following the transfer from their small tank into the experimental setup (where they acted rather keen to be released into the next trial), sailfin mollies required an acclimatization phase of up to 10 min until they showed a similar behavior. Guppies showed some kind of “intermediate behavior” compared with both molly species. To support these observations, we determined an individual’s neophobia, that is, the latency until its first decision in the first initial training trial. While the longest average latencies were observed in male sailfin mollies (32.27 ± 12.18 s), medium latencies were observed in their female conspecifics (24.35 ± 15.88 s) and guppy females (20.49 ± 9.88 s). Conversely, the shortest latencies were determined for Atlantic molly females (13.18 ± 4.47 s) and, particularly, their male conspecifics (11.68 ± 2.35 s). For instance, it took Atlantic molly males only about a third of the time to make a decision compared with male sailfin mollies. Therefore, Atlantic mollies were expected to learn the initial color discrimination task quickly, but to make more errors in reversals, whereas sailfin mollies and guppies were expected to perform considerably better in the serial reversal tasks. But despite their eagerness and high motivation to participate in the experiments observed in all individuals (which suggested a higher susceptibility to errors in the reversals), male Atlantic mollies were the most successful group in reversal learning. Contrary to expectations, female Atlantic mollies did not fit into this scheme at all. Similar, apparently contradictory results have also been found in other studies. Shyer individuals of several species performed better in both avoidance learning (Exnerová et al. 2010; Budaev and Zhuikov 1998) and reversal learning (Guillette et al. 2011; Guenther et al. 2014), whereas in other species there were no differences (Amy et al. 2012; Guillette et al. 2015; Sommer-Trembo and Plath 2018). Present results suggest that the relationship between learning speed and personality seems to be more complex than previously thought and, potentially, species-specific. In addition, a trade-off between learning speed and accuracy apparently does not always support associations between cognitive ability and personality.
Concluding remarks
Our results seem to contradict previous data obtained in primates, rodents, domestic fowl, and teleosts, where females showed a greater behavioral flexibility and appeared to be more apt to change their response when a learned rule becomes invalid in a new context (Rogers 1974; Guillamón et al. 1986; Ha et al. 2011; Lucon-Xiccato and Bisazza 2014). Hence, this hitherto apparently universal behavioral pattern seems to be inverted in the 2 examined molly species. However, the evolutionary account of this pattern remains highly speculative. Hypothetically, sex differences in behavioral flexibility may possibly be explained in terms of the different roles that males and females play in mating competition, mate choice, and reproduction or, more generally, in complex social interactions. Each of these characteristics differed between the closely related mollies in comparison to the more distantly related guppies (e.g., Croft et al. 2004; Allan et al. 2012). Thereby, the observed differences between species may possibly reflect an ability status that arose phylogenetically together with the traits that led to the formation of the 3 examined species. Although it is not known whether differences in cognitive abilities that have arisen in a particular context (e.g., reproductive behavior) influences other behavior (e.g., foraging), it nevertheless appears to be conceivable that sex differences in behavioral flexibility are a consequence of different selection pressures on sexes in the context of sexual selection.
Acknowledgments
The authors would like to thank Sara Hartmann for measuring the color spectra of the 4 chips. We thank Dr Nils Krützfeldt for proofreading the article.
Appendix
Figure A1.
Reflectance (%) of the red, green, blue and yellow chips used in experiments (measured with AvaSpec 2048 spectrometer, Avantes, the Netherlands).
Funding
No funding.
Conflict of Interest Statement
None declared.
References
- Allan K, Jones BC, DeBruine LM, Smith DS, 2012. Evidence of adaptation for mate choice within women’s memory. Evol Hum Behav 33:193–199. [Google Scholar]
- Amy M, van Oers K, Naguib M, 2012. Worms under cover: relationships between performance in learning tasks and personality in great tits (Parus major). Anim Cogn 15:763–770. [DOI] [PubMed] [Google Scholar]
- Bebus SE, Small TW, Jones BC, Elderbrock EK, Schoech SJ, 2016. Associative learning is inversely related to reversal learning and varies with nestling corticosterone exposure. Anim Behav 111:251–260. [Google Scholar]
- Bissonette GB, Powell EM, 2012. Reversal learning and attentional set–shifting in mice. Neuropharm 62:1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond AB, Kamil AC, Balda RP, 2007. Serial reversal learning and the evolution of behavioural flexibility in three species of North American corvids (Gymnorhinus cyanocephalus, Nucifraga columbiana, Aphelocoma californica). J Comp Psychol 121:372–379. [DOI] [PubMed] [Google Scholar]
- Boogert NJ, Monceau K, Lefebvre L, 2010. A field test of behavioural flexibility in Zenaidadoves (Zenaidaaurita). Behav Process 85:135–141. [DOI] [PubMed] [Google Scholar]
- Bousquet CAH, Petit O, Arrive M, Robin J-P, Sueur C, 2015. Personality tests predict responses to a spatial-learning task in mallards Anas platyrhynchos. Anim Behav 110:145–154. [Google Scholar]
- Brown VJ, Tait DS, 2015. Attentional set-shifting across species In: Robbins TW, Sahakian BJ, editors. Translational Neuropsychopharmacology: Current Topics in Behavioral Neurosciences. Vol. 28 Cham: Springer; 363–395. Available from: 10.1007/7854_2015_5002. [DOI] [PubMed] [Google Scholar]
- Brown GE, Chuard PJ, Demers EE, Ramnarine IW, Chivers DP. et al. , 2018. Personality and the retention of neophobic predator avoidance in wild caught Trinidadian guppies. Behaviour 155:265–278. [Google Scholar]
- Brust V, Wuerz Y, Krüger O, 2013. Behavioural flexibility and personality in zebra finches. Etholology 119:559–569. [Google Scholar]
- Bublitz A, Weinhold SR, Strobel S, Dehnhardt G, Hanke FD, 2017. Reconsideration of serial visual reversal learning in octopus (Octopus vulgaris) from a methodological perspective. Front Physiol 8:54.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budaev SV, Zhuikov AY, 1998. Avoidance learning and ‘personality’ in the guppy (Poecilia reticulata). J Comp Psychol 112(1):92–94. [Google Scholar]
- Carazo P, Noble DWA, Chandrasoma D, Whiting MJ, 2014. Sex and boldness explain individual differences in spatial learning in a lizard. Proc R Soc B 281:20133275.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caves EM, Green PA, Zipple MN, Peters S, Johnsen S. et al. , 2018. Categorical perception of color signals in a songbird. Nature 560:365–267. [DOI] [PubMed] [Google Scholar]
- Colwill RM, Raymond MP, Ferreira L, Escudero H, 2005. Visual discrimination learning in zebrafish Danio rerio. Behav Proc 70:19–31. [DOI] [PubMed] [Google Scholar]
- Coppens CM, de Boer SF, Koolhaas JM, 2010. Coping styles and behavioural flexibility: towards underlying mechanisms. Proc R Soc Lond B 365:4021–4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft DP, Krause J, James R, 2004. Social networks in the guppy (Poecilia reticulata). Proc R Soc Lond B 271(Suppl 6):S516–S519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings ME, 2018. Sexual conflict and sexually dimorphic cognition- reviewing their relationship in poeciliid fishes. Behav Ecol Sociobiol 72:73–86. [Google Scholar]
- Dall SRX, Houston AI, McNamara JM, 2004. The behavioural ecology of personality: consistent individual differences from an adaptive perspective. Ecol Lett 7:734–739. [Google Scholar]
- Day L, Ismail N, Wilczynski W, 2003. Use of position and feature cues in discrimination learning by the whiptail lizard Cnemidophorus inornatus. J Comp Psychol 117:440–448. [DOI] [PubMed] [Google Scholar]
- Dodson JJ, 1988. The nature and role of learning in the orientation and migratory behaviour of fishes. Environ Biol Fish 23:161–182. [Google Scholar]
- Dougherty LR, Guilliette LM, 2018. Linking personality and cognition: a meta-analysis. Philos TR Soc B 373(1756):20170282. Available from: 10.1098/rstb.2017.0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugatkin LA, Alfieri MS, 2003. Boldness, behavioral inhibition and learning. Ethol Ecol Evol 15:43–49. [Google Scholar]
- Dukas R, 2004. Evolutionary biology of animal cognition. Annu Rev Ecol Syst 35:347–374. [Google Scholar]
- Etheredge RI, Avenas C, Armstrong MJ, Cummings ME, 2018. Sex–specific cognitive–behavioural profiles emerging from individual variation in numerosity discrimination in Gambusia affinis. Anim Cogn 21:37–53. [DOI] [PubMed] [Google Scholar]
- Exnerová A, Svádová KH, Fučíková E, Drent P, Štys P, 2010. Personality matters: individual variation in reactions of naive bird predators to aposematic prey. Proc R Soc Lond B 277:723–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuss T, Bleckmann H, Schluessel V, 2014. Visual discrimination abilities in grey bamboo sharks Chiloscyllium griseum. Zoology (Jena) 117:104–111. [DOI] [PubMed] [Google Scholar]
- Godfrey-Smith P, 2002. Environmental complexity and the evolution of cognition In: Sternberg R, Kaufman J, editors. The Evolution of Intelligence. Mahwah (NJ: ): Lawrence Erlbaum Associates; 233–249. [Google Scholar]
- Gosling SD, 2001. From mice to men: what can we learn about personality from animal research? Psychol Bull 127:45–86. [DOI] [PubMed] [Google Scholar]
- Griffin AS, Guillette LM, Healy SD, 2015. Cognition and personality: an analysis of an emerging field. Trends Ecol Evol 30:207–214. [DOI] [PubMed] [Google Scholar]
- Guenther A, Brust V, Dersen M, Trillmich F, 2014. Learning and personality types are related in cavies Cavia aperea. J Comp Psychol 128:74–81. [DOI] [PubMed] [Google Scholar]
- Guillamón A, Valencia A, Calés J, Segovia S, 1986. Effects of early postnatal gonadal steroids on the successive conditional discrimination reversal learning in the rat. Physiol Behav 38:845–849. [DOI] [PubMed] [Google Scholar]
- Guillette LM, Reddon AR, Hoeshele M, Sturdy CB, 2011. Sometimes slower is better: slow-exploring birds are more sensitive to changes in a vocal discrimination task. Proc R Soc B Lond 278:767–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillette LM, Hahn AH, Hoeschele M, Przyslupski A-M, Sturdy CB, 2015. Individual differences in learning speed, performance accuracy and exploration behaviour in black-capped chickadees. Anim Cogn 18:165–178. [DOI] [PubMed] [Google Scholar]
- Ha JC, Mandell DJ, Gray J, 2011. Two-item discrimination and Hamilton search learning in infant pigtailed macaque monkeys. Behav Process 86:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern DF, 2012. Sex Differences in Cognitive Abilities. 4th edn. New York: Psychology Press. [Google Scholar]
- Hamilton DA, Rosenfelt CS, Whishaw IQ, 2004. Sequential control of navigation by locale and taxon cues in the Morris water task. Behav Brain Res 154:385–397. [DOI] [PubMed] [Google Scholar]
- Hoskins EA, 2018. Within-Species Variation in Cognition in Cichlid Fishes: Influences of Social Status and Personality [doctoral dissertation]. The Ohio State University. [Google Scholar]
- Hughes RN, Blight CM, 2000. Two intertidal fish species use visual association learning to track the status of food patches in a radial maze. Anim Behav 59:613–621. [DOI] [PubMed] [Google Scholar]
- Huntingford FA, 2004. Implications of domestication and rearing conditions for the behavior of cultivated fishes. J. Fish Biol 65:122–142. [Google Scholar]
- Jenkin SE, Laberge F, 2010. Visual discrimination learning in the fire-bellied toad Bombina orientalis. Learn Behav 38:418–425. [DOI] [PubMed] [Google Scholar]
- Jones KA, Godin JGJ, 2010. Are fast explorers slow reactors? Linking personality type and anti-predator behaviour. Proc R Soc B Biol Sci 277:625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B, Mishkin M, 1972. Limbic lesions and the problem of stimulus-reinforcement associations. Exp Neurol 36:362–377. [DOI] [PubMed] [Google Scholar]
- Keagy J, Minter R, Tinghitella RM, 2019. Sex differences in cognition and their relationship to male mate choice. Curr Zool. 10.1093/cz/zoz014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolhaas JM, De Boer SF, Coppens CM, Buwalda B, 2010. Neuroendocrinology of coping styles: towards understanding the biology of individual variation. Front Neuroendocrinol 31:307–321. [DOI] [PubMed] [Google Scholar]
- Laland KN, Reader SM, 1999. Foraging innovation in the guppy. Anim Behav 57:331–340. [DOI] [PubMed] [Google Scholar]
- Leal M, Powell BJ, 2012. Behavioural flexibility and problem-solving in a tropical lizard. Biol Lett 8:28–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke J, Schneider JM, 2014. Association and reversal learning abilities in a jumping spider. Behav Proc 103:192–198. [DOI] [PubMed] [Google Scholar]
- Liu Y, Day LB, Summers K, Burmeister SS, 2016. Learning to learn: advanced behavioural flexibility in a poison frog. Anim Behav 111:167–172. [Google Scholar]
- Lopez JC, Broglio C, Rodriguez F, Thinus-Blanc C, Salas C, 1999. Multiple spatial learning strategies in goldfish Carassius auratus. Anim Cogn 2:109–120. [Google Scholar]
- Lucon-Xiccato T, Bisazza A, 2014. Discrimination reversal learning reveals greater female behavioural flexibility in guppies. Biol Lett 10:20140206. Available from: 10.1098/rsbl.2014.0206. [DOI] [Google Scholar]
- Lucon-Xiccato T, Bisazza A, 2016. Male and female guppies differ in speed but not in accuracy in visual discrimination learning. Anim Cogn 19:733–744. [DOI] [PubMed] [Google Scholar]
- Lucon-Xiccato T, Bisazza A, 2017. Sex differences in spatial abilities and cognitive flexibility in the guppy. Anim Behav 123:53–60. [Google Scholar]
- Maharaj G, Horack P, Yoder M, Dunlap AS, 2018. Influence of preexisting preference for color on sampling and tracking behavior in bumble bees. Behav Ecol 30:150–158. [Google Scholar]
- Matzel LD, Townsend DA, Grossman H, Han YR, Hale G. et al. , 2006. Exploration in outbred mice covaries with general learning abilities irrespective of stress reactivity, emotionality, and physical attributes. Neurobiol Learn Mem 86:228–240. [DOI] [PubMed] [Google Scholar]
- Meffe GK, Snelson FF, 1989. An ecological overview of poeciliid fishes In: Meffe GK, Snelson FF, editors. Ecology and Evolution of Livebearing Fishes (Poeciliidae). Englewood Cliffs (NJ: ): Prentice Hall; 13–31. [Google Scholar]
- Moore A, Malinowski P, 2009. Mediation, mindfulness, and cognitive flexibility. Conscious Cogn 18:176–186. [DOI] [PubMed] [Google Scholar]
- Parker MO, Gaviria J, Haigh A, Millington ME, Brown VJ. et al. , 2012. Discrimination reversal and attentional sets in zebrafish Danio rerio. Behav Brain Res 232:264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrazzini MEM, Bisazza A, Agrillo C, Lucon-Xiccato T, 2017. Sex differences in discrimination reversal learning in the guppy. Anim Cogn 20:1081–1091. [DOI] [PubMed] [Google Scholar]
- Raine NE, Chittka L, 2012. No trade-off between learning speed and associative flexibility in bumblebees: a reversal learning test with multiple colonies. PLoS ONE 7:e45096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Range F, Bugnyar K, Kotrschal K, 2008. The performance of ravens on simple discrimination tasks: a preliminary study. Acta Ethol 11:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Range F, Bugnyar T, Schlögl C, Kotrschal K, 2006. Individual and sex differences in learning abilities of ravens. Behav Process 73:100–106. [DOI] [PubMed] [Google Scholar]
- Rayburn-Reeves RM, Qadri MA, Brooks DI, Keller AM, Cook RG, 2017a. Dynamic cue use in pigeon mid-session reversal. Behav Proc 137:53–63. [DOI] [PubMed] [Google Scholar]
- Rayburn-Reeves RM, James BT, Beran MJ, 2017b. Within-session reversal learning in rhesus macaques Macaca mulatta. Anim Cogn 20:975–983. [DOI] [PubMed] [Google Scholar]
- Rogers LJ, 1974. Persistence and search influenced by natural levels of androgens in young and adult chickens. Physiol Behav 12:197–204. [DOI] [PubMed] [Google Scholar]
- Řežucha R, Reichard M, 2016. The association between personality traits, morphological traits and alternative mating behaviour in male Endler’s guppies Poecilia wingei. Ethology 122:456–467. [Google Scholar]
- Rolls ET, 2000. Memory systems in the brain. Annu Rev Psychol 51:599–630. [DOI] [PubMed] [Google Scholar]
- Roth G, Dicke U, 2005. Evolution of the brain and intelligence. Trend Cogn Sci 9:250–257. [DOI] [PubMed] [Google Scholar]
- Roelofs S, Nordquist RE, van der Staay FJ, 2017. Female and male pigs’ performance in a spatial holeboard and judgment bias task. Appl Anim Behav Sci. https://doi:10.1016/j.applanim.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott WA, 1962. Cognitive complexity and cognitive flexibility. Sociometry 25:405–414. [Google Scholar]
- Shettleworth SJ, 2010. Cognition, Evolution, and Behavior. Oxford: Oxford University Press. [Google Scholar]
- Sih A, Del Giudice M, 2012. Linking behavioural syndromes and cognition: a behavioural ecology perspective. Phil Trans R Soc B 367:2762–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sih A, Bell AM, Johnson JC, Ziemba RE, 2004. Behavioral syndromes: an integrative overview. Q Rev Biol 79:241–558. [DOI] [PubMed] [Google Scholar]
- Sommer-Trembo C, Plath M, 2018. Consistent individual differences in associative learning speed are not linked to boldness in female Atlantic mollies. Anim Cogn 21:661–670. [DOI] [PubMed] [Google Scholar]
- Sommer-Trembo C, Bierbach D, Arias-Rodriguez L, Verel Y, Jourdan J. et al. , 2016. Does personality affect premating isolation between locally–adapted populations? BMC Evol Biol 16:138.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland NS, 1964. Visual discrimination of animals. Br Med Bull 20:54–59. [DOI] [PubMed] [Google Scholar]
- Thornton A, Lukas D, 2012. Individual variation in cognitive performance: developmental and evolutionary perspectives. Phil Trans R Soc 367:2773–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JM, 1960. Reversal learning by paradise fish (Macropodus opercularis). J Comp Physiol Psych 53(4):376–378. [DOI] [PubMed] [Google Scholar]
- Warren JM, 1966. Reversal learning and the formation of learning sets by cats and rhesus monkeys. J Comp Physiol Psychol 61:421–428. [DOI] [PubMed] [Google Scholar]
- White SJ, 2017. The Evolutionary Genetics of Behavioural Variation: Multivariate Perspectives on Personality in the Trinidadian Guppy [PhD thesis]. University of Exeter; [cited 2017 May 15]. Available from: http://hdl.handle.net/10871/30848. [Google Scholar]