Abstract
Pomacea canaliculata is a successful invader and also a competitor and predator of other snails and may play a key role in structuring freshwater snail communities both in its native and invaded range. In the present study we evaluated the contact and distant effects of P. canaliculata in its native range on exotic (Melanoides tuberculata and Physa acuta) and native snails (Heleobia parchappii, Biomphalaria peregrina, and Chilina parchappii). Habitat use was affected in P. acuta, H. parchappii, and B. peregrina by contact effects of P. canaliculata, whereas survival was only affected in P. acuta through combined contact and distant effects. Fecundity was reduced in P. acuta and B. peregrina by combined contact and distant effects; evidence of egg mass predation was also observed in both species. Melanoides tuberculata was not affected at all by P. canaliculata. The snail species with higher withdrawal responses to contacts with P. canaliculata were those that suffered less mortality by corporal contact, whereas snails with high crawling away responses suffered from higher mortality. The effects of P. canaliculata seem to be highly negative to small nonoperculate snails that lay gelatinous egg masses, whereas large operculate ovoviviparous snails are not affected in their survival and reproduction. This apple snail may exert biotic resistance against P. acuta but could favor the establishment of M. tuberculata and other functionally similar species in new habitats in South America.
Keywords: Ampullariidae, behavior, biotic resistance, interspecific interactions
Biological invasions are considered one of the main threats to biodiversity and also a source of impacts ranging from ecological and veterinary to medical, economic, and social (Simberloff et al. 2013). Interspecific interactions are in the very base of many mechanisms like enemy release, biotic resistance, and invasional meltdown that may determine the failure or success and the sign and extent of impacts of biological invasions (Jeschke et al. 2012; Traveset and Richardson 2014; Jackson 2015). Biotic resistance, the combined negative effects of a resident species in a community, either native or exotic, on the new species that arrives in that community (Simberloff and Von Holle 1999; Levine et al. 2004; Parker and Hay 2005) is a determinant of its invasibility (Byers and Noonburg 2003; Alofs and Jackson 2014). In aquatic environments, the effect of predation on biotic resistance is generally more important than competition but the effects vary between habitats and taxa (Alofs and Jackson 2014). The role of some species in biotic resistance is expected to be enhanced if they combine two antagonist interactions over the newcomers (e.g., intraguild predation; Britton 2012).
Underlying biotic resistance are antagonistic interspecific interactions that may explain the failure of many species to establish, spread, or become an invader in recipient ecosystems. Interspecific interactions have a strong structuring role in freshwater snail communities (Turner et al. 2007; Larson and Black 2016; Früh et al. 2017) and the potential effects of these interactions on survival, growth, and fecundity may be negative (Anto et al. 2005; Kwong et al. 2009; Posch et al. 2013) or positive (Kawata and Ishigami 1992; Cope and Winterbourn 2004; Zukowski and Walker 2009) for at least one of the species involved. Whatever the type of interactions, the underlying mechanisms are diverse and they can be categorized as occurring either by contact or at a distance. The interactions by contact require that the snails have been in the same place, either at the same moment (e.g., corporal contacts, like in bulldozing or predation; Turner et al. 2007; Kwong et al. 2009) or at different moments (e.g. competitive interference by feces or mucus trails; Karowe et al. 1993). Distant interactions do not require that the snails are or have been in the same place and they are mediated by dissolved substances, either depleted resources, like oxygen, or metabolic products, like urea that can foul the water or be used as infochemicals (Kawata and Ishigami 1992; Anto et al. 2005); a few freshwater snails have evolved secondary metabolites that repel many other snails species (Raw et al. 2013, 2015). According to this scheme, exploitative competition over shared resources can occur either by contact (food or refuges) or at a distance (dissolved oxygen or calcium).
The apple snail Pomacea canaliculata (Ampullariidae) is a native freshwater snail from the Río de la Plata basin in South America (Martín et al. 2001; Hayes et al. 2012). This apple snail is considered a hyper-successful invasive species (Carlsson et al. 2009) that has established feral populations in different regions around the world, especially East and Southeast Asia, and has caused strong ecological impacts in wetlands and huge economic losses in aquatic crops (Nghiem et al. 2013; Horgan et al. 2014). Due to its relatively larger size, high trophic flexibility, high feeding rates, and reproductive potential (Morrison and Hay 2011; Hayes et al. 2015; Saveanu et al. 2017; Tiecher et al. 2017), it will probably be able to outcompete with other species of snails (Tan et al. 2013; Chaichana and Sumpan 2014; Horgan et al. 2014; Tricarico et al. 2016). On the other hand, it has been shown that P. canaliculata is also able to prey on adults, newborn, and egg masses of other snails (Cazzaniga 1990; Kwong et al. 2009). In particular, intraguild predation on egg masses has been shown to be an important interspecific interaction among freshwater pulmonate snails of similar sizes (Turner et al. 2007) and is expected to be even more important in the case of P. canaliculata, due to its powerful radula and jaws and its larger size. The effects of P. canaliculata on other snails are expected to be strong in the areas invaded, where it can reach high densities, monopolize secondary production, and provoke ecosystem changes (Carlsson et al. 2004; Kwong et al. 2010; Horgan et al. 2014; Tricarico et al. 2016).
The negative effects of Pomacea apple snails on the growth and survivorship of other snails have mostly been studied in the areas where the former are exotic (Conner et al. 2008; Kwong et al. 2009; Posch et al. 2013; Chaichana and Sumpan 2014). However, in its native range, the predation of P. canaliculata has only been studied on a native snail (Cazzaniga 1990). Pomacea canaliculata is very abundant in its native range and, at least in Argentina, it has been anthropogenically dispersed across natural hydrographic barriers (Martín et al. 2001; Martín and De Francesco 2006). As it has become established beyond the Río de la Plata basin, there are potential effects on the structure of the snail communities in these new areas but this has not been studied yet. Being a hyper-successful invader worldwide (Carlsson et al. 2009), P. canaliculata may also have negative effects on exotic snails that have invaded its own native range and hence may contribute to the biotic resistance of the communities in freshwater habitats.
Contact and distant effects (henceforth CEs and DEs, respectively) of Pomacea on other freshwater snails have not been discriminated (Conner et al. 2008; Kwong et al. 2009; Posch et al. 2013). Changes in habitat use or behavior are among the diverse effects that predators and competitors exert on freshwater snails (Karowe et al. 1993; Turner 1996; Raw et al. 2013, 2015), but the effect of apple snails on habitat use by other snails has not been studied. In the present study, we evaluate the effects of P. canaliculata on the habitat use, survival, fecundity, and behavior of two exotic and three native snails in southern South America and discriminate the influence of contact and distant interactions The evidence obtained would help to evaluate its role in biotic resistance to exotic snails and also the possibility of interactions among invaders.
Materials and Methods
Study zone and snail collection
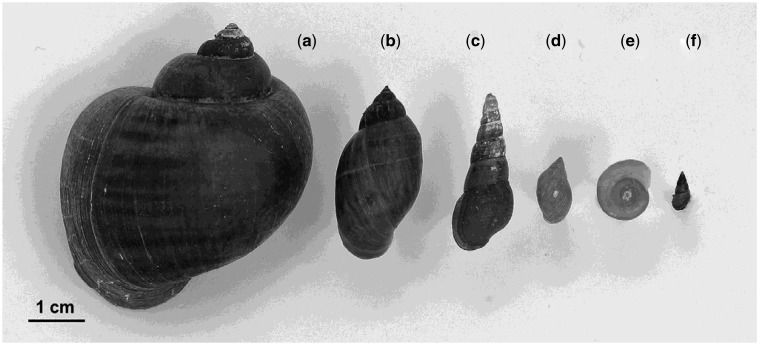
The specimens of P. canaliculata (Figure 1a) were hand-collected at an earthen channel connecting the Pigüe and Venado streams (37° 11' 26” S, 62° 40' 25'' W, Buenos Aires province, Argentina). These streams belong to the Encadenadas del Oeste basin where P. canaliculata is naturally and widely distributed (Seuffert and Martín 2013).
Figure 1.
Shells of the snails species used in the study (scale bar: 1 cm), (a)P. canaliculata, (b)C. parchappii, (c)M. tuberculata, (d)P. acuta, (e)B. peregrina, (f)H. parchappii. The shells portrayed belong to the largest snails found in the source population of each species.
We studied Physa acuta Draparnaud 1805 (Physidae) and Melanoides tuberculata (Müller 1774) (Thiaridae) as representatives of exotic snails in Argentina (Rumi et al. 2006) (Figure 1c,d) and also three native species of snails: Chilina parchappii (d'Orbigny 1835), Biomphalaria peregrina (d'Orbigny 1835) and Heleobia parchappii (d'Orbigny 1835), belonging to the families Chilinidae, Planorbidae and Cochliopidae, respectively (Figure 1b,e,f). These 5 species are the most abundant and widespread in the area among the 7 present in southern Buenos Aires province (Martín et al. 2016) and the most adaptable to laboratory conditions. The snails were searched for and collected by hand or with a hand net in a reach of the Napostá Grande stream (38° 40' 48′ S, 62° 14′ 01'' W to 38° 41' 44'' S, 62° 15' 51'' W), upstream from Bahía Blanca. The specimens of M. tuberculata were obtained from a small earthen channel that discharges into the Napostá Grande stream in the same reach. Biomphalaria peregrina was not found in this area during the present sampling so we searched nearby streams. A few specimens found in the Ventana stream (38° 02' 52'' S, 62° 07' 41'' W) were taken to the laboratory and reared for 4 months to increase the numbers. Pomacea canaliculata is not naturally present in the basins of these 2 streams (Martín et al. 2001). The 3 native species and P. acuta are present in the channel where P. canaliculata was collected and also in many waterbodies of the Encadenadas del Oeste basin.
Snail maintenance and rearing
The snails were taken to the laboratory in water from the collection site and acclimatized to laboratory conditions in plastic trays (53 × 34 × 10 cm). The snails of different species were maintained in different aquaria in a rearing room at 25 ± 2°C and under a 14 h light/10 h dark photoperiod. The snails were fed daily with lettuce and fish food flakes (VitaFish®) and once a week the water was changed and the aquaria cleaned. The apple snails were maintained for at least 1 month in the laboratory before they were used in the experiments; the other snails were collected at least 1 week before use. No individual apple snail or non-apple snail was used more than once in the entire series of experiments (see below CE Experiment).
Juveniles and adults of the 5 non-apple snail species were included in the experiments. Before they were used, the presence of egg masses (P. acuta, C. parchappii, and B. peregrina), eggs (H. parchappii), or newborns (M. tuberculata) was confirmed in the aquaria to assure the possibility of reproductive activity during the experiments. The minimum and maximum shell lengths were 4.2–4.8 mm for H. parchappii, 10.2–22.5 mm for M. tuberculata, 6.8–10.9 mm for P. acuta, 10.9–11.7 mm for C. parchappii, and 4.6–9.5 mm for B. peregrina. In the case of P. canaliculata only adult males (34.0–44.5 mm) were used because of the females’ habit of laying eggs out of water for several hours (Estebenet and Martín 2002) would have interfered with the progress of the experiments.
Experiments to test the CEs of P. canaliculata on non-apple snails (CE Experiments)
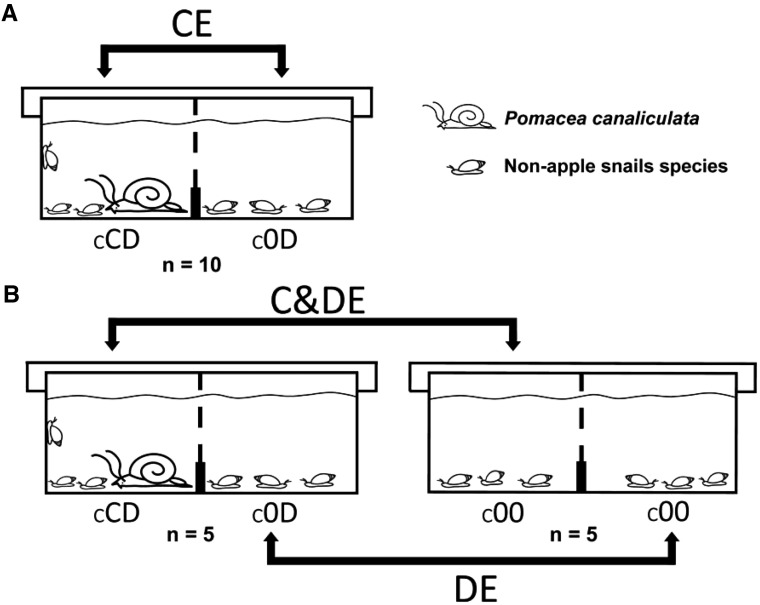
The effects of P. canaliculata were tested separately for each of the 5 non-apple snail species in 5 experiments with the same design (Figure 2a). Ten plastic aquaria were used in each of these experiments. Each aquarium was divided into two equal compartments (28 × 21 × 18.5 cm) by a vertical plastic grid with aperture size of 0.8 cm that allowed all the snail species to move from 1 compartment to the other, except for P. canaliculata. A 2-cm-tall plastic barrier was installed at the bottom end of the grid to avoid the movement of food debris, feces, and remains of dead snails or egg masses from 1 compartment to the other. The aquaria were filled with tap water up to a depth of 14 cm (total water volume: 17 L) and provided with a plastic lid to avoid the escape of the snails.
Figure 2.
Scheme of the experimental design and statistical comparisons. Aquaria compartments where different effects of P. canaliculata can occur: cCD (contact and distant effects), c0D (distant effects only), and c00 (control, no effects). (A) CE Experiments: only aquaria cCD-c0D were used, (B) C&DE and DE Experiments: both cCD-c0D and c00-c00 aquaria (control) were used. Black arrows indicate the effects that could be detected in each statistical comparison between compartments: CE (contact effects), DE (distant effects) and C&DE (contact and distant effects).
One individual of P. canaliculata was placed at random in one of the compartments and the other one served as control. Ten snails of only one of the non-apple snail species were put in each compartment of each aquarium (8 in the case of C. parchappii, the species with largest adults and the most sensitive to crowding; P.R.M., personal observation). The sizes of the apple snails prevented them from passing through the plastic grid to the control compartment. On the other hand, the maximum sizes of non-apple snail species allowed them to move freely through the grid in both directions.
Each of the 5 experiments lasted for 2 weeks during which lettuce and fish food flakes were provided ad libitum in both compartments to avoid competition for trophic resources. Five times a week the number of live and dead individuals of the non-apple snail species were recorded and also their position (in the compartment with P. canaliculata, or in the control compartment, or on the plastic grid). At the end of each week the water was changed to avoid fouling. Aquaria were slowly emptied with a siphon in order to avoid the mixing of the bottom materials from the compartments; these materials were collected separately for each compartment and inspected under a stereoscopic microscope in search of pieces of shell, remains of snail bodies, egg masses, or newborn (see below in this same section). The number of live and dead snails, egg masses or fragments, eggs and live newborn on the walls, on the grid and on the bottom of each compartment of the empty aquarium were recorded. The original M. tuberculata snails and their newborn were easily discriminated since there was always a very notable size difference given the initial size of the original snails and the short duration of the trials; on the other hand, there was not enough time for the eggs of oviparous snails to hatch before the aquaria were cleaned at the end of each week.
As the eggs of H. parchappii are very small (300 um in diameter; Martín 2002) and are laid isolated it was not possible to count them on the aquarium walls. A half-cylinder of PVC (diameter: 9 cm, length: 6.5 cm) was deployed horizontally at the bottom of each compartment as an oviposition substratum. The half-cylinder allowed P. canaliculata snails to crawl and graze underneath it and it was retrieved at the end of each week. The eggs on both sides were counted using a magnifying lens.
For each compartment 4 variables were estimated in each experiment: habitat use, survival, fecundity, and damage to egg masses. Habitat use (%) was calculated as 100 times the total number of snails (alive and dead) in each compartment, divided by the total number of snails (alive and dead) in all the aquaria; the dead snails were counted because it was assumed that they were using it until they died and they were not displaced post-mortem due to the plastic barrier at the bottom. The habitat use (%) of the 2 compartments did not always add up 100% since the snails on the grid were not counted as being located in either compartment.
Survival, fecundity, and damage to egg masses in each compartment were calculated relative to the number of snails or egg masses in that compartment, as the snails were able to move from 1 compartment to the other, and hence there were differences in the density between compartments. Therefore, survival (%) was calculated as 100 times the number of live snails in each compartment divided by the total number of snails (alive and dead) that used that compartment. Fecundity was calculated on a per capita basis, as the total number of egg masses (intact or damaged), eggs H. parchappii, or live newborn M. tuberculata in each compartment divided by the number of live snails that used that compartment during the 2 weeks of the experiment. The damage to egg masses (%) was calculated as 100 times the number of damaged egg masses in a compartment divided by the total number of egg masses (damaged or not) in the same compartment. The damage was not calculated for C. parchappi because only 4 egg masses were found during the experiments (see section “Results”).
For each of the 4 variables paired t-tests were performed to compare the compartments with and without P. canaliculata in the same aquarium (Figure 2a). The average of the 10 records of habitat use and survival obtained during the 2 weeks was used as the datum for each compartment; in the case of both fecundity and damaged egg masses, the average of the 2 records obtained at the end of each week was used. Assuming that in the compartment with P. canaliculata (cCD) there are both CEs and DEs and that in the one without P. canaliculata (c0D) there are only DEs, any significant differences between compartments can be attributed to the contact effects (CEs) of P. canaliculata. To allow a comparison of the effects of P. canaliculata on the different species of non-apple snails, or between the different variables for the same species, we estimated the effect sizes through Cohen’s d .The effect sizes were estimated as the difference between the means of the compartments divided by the average of their standard deviations (SDs) (Cohen’s dav), as suggested by Lakens (2013) for paired comparisons.
Experiments to test DEs alone or combined with CEs of P. canaliculata on non-apple snails (C&DE and DE Experiments)
The experiments for each non-apple snail species were performed and tested separately, as in CE Experiments. Five aquaria identical to those of the CE Experiments were used; in addition, 5 aquaria with 16–20 individuals of only 1 of the other non-apple snail species were used as controls in each experiment (Figure 2b).
The procedures of feeding, cleaning, and recording of the events were all the same as in the CE Experiments. As in the CE experiment the differences in the density between compartments due to avoidance behavior were taken into account for the calculation of survival, fecundity, and damage to egg masses in each compartment, which were calculated relative to the number of snails or egg masses in that compartment. However, habitat use (%) was not estimated for C&DE and DE Experiments because this variable needs to be calculated comparing the 2 compartments in the same aquarium and in the present experiments all the comparisons were between the compartments of 2 different aquaria (Figure 2b).
To test the combined distant and CEs the 5 compartments with P. canaliculata, where both contact and DEs may occur (cCD), were compared through independent t-tests with 5 compartments taken at random from the each of the 5 control aquaria (c00), where no effect was present (Figure 2b). Levene’s tests were performed to check the equality of the variances; then if this assumption was rejected, Welch’s tests for unequal variances were performed (Zar 1984). Any significant difference between these compartments can be attributed to the combination of contact and DEs of P. canaliculata (C&DE). The effect sizes of the 3 variables were estimated using Cohen’s ds as the difference between the means of the compartments divided by their pooled SD (Lakens 2013).
Finally, to test the DEs alone, the 5 compartments without P. canaliculata from the aquaria with P. canaliculata (c0D), where only DEs may occur, were compared through independent t-tests with the other five compartments of the five control aquaria (c00), where no effect of P. canaliculata was present; then when the equality of the variances was rejected, Welch’s tests were performed. When the difference between c0D and c00 was significant it was attributed to the DEs of P. canaliculata (DE, Figure 2b). The effect sizes in the three variables were estimated using Cohen’s ds as above.
Experiment to test behavioral responses of non-apple snails to contacts with P. canaliculata
In this experiment, glass aquaria (25 × 15 × 14.5 cm) filled with tap water up to a depth of 11 cm were used. Five aquaria with 1 P. canaliculata and 3 individuals of only 1 of each of the 5 non-apple species were tested simultaneously. The scheme was repeated on 5 consecutive days, each time with different individuals of the 6 snail species.
Up to the time of the trials, the procedures of feeding and cleaning were the same as in the previous experiments. The P. canaliculata snails were maintained without food for 24 h before the trials. The snails were not fed during the trials and the water was maintained at 25°C. The trials were all done between 3.00 and 7.00 p.m. under diffuse natural light from 2 windows supplemented with an artificial light of 100 W located at 1.5 m above the aquaria.
The apple snail and the 3 non-apple snails were put simultaneously on to the bottom of aquaria. The duration of the observation period was 1 h, starting when the P. canaliculata foot first made contact with the bottom. The snails were checked at periods of 5 s to record all the corporal contacts between the individual of P. canaliculata and the individuals of the other snail species. The behavioral responses of these snails to contacts with the shell, foot, or tentacles of P. canaliculata were categorized as: indifference (the snail did not change its previous state or activity after the contact), withdrawal (the snail retracted the cephalopodium within the shell after the contact), and crawling (the snail started crawling after the contact). To test if the frequency of the different responses varied among species an χ2 test was performed.
Results
Experiments to test CEs of P. canaliculata on non-apple snails (CE Experiments)
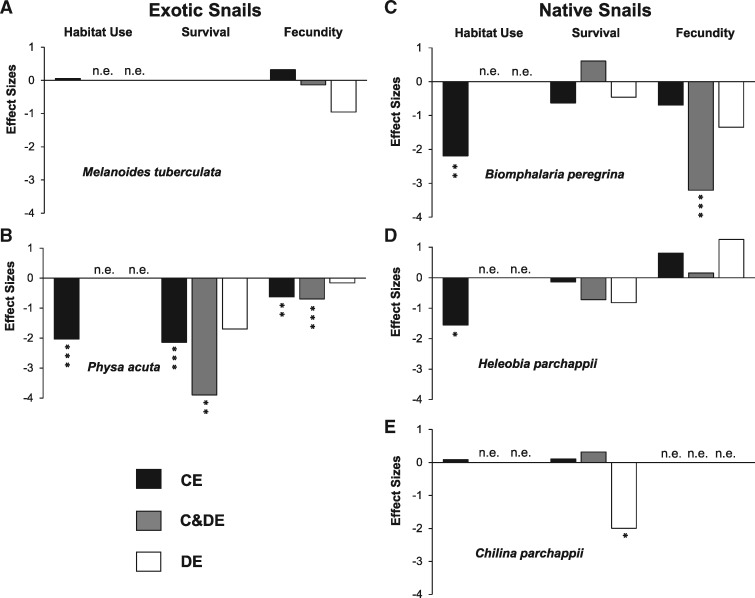
Habitat use was only significantly affected in the exotic P. acuta and in 2 of the native snails (B. peregrina and H. parchappii) (Table 1); in all these cases the use of the compartment with P. canaliculata was reduced by 21.2, 33.1, and 25.3%, respectively, relative to the other compartment. However, taking into account the variability in the treatments, the greatest negative effect sizes on habitat use was observed in B. peregrina and P. acuta and the lowest in H. parchappii (Figure 3).
Table 1.
Paired t-tests to measure CEs of P. canaliculata on habitat use (%), survival (%), fecundity (egg masses, eggs, or newborn per live snail) and damaged egg masses (%) of the 2 exotic and the 3 native non apple-snails species (CE experiments)
| Non apple-snails | Variable | CE |
cCD |
c0D |
|
|---|---|---|---|---|---|
| t9 | P-value | Mean ± SD (n = 10) | Mean ± SD (n = 10) | ||
| Melanoides tuberculata | Habitat use | 0.582 | 0.575 | 49.950 ± 7.365 | 49.550 ± 6.885 |
| Survival | – | – | 100 | 100 | |
| Fecundity | 0.815 | 0.436 | 1.978 ± 0.607 | 1.737 ± 0.884 | |
| Physa acuta | Habitat use | −3.284 | 0.009 | 42.218± 4.972 | 53.590 ± 6.208 |
| Survival | −4.662 | 0.001 | 75.078 ± 13.059 | 94.151 ± 4.770 | |
| Fecundity | −3.652 | 0.005 | 1.736 ± 0.845 | 3.256 ± 0.642 | |
| Damaged egg masses | 7.613 | <0.001 | 53.108 ± 20.598 | 2.551 ± 3.621 | |
| Biomphalaria peregrina | Habitat use | −3.476 | 0.007 | 37.771± 8.775 | 56.474 ± 8.303 |
| Survival | −1.165 | 0.273 | 98.277 ± 4.259 | 99.750 ± 0.420 | |
| Fecundity | −2.047 | 0.071 | 2.119 ± 1.206 | 2.986 ± 1.292 | |
| Damaged egg masses | 9.141 | <0.001 | 78.785 ± 20.841 | 11.453 ±13.067 | |
| Heleobia parchappii | Habitat use | −2.511 | 0.033 | 38.787 ± 8.479 | 51.970 ± 8.501 |
| Survival | −0.320 | 0.756 | 97.170 ± 2.647 | 97.747 ± 5.580 | |
| Fecundity | 3.431 | 0.164 | 2.762 ± 2.491 | 1.384 ± 0.938 | |
| Chilina parchappii | Habitat use | 0.145 | 0.888 | 40.396 ± 8.365 | 39.725 ± 6.778 |
| Survival | 0.251 | 0.808 | 95.322 ± 5.863 | 94.769 ± 4.091 | |
| Fecundity | – | – | 0.016 ± 0.051 | 0.044 ± 0.080 | |
cCD: compartment with P. canaliculata (CEs and DEs); c0D: compartment without P. canaliculata (DEs only).
Figure 3.
Effect sizes on habitat use, survival and fecundity of the two exotic and the three native non apple-snails species due to CEs, DEs, and C&DE of P. canaliculata (CE, C&DE and DE Experiments). (A) M. tuberculata, (B) P. acuta, (C) B. peregrina, (D) H. parchappii, (E) C. parchappii. Asterisks indicate the significance of the effects from Tables 1–3 (*P < 0.05; **P < 0.01; ***P < 0.001). n.e. indicates that the effect was not estimated or that it was not possible to evaluate it.
The exotic M. tuberculata was the only snail in which survival was 100% after the 2 weeks of experiment. Survival was only significantly affected by P. canaliculata in the exotic P. acuta (Figure 3). Survival decreased by 20.3% in the compartment with P. canaliculata.
Fecundity showed no significant CEs of P. canaliculata in most snails; only in the exotic P. acuta a 46.8% decrease was observed in the fecundity (Figure 3a). In the case of C. parchappii only 4 egg masses were detected during the experiment (1 in the compartment with P. canaliculata and 3 in the 1 without it) so the effect on fecundity was not tested.
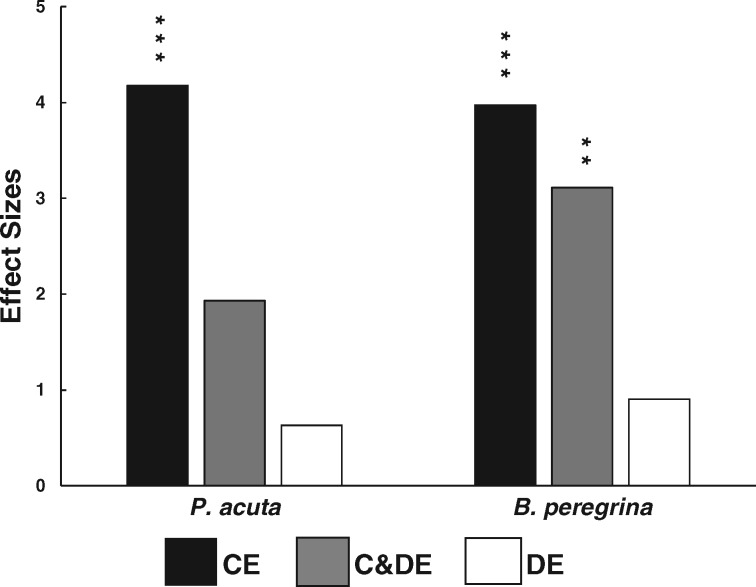
During the analyses of the material from the bottom of the aquaria, remains of soft parts, shell, and egg masses of P. acuta and B. peregrina were found in the feces of P. canaliculata and remains of egg masses were also found attached to the aquarium walls. The percentage of damaged egg masses showed highly significant CEs of P. canaliculata on both species (Table 1). The percentage of damage in the compartment with P. canaliculata increased 20.8 times in P. acuta and 6.9 times in B. peregrina relative to the compartment without it but taking into account the high variability of the data that these two effects were almost the same size (4.17 and 3.97, respectively; Figure 4).
Figure 4.
Effect sizes on the percentage of damaged egg masses of the exotic P. acuta and the native B. peregrina due to the CEs, DEs, and C&DE of P. canaliculata (CE, C&DE, and DE Experiments). Asterisks indicate the significance of the effects from Tables 1–3 (*P < 0.05; **P < 0.01; ***P < 0.001).
Experiments to test DEs alone or combined with CEs of P. canaliculata on non-apple snails (C&DE and DE Experiments)
The only snail whose survival showed a significant combination of contact and DEs of P. canaliculata was the exotic P. acuta (Table 2; Figure 3). The survival of P. acuta decreased by 30.5% in the compartment with P. canaliculata relative to the compartment in the control aquarium without it (Figure 3a). The survival of the exotic M. tuberculata was 100% at the end of the experiment (Figure 3e).
Table 2.
Independent t-tests to measure combined contact and distant effects (C&DE) of P. canaliculata on survival (%), fecundity (egg masses, eggs, or newborn per live snail), and damaged egg masses (%) of the 2 exotic and the 3 native non-apple snail species (C&DE and DE experiments)
| Non apple-snails | Variable | C&DE |
cCD |
c00 |
|
|---|---|---|---|---|---|
| t8 | P-value | Mean ± SD (n = 5) | Mean ± SD (n = 5) | ||
| Melanoides tuberculata | Survival | – | – | 100 | 100 |
| Fecundity | −0.216 | 0.834 | 1.412 ± 0.449 | 1.489 ± 0.667 | |
| Physa acuta | Survival | −6.158 (4.232) | 0.003 | 68.606 ± 10.782 | 98.729 ± 1.837 |
| Fecundity | −6.364 | < 0.001 | 0.778 ± 0.649 | 5.803 ± 1.641 | |
| Damaged egg masses | 2.732 (3.002) | 0.72 | 48.33 ± 35.119 | 0.34 ± 0.771 | |
| Biomphalaria peregrina | Survival | 1.000 (4.000) | 0.374 | 100 ± 0 | 99.833 ± 0.372 |
| Fecundity | −5.059 | 0.001 | 2.466 ± 1.433 | 7.131 ± 1.482 | |
| Damaged egg masses | 4.922 (4.156) | 0.007 | 41.741 ± 18.267 | 1.142 ± 2.555 | |
| Heleobia parchappii | Survival | −1.136 | 0.289 | 98.622 ± 0.690 | 99.267 ± 1.065 |
| Fecundity | 0.253 | 0.807 | 0.916 ± 0.740 | 0.822 ± 0.391 | |
| Chilina parchappii | Survival | 0.499 | 0.632 | 82.590 ± 12.829 | 78.041 ± 15.864 |
| Fecundity | – | – | 0 | 0 | |
Note: When the variances were significantly different the numbers between parentheses indicate the degrees of freedom of the welch t-test.
cCD: compartments with P. canaliculata (contact and distant effects); c00: control compartment (no effects; n = 5).
There was a significant decrease in fecundity relative to the controls in P. acuta and in B. peregrina (86.7 and 65.5%, respectively). However, the size of the combined negative effects of P. canaliculata was 4.6 times higher in B. peregrina than in P. acuta (Figures 3a,b). No egg masses were laid by C. parchappii in this experiment. Scarce remains of soft parts, shell, and egg masses of P. acuta and B. peregrina were found in the feces of P. canaliculata. A few damaged egg masses were found in the control aquaria of P. acuta and B. peregrina. The percentage of egg mass damage in the compartment with P. canaliculata only showed a significant increase relative to the control aquaria in B. peregrina (35.6%; Table 2; Figure 4).
The tests performed to detect DE alone by comparing a compartment without P. canaliculata relative to a random compartment of the control aquaria showed significant and marginally significant decreases, respectively, on the survival of P. acuta and C. parchappii (4.6 and 14.4%; Table 3). The size of the effects in these 2 species was similar (Figures 3a,d).
Table 3.
Independent t-tests to measure DEs of P. canaliculata on survival (%), fecundity (egg masses, eggs, or newborn per live snail), and damaged egg masses (%) of the 2 exotic and the 3 native non-apple snail species (C&DE and DE experiments)
| Non apple-snails | Variable | DE |
c0D |
c00 |
|
|---|---|---|---|---|---|
| t8 | P-value | Mean ± SD (n = 5) | Mean ± SD (n = 5) | ||
| Melanoides tuberculata | Survival | – | – | 100 | 100 |
| Fecundity | −1.502 | 0.172 | 0.827 ± 0.452 | 1.182 ± 0.271 | |
| Physa acuta | Survival | −2.692 (4.057) | 0.054 | 95.284 ± 3.785 | 99.857 ± 0.319 |
| Fecundity | −1.571 | 0.155 | 3.936 ± 1.515 | 6.188 ± 2.824 | |
| Damaged egg masses | 1.000 (4.000) | 0.374 | 2.22 ± 4.969 | 0 | |
| Biomphalaria peregrina | Survival | −0.729 | 0.487 | 98.996 ± 2.243 | 99.750 ± 0.559 |
| Fecundity | −2.129 | 0.066 | 5.087 ± 0.498 | 6.745 ± 1.669 | |
| Damaged egg masses | 1.435 | 0.189 | 3.508 ± 4.727 | 0.416 ± 0.931 | |
| Heleobia parchappii | Survival | −1.297 | 0.231 | 99.453 ± 0.649 | 99.867 ± 0.298 |
| Fecundity | 1.987 | 0.082 | 0.935 ± 0.707 | 0.297 ± 0.122 | |
| Chilina parchappii | Survival | −3.150 | 0.014 | 77.835 ± 5.449 | 90.973 ± 7.568 |
| Fecundity | – | – | 0 | 0 | |
Note: When the variances were significantly different the numbers between parentheses indicate the degrees of freedom of the welch t-test.
c0D: compartment without P. canaliculata (distant effects only); c00: control compartment (no effects).
The percentage of damaged egg mass in the compartment with only DEs of P. canaliculata did not increase significantly relative to the control aquaria in P. acuta and in B. peregrina (Table 3; Figure 4). A few damaged egg masses were found in the control aquaria of B. peregrina but none in those of P. acuta.
Experiment to test the behavioral responses of non-apple snails to contacts with P. canaliculata
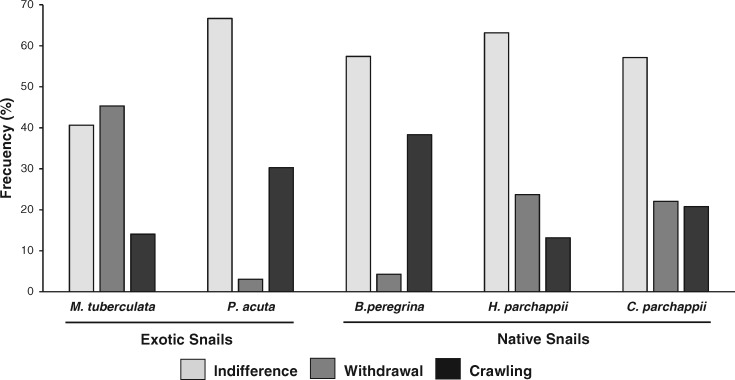
In most (55.2%) of the corporal contacts with an individual of P. canaliculata (n = 259) the snails showed no change in their state or activity. The 2 active responses to corporal contact (crawling and withdrawal) were equally frequent on the whole (22.4%). However, the frequency of these three responses was significantly different between the 5 non-apple snails species (χ28 = 41.0, P > 0.001; Figure 5).
Figure 5.
Frequency (%) of indifference, withdrawal, and crawling to corporal contacts with P. canaliculata for each of the 2 exotic and the 3 native non-apple snail species (behavioral responses Experiment).
The exotic M. tuberculata was the snail with the lowest frequency of indifference to corporal contacts with P. canaliculata (40.6%). In the other 4 species indifference was by far the most frequent response to corporal contact (57.1–66.6%). Melanoides tuberculata showed the highest frequency of withdrawal after corporal contact (45.3%) whereas the other exotic snail (P. acuta) and 1 of the natives (B. peregrina) showed the lowest values (3.0 and 4.2%, respectively); the other 2 native snails showed values of 22.1 and 23.7%. The highest and the lowest frequency of crawling upon corporal contact were observed in 2 native snails: B. peregrina (38.3%) and H. parchappii (13.1%). On 2 occasions, 1 individual of H. parchappii entered into the shell umbilicus of P. canaliculata and remained there until the end of the observation period. Specimens of H. parchappii, P. acuta, and B. peregrina crawled onto the shell of P. canaliculata on rare occasions.
Discussion
Our experiments showed that all the species of freshwater snails tested can be negatively affected by contact interactions or at a distance with P. canaliculata, except for the exotic M. tuberculata. The possibility of facilitative interactions cannot be ruled out a priori, for instance, in coprophagous or detrivorous snail species that may benefit from the large amount of particulate organic matter that P. canaliculata produces as feces (Castro-Vazquez et al. 2002). Notwithstanding, in the present study any positive effects were overcome by negative ones. However, there were important differences between the snail species in which the traits were negatively affected by the apple snail and in how and by how often they responded upon corporal contact with it. On the whole, the negative effects and behavioral responses to P. canaliculata varied strongly between the 2 exotic snails and also between the 3 native ones.
The P. canaliculata snails that we used in our experiences have been in contact with all the snail species (except for M. tuberculata) in the channel in which they were collected. Consequently, the interspecific differences in the effects of P. canaliculata were not attributable to the lack of previous experience with some of them and not with others. However, it is possible that previous experience enhanced the predatory attacks of P. canaliculata on some snail species or their egg masses more than on others. Pomacea canaliculata exhibits associative learning but this capacity is significantly weakened after a few days (Aizaki and Yusa 2010). As the apple snails were fed on lettuce and fish food for at least a month before using them, any learnt response against a given snail species had probably vanished by the time of the experiments. On the other hand, all the individuals of the 5 species used in the experiments were naïve about the behavior of P. canaliculata, since the latter is not present in streams where they were collected. Hence the interspecific differences in the behavioral responses to P. canaliculata did not depend on the occurrence of previous contacts between them but were probably due to innate unspecific responses. However, these innate responses cannot be attributed to the existence or lack of a coevolutive history since the responses were very variable between both natives and exotics.
The effects of P. canaliculata on the habitat use of the other snail species were not associated with their exotic or native status but with their specific behavioral responses and shell sizes. Habitat use was negatively affected in the exotic P. acuta and the native B. peregrina and H. parchappii with similar intensity. On the contrary, it was not affected in the native C. parchappii and in the exotic M. tuberculata, which are the two largest species that also showed infrequent crawling responses upon contact with P. canaliculata. In contrast, the high frequency of crawling responses of P. acuta and B. peregrina upon corporal contacts (30 and 38%, respectively) may explain their reduced use of the compartment with P. canaliculata, as snails with this type of response would tend to move away. In addition, as remains of shells and soft parts were found in the feces of P. canaliculata in both cases, the avoidance response may be related to the release of chemical cues from injured or eaten conspecifics, a frequent response in freshwater snails (e.g., Alexander and Covich 1991; Turner 1996; Ueshima and Yusa 2015). The remaining species in which habitat use was negatively affected (H. parchappii) showed a very low frequency of crawling response upon corporal contact with P. canaliculata (13%). Perhaps the change in habitat use in this species, the smallest in our study, was not related to the corporal contacts with the snail itself, but to the avoidance of mucus trails of P. canaliculata, as has been reported in another small freshwater snail as related to a large one (Karowe et al. 1993). The mucus trails of P. canaliculata are very wide (2–3 cm) and after a few days probably most of the compartment surfaces would be covered by its mucus.
The scarce CEs of P. canaliculata on the survival of other snails in its native area found here are at odds with the strong effects detected in an invaded area. In Hong Kong, Kwong et al. (2009) reported significant mortalities of the adults of the 3 species of pulmonates due to P. canaliculata predation, but not of those of 2 large-sized prosobranchs. The overall differences may be related to a 60% higher density of prey (1.875 vs. 1.176 snails per liter) or to a 6.4 times higher density of P. canaliculata (0.375 vs. 0.059 snails per liter) in the study in Hong Kong relative to the present one. Moreover, the snails in that study were not able to escape from P. canaliculata predation as there were no refuges where the apple snail was excluded. Even though the duration of our trials was 5 times longer, all these differences combined probably increased the predation chances relative to our study.
The effects of P. canaliculata on the survival of the other snail species were not consistently different between natives and exotics, since the survival of the exotic P. acuta was only significantly reduced by contact effects, either alone or in combination with DEs. The lack of effects on the exotic M. tuberculata was probably related to a combination of a large strong shell and a greater frequency of withdrawal upon contact with P. canaliculata. The remaining parts of shells and soft parts of P. acuta in the feces of P. canaliculata indicates that some snails were eaten but it does not show whether it was due to predation, opportunistic predation of moribund snails, or necrophagy. On the other hand, the fact that survival was very high in the controls (94.1 and 98.7%) indicates that at least part of these decreases in survival was due to corporal contact with P. canaliculata, probably by attacking the P. acuta snails with its jaws and radula, as was observed by Kwong et al. (2009).
The fact that only the survival of the exotic P. acuta was affected by P. canaliculata contacts cannot be explained solely by its behavior. The native B. peregrina exhibited a very similar pattern of behavioral responses and of changes in habitat use and notwithstanding showed no CEs on mortality. In contrast to P. acuta, the shell of B. peregrina possesses a much narrower aperture that renders it less susceptible to the attack of its soft parts by P. canaliculata, which needs to perforate the shell of this species to reach the flesh (Cazzaniga 1990). The possession of an operculum, in addition to narrow apertures (Kelly and Cory 1987; Kwong et al. 2009), probably further reduces the susceptibility of M. tuberculata and H. parchappii relative to P. acuta. The shell and aperture shapes of C. parchappii and P. acuta are similar, but the former is twice as large in size and possesses a very wide strong foot that allows for a very much higher tenacity than in P. acuta, that has a long narrow foot. The relative size of the snails is relevant to the mortality rates since P. canaliculata juveniles are always less lethal than adults when preying on other snails (Kwong et al. 2009).
DEs of P. canaliculata per se on the other snail species were neither frequent nor strong. They were marginally significant in the exotic P. acuta survival and they were significant in combination with contact ones. These apparently additive effects may be mediated by dissolved substances, probably urea (see below), since oxygen was not depleted in the aquaria and P. acuta had plenty access to the surface for using atmospheric oxygen.
The effects of P. canaliculata on reproduction did not differ between the exotic and native snails. P. canaliculata only negatively affected the fecundity of the 2 species of the pulmonates, 1 of them exotic (P. acuta), and the other native (B. peregrina), that laid gelatinous egg masses in our study. In both cases, CEs alone or in combination with those at a distance were significant but DEs per se were never significant. In our experiments we calculated fecundity on the basis of the number of eggs, egg masses, or newborn that were produced and lasted up to the end of each week of the experiment. Thus, the CEs on the fecundity of these 2 species may be related to the avoidance of oviposition on substrates with mucus or feces of P. canaliculata but also to the ingestion of whole egg masses. In the exotic P. acuta the CEs seem to be more important in reducing fecundity and the distant ones only worsened them slightly.
The negative DEs of P. canaliculata on the fecundity of B. peregrina, although not significant per se, were clearly additive or synergic with the contact ones and may be mediated by an increase in the concentration of urea (Smith et al. 1994), which is the second nitrogenous compound in importance in the soluble excreta of P. canaliculata (Vega et al. 2007).
A much higher percentage of damaged egg masses was found in compartments in which P. canaliculata was present, irrespective of the native (B. peregrina) or exotic status (P. acuta). The damaged egg masses were both uneaten pieces of egg masses and others that had passed through the digestive system of P. canaliculata, which indicates that egg mass predation had in fact occurred and it was not just the result of bulldozing. According to Turner et al. (2007), the globose egg masses of P. acuta are highly vulnerable to predation even by small pulmonate snails. However, in the present study the predation rates by P. canaliculata were similar to those on the more compact and flat egg masses of B. peregrina, indicating that the traits that allow resistance to predation by pulmonates (Turner et al. 2007) are not effective against the powerful jaws and radula of P. canaliculata. The only oviparous species in which no significant effect of P. canaliculata was detected on fecundity was H. parchappii. The lack of CEs like predation may be due the small size of the eggs and to the fact that they are deposited in isolation (Martín 2002) and not as egg masses, which would make them harder to detect or to detach than the gelatinous egg masses of the 2 pulmonates.
The fecundity of the exotic M. tuberculata, which combines the release of large, strong-shelled newborn with their survival, was not affected by P. canaliculata in our study. However, in Hong Kong, Kwong et al. (2009) found that adult P. canaliculata consumed 42% of the newborn of M. tuberculata, probably due to the higher ratio of M. tuberculata newborns to P. canaliculata in that study as compared to ours (20:1 vs. 7.23:1).
The negative CEs of P. canaliculata on the survival of P. acuta adults and the egg masses of P. acuta and B. peregrina were probably due to predation and occurred even when very palatable vegetal food (lettuce) was provided in excess. This probably indicates that the egg masses of pulmonate snails, and perhaps the adults of some species, are a normal trophic resource for P. canaliculata, as also occurs in other apple snails (e.g., Hubendick 1966; Cazzaniga and Estebenet 1984; Hofkin et al. 1991; Stryker et al. 1991; Aditya and Raut 2002).
On the whole, we found no consistent differences between exotic and native snails in the negative effects and behavioral responses to P. canaliculata. The native species differed in the degree in which they were affected by P. canaliculata, with B. peregrina being the most and C. parchappii the least affected. Regarding the 2 exotic species, they differed even more: P. acuta was the one with more profound harmful effects in our study whereas M. tuberculata was not affected at all. This indicates that the harmful effects of the apple snail are more related to specific traits of the other snails than to a shared or different geographic origin. A similar situation was reported recently for the grazing effects of apple snails on aquatic macrophytes (Grutters et al. 2017), which stresses the importance of specific defensive traits against generalist consumers.
The role of some species in the biotic resistance is expected to be enhanced if they combine competition and predation over the newcomers (intraguild predation; Britton 2012; Takizawa and Snyder 2012). The abundance of P. canaliculata in its native range and the bioecological traits that make it a hyper-successful invader indicate that competition and intraguild predation by this species may play an important role in the biotic resistance to exotic snails in its native range. However, our results indicate that the biotic resistance exerted by P. canaliculata is species-specific, being strong against P. acuta but extremely weak against M. tuberculata. The results obtained in both the native and exotic range (this study; Kwong et al. 2009) suggest that in general its biotic resistance would be higher against small snails without an operculum that lay gelatinous egg masses than that exerted on large operculate snails that give birth to their offspring.
The co-occurrence of multiple invaders is becoming increasingly common and this prompted the study of the interactions between these species since their negative, neutral, or positive characters may have profound effects on the impacts or their chances of establishment (Simberloff and Von Holle 1999; Kuebbing and Nuñez 2015; Jackson 2015). Our results show that P. canaliculata can negatively affect other snail species, and could influence the structure of snail communities both in its native and invaded ranges through differential effects on other species. Melanoides tuberculata is a widespread invader in tropical and subtropical areas and a superior competitor of freshwater pulmonate snails in stable habitats but not in unstable ones (Duggan 2002; Facon et al. 2003). Due to the fact that the negative effects of P. canaliculata on M. tuberculata are much weaker than on pulmonate snails, it is possible that it would displace the competitive balance to favor the latter and facilitate its establishment and dominance in a wider range of habitats.
The invasion process of M. tuberculata is difficult to trace to its origins but nonetheless, nowadays it has reached a pantropical distribution (Facon et al. 2003) whereas that of apple snails is much more recent and still in progress (Gilioli et al. 2017). Co-occurring invaders can either mitigate or enhance each other’s impacts on individual species or ecosystem processes (Kuebbing and Nuñez 2015; Jackson 2015). For instance, the negative interaction between 2 invaders may result in nonadditive impacts on native species, with 1 of the invaders mitigating the negative effects of the other 1 when occurring at high densities (Liu et al. 2018). The indirect facilitation of P. canaliculata on M. tuberculata may show that the negative impacts of these 2 widespread invasive snails on pulmonates may not be additive but synergic. The situations in which the apple snail becomes established in habitats already invaded by M. tuberculata will probably escalate in future decades, thus increasing the chances of enhancing harmful effects on native species.
Acknowledgments
We are grateful to Enzo Manara for his help with snail collection. We also wish to thank the criticisms and suggestions of two anonymous reviewers.
Funding
This work was funded with grants from UNS (“Universidad Nacional del Sur”, PGI 24/B185 and 24/B232) and ANPCyT (“Agencia Nacional de Promoción Científica y Tecnológica, PICT 2012-1956). MAM is a Doctoral fellow in ANPCyT and CONICET. PRM is a researcher in CONICET.
References
- Aditya G, Raut SK, 2002. Destruction of Indoplanorbis exustus (Planorbidae) eggs by Pomacea bridgesi (Ampullariidae). Molluscan Res 22:87–90. [Google Scholar]
- Aizaki K, Yusa Y, 2010. Learned predator recognition in a freshwater snail Pomacea canaliculata. Malacologia 52:21–29. [Google Scholar]
- Alexander JE, Covich A, 1991. Predator avoidance by the freshwater snail Physella virgate in response to the crayfish Procambarus simulans. Oecología 87:435–442. [DOI] [PubMed] [Google Scholar]
- Alofs KM, Jackson DA, 2014. Meta-analysis suggests biotic resistance in freshwater environments is driven by consumption rather than competition. Ecology 95:3259–3270. [Google Scholar]
- Anto F, Bosompem K, Kpikpi J, Adjuik M, Edoh D, 2005. Experimental control of Biomphalaria pfeifferi, the intermediate host of Schistosoma mansoni, by the ampullariid snail Lanistes varicus. Ann Trop Med Parasit 93:203–209. [DOI] [PubMed] [Google Scholar]
- Britton JR, 2012. Testing strength of biotic resistance against an introduced fish: inter-specific competition or predation through facultative piscivory? PLoS ONE 7: e31707.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers JE, Noonburg EG, 2003. Scale dependent effects of biotic resistance to biological invasion. Ecology 84:1428–1433. [Google Scholar]
- Carlsson NO, Brönmark C, Hanson LA, 2004. Invading herbivory: the golden apple snail alters ecosystem functioning in Asian wetlands. Ecology 85:1575–1580. [Google Scholar]
- Carlsson NO, Sarnelle O, Strayer DL, 2009. Native predators and exotic prey-an acquired taste? Front Ecol Environ 7:525–532. [Google Scholar]
- Castro-Vazquez A, Albrecht EA, Vega IA, Koch E, Gamarra-Luques C, 2002. Pigmented corpuscles in the midgut gland of Pomacea canaliculata and other Neotropical apple-snails (Prosobranchia, Ampullariidae): a possible symbiotic association. Biocell 26:101–109. [PubMed] [Google Scholar]
- Cazzaniga NJ, 1990. Predation of Pomacea canaliculata (Ampullariidae) on adult Biomphalaria peregrina (Planorbidae). Ann Trop Med Parasit 84:97–100. [DOI] [PubMed] [Google Scholar]
- Cazzaniga NJ, Estebenet AL, 1984. Revisión y notas sobre los hábitos alimentarios de los Ampullariidae (Gastropoda). Historia Natural 4:213–224. [Google Scholar]
- Chaichana R, Sumpan T, 2014. The potential ecological impact of the exotic snail Pomacea canaliculata on the Thai native snail Pila scutata. Science Asia 40:11–15. [Google Scholar]
- Conner SL, Pomory CM, Darby PC, 2008. Density effects of native and exotic snails on growth in juvenile apple snails Pomacea paludosa (Gastropoda: Ampullariidae): a laboratory experiment. J Mollus Stud 74:355–362. [Google Scholar]
- Cope NJ, Winterbourn MJ, 2004. Competitive interactions between two successful molluscan invaders of freshwaters: an experimental study. Aquat Ecol 38:83–91. [Google Scholar]
- Duggan IC, 2002. First record of a wild population of the tropical snail Melanoides tuberculata in New Zealand natural waters. New Zeal. J Mar Fresh 36:825–829. [Google Scholar]
- Estebenet AL, Martín PR, 2002. Pomacea canaliculata (Gastropoda: Ampullariidae): life-history traits and their plasticity. Biocell 26:83–89. [PubMed] [Google Scholar]
- Facon B, Pointier JP, Glaubrecht M, Poux C, Jarne P. et al. 2003. A molecular phylogeography approach to biological invasions of the New World by parthenogenetic Thiarid snails. Mol Ecol 12:3027–3039. [DOI] [PubMed] [Google Scholar]
- Früh D, Haase P, Stoll S, 2017. Temperature drives asymmetric competition between alien and indigenous freshwater snail species, Physa acuta and Physa fontinalis. Aquat Sci 79:187–195. [Google Scholar]
- Gilioli G, Pasquali S, Martín PR, Carlsson N, Mariani L, 2017. A temperature-dependent physiologically based model for the invasive apple snail Pomacea canaliculata. Int J Biometeorol 61:1899–1911. [DOI] [PubMed] [Google Scholar]
- Grutters B, Roijendijk YO, Verberk WC, Bakker ES, 2017. Plant traits and plant biogeography control the biotic resistance provided by generalist herbivores. Funct Ecol 31:1184–1192. [Google Scholar]
- Hayes KA, Cowie RH, Thiengo SC, Strong EE, 2012. Comparing apples with apples: clarifying the identities of two highly invasive Neotropical Ampullariidae (Caenogastropoda). Zool J Linn Soc 166:723–753. [Google Scholar]
- Hayes KA, Burks RL, Castro-Vázquez A, Darby PC, Heras H. et al. 2015. Insights from an integrated view of the biology of apple snails (Caenogastropoda: Ampullariidae). Malacologia 58:245–302. [Google Scholar]
- Hofkin BV, Stryker GA, Koech DK, Loker ES, 1991. Consumption of Biomphalaria glabrata egg masses and juveniles by the ampullariid snails Pila ovata, Lanistes carinatus and Marisa cornuarietis. Acta Trop 49:37–44. [DOI] [PubMed] [Google Scholar]
- Horgan FG, Stuart AM, Kudavidanage EP, 2014. Impact of invasive apple snails on the functioning and services of natural and managed wetlands. Acta Oecol 54:90–100. [Google Scholar]
- Hubendick B, 1966. Some aspects of vector snail control. Malacologia 5:31–32. [Google Scholar]
- Jackson MC, 2015. Interactions among multiple invasive animals. Ecology 96:2035–2041. [DOI] [PubMed] [Google Scholar]
- Jeschke J, Aparicio LG, Haider S, Heger T, Lortie C. et al. 2012. Support for major hypotheses in invasion biology is uneven and declining. NeoBiota 14:1–20. [Google Scholar]
- Karowe DN, Pearce TA, Spaller WR, 1993. Chemical communication in freshwater snails: behavioral responses of Physa parkeri to mucous trails of P. parkeri (Gastropoda: Pulmonata) and Campeloma decisum (Gastropoda: Prosobranchia). Malacol Rev 26:9–14. [Google Scholar]
- Kawata M, Ishigami H, 1992. The growth of juvenile snails in water conditioned by snails of a different species. Oecologia 91:245–248. [DOI] [PubMed] [Google Scholar]
- Kelly PM, Cory JS, 1987. Operculum closing as a defense against predatory leeches in four British freshwater prosobranch snails. Hydrobiologia 144:121–124. [Google Scholar]
- Kuebbing SE, Nuñez MA, 2015. Negative, neutral, and positive interactions among nonnative plants: patterns, processes, and management implications. Global Change Biol 21:926–934. [DOI] [PubMed] [Google Scholar]
- Kwong KL, Chan RKY, Qiu JW, 2009. The potential of the invasive snail Pomacea canaliculata as a predator of various life-stages of five species of freshwater snails. Malacologia 51:343–356. [Google Scholar]
- Kwong KL, Dudgeon D, Wong PK, Qiu JW, 2010. Secondary production and diet of an invasive snail in freshwater wetlands: implications for resource utilization and competition. Biol Invasions 12:1153–1164. [Google Scholar]
- Lakens D, 2013. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol 4:863.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson MD, Black AR, 2016. Assessing interactions among native snails and the invasive New Zealand mud snail Potamopyrgus antipodarum using grazing experiments and stable isotope analysis. Hydrobiologia 763:147–159. [Google Scholar]
- Levine JM, Adler PB, Yelenik SG, 2004. A meta-analysis of biotic resistance to exotic plant invasions. Ecol Lett 7:975–989. [Google Scholar]
- Liu X, Wang S, Ke Z, Cheng C, Wang Y. et al. 2018. More invaders do not result in heavier impacts: the effects of non-native bullfrogs on native anurans are mitigated by high densities of non-native crayfish. J Anim Ecol 87:850–862. [DOI] [PubMed] [Google Scholar]
- Martín PR, 2002. Evidence for parthenogenesis and natural imposes in the Patagonian freshwater snail Heleobia hatcheri (Gastropoda: Hydrobiidae). J Mollus Stud 68:291–295. [Google Scholar]
- Martín PR, De Francesco CG, 2006. Fossil record of Pomacea (Caenogastropoda: ampullariidae) in Argentina and its paleoenvironmental implications. Biocell 30:337–343. [PubMed] [Google Scholar]
- Martín PR, Estebenet AL, Cazzaniga NJ, 2001. Factors affecting the distribution of Pomacea canaliculata (Gastropoda: ampullariidae) along its southernmost natural limit. Malacologia 43:13–23. [Google Scholar]
- Martín PR, Ovando XMC, Seuffert ME, 2016. First record of the freshwater snail Pseudosuccinea columella (Gastropoda: lymnaeidae) in southern Pampas (Argentina) and assessment of future spread. Molluscan Res 36:213–221. [Google Scholar]
- Morrison WE, Hay ME, 2011. Feeding and growth of native, invasive and non-invasive alien apple snails (Ampullariidae) in the United States: invasives eat more and grow more. Biol Invasions 13:945–955. [Google Scholar]
- Nghiem LT, Soliman T, Yeo DC, Tan HT, Evans TA. et al. 2013. Economic and environmental impacts of harmful non-indigenous species in Southeast Asia. PLoS ONE 8:e71255. doi: 10.1371/journal.pone.0071255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JD, Hay ME, 2005. Biotic resistance to plant invasions? Native herbivores prefer non-native plants. Ecol Lett 8:959–967. [DOI] [PubMed] [Google Scholar]
- Posch H, Garr AL, Reynolds E, 2013. The presence of an exotic snail Pomacea maculata inhibits growth of juvenile Florida apple snails, Pomacea paludosa. J Mollus Stud 79:383–385. [Google Scholar]
- Raw JL, Miranda NA, Perissinotto R, 2013. Chemical cues released by an alien invasive aquatic gastropod drive its invasion success. PLoS ONE 8: e64071.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raw JL, Miranda NA, Perissinotto R, 2015. Chemical cues released by heterospecific competitors: behavioural responses of native and alien invasive aquatic gastropods. Aquat Sci 77:655–666. [Google Scholar]
- Rumi A, Gutiérrez Gregoric DE, Núñez V, César II. et al. 2006. Freshwater Gastropoda from Argentina: species richness, distribution patterns, and an evaluation of endangered species. Malacologia 49:189–208. [Google Scholar]
- Saveanu L, Manara E, Martín PR, 2017. Carrion consumption and its importance in a freshwater trophic generalist: the invasive apple snail Pomacea canaliculata. Mar Freshwater Res 68:752–759. [Google Scholar]
- Seuffert ME, Martín PR, 2013. Distribution of the apple snail Pomacea canaliculata in Pampean streams (Argentina) at different spatial scales. Limnologica 43:91–99. [Google Scholar]
- Simberloff D, Von Holle B, 1999. Positive interactions of nonindigenous species: invasional meltdown? Biol Invasions 1:21–32. [Google Scholar]
- Simberloff D, Martin JL, Genovesi P, Maris V, Wardle DA. et al. 2013. Impacts of biological invasions: what's what and the way forward. Trends Ecol Evol 28:58–66. [DOI] [PubMed] [Google Scholar]
- Smith ME, Steiner SA, Isseroff H, 1994. Urea: inhibitor of growth and reproduction in Bulinus truncatus. Comp Biochem Phys A 108:569–577. [Google Scholar]
- Stryker GA, Koech DK, Loker ES, 1991. Growth of Biomphalaria glabrata populations in the presence of the ampullariid snails Pila ovata, Lanistes carinatus and Marisa cornuarietis. Acta Trop 49:137–147. [DOI] [PubMed] [Google Scholar]
- Takizawa T, Snyder WE, 2012. Alien vs. predator: could biotic resistance by native generalist predators slow lady beetle invasions? Biol Control 63:79–86. [Google Scholar]
- Tan SK, Lee YL, Ng TH, 2013. The status of the apple snail Pila scutata (Gastropoda: Ampullariidae) in Singapore. Nature in Singapore 6:135–141. [Google Scholar]
- Tiecher MJ, Seuffert ME, Burela S, Martín PR, 2017. Life table and demographic parameters of the Neotropical apple snail Asolene platae (Caenogastropoda, Ampullariidae). Am Malacol Bull 35:1–7. [Google Scholar]
- Traveset A, Richardson DM, 2014. Mutualistic interactions and biological invasions. Annu Rev Ecol Evol Syst 45:89–113. [Google Scholar]
- Tricarico E, Junqueira AO, Dudgeon D, 2016. Alien species in aquatic environments: a selective comparison of coastal and inland waters in tropical and temperate latitudes. Aquat Conserv 26:872–891. [Google Scholar]
- Turner AM, 1996. Freshwater snails alter habitat use in response to predation. Anim Behav 51:747–756. [Google Scholar]
- Turner AM, Turner RR, Ray SR, 2007. Competition and intraguild egg predation among freshwater snails: re-examining the mechanism of interspecific interactions. Oikos 116:1895–1903. [Google Scholar]
- Ueshima E, Yusa Y, 2015. Antipredator behaviour in response to single or combined predator cues in the apple snail Pomacea canaliculata. J Mollus Stud 81:51–57. [Google Scholar]
- Vega IA, Giraud-Billoud M, Koch E, Gamarra-Luques C, Castro-Vazquez A, 2007. Uric acid accumulation within intracellular crystalloid corpuscles of the midgut gland in Pomacea canaliculata (Caenogastropoda, Ampullariidae). Veliger 48:276–283. [Google Scholar]
- Zar JH, 1984. Biostatistical Analysis. 2nd edn. New Jersey: Prentice Hall International. [Google Scholar]
- Zukowski S, Walker KF, 2009. Freshwater snails in competition: alien Physa acuta (Physidae) and native Glyptophysa gibbosa (Planorbidae) in the River Murray, South Australia. Mar Freshwater Res 60:999–1005. [Google Scholar]