Abstract
Nanomotors in nanotechnology are as important as engines in daily life. Many ATPases are nanoscale biomotors classified into three categories based on the motion mechanisms in transporting substrates: linear, rotating, and the recently discovered revolving motion. Most biomotors adopt a multisubunit ring-shaped structure that hydrolyzes ATP to generate force. How these biomotors control the motion direction and regulate the sequential action of their multiple subunits is intriguing. Many ATPases are hexameric with each monomer containing a conserved arginine finger. This review focuses on recent findings on how the arginine finger controls motion direction and coordinates adjacent subunit interactions in both revolving and rotating biomotors. Mechanisms of intersubunit interactions and sequential movements of individual subunits are evidenced by the asymmetrical appearance of one dimer and four monomers in high-resolution structural complexes. The arginine finger is situated at the interface of two subunits and extends into the ATP binding pocket of the downstream subunit. An arginine finger mutation results in deficiency in ATP binding/hydrolysis, substrate binding, and transport, highlighting the importance of the arginine finger in regulating energy transduction and motor function. Additionally, the roles of channel chirality and channel size are discussed as related to controlling one-way trafficking and differentiating the revolving and rotating mechanisms. Finally, the review concludes by discussing the conformational changes and entropy conversion triggered by ATP binding/hydrolysis, offering a view different from the traditional concept of ATP-mediated mechanochemical energy coupling. The elucidation of the motion mechanism and direction control in ATPases could facilitate nanomotor fabrication in nanotechnology.
Keywords: ATPase, biomotor mechanism, arginine finger, Walker A motif, Walker B motif, channel size, channel chirality, entropy driven
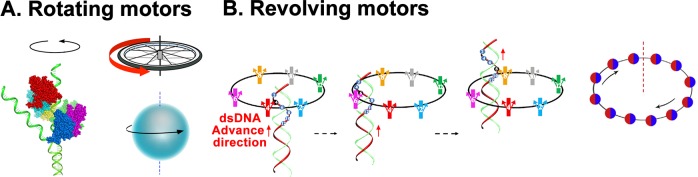
Biomotors, also known as biological motors, are nanoscale machines ubiquitous in many biological processes,1−3 such as cell mitosis, bacterial binary fission,4,5 DNA replication,6−8 DNA repair,9−12 homologous recombination,13−15 RNA transcription,16 macromolecule trafficking,17 and viral genome packaging.18−41 Biomotors are essential protein devices that convert an energy source into different kinds of mechanical motions essential to cellular functions.42 Many of them display a hexameric ring structure.41,43−55 With the recent discovery of a revolving biomotor,56,57 biological motors can be classified into three categories1−3 based on the movement mode of transporting their substrates: linear, rotating, and revolving.1,5,8,58,59 Specifically, in a rotating motor, the substrate rotates around its own axis, while in a revolving motor, the substrate revolves around the second object (Figure 1). The way that revolving motors work is distinct from rotating motors in that among the multiple parts, only the substrate is circumnavigating. Rotating refers to the action similar to the Earth turning around its own axis every 24 h, while revolving is akin to how the Earth circumnavigates around the Sun every 365 days but without self-rotation. Revolving rather than rotating avoids the coiling and tangling of long polymer chains, such as genomic dsDNAs during translocation. The well-studied rotating motors include F1/F0 ATPase,43−47 DNA helicase,48,49 Rho transcription termination factor,50−53 TrwB,60−65 MCM,66,67 and RepA or RuvB,68−73 all of which have a channel diameter of 1–2 nm.74 Revolving motors include the DNA translocases Ftsk in Gram-negative bacteria,54 SpoIIIE or SftA (YtpS) in Gram-positive bacteria,75 A32 ATPase of poxvirus,76−80 DNA packaging enzyme of adenovirus,81−83 the genome segregation enzymes of mimivirus,2,84−87 as well as the DNA packaging motors of herpesvirus,88−103 SPP1,27 T7,104 HK97,105 P22,106 and Phi29.107 The three classes of biomotors differ in structure and function, but utilize similar mechanisms for force generation to perform mechanical work. More information about the linear, rotating, and revolving motors can be found in recent reviews.1−3
Figure 1.
Illustration of two different types of motors. (A) Rotating motors are like a wheel and like the Earth rotating on its own axis round per day. Reprinted with permission from ref (3), Copyright 2016, American Society for Microbiology, and adapted with permission from ref (74). Copyright 2014 Springer Nature. (B) Revolving motors resemble the Earth revolving around the Sun one round per year without self-rotation. Reprinted with permission from ref (220). Copyright 2014 Elsevier.
The common feature of a multisubunit ring-shaped structure of ATPase motors108,109 has raised an intriguing question on how these biomotors control the direction of their motion and how the sequential action of their individual subunits is regulated. The key driving force in a viral DNA packaging motor is a DNA-dependent ATPase. Although this was first reported more than 30 years ago,18 literature on mechanisms of directional control of ATPase motors has just begun to emerge.31,110−115 The common ATP binding domain116,117 contains highly conserved motifs that form an ATPase activity pocket.117 Previous modeling work on the phi29 gp16 ATPase118 suggested that a conserved arginine residue plays a critical allosteric role in coordinating the sequential hydrolysis on the multisubunit ring, as found in both RNA and DNA packaging motors.110,119−124 This arginine residue was defined as the arginine finger. In this review, we summarize the most recent discoveries on the arginine finger, focusing on its role in motion direction control, sequential intersubunit coordination, and asymmetrical multimer assembly. We also discuss the chirality and size of the DNA transport channel, conformational changes, and entropy conversion of the motors involved in the revolving mechanism. Finally, we present a different perception on ATP chemical energy conversion into physical motion in the hexameric biomotors. The understanding of motor structure, motion mechanism, and direction control of oligomeric ATPases will provide a prototype model for future manufacturing of nanomotors in nanotechnology.110,125,126
Definition and Location of the Arginine Finger in ATP Regulating Complexes
Characteristics of ATP-Activity Pocket
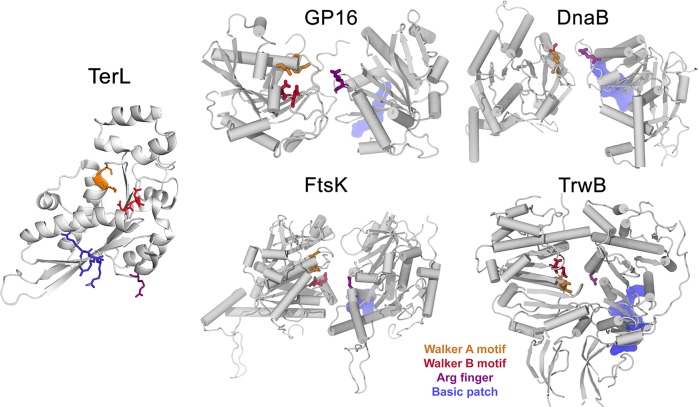
The ATP activity pocket in an ATPase complex typically comprises the following components: arginine finger, Walker A motif, Walker B motif, P-loop, and lid subdomain (Figure 2).127 Hexameric ATPases each contain a conserved core domain, which consists of two conserved sequence motifs termed Walker A and Walker B45,128,129 with a sequence of GXXGXGKS/T and hhhhDE (Figure 3),18 respectively. The Walker A and Walker B motifs have been identified to be responsible for the ATP binding and ATP hydrolysis.18,109,116 Given the conservation of the Walker motifs, it is not surprising that most residues interacting with ATP are intolerant of amino acid alterations. The invariant lysines in the Walker A motif have been intensively studied, revealing their roles in coordinating the ATP β and γ phosphates and structuring the P-loop in related NTPases.43,130 Mutation of lysine to a polar amino acid generally will eliminate the wild-type ATPase function.96 As for the Walker B motif, the conserved negatively charged residues such as glutamate and aspartate act to polarize a water molecule to nucleophilically attack the γ phosphate group of ATP. Most commonly studied mutations are substitutions of glutamate and aspartate by glutamine or alanine.56,110,125,131,132 Upon these mutations, ATP hydrolysis is prevented, but ATP binding is retained.
Figure 2.
Structures of some ATPase domains in their dimer form. The Walker A and Walker B motifs, which form the active site, are colored in orange and red, respectively. The arginine finger is colored in purple. A solvent-exposed basic patch composed of positively charged residues is colored in blue. Adapted with permission from ref (29). Copyright 2015 National Academy of Sciences.
Figure 3.
Sequence alignment reveals conserved motifs18,110 (Walker A motif, Walker B motif,116,117 and Arg finger110,125) across different types of ATPases. Highly conserved residues are highlighted as follows: Orange for Walker A with darkness representing the rate of homology; red for Walker B; and purple for Arg finger. The letter h above the column denotes conserved hydrophobic residues.
For some ATPases, the sensor 1 and 2 motifs have also been reported to play important roles in the ATPase function; however, sensor 1 is not strictly conserved in ATPase proteins, thus whether these two sensors are common features of all ATPases or just an alternate description of the arginine finger requires future verification.29,133−138 Sensor 1 motif is located in the loop connecting the β4 strand to α4 helix. It is often a polar residue thought to interact with the γ-phosphate of ATP. Due to this interaction, it is believed that the sensor 1 motif senses the binding of ATP and orients a water molecule for a nucleophilic attack on the γ-phosphate of the bound ATP molecule. It has been shown in p97 D2 that the shift of the sensor 1 residues, upon nucleotide binding, induces displacements at the distal end of the ATP binding domain.3 Sensor 2, located near the beginning of α7, is conserved in many ATPase proteins. It contains a conserved arginine residue, which, together with the Walker B motif, engages the bound ATP and mediates conformational changes that sequester the catalytic site from water.33,133 Mutations of the sensor 2 residues led to a loss or decrease of ATP binding and/or ATP hydrolysis.110,125,135−138
Besides the Walker A, Walker B, and sensors 1 and 2, another common component in the ATP-regulating complexes is the lid domain (Figure 2). The lid subdomain (residues 221–251 in the TerL ATPase) is a short peptide with negatively charged amino acids that might interact with the positively charged arginine residues. Crystal structure revealed that the lid is displayed adjacent to the ATP binding site on the surface of the ATPase protein.29 Despite its relevance to ATPase activity, the study on the lid domain has been limited, probably due to its low degree of conservation among ATPases.
ATP binding and hydrolysis in ATPases are attributed to residues occupying two or more of the four key sites near the ATP molecule. These residues are located in the ATP-binding pocket or at the adjacent intersubunit interface. Among them, arginine possesses an extended and flexible side chain with a planar and positively charged guanidine group at its extremity. The positive charge is distributed over the three side-chain nitrogens, which is advantageous for hydrogen-bond and electrostatic interactions with groups of opposite charge and polarity, for example, ATP phosphate groups. The multidentate character of arginine allows for strong inter- and intraprotein interactions, as seen in phosphorylation-driven signal transduction pathways.139
Definition and Location of the Arginine Finger
“Arginine finger” means a particular arginine residue coordinated to the β- or γ-phosphate of ATP in the ATPase catalytic reaction center.140 The location of representative arginine fingers in a certain monomer (Figure 2 left), dimer (Figure 2 right), and hexamer (Figure 4) is illustrated. Although there are multiple arginine residues present throughout the ATPase protein, the arginine finger can be identified using knockout experiments.141 For example, an arginine finger knockout study was used to determine the role and necessity of the arginine finger in F1-ATPase.43 It was found that the substitution of the arginine residue in the arginine finger motif by a lysine analogue called Lyk resulted in reduced catalytic function. In another case, the identification of the arginine finger was achieved via the mutation of the basic arginine residue to a neutral residue, alanine, in phi29 motor ATPase gp16. Mutated gp16 was found to lose the capability to incorporate into the hexameric ring, to bind dsDNA, or to package DNA.110,125
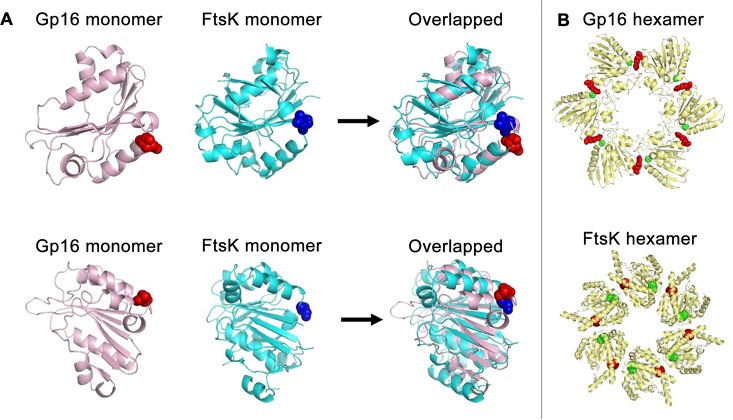
Figure 4.
Adjacent location of arginine fingers and Walker A motifs within gp16 and FtsK ATPases. (A) Comparison of the crystal structure of FtsK and the computed gp16 monomers, which represent a single subunit of a hexameric ATPase, revealing the alignment of the two ATPase subunits even though they are from different species. The arginine finger (red sphere) and the Walker A (blue sphere) overlap when the gp16 and FtsK ATPases are superimposed. (B) Comparison of gp16 and FtsK hexamer models. The green sphere represents Walker domains. The red sphere represents the arginine finger. Based on the proximity of the green and red spheres, the figure reveals that the arginine finger interacts with the adjacent ATPase subunit. Reprinted with permission from ref (110). Copyright 2016 American Society of Microbiology.
The arginine residue is a recurrent characteristic of the active sites and subunit interfaces of many ATPase proteins.139 Sequence alignment of different ATPases shows that the arginine finger motif is conserved across the ATPase families, but its location varies. Depending on the family it belongs to, the ATPase protomers can orient differently in the oligomeric assembly, leading to a different location of the arginine finger. In most ATPase proteins, the arginine finger contains one or more arginine residues and is often found at the end of the α4 helix.135 In order to identify the location of the arginine finger in TerL ATPase, investigators screened mutants of surface-exposed arginine residues for ATPase activity.29 This experiment identified residue Arg139 as the arginine finger that interacts with the γ-phosphate of the ATP molecule binding in the adjacent subunit and helps catalyze ATP hydrolysis. The arginine finger in the gp16 ATPase was identified to be Arg146, located after α4, as is the case in other ATPases in the same family with consensus sequence and confirmed structural information (Figures 2 and 3).110,142 This motif interacts with ATP in a highly specific fashion, binding to the γ-phosphate of ATP that is also coordinated by the neighboring subunit. The arginine finger is essential for ATP hydrolysis, as even conservative mutations led to the abolition of ATPase activity.143,144
General Function of the Arginine Finger
The conserved arginine finger plays essential functional roles in many ATPases.145−156 Positive residues in the active site are necessary for ATP hydrolysis, which are involved in stabilizing the transition state during the reaction.157 The arginine finger provides some of this necessary charge. Substitution mutations that replace the arginine residue with neutral residues result in the loss of ATPase function.29,110,125
Although more detailed structural, biophysical, and biochemical characterization of the arginine finger in motor ATPases is needed, significant evidence has led to the speculation that the arginine residue is part of the Walker A and Walker B domains. The characteristic Walker A and B motifs in ATPases are involved in coordinating the β and γ phosphates of ATP and a water-activating magnesium ion during ATP hydrolysis. The ATP hydrolysis is also aided by sensor 1 and 2 motifs. Crystal structures of the biomotor ATPase domains reveal a highly conserved arginine residue in the proximity of the sensor 2 motif (Figure 2). In these structures, an ATP molecule comes into contact with the Walker A and B motifs of one subunit, while the arginine finger coordinates the ATP from the adjacent subunit (Figure 4).158
Perception on trans Action but Not cis Action of the Arginine Fingers in ATPases or Motor Complexes
It is believed that the arginine finger facilitates the ATP hydrolysis in a trans manner.159 The term trans originates from the Latin root “trans” meaning “across from”, which is relative to “cis”, meaning “the same side as”. Specifically, a trans-acting arginine finger refers to an arginine residue from one ATPase subunit that regulates the ATP hydrolysis in the adjacent subunit. A cis-acting arginine finger, on the other hand, refers to an arginine residue that regulates the ATP hydrolysis in the same subunit. The classification of the trans-acting arginine finger is important for both understanding the ATPase mechanism and defining the structure of the active ring assembly.29 To investigate this, the crystal structures of the ATPase activity domains of biomotors have been employed for comparison and analysis. The overall structural features of the core domains are conserved in all ATPases of the superfamily with a conserved arginine residue near the sensor 2 motif; however, the helicase superfamily III proteins lack the sensor 2 arginine due to an atypically formed α-helical domain. A majority of ATPases are arranged in such a way that the nucleotide binding pocket is positioned at the interface between two protomers.110,160 This structural arrangement supports the notion that, in an active ATPase complex, the arginine finger of one subunit should be positioned near the nucleotide bound in the neighboring subunit. A structure of the hexameric ring of phi29 gp16 ATPase was modeled by aligning with the hexameric FtsK DNA translocase of Escherichia coli.110 The arginine finger of one subunit was shown to outstretch to the active site of the adjacent subunit, in agreement with other ATPases, such as TerL and ClpX, in which the arginine finger is positioned in the ATP binding pocket for cooperative behavior among subunits.29,161 This structural feature is evident in various ATPase hexamers (Figure 2). Mutants that showed no ATPase activity were tested to determine if proper function could be restored by adding ATPase monomers with an intact arginine finger. Biochemical complementation assays thus revealed that the mutant whose arginine is disabled in cis (within the same subunit) does not restore activity, but that disabled in trans (not in the same subunit) does restore activity.
Nonetheless, the literature on the arginine finger is still inconsistent. Some reports suggest that the arginine finger is a cis-acting component that functions within a single subunit of the ATPase ring,43 while others report that the arginine finger is a trans-acting factor that bridges two adjacent subunits.29,110,125,159,162−164 Some studies even suggest that there are two arginine fingers in each ATPase subunit.109,139 It has also been reported that the reduction in ATPase activity upon arginine finger mutation is due to an effect on catalysis but not ATP binding.143 The complexity and the controversy may be due to the fact that some ATPases are a circular-shaped, multiple component ring,47,165−169 but some ATPases are present as a single subunit.170,171
Comparison of the Arginine Fingers Across Various ATPase Types
Arginine fingers are mostly conserved in ATPase proteins (Figure 3). Oligomeric ATPases contain one arginine finger per monomer subunit. It has been shown that ATPases from SF1 and SF2 contain a tandem fold and bind the nucleotide at the interface between two domains. Similar to many ATPases,172,173 the N-terminal provides the Walker A and Walker B motifs, and the C-terminal provides other elements, some of which are for binding of the substrate, such as dsDNA. Mutations of these arginine residues are lethal and lead to loss of in vivo and/or in vitro activity, suggesting that these residues are imperative in ATP metabolism.110,139,159,162,174
Although the arginine finger is, in general, involved in the proper functioning of ATPases, its function may vary slightly across different ATPase types. The arginine residue is able to interact with the γ-phosphate of ATP and is required for ATP binding, hydrolysis, and intersubunit communication. It is positioned near the γ-phosphate of ATP and plays a catalytic role in properly positioning the ATP molecule within the ATP binding site.175 It is believed that the arginine finger may also play a role in stabilizing the transition state during hydrolysis.176 Mutational studies concluded that the main role of the arginine finger in the F1-ATPase is to catalyze ATP hydrolysis and mediate efficient energy conversion.43,44 Mutations of Walker A and arginine finger yield a similar phenotype, indicating that the arginine finger is also involved in nucleotide binding.110,159
Additionally, the arginine finger may also aid in stabilizing the ATPase hexamers135 due to its role in dimer formation and intersubunit interaction.110,125,177 Arginine finger mutations178 in HslU, p97 VCP, ClpB D1, ClpC D1, and Hsp104 D1 prevent oligomer formation even in the presence of ATP, supporting the proposal that the arginine finger is involved in formation of a dimer in the hexameric ring.110 Nevertheless, arginine finger mutations have led to different results from those in Ras/RasGAP proteins, where complex formation was not affected upon arginine subsititution. This mystery suggests that the identification of the arginine finger in Ras/RasGAP requires rescrutiny.
Another ATPase, which differs in its role in the cell but contains similar structural motifs and ATP hydrolysis mechanisms, is RuvB ATPase.137 RuvB and motor ATPases are both hexameric proteins. In E. coli, the cross-shaped Ruv family proteins function in genetic recombination through processing Holliday junctions. RuvB contains an arginine residue (Arg174) that is located between sensor 1 and 2 motifs. Mutagenesis experiments reveal that this arginine residue is essential for ATP hydrolysis and proper ATPase function.70−73 The arginine finger in RuvB is also responsible for intersubunit interaction during the ATP hydrolytic cycle, similar to that of the arginine finger in the phi29 biomotor.
An Asymmetric ATPase Hexamer Made Up of One “Dimer” and Four Monomers
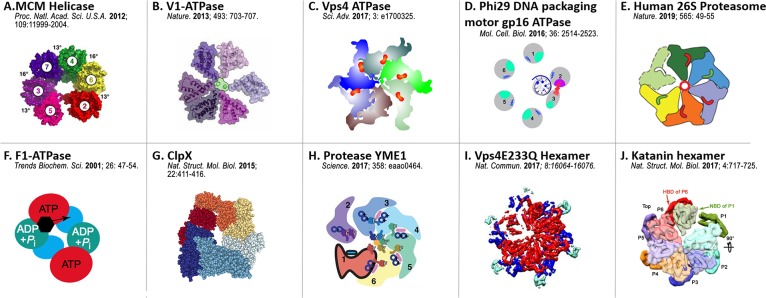
In many hexameric ring-shaped ATPases, the arginine finger serves as the bridge between two of the ATPase subunits; the two adjacent subunits thus form a more compact dimer configuration that may appear as a monomer in low-resolution cryo-EM images. This caused the hexameric ring to appear asymmetrical, as shown for the phi29 motor ATPase by the Guo group.56,57 This asymmetric hexameric structure has been observed in X-ray diffraction and cryo-EM imaging of many ATPases in addition to the phi29 motor ATPase gp16 (Figure 5).110,179−187 This hypothesis of one interchanging dimer and four monomers is supported by the profile of gp16 in ultracentrifugation, showing the presence of both monomers and dimers in the mixture. However, ATPase motors have for a long time been reported as a pentameric configuration by cryo-EM, probably due to the interchanging dimers that display close contact between two adjacent ATPase subunits. Traditional cryo-EM is an ensemble measurement by averaging over many configurations, thus the dimer with close contact might show as one instead of two subunits. Moreover, the low and featureless EM density maps of gp16 in recent cryo-EM imaging of the entire motor complex115 have precluded the possibility of obtaining an unambiguous fit for five or six copies of gp16, adding another layer of ambiguity to the ongoing debate.
Figure 5.
Asymmetrical crystal or Cryo-EM structures of various ATPase hexamers: (A) MCM helicase. Reprinted in part with permission from ref (179). Copyright 2012 National Academy of Science. (B) V1-ATPase. Reprinted by permission from ref (180). Copyright 2013 Springer Nature. (C) Vps4 ATPase. Illustration adapted from ref (181). (D) Phi29 DNA packaging motor gp16 ATPase. Reprinted in part with permission from ref (110). Copyright 2016 American Society for Microbiology. (E) Human 26S Proteasome ATPase. Reprinted with permission from ref (182). Copyright 2019 Springer Nature. (F) F1-ATPase. Reprinted with permission from ref (183). Copyright 2001 Elsevier. (G) ClpX. Illustration adapted from ref (110). (H) Protease YME1. Reprinted with permission from ref (185). Copyright 2017 American Association for the Advancement of Science. (I) Vps4E233Q Hexamer. Reprinted in part with permission from ref (186). Copyright 2017 Springer Nature. (J) Katanin hexamer. Reprinted with permission from ref (187). Copyright 2017 Springer Nature.
Each subunit of the ATPase hexamer has the capability of binding an ATP molecule; however, saturation of the ATPase with ATP reveals that at least two of the subunits are not bound with ATP. Even when not all subunits are able to bind ATP, the ATPase function is retained.188 These observations suggest that the functional ATPase hexamer is asymmetrical and the subunits in the ATPase vary in conformation during ATP hydrolysis. To investigate the role of the arginine finger in the dimer formation within the hexameric ATPases, arginine knockout experiments were performed. It was found that mutation of the arginine finger in phi29 gp16 resulted in loss of dimer assembly and DNA packaging ability; however, dimer formation was rescued with the addition of either a wild-type gp16, a Walker A mutant, or a Walker B mutant, which all contain a functional arginine finger. An inhibition assay in which the arginine finger function is knocked out revealed that a single arginine mutant subunit led to inactivation of the entire ATPase ring. These results suggest that the arginine finger is a necessary component for coordinating the formation of the ATP binding pocket and intersubunit communication in the revolving motor ATPases.110,139
Further evidence that the arginine finger motif drives the formation of dimers is provided by glycerol gradient centrifugation and electromobility shift assays (EMSA) experiments, where both monomeric and dimeric subunits are present in the ATPase population.110,125 In order to determine the ratio of monomer to dimer during DNA packaging, investigators tested the packaging activity of the different fractions recovered from the sucrose gradient. It was observed that the fraction containing the dimer alone did not have DNA packaging activity, while DNA packaging activity was retained in the fractions that also contained monomers. This finding is also supported by a previous report that the addition of fresh gp16 monomer and ATP is necessary for re-initiating the activity of DNA packaging intermediates, which contained gp16 dimers, into an infection virus.189
Outstretching to Adjacent ATP Pockets and Formation of Dimeric Subcomplexes To Regulate Sequential Action of ATPases
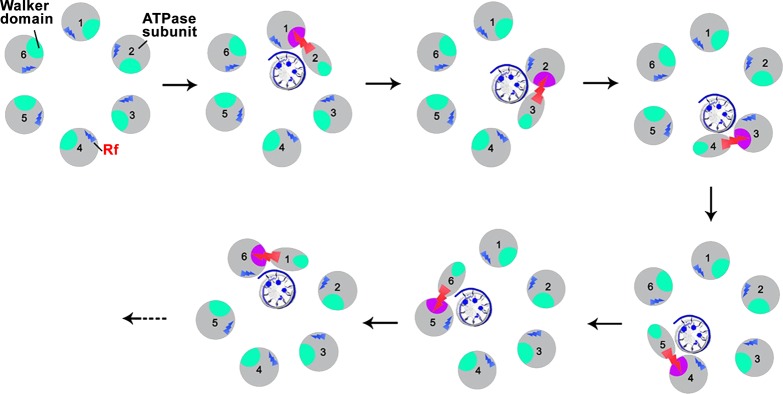
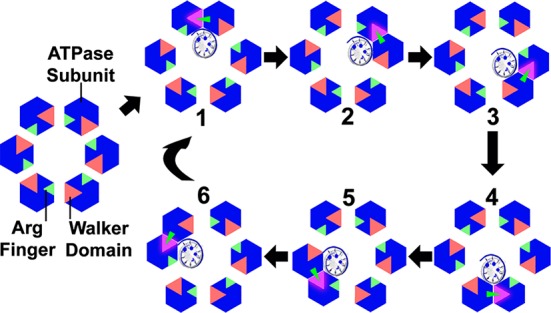
Recently, the way the arginine finger regulates the motion direction of the ATPase within the phi29 DNA packaging motor has been proposed.110 The model assumes that ATP/DNA binding and ATP hydrolysis are coupled with conformational changes of the gp16 ATPase. These changes occur in a sequential manner and are coordinated by the arginine finger. The arginine finger acts as a bridge between two adjacent subunits, leading to the formation of a transient dimer. The conformational changes of the ATPase subunit will in turn lead to the displacement of the dsDNA to the adjacent monomer. During this process, the formation of the dimer results in an asymmetric hexamer, which explains why many previous studies showed asymmetric structures of various ATPase hexamer models (Figure 5).110,179−187 The arginine finger functions in intersubunit interaction by extending from one subunit to the adjacent one, which facilitates the formation of a dimer.
The demonstration of a sequential mechanism raises the question of how the different subunits of the ATPase can sense the ATP/DNA binding state of the adjacent subunits. Investigators addressed this question by studying the behavior of gp16 mutants in which the arginine fingers were mutated. Mutated gp16 eliminated its capability to assemble into dimers, and the mutant was unable to hydrolyze ATP, bind DNA, or package DNA in an ATP-dependent manner.110,125 Thus, the arginine finger was implicated to regulate conformational changes, dimer formation, DNA binding, and ATP hydrolysis and thus eventually to orchestrate force generation for DNA translocation in the phi29 motor.110,125,190,191 This finding was further confirmed recently, reported as a switch-like regulator.192
In the sequential mechanism of gp16 action (Figure 6), it was proposed that the hydrolysis of ATP causes a conformational change to the ATPase subunit, which destabilizes the “active” ATPase dimer and may simultaneously trigger a conformational change (strike) of the arginine finger. This outstretch of the arginine finger to the adjacent ATP binding pocket facilitates the formation of the next in-line “active” dimer. Mutagenesis studies were conducted with phi29 ATPase, HslU, p97 VCP, and others in which both ATP hydrolysis and oligomer formation were impaired due to mutation of the arginine finger. This leads to the conclusion that these arginine fingers function in motor subunit communication as well as ATP hydrolysis.109,110,125,138 The mutation of the arginine finger in gp16 led to impaired function in DNA binding in the presence of γ-S-ATP. Hence, the arginine finger appears to regulate the sequential action of the gp16 ATPases by carrying the ATP/DNA binding/hydrolysis information from one subunit to another, adding an extra level of cooperativity in gp16 as seen in other mutants, such as in Walker B mutations.56,57,110
Figure 6.
Arginine finger (red arrow) regulates dimer formation and sequential action during ATP hydrolysis. Green: The five inactive Walker domains for ATP-binding (the P-loop, Walker A and B motifs). Pink: The one active ATP-binding center after activation by arginine finger. The trans-acting arginine finger acts as a bridge between two ATPase subunits when ATP is bound. As hydrolysis continues, ATP binds to the subsequent subunit and dimer formation occurs in a sequential manner. Reprinted with permission from ref (110). Copyright 2016 American Society for Microbiology.
Interestingly, it was reported that hydrophobic residues in the catalytic site of an ATPase may play a role in controlling the motor speed.193 These hydrophobic residues are thought to be responsible for controlling the number of water molecules within the catalytic space and altering the network of water interactions. Natural evolution has selected the optimal speed variants that ultimately improve the fitness of organisms or phages, which may be the reason why these hydrophobic residues are considered nonconserved motifs.
Entropy-Driven Prohead- and DNA-Dependent Conformational Changes of ATPases To Trigger ATP Hydrolysis and Motion in Relevance to Allosteric Effects of the Arginine Finger
Besides providing necessary positive charges for ATP binding and hydrolysis, the arginine finger plays an indispensable role in regulating the conformational changes and coordinating the sequential motions in the ATPase complexes.194,195 In 1986, Guo et al. reported18 that viral DNA packaging enzymes, including gp16 of phi29, gp19 of T7, gp17 of T4 and gpA of λ, all contain a conserved A-type sequence of “basic-hydrophobic region-G-X2-G-X-G-K-S-X7-hydrophobic” (X represents any amino acid) for ATP binding. After the construction of the first defined in vitro DNA packaging system with all purified components40 and the discovery of the pRNA as the motor-gearing component,38 they were able to elucidate that the two enzymes involved in DNA packaging have distinct functions; the enzyme with larger molecular weight is a prohead and DNA-dependent ATPase, while the other with smaller molecular weight is responsible for DNA binding. In the same paper, it was reported that the gp16 of bacteriophage phi29 DNA packaging motor is a prohead and DNA-dependent ATPase.18 The mechanism of “prohead and DNA-dependent ATPase” has been scrutinized for 30 years and is now clear. It suggests that the interaction of the gp16 ATPase with other motor components leads to a change in conformation (entropy) of the ATPase subunit, resulting in a higher affinity for dsDNA. The subsequent DNA binding leads to a second conformational change of the ATPase subunit that is activated to hydrolyze the bound ATP. Hydrolysis of ATP leads to another conformational change of the ATPase (higher entropy) that resumes a low affinity for dsDNA, thus pushing the DNA to the adjacent ATP-bound subunit of a high affinity for dsDNA. Such alternative high and low affinities for DNA are the forces that drive the motion of the dsDNA substrate in the DNA packaging motor.
In the ATPase catalytic cycle, ATP binding causes the first round of conformational (entropy) changes of the ATPase subunits, a positive allosteric effect that results in a higher affinity for dsDNA. The interaction of ATP and ATPase has been studied using a variety of assays. EMSA demonstrated that nonhydrolyzable γ-S-ATP qualitatively stalls and fastens the formation of ATPase/dsDNA complex, indicating that ATPase undergoes conformational (entropy) changes upon ATP binding and leads to a higher affinity for dsDNA.56,57,110 Similar results were observed from Förster resonance energy transfer (FRET) analysis, showing increased energy transfer from eGFP-ATPase to Cy3-dsDNA upon addition of γ-S-ATP.56 Sedimentation studies also revealed a high prevalence of the gp16-dsDNA complex with γ-S-ATP. As expected, such conformational changes are abolished by the site-directed mutation of the Walker A motif,165 which has been identified18 and confirmed18,56,172 to be responsible for ATP binding.133
ATP is hydrolyzed only after dsDNA binding, which then causes a conformational (entropy) setback of the ATPase subunit, a negative allosteric effect on the ATPase subunit that leads to a lower affinity toward dsDNA, pushing the dsDNA toward the next adjacent ATPase subunit that has already bound with an ATP. The dsDNA advances by dsDNA by 0.54 nm or 0.27 nm for each of the 12 steps in the connector channel. That is, each ATP molecule packages 1.75 bp of dsDNA. The process repeats six times as the DNA moves by a helical pitch, that is six ATP molecules are consumed for one DNA revolving cycle, corresponding to the packaging of 10.6 bp.56 The translocation from one subunit to another subunit is regulated by the action of the arginine finger. The hydrolysis of ATP was confirmed by adding ATP to the purified ATPase/dsDNA/γ-S-ATP complex. ATP replaced the γ-S-ATP, leading to the release of dsDNA from the complex. ADP had a lesser effect on dsDNA release, whereas AMP was incapable of releasing dsDNA from ATPase.56 The release of inorganic phosphate from the P-loop stimulates an entropy gain in ATPase, which is accompanied by a conformational shift that forces the substrate DNA away from the interior pocket of the ATPase, resulting in the movement of the genomic DNA toward the next ATPase subunit. Given that Walker B mutants bind ATP but do not hydrolyze ATP,165 introduction of a mutation to the Walker B motif eliminates the catalytic step and thus halts DNA translocation.
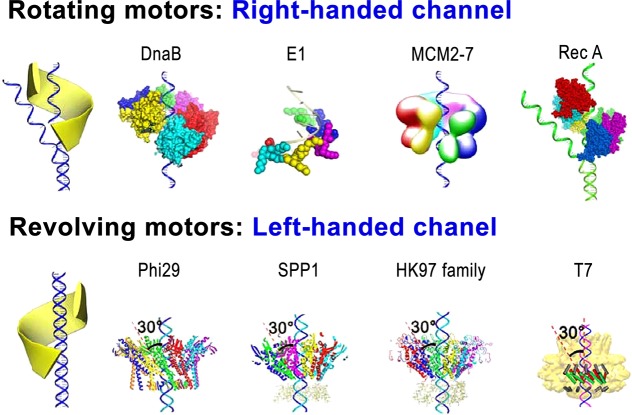
The Left- and Right-Handed Chirality between the Revolving and Rotating Motors Offers Additional Direction Control Coupled with the Arginine Finger
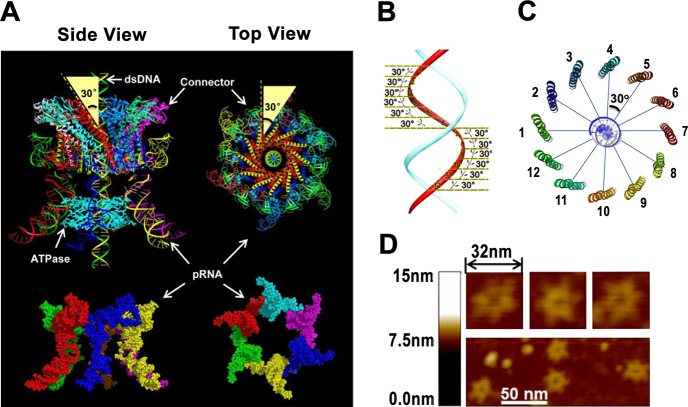
Sequential action of the arginine finger is critical for controlling the motion direction in the phi29 DNA package motor; however, an additional component, the 12-subunit connector, also plays a significant role in controlling the motion direction. For example, the chirality is the way to ensure “the push through a one-way valve”.193,196 Revolving motors show left-handed chirality, which is distinct from the right-handed chirality of rotating motors. Genomic dsDNA generally is B-type right-handed. The revolving of the dsDNA along the channel wall without self-rotating requires the surrounding track to have an opposite chirality to match contours of the DNA and the channel (Figure 8).1,74 Hence, the left-handed channel wall is a necessary factor for a revolving motor, as it facilitates the threading motion of one strand of the dsDNA. This antichiral arrangement between the DNA helix and the channel is also seen in SPP1, T7, HK97, and P22 motors.27,104−107 The connector channels in these motors are made up of 12 subunits that are oriented in a 30° tilt, leading to the opposite chiral arrangement to reach a configuration match during DNA translocation. Since the phage genome moves along the channel wall via only one strand in the 5′-3′ direction, as seen in Phi29,2,37 the 30° tilt to the left ensures the continuous engagement and contact of this strand when the DNA shifts to the next subunit of the dodecamer. Three hundred sixty degrees in one turn during the 12-step motion results in 30° per step (360°/12 = 30°) (Figure 7).1,74 This configuration avoids coiling and torsional forces as seen in rotating motors. Taken together, the left-handed antichiral arrangement of the motor channels of the revolving motor leads to a controlled threading motion of the substrate, supporting a revolving motor model (Figure 8 bottom).74
Figure 8.
Different chiralities of rotating and revolving motors. Rotating biomotors exhibit right-handed chirality to drive the right-handed dsDNA similar to the nut driving the bolt or the screw driver turning the screw, whereas revolving biomotors exhibit left-handed chirality within the channel. Crystal structure analysis of viral DNA packaging motors reveals that this class of biomotors package DNA using the revolving mechanism. Reprinted with permission from ref (5). Copyright 2014 Springer Nature. Reprinted in part with permission from ref (74). Copyright 2014 Springer Nature.
Figure 7.
Structure of phi29 DNA packaging motor. (A) Structure of hexameric pRNA and the connector showing a 30° tilt. (B, C) dsDNA showing the shift of 30° angle between two adjacent connector subunits. Reprinted with permission from ref (56). Copyright 2013 Elsevier. (D) AFM images of hexameric pRNA with 7-nucleotide loops. Adapted with permission from ref (219). Copyright 2013 RNA Society.
Although bacteriophage portal proteins from various families do not show significant sequence alignment nor similar size, they assemble to a similar overall structure. For example, bacteriophage Phi29, SPP1, and T7104 have protein sizes of 36 kDa (Phi29 gp10),107 57 kDa (SPP1 gp6),27 and 59 kDa (T7 gp8),104 respectively. Bacteriophage P22 has a protein component, gp1, which is 94 kDa.106 These portal proteins are all arranged in a propeller-like, 12-subunit structure with a central channel acting as a valve for DNA translocation. In addition to sharing similar three-dimensional structures, these bacteriophage motor proteins have analogous conserved regions that function in viral genome packaging. In nearly all portal proteins, the sequence stretch of α-β-α-β-β-α exists with a similar pattern of strands and helices and with similar spacing and length.
Analysis of the quaternary structures of various bacteriophages has revealed that the 30° tilted helix exists in all portal proteins. Evidence that the antichiral arrangement is integral in dsDNA packaging is seen in mapping studies, revealing that the 30° tilt occurs in the same conserved sequences in the last α helix of the α-β-α-β-β-α stretch. This highlights the importance of this 30° antichiral arrangement, as it has been conserved by evolution.1 According to a parallel threading mechanism of bolt and nut,1,74 rotating motors need to have right-handed channels in order to accommodate right-handed dsDNAs (Figure 8, top). Verification of the right-handed rotating motor is provided by crystal structures of helicase-DNA complexes that have a right-handed spiral configuration.48 This is seen in RecA filament and DnaB, which function in a nonplanar hexameric conformation.48 In this rotating-like mechanism, for example, RecA monomers assemble into an open washer shape in a concatemeric arrangement.197 ATPases, however, remain as a symmetrical closed ring in the absence of dsDNA.110 E1 helicase also adopts a right-handed staircase conformation when bound with dsDNA.198 Crystallographic studies provide evidence that right-handed motor complexes use the rotating mechanism.197
The mechanism for the packaging of viral double-stranded genome into the protein shell with the aid of an elegant motor is an intriguing subject.1−3,20,199−204 Significant progress on the study of the mechanisms of viral DNA packaging motors has been achieved in the poxvirus,76−80 adenovirus,81−83 herpesvirus,88−103 and minivirus.2,84−87 Studies have revealed that the revolving mechanism is a common feature shared by all the dsDNA packaging motors, including SPP1, P22, T7, the HK97 family phage, and poxvirus evidenced by the results from both structural and biochemical studies. Analysis of crystal structures of the motor channels (the connectors) of SPP1,27 T7,104 HK97,105 P22,106 and Phi29107 revealed that all of the motor channels displayed an antichiral arrangement between the channel and the DNA helices. The primary amino acid sequences are not conserved; however, the 3D structures of the swivels are both conserved and aligned.1,74 Structural analysis of the SPP1 and Phi29 channels reveals unidirectional flow loops that function in the one-way trafficking of dsDNA. Layers of positively charged lysine residues,193 representative of all phage channels, interact with the electronegative phosphate backbone of a single DNA strand. The effectiveness of the viral DNA packaging motor is due in part to the coordination of these complementary forces.
Revolving ATPase motors move along one strand of the dsDNA in the 5′ → 3′ direction.3,37,74,110,205 RecA ATPases also move along in the 5′ → 3′ direction. Unlike the revolving motors and RecA ATPases, some rotating ATPases move in the 3′ → 5′ direction.206 Whether the DNA strand polarity is relevant to the revolving or rotating mechanism remains to be elucidated.
Revolving and Rotating Motors Can Be Distinguished by Their Channel Size
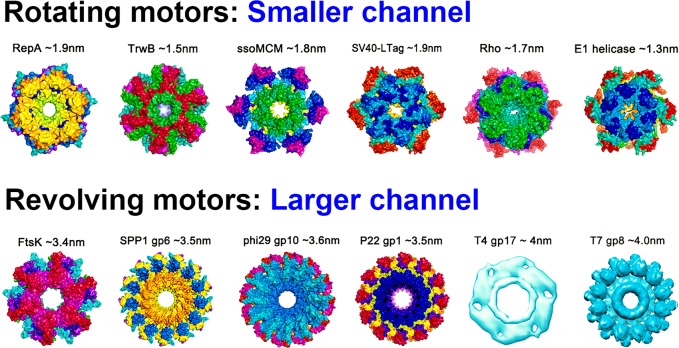
The arginine finger is critical for controlling the motion direction; however, how could the similar arginine finger control the two kinds of motors (rotating and revolving) that are very different in motion mechanism? The two differential motion mechanisms are also dictated by an additional motor structure factor: the channel size, which can be used to distinguish revolving motors from rotating ones. Channel size also plays an important role in controlling the one way motion and the motion direction. For rotating motors, their channel diameter should be no larger than 2 nm (the diameter of a dsDNA) to allow for close contact between a DNA and the channel wall for threading, since a ssDNA within the channel displays an A form helical structure and is smaller than 2 nm in diameter.48 Examples include rotating motors of DnaB,48 Rho factor,50−52 TrwB,60−65 MCM,66 and RepA or RuvB,68−73 all of which have a channel diameter of 1–2 nm.74
For revolving motors, such as a Phi29 DNA packaging motor, their channel diameter is generally larger than 3 nm. The larger channel size of the revolving motors allows a dsDNA to revolve around the channel wall, while precluding the possibility of a bolt and nut tracing mechanism, characteristic of rotating motors. Cryo-EM images of a tilted T7 dsDNA core relative to its axis reveal that in revolving motors, dsDNA advances by touching the channel wall rather than passing through the center of the channel.207,208
The difference in channel size has been confirmed by crystal structure analysis, cryo-EM measurement, and single-channel conductance assays. The diameter of dsDNA is 2 nm, while the diameters of the narrowest region of the connector channels of Phi29,107 SPP1,27 HK97,105 the ATPase ring of T4,33,205 as well as the dsDNA translocase FtsK54 of bacteria, are all larger than 3 nm (Figure 9). To prove the revolving mechanism, the connector of bacteriophage Phi29 DNA packaging motor was used as the channel for the single pore translocation of folded, double-stranded, or tetra-stranded DNA. A current blockage of 32% was observed for translocation of dsDNA through the connector channel,209 consistent with the ratio of the cross-sectional areas of dsDNA; A = πr2, dsDNA ((2/2)2 × 3.14 = 3.14 nm2), and channel ((3.6/2)2 × 3.14 = 10.2 nm2, 10.2 nm2/3.14 nm2 = 32%). While for tetra-stranded DNA, passage through the connector channel of Phi29 yields a blockage of ∼64%. This blockage data show that the cross-sectional area at the narrowest region of the Phi29 funnel is 3-fold the area of the dsDNA. The much larger width of the nut, in comparison to the bolt, precludes the possibility of a bolt and nut threading mechanism, but rather suggests that, at any translocation step, the dsDNA can be in contact with only one (or two) ATPase subunit.74
Figure 9.
Channel size to differentiate rotating and revolving mechanism. Rotating motors have channel sizes all ≤2.0 nm in diameter to ensure full contact between DNA and channel wall similar to the nut driving the bolt, while revolving motors have channel sizes ≥3 nm to have room to accommodate the revolving motion. Reprinted in part with permission from ref (5). Copyright 2014 Springer Nature.
In contrast, the channels of rotating motors, such as replicative DNA helicases TrwB, E1, and DnaB,48,64,167,210−212 are smaller than 2 nm in diameter (Figure 9). For these motors, the channel is expected to have a width similar to that of ssDNA. Nonetheless, for certain rotating motors, local unwinding fluctuations of the dsDNA lead to separation of the double helix, and thus only one strand enters the channel, while the other remains outside.168,213−218 Given that the ssDNA within the channel displays an A form helical structure,48 the channel diameter should be no larger than 2 nm so that the ssDNA can make full contact with the channel. Overall, the above data indicate that the revolving motor can be distinguished from the rotating motor by the size of their motor channels.
Conclusion
The arginine finger is an indispensable part of the ATP-activity pocket of the ring-shaped ATPase motors with revolving or rotating mechanisms. It is believed to be involved in initiating and coordinating the sequential action within the motor, which eventually leads to the pulling and pushing motions of the substrate during translocation. The arginine finger is also implicated to play a role in controlling the motion direction of the motor. All of these are achieved through a trans-action mechanism in promoting dimer formation, direct involvement in regulating ATP binding and hydrolysis, and allosteric effects associated with protein conformational changes.
Acknowledgments
We thank P. Li of NY University at Albany, J. Yu at Beijing Computational Science Research Center, J. Li at Zhejiang University, I. Molineux at UT Austin for constructive comments, and L. McBride, D. Binzel, X. Li, and C. Ghimire and Guo Lab members for the manuscript preparation. The work was supported by NIH grant nos. R01-EB012135 and R01-EB019036 to P.G. Funding to P.G.’s Sylvan G. Frank Endowed Chair position in Pharmaceutics and Drug Delivery is by the CM Chen Foundation.
Glossary
Abbreviations
- ATPase
a class of enzymes that catalyze the hydrolysis of ATP to provide the driving force for different kinds of mechanical motions essential to cellular functions
- biomotor mechanism
concerns how biomotor proteins harness energy to drive the mechanical motions of their substrates
- arginine finger
a particular arginine residue coordinated to the β- or γ-phosphate of ATP or interacting with some components/motifs in the ATPase catalytic reaction center
- Walker A motif and Walker B motif
two conserved sequence motifs in ATPases responsible for ATP binding and ATP hydrolysis
- channel size
refers to the diameter of the substrate translocation pore in biomotors, which can be used to distinguish revolving motors from rotating ones
- channel chirality
the orientation of the pore-lining secondary structures tilted to either the left or the right, which is an attribute of asymmetry in many channel structures
- entropy driven
the biological processes are driven by the increase or the decrease of entropy. High entropy refers to the product state that is conformationally more dynamic or disordered.
The authors declare the following competing financial interest(s): P.G. is a consultant of Oxford Nanopore Technologies, Inc., the cofounder of Shenzhen P&Z Bio-medical Co. Ltd. and its subsidiary US P&Z Biological Technology LLC, as well as cofounder of ExonanoRNA, LLC and its subsidiary Weina Biomedical LLC in Foshan.
References
- Guo P.; Schwartz C.; Haak J.; Zhao Z. Discovery of a New Motion Mechanism of Biomotors Similar to the Earth Revolving around the Sun without Rotation. Virology 2013, 446, 133–143. 10.1016/j.virol.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo P.; Zhao Z.; Haak J.; Wang S.; Wu D.; Meng B.; Weitao T. Common Mechanisms of DNA Translocation Motors in Bacteria and Viruses Using One-Way Revolution Mechanism without Rotation. Biotechnol. Adv. 2014, 32, 853–872. 10.1016/j.biotechadv.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo P.; Noji H.; Yengo C. M.; Zhao Z.; Grainge I. Biological Nanomotors with a Revolution, Linear, or Rotation Motion Mechanism. Microbiol. Mol. Biol. Rev. 2016, 80, 161–186. 10.1128/MMBR.00056-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Yehuda S.; Rudner D. Z.; Losick R. Assembly of the SpoIIIE DNA Translocase Depends on Chromosome Trapping in Bacillus subtilis. Curr. Biol. 2003, 13, 2196–2200. 10.1016/j.cub.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Guo P.; Grainge I.; Zhao Z.; Vieweger M. Two Classes of Nucleic Acid Translocation Motors: Rotation and Revolution without Rotation. Cell Biosci. 2014, 4, 54–59. 10.1186/2045-3701-4-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowiec J. A.; Dean F. B.; Bullock P. A.; Hurwitz J. Binding and Unwinding—How T Antigen Engages the SV40 Origin of DNA Replication. Cell 1990, 60, 181–184. 10.1016/0092-8674(90)90730-3. [DOI] [PubMed] [Google Scholar]
- Chakraverty R. K.; Hickson I. D. Defending Genome Integrity During DNA Replication: A Proposed Role for RecQ Family Helicases. BioEssays 1999, 21, 286–294. . [DOI] [PubMed] [Google Scholar]
- Tran N. Q.; Dang H. Q.; Tuteja R.; Tuteja N. A Single Subunit MCM6 from Pea Forms Homohexamer and Functions as DNA Helicase. Plant Mol. Biol. 2010, 74, 327–336. 10.1007/s11103-010-9675-7. [DOI] [PubMed] [Google Scholar]
- Li G.-M.; Modrich P. Restoration of Mismatch Repair to Nuclear Extracts of H6 Colorectal Tumor Cells by a Heterodimer of Human Mutl Homologs. Proc. Natl. Acad. Sci. U. S. A. 1995, 92, 1950–1954. 10.1073/pnas.92.6.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.; Hanne J.; Britton B. M.; Bennett J.; Kim D.; Lee J. B.; Fishel R. Cascading MutS and MutL Sliding Clamps Control DNA Diffusion to Activate Mismatch Repair. Nature 2016, 539, 583–587. 10.1038/nature20562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.; Kim J.; Hur J. K.; Been K. W.; Yoon S.-h.; Kim J.-S. Genome-Wide Analysis Reveals Specificities of Cpf1 Endonucleases in Human Cells. Nat. Biotechnol. 2016, 34, 863–888. 10.1038/nbt.3609. [DOI] [PubMed] [Google Scholar]
- Sakato M.; O’Donnell M.; Hingorani M. M. A Central Swivel Point in the RFC Clamp Loader Controls PCNA Opening and Loading on DNA. J. Mol. Biol. 2012, 416, 163–175. 10.1016/j.jmb.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos S. J.; Heyer W.-D. Functions of the Snf2/Swi2 Family Rad54 Motor Protein in Homologous Recombination. Biochim. Biophys. Acta, Gene Regul. Mech. 2011, 1809, 509–523. 10.1016/j.bbagrm.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazin A. V.; Mazina O. M.; Bugreev D. V.; Rossi M. J. Rad54, The Motor of Homologous Recombination. DNA Repair 2010, 9, 286–302. 10.1016/j.dnarep.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinagawa H.; Iwasaki H. Processing the Holliday Junction in Homologous Recombination. Trends Biochem. Sci. 1996, 21, 107–111. 10.1016/S0968-0004(96)10014-1. [DOI] [PubMed] [Google Scholar]
- Gogol E. P.; Seifried S. E.; Von Hippel P. H. Structure and Assembly of the Escherichia coli Transcription Termination Factor Rho and Its Interactions with RNA I. Cryoelectron Microscopic Studies. J. Mol. Biol. 1991, 221, 1127–1138. 10.1016/0022-2836(91)90923-T. [DOI] [PubMed] [Google Scholar]
- Miyata H.; Nishiyama S.; Akashi K.-I.; Kinosita K. Protrusive Growth from Giant Liposomes Driven by Actin Polymerization. Proc. Natl. Acad. Sci. U. S. A. 1999, 96, 2048–2053. 10.1073/pnas.96.5.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo P.; Peterson C.; Anderson D. Prohead and DNA-gp3-Dependent ATPase Activity of the DNA Packaging Protein gp16 of Bacteriophage Φ29. J. Mol. Biol. 1987, 197, 229–236. 10.1016/0022-2836(87)90121-5. [DOI] [PubMed] [Google Scholar]
- Guo P.; Lee T. J. Viral Nanomotors for Packaging of dsDNA and dsRNA. Mol. Microbiol. 2007, 64, 886–903. 10.1111/j.1365-2958.2007.05706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe A.; Phipps K.; Weitao T. Viral and Cellular Sos-Regulated Motor Proteins: dsDNATranslocation Mechanisms with Divergent Functions. Cell Biosci. 2014, 4, 31–40. 10.1186/2045-3701-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits C.; Chechik M.; Kovalevskiy O. V.; Shevtsov M. B.; Foster A. W.; Alonso J. C.; Antson A. A. Structural Basis for the Nuclease Activity of a Bacteriophage Large Terminase. EMBO Rep. 2009, 10, 592–598. 10.1038/embor.2009.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao V. B.; Feiss M. The Bacteriophage DNA Packaging Motor. Annu. Rev. Genet. 2008, 42, 647–681. 10.1146/annurev.genet.42.110807.091545. [DOI] [PubMed] [Google Scholar]
- Dasgupta A.; Wilson D. W. ATP Depletion Blocks Herpes Simplex Virus DNA Packaging and Capsid Maturation. J. Virol. 1999, 73, 2006–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira L.; Henriques A. O.; Tavares P. Modulation of the Viral ATPase Activity by the Portal Protein Correlates with DNA Packaging Efficiency. J. Biol. Chem. 2006, 281, 21914–21923. 10.1074/jbc.M603314200. [DOI] [PubMed] [Google Scholar]
- Němeček D.; Gilcrease E. B.; Kang S.; Prevelige P. E. Jr; Casjens S.; Thomas G. J. Jr Subunit Conformations and Assembly States of a DNA-Translocating Motor: The Terminase of Bacteriophage P22. J. Mol. Biol. 2007, 374, 817–836. 10.1016/j.jmb.2007.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. J.; Zhang H.; Liang D.; Guo P. Strand and Nucleotide-Dependent ATPase Activity of gp16 of Bacterial Virus Phi29 DNA Packaging Motor. Virology 2008, 380, 69–74. 10.1016/j.virol.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebedev A. A.; Krause M. H.; Isidro A. L.; Vagin A. A.; Orlova E. V.; Turner J.; Dodson E. J.; Tavares P.; Antson A. A. Structural Framework for DNA Translocation via the Viral Portal Protein. EMBO J. 2007, 26, 1984–1994. 10.1038/sj.emboj.7601643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. L.; Hsu W. L.; Wang C. Y.; Chen H. Y.; Lin F. Y.; Chang M. H.; Chang H. Y.; Wong M. L.; Chan K. W. Goatpoxvirus ATPase is Increased by dsDNA and Decreased by Zinc Ion. Virus Genes 2016, 52, 625–632. 10.1007/s11262-016-1349-3. [DOI] [PubMed] [Google Scholar]
- Hilbert B. J.; Hayes J. A.; Stone N. P.; Duffy C. M.; Sankaran B.; Kelch B. A. Structure and Mechanism of the ATPase That Powers Viral Genome Packaging. Proc. Natl. Acad. Sci. U. S. A. 2015, 112, E3792–E3799. 10.1073/pnas.1506951112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q.; Catalano C. E.; Maluf N. K. Kinetic Analysis of the Genome Packaging Reaction in Bacteriophage Lambda. Biochemistry 2009, 48, 10705–10715. 10.1021/bi901016n. [DOI] [PubMed] [Google Scholar]
- Happonen L. J.; Erdmann S.; Garrett R. A.; Butcher S. J. Adenosine Triphosphatases of Thermophilic Archaeal Double-Stranded DNA Viruses. Cell Biosci. 2014, 4, 37–52. 10.1186/2045-3701-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes S.; Ma S.; Gao J.; Atz R.; Jardine P. J. Role of Φ29 Connector Channel Loops in Late-Stage DNA Packaging. J. Mol. Biol. 2011, 410, 50–59. 10.1016/j.jmb.2011.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit A. B.; Ray K.; Black L. W. Compression of the DNA Substrate by a Viral Packaging Motor Is Supported by Removal of Intercalating Dye During Translocation. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, 20419–20424. 10.1073/pnas.1214318109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T. Y.; Schaefer J. REDOR NMR Characterization of DNA Packaging in Bacteriophage T4. J. Mol. Biol. 2008, 382, 1031–1042. 10.1016/j.jmb.2008.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano C. The Terminase Enzyme from Bacteriophage Lambda: A DNA-Packaging Machine. Cell. Mol. Life Sci. 2000, 57, 128–148. 10.1007/s000180050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J. S.; Kregler O.; Schilf R.; Bannert N.; Drach J. C.; Townsend L. B.; Bogner E. Identification of Acetylated, Tetrahalogenated Benzimidazole D-ribonucleosides With Enhanced Activity Against Human Cytomegalovirus. J. Virol. 2007, 81, 11604–11611. 10.1128/JVI.01130-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aathavan K.; Politzer A. T.; Kaplan A.; Moffitt J. R.; Chemla Y. R.; Grimes S.; Jardine P. J.; Anderson D. L.; Bustamante C. Substrate Interactions and Promiscuity in a Viral DNA Packaging Motor. Nature 2009, 461, 669–682. 10.1038/nature08443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo P.; Erickson S.; Anderson D. A Small Viral RNA Is Required for In Vitro Packaging of Bacteriophage Phi 29 DNA. Science 1987, 236, 690–694. 10.1126/science.3107124. [DOI] [PubMed] [Google Scholar]
- Guo P.; Peterson C.; Anderson D. Initiation Events in In-Vitro Packaging of Bacteriophage Φ29 DNA-gp3. J. Mol. Biol. 1987, 197, 219–228. 10.1016/0022-2836(87)90120-3. [DOI] [PubMed] [Google Scholar]
- Guo P.; Grimes S.; Anderson D. A Defined System for In Vitro Packaging of DNA-gp3 of the Bacillus subtilis Bacteriophage Phi 29. Proc. Natl. Acad. Sci. U. S. A. 1986, 83, 3505–3509. 10.1073/pnas.83.10.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo P.; Zhang C.; Chen C.; Garver K.; Trottier M. Inter-RNA Interaction of Phage Φ29 pRNA to Form a Hexameric Complex for Viral DNA Transportation. Mol. Cell 1998, 2, 149–155. 10.1016/S1097-2765(00)80124-0. [DOI] [PubMed] [Google Scholar]
- Sauna Z. E.; Ambudkar S. V. About a Switch: How P-Glycoprotein (ABCB1) Harnesses the Energy of ATP Binding and Hydrolysis to Do Mechanical Work. Mol. Cancer Ther. 2007, 6, 13–23. 10.1158/1535-7163.MCT-06-0155. [DOI] [PubMed] [Google Scholar]
- Yukawa A.; Iino R.; Watanabe R.; Hayashi S.; Noji H. Key Chemical Factors of Arginine Finger Catalysis of F1-ATPase Clarified by an Unnatural Amino Acid Mutation. Biochemistry 2015, 54, 472–480. 10.1021/bi501138b. [DOI] [PubMed] [Google Scholar]
- Mitome N.; Ono S.; Sato H.; Suzuki T.; Sone N.; Yoshida M. Essential Arginine Residue of the F(o)-a Subunit in F(o)F(1)-ATP Synthase Has a Role to Prevent the Proton Shortcut without C-Ring Rotation in the F(o) Proton Channel. Biochem. J. 2010, 430, 171–177. 10.1042/BJ20100621. [DOI] [PubMed] [Google Scholar]
- Abrahams J. P.; Leslie A. G.; Lutter R.; Walker J. E. Structure at 2.8 Å Resolution of F1-ATPase from Bovine Heart Mitochondria. Nature 1994, 370, 621–628. 10.1038/370621a0. [DOI] [PubMed] [Google Scholar]
- Noji H.; Yasuda R.; Yoshida M.; Kinosita K. Jr Direct Observation of the Rotation of F1-ATPase. Nature 1997, 386, 299–302. 10.1038/386299a0. [DOI] [PubMed] [Google Scholar]
- Hayashi S.; Ueno H.; Shaikh A. R.; Umemura M.; Kamiya M.; Ito Y.; Ikeguchi M.; Komoriya Y.; Iino R.; Noji H. Molecular Mechanism of ATP Hydrolysis in F1-ATPase Revealed by Molecular Simulations and Single-Molecule Observations. J. Am. Chem. Soc. 2012, 134, 8447–8454. 10.1021/ja211027m. [DOI] [PubMed] [Google Scholar]
- Itsathitphaisarn O.; Wing R. A.; Eliason W. K.; Wang J.; Steitz T. A. The Hexameric Helicase DnaB Adopts a Nonplanar Conformation During Translocation. Cell 2012, 151, 267–277. 10.1016/j.cell.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebarth T. D.; Farr C. L.; Kaguni L. S. Modular Architecture of the Hexameric Human Mitochondrial DNA Helicase. J. Mol. Biol. 2007, 367, 1382–1391. 10.1016/j.jmb.2007.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skordalakes E.; Berger J. M. Structural Insights into RNA-Dependent Ring Closure and ATPase Activation by the Rho Termination Factor. Cell 2006, 127, 553–564. 10.1016/j.cell.2006.08.051. [DOI] [PubMed] [Google Scholar]
- Burgess B. R.; Richardson J. P. RNA Passes through the Hole of the Protein Hexamer in the Complex with the Escherichia coli Rho Factor. J. Biol. Chem. 2001, 276, 4182–4189. 10.1074/jbc.M007066200. [DOI] [PubMed] [Google Scholar]
- Stitt B. L.; Xu Y. Sequential Hydrolysis of ATP Molecules Bound in Interacting Catalytic Sites of Escherichia coli Transcription Termination Protein Rho. J. Biol. Chem. 1998, 273, 26477–26486. 10.1074/jbc.273.41.26477. [DOI] [PubMed] [Google Scholar]
- Adelman J. L.; Jeong Y.-J.; Liao J.-C.; Patel G.; Kim D.-E.; Oster G.; Patel S. S. Mechanochemistry of Transcription Termination Factor Rho. Mol. Cell 2006, 22, 611–621. 10.1016/j.molcel.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Massey T. H.; Mercogliano C. P.; Yates J.; Sherratt D. J.; Löwe J. Double-Stranded DNA Translocation: Structure and Mechanism of Hexameric FtsK. Mol. Cell 2006, 23, 457–469. 10.1016/j.molcel.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Endrizzi J. A.; Shu Y.; Haque F.; Sauter C.; Shlyakhtenko L. S.; Lyubchenko Y.; Guo P.; Chi Y.-I. Crystal Structure of 3WJ Core Revealing Divalent Ion-Promoted Thermostability and Assembly of the Phi29 Hexameric Motor pRNA. RNA 2013, 19, 1226–1237. 10.1261/rna.037077.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz C.; De Donatis G. M.; Zhang H.; Fang H.; Guo P. Revolution Rather Than Rotation of AAA+ Hexameric Phi29 Nanomotor for Viral dsDNA Packaging without Coiling. Virology 2013, 443, 28–39. 10.1016/j.virol.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz C.; Fang H.; Huang L.; Guo P. Sequential Action of ATPase, ATP, ADP, Pi and dsDNA in Procapsid-Free System to Enlighten Mechanism in Viral dsDNA Packaging. Nucleic Acids Res. 2012, 40, 2577–2586. 10.1093/nar/gkr841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno H.; Suzuki T.; Kinosita K.; Yoshida M. ATP-Driven Stepwise Rotation of Fof1-ATP Synthase. Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 1333–1338. 10.1073/pnas.0407857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura H.; Nakano M.; Noji H.; Muneyuki E.; Ohkuma S.; Yoshida M.; Yokoyama K. Evidence for Rotation of V1-ATPase. Proc. Natl. Acad. Sci. U. S. A. 2003, 100, 2312–2315. 10.1073/pnas.0436796100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matilla I.; Alfonso C.; Rivas G.; Bolt E. L.; De la Cruz F.; Cabezon E. The Conjugative DNA Translocase TrwB Is a Structure-Specific DNA-Binding Protein. J. Biol. Chem. 2010, 285, 17537–17544. 10.1074/jbc.M109.084137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tato I.; Matilla I.; Arechaga I.; Zunzunegui S.; De la Cruz F.; Cabezon E. The ATPase Activity of the DNA Transporter TrwB Is Modulated by Protein TrwA: Implications for a Common Assembly Mechanism of DNA Translocating Motors. J. Biol. Chem. 2007, 282, 25569–25576. 10.1074/jbc.M703464200. [DOI] [PubMed] [Google Scholar]
- Cabezon E.; De la Cruz F. TrwB: An F1-ATPase-Like Molecular Motor Involved in DNA Transport During Bacterial Conjugation. Res. Microbiol. 2006, 157, 299–305. 10.1016/j.resmic.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Tato I.; Zunzunegui S.; De la Cruz F.; Cabezon E. TrwB, the Coupling Protein Involved in DNA Transport During Bacterial Conjugation, Is a DNA-Dependent ATPase. Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 8156–8161. 10.1073/pnas.0503402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomis-Rüth F. X.; Coll M. Structure of TrwB, a Gatekeeper in Bacterial Conjugation. Int. J. Biochem. Cell Biol. 2001, 33, 839–843. 10.1016/S1357-2725(01)00060-7. [DOI] [PubMed] [Google Scholar]
- Gomis-Rüth F. X.; Moncalián G.; Pérez-Luque R.; González A.; Cabezón E.; De la Cruz F.; Coll M. The Bacterial Conjugation Protein TrwB Resembles Ring Helicases and F 1-ATPase. Nature 2001, 409, 637–641. 10.1038/35054586. [DOI] [PubMed] [Google Scholar]
- McGeoch A. T.; Trakselis M. A.; Laskey R. A.; Bell S. D. Organization of the Archaeal MCM Complex on DNA and Implications for the Helicase Mechanism. Nat. Struct. Mol. Biol. 2005, 12, 756–762. 10.1038/nsmb974. [DOI] [PubMed] [Google Scholar]
- Bochman M. L.; Schwacha A. The Saccharomyces cerevisiae Mcm6/2 and Mcm5/3 ATPase Active Sites Contribute to the Function of the Putative Mcm2–7’ ‘ ‘Gate’. Nucleic Acids Res. 2010, 38, 6078–6088. 10.1093/nar/gkq422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedenzu T.; Röleke D.; Bains G.; Scherzinger E.; Saenger W. Crystal Structure of the Hexameric Replicative Helicase RepA of Plasmid RSF1010. J. Mol. Biol. 2001, 306, 479–487. 10.1006/jmbi.2000.4398. [DOI] [PubMed] [Google Scholar]
- Xu H.; Frank J.; Niedenzu T.; Saenger W. DNA Helicase RepA: Cooperative ATPase Activity and Binding of Nucleotides. Biochemistry 2000, 39, 12225–12233. 10.1021/bi0008938. [DOI] [PubMed] [Google Scholar]
- Matias P. M.; Gorynia S.; Donner P.; Carrondo M. A. Crystal Structure of the Human AAA+ Protein RuvBL1. J. Biol. Chem. 2006, 281, 38918–38929. 10.1074/jbc.M605625200. [DOI] [PubMed] [Google Scholar]
- Ohnishi T.; Hishida T.; Harada Y.; Iwasaki H.; Shinagawa H. Structure-Function Analysis of the Three Domains of RuvB DNA Motor Protein. J. Biol. Chem. 2005, 280, 30504–30510. 10.1074/jbc.M502400200. [DOI] [PubMed] [Google Scholar]
- Mézard C.; Davies A. A.; Stasiak A.; West S. C. Biochemical Properties of RuvBD113N: A Mutation in Helicase Motif II of the RuvB Hexamer Affects DNA Binding and ATPase Activities. J. Mol. Biol. 1997, 271, 704–717. 10.1006/jmbi.1997.1225. [DOI] [PubMed] [Google Scholar]
- Stasiak A.; Tsaneva I. R.; West S. C.; Benson C.; Yu X.; Egelman E. H. The Escherichia coli RuvB Branch Migration Protein Forms Double Hexameric Rings around DNA. Proc. Natl. Acad. Sci. U. S. A. 1994, 91, 7618–7622. 10.1073/pnas.91.16.7618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De-Donatis G. M.; Zhao Z.; Wang S.; Huang L. P.; Schwartz C.; Tsodikov O. V.; Zhang H.; Haque F.; Guo P. Finding of Widespread Viral and Bacterial Revolution dsDNA Translocation Motors Distinct from Rotation Motors by Channel Chirality and Size. Cell Biosci. 2014, 4, 30–43. 10.1186/2045-3701-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Najjar N.; Kaimer C.; Rösch T.; Graumann P. L. Requirements for Septal Localization and Chromosome Segregation Activity of the DNA Translocase SftA from Bacillus subtilis. J. Mol. Microbiol. Biotechnol. 2017, 27, 29–42. 10.1159/000450725. [DOI] [PubMed] [Google Scholar]
- Mantynen S.; Sundberg L. R.; Oksanen H. M.; Poranen M. M. Half a Century of Research on Membrane-Containing Bacteriophages: Bringing New Concepts to Modern Virology. Viruses 2019, 11, 76–93. 10.3390/v11010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mun K.; Punga T. Cellular Zinc Finger Protein 622 Hinders Human Adenovirus Lytic Growth and Limits Binding of the Viral pVII Protein to Virus DNA. J. Virol. 2019, 93, e01628-18 10.1128/JVI.01628-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassetti M. C.; Merchlinsky M.; Wolffe E. J.; Weisberg A. S.; Moss B. DNA Packaging Mutant: Repression of the Vaccinia Virus A32 Gene Results in Noninfectious, DNA-Deficient, Spherical, Enveloped Particles. J. Virol. 1998, 72, 5769–5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B. Poxvirus DNA Replication. Cold Spring Harbor Perspect. Biol. 2013, 5, a010199 10.1101/cshperspect.a010199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z.; Reynolds S. E.; Martens C. A.; Bruno D. P.; Porcella S. F.; Moss B. Expression Profiling of the Intermediate and Late Stages of Poxvirus Replication. J. Virol. 2011, 85, 9899–9908. 10.1128/JVI.05446-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechman S. L.; Rao X. M.; McMasters K. M.; Zhou H. S. Adenovirus with DNA Packaging Gene Mutations Increased Virus Release. Viruses 2016, 8, 333–350. 10.3390/v8120333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostapchuk P.; Suomalainen M.; Zheng Y.; Boucke K.; Greber U. F.; Hearing P. The Adenovirus Major Core Protein VII Is Dispensable for Virion Assembly but Is Essential for Lytic Infection. PLoS Pathog. 2017, 13, e1006455 10.1371/journal.ppat.1006455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K.; Guimet D.; Hearing P. The Adenovirus L4–33k Protein Regulates Both Late Gene Expression Patterns and Viral DNA Packaging. J. Virol. 2013, 87, 6739–6747. 10.1128/JVI.00652-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi F.; Zhao Z.; Chelikani V.; Yoder K.; Kvaratskhelia M.; Guo P. Development of Potent Antiviral Drugs Inspired by Viral Hexameric DNA-Packaging Motors with Revolving Mechanism. J. Virol. 2016, 90, 8036–8046. 10.1128/JVI.00508-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelikani V.; Ranjan T.; Kondabagil K. Revisiting the Genome Packaging in Viruses with Lessons from the “Giants”. Virology 2014, 466–467, 15–26. 10.1016/j.virol.2014.06.022. [DOI] [PubMed] [Google Scholar]
- Chelikani V.; Ranjan T.; Zade A.; Shukla A.; Kondabagil K. Genome Segregation and Packaging Machinery in Acanthamoeba polyphaga Mimivirus Is Reminiscent of Bacterial Apparatus. J. Virol. 2014, 88, 6069–6075. 10.1128/JVI.03199-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zauberman N.; Mutsafi Y.; Halevy D. B.; Shimoni E.; Klein E.; Xiao C.; Sun S.; Minsky A. Distinct DNA Exit and Packaging Portals in the Virus Acanthamoeba polyphaga Mimivirus. PLoS Biol. 2008, 6, e114 10.1371/journal.pbio.0060114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L.; Yang Q.; Wang M.; Jia R.; Chen S.; Zhu D.; Liu M.; Wu Y.; Zhao X.; Zhang S.; Liu Y.; Yu Y.; Zhang L.; Chen X.; Cheng A. Terminase Large Subunit Provides a New Drug Target for Herpesvirus Treatment. Viruses 2019, 11, 219–237. 10.3390/v11030219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn-Kittenplon D. D.; Kalt I.; Lellouche J. M.; Sarid R. The KSHV Portal Protein ORF43 Is Essential for the Production of Infectious Viral Particles. Virology 2019, 529, 205–215. 10.1016/j.virol.2019.01.028. [DOI] [PubMed] [Google Scholar]
- Uppal T.; Meyer D.; Agrawal A.; Verma S. C. The DNase Activity of KSHV SOX Protein Serves an Important Role in Viral Genome Processing During Lytic Replication. J. Virol. 2019, 93, e01983-18 10.1128/JVI.01983-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visalli R. J.; Schwartz A. M.; Patel S.; Visalli M. A. Identification of the Epstein Barr Virus Portal. Virology 2019, 529, 152–159. 10.1016/j.virol.2019.01.003. [DOI] [PubMed] [Google Scholar]
- Li M.; Chen T.; Zou X.; Xu Z.; Wang Y.; Wang P.; Ou X.; Li Y.; Chen D.; Peng T.; Wang Y.; Cai M. Characterization of the Nucleocytoplasmic Transport Mechanisms of Epstein-Barr Virus BFLF2. Cell. Physiol. Biochem. 2018, 51, 1500–1517. 10.1159/000495641. [DOI] [PubMed] [Google Scholar]
- Miller J. T.; Zhao H.; Masaoka T.; Varnado B.; Cornejo Castro E. M.; Marshall V. A.; Kouhestani K.; Lynn A. Y.; Aron K. E.; Xia A.; Beutler J. A.; Hirsch D. R.; Tang L.; Whitby D.; Murelli R. P.; Le Grice S. F. J. Sensitivity of the C-Terminal Nuclease Domain of Kaposi’s Sarcoma-Associated Herpesvirus ORF29 to Two Classes of Active-Site Ligands. Antimicrob. Agents Chemother. 2018, 62, e00233-18 10.1128/AAC.00233-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkosi D.; Howell L. A.; Cheerathodi M. R.; Hurwitz S. N.; Tremblay D. C.; Liu X.; Meckes D. G. Jr. Transmembrane Domains Mediate Intra- and Extracellular Trafficking of Epstein-Barr Virus Latent Membrane Protein 1. J. Virol. 2018, 92, e00280-18 10.1128/JVI.00280-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump C. Virus Assembly and Egress of HSV. Adv. Exp. Med. Biol. 2018, 1045, 23–44. 10.1007/978-981-10-7230-7_2. [DOI] [PubMed] [Google Scholar]
- Gardner M. R.; Glaunsinger B. A. Kaposi’s Sarcoma-Associated Herpesvirus ORF68 Is a DNA Binding Protein Required for Viral Genome Cleavage and Packaging. J. Virol. 2018, 92, e00840-18 10.1128/JVI.00840-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeind E. M.; Visalli R. J. Human Herpesvirus Portal Proteins: Structure, Function, and Antiviral Prospects. Rev. Med. Virol. 2018, 28, e1972 10.1002/rmv.1972. [DOI] [PubMed] [Google Scholar]
- Ligat G.; Cazal R.; Hantz S.; Alain S. The Human Cytomegalovirus Terminase Complex as an Antiviral Target: A Close-up View. FEMS Microbiol. Rev. 2018, 42, 137–145. 10.1093/femsre/fuy004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth R.; Horniblow R. D.; Forrest C.; Stewart G. S.; Grand R. J. Localization of Double-Strand Break Repair Proteins to Viral Replication Compartments Following Lytic Reactivation of Kaposi’s Sarcoma-Associated Herpesvirus. J. Virol. 2017, 91, e00930-17 10.1128/JVI.00930-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuhlsdorf M.; Hinrichs W. Assemblins as Maturational Proteases in Herpesviruses. J. Gen. Virol. 2017, 98, 1969–1984. 10.1099/jgv.0.000872. [DOI] [PubMed] [Google Scholar]
- Yang K.; Dang X.; Baines J. D. A Domain of Herpes Simplex Virus pUL33 Required to Release Monomeric Viral Genomes from Cleaved Concatemeric DNA. J. Virol. 2017, 91, e00854-17 10.1128/JVI.00854-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi R.; Kato A.; Sagara H.; Watanabe M.; Maruzuru Y.; Koyanagi N.; Arii J.; Kawaguchi Y. Herpes Simplex Virus 1 Small Capsomere-Interacting Protein VP26 Regulates Nucleocapsid Maturation. J. Virol. 2017, 91, e01068-17 10.1128/JVI.01068-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijder J.; Radtke K.; Anderson F.; Scholtes L.; Corradini E.; Baines J.; Heck A. J. R.; Wuite G. J. L.; Sodeik B.; Roos W. H. Vertex-Specific Proteins pUL17 and pUL25 Mechanically Reinforce Herpes Simplex Virus Capsids. J. Virol. 2017, 91, e00123-17 10.1128/JVI.00123-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agirrezabala X.; Martín-Benito J.; Valle M.; González J. M.; Valencia A.; Valpuesta J. M.; Carrascosa J. L. Structure of the Connector of Bacteriophage T7 at 8 Å Resolution: Structural Homologies of a Basic Component of a DNA Translocating Machinery. J. Mol. Biol. 2005, 347, 895–902. 10.1016/j.jmb.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Juhala R. J.; Ford M. E.; Duda R. L.; Youlton A.; Hatfull G. F.; Hendrix R. W. Genomic Sequences of Bacteriophages HK97 and HK022: Pervasive Genetic Mosaicism in the Lambdoid Bacteriophages. J. Mol. Biol. 2000, 299, 27–51. 10.1006/jmbi.2000.3729. [DOI] [PubMed] [Google Scholar]
- Olia A. S.; Prevelige P. E. Jr; Johnson J. E.; Cingolani G. Three-Dimensional Structure of a Viral Genome-Delivery Portal Vertex. Nat. Struct. Mol. Biol. 2011, 18, 597–615. 10.1038/nsmb.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guasch A.; Pous J.; Ibarra B.; Gomis-Rüth F. X.; Valpuesta J. M.; Sousa N.; Carrascosa J. L.; Coll M. Detailed Architecture of a DNA Translocating Machine: The High-Resolution Structure of the Bacteriophage Φ29 Connector Particle. J. Mol. Biol. 2002, 315, 663–676. 10.1006/jmbi.2001.5278. [DOI] [PubMed] [Google Scholar]
- Snider J.; Houry W. A. AAA+ Proteins: Diversity in Function, Similarity in Structure. Biochem. Soc. Trans. 2008, 36, 72–77. 10.1042/BST0360072. [DOI] [PubMed] [Google Scholar]
- Hanson P. I.; Whiteheart S. W. AAA+ Proteins: Have Engine, Will Work. Nat. Rev. Mol. Cell Biol. 2005, 6, 519–529. 10.1038/nrm1684. [DOI] [PubMed] [Google Scholar]
- Zhao Z.; De-Donatis G. M.; Schwartz C.; Fang H.; Li J.; Guo P. An Arginine Finger Regulates Sequential Action of Asymmetrical Hexameric ATPase in dsDNA Translocation Motor. Mol. Cell. Biol. 2016, 36, 2514–2523. 10.1128/MCB.00142-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz D.; DelToro D.; Ordyan M.; Pajak J.; Sippy J.; Catala A.; Oh C.-S.; Vu A.; Arya G.; Feiss M.; et al. Evidence That a Catalytic Glutamate and an ‘Arginine Toggle’ act in Concert to Mediate ATP Hydrolysis and Mechanochemical Coupling in a Viral DNA Packaging Motor. Nucleic Acids Res. 2019, 47, 1404–1415. 10.1093/nar/gky1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R.-G.; Jenkins H. T.; Antson A. A.; Greive S. J. Structure of the Large Terminase from a Hyperthermophilic Virus Reveals a Unique Mechanism for Oligomerization and ATP Hydrolysis. Nucleic Acids Res. 2017, 45, 13029–13042. 10.1093/nar/gkx947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T.-C.; Ortiz D.; Yang Q.; De Angelis R. W.; Sanyal S. J.; Catalano C. E. Physical and Functional Characterization of a Viral Genome Maturation Complex. Biophys. J. 2017, 112, 1551–1560. 10.1016/j.bpj.2017.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbert B. J.; Hayes J. A.; Stone N. P.; Xu R.-G.; Kelch B. A. The Large Terminase DNA Packaging Motor Grips DNA with Its ATPase Domain for Cleavage by the Flexible Nuclease Domain. Nucleic Acids Res. 2017, 45, 3591–3605. 10.1093/nar/gkw1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao H.; Saha M.; Reyes-Aldrete E.; Sherman M. B.; Woodson M.; Atz R.; Grimes S.; Jardine P. J.; Morais M. C. Structural and Molecular Basis for Coordination in a Viral DNA Packaging Motor. Cell Rep. 2016, 14, 2017–2029. 10.1016/j.celrep.2016.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. E.; Saraste M.; Runswick M. J.; Gay N. J. Distantly Related Sequences in the Alpha and Beta Subunits of ATP Synthase, Myosin, Kinases and Other ATP Requiring Enzymes and a Common Nucleotide Binding Fold. EMBO J. 1982, 1, 945–951. 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. E.; Saraste M.; Gay N. J. E. Coli F1-ATPase Interacts with a Membrane Protein Component of a Proton Channel. Nature 1982, 298, 867–869. 10.1038/298867a0. [DOI] [PubMed] [Google Scholar]
- Yu J.; Moffitt J.; Hetherington C. L.; Bustamante C.; Oster G. Mechanochemistry of a Viral DNA Packaging Motor. J. Mol. Biol. 2010, 400, 186–203. 10.1016/j.jmb.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Lísal J.; Tuma R. Cooperative Mechanism of RNA Packaging Motor. J. Biol. Chem. 2005, 280, 23157–23164. 10.1074/jbc.M502658200. [DOI] [PubMed] [Google Scholar]
- Schwartz C.; Guo P. Ultrastable pRNA Hexameric Ring Gearing Hexameric Phi29 DNA-Packaging Motor by Revolving without Rotating and Coiling. Curr. Opin. Biotechnol. 2013, 24, 581–590. 10.1016/j.copbio.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirunavukarasu D.; Shi H. An RNA Aptamer Specific to Hsp70-ATP Conformation Inhibits Its ATPase Activity Independent of Hsp40. Nucleic Acid Ther. 2015, 25, 103–112. 10.1089/nat.2014.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M. M.; Faris R.; Van Schaik E. J.; Samuel J. E. Identification and Characterization of Arginine Finger-Like Motifs, and Endosome-Lysosome Basolateral Sorting Signals within the Coxiellaburnetii Type IV Secreted Effector Protein CirA. Microbes Infect. 2018, 20, 302–307. 10.1016/j.micinf.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobruk-Serkowska A.; Caccamo M.; Rodríguez-Castañeda F.; Wu M.; Bryce K.; Ng I.; Schumacher M. A.; Barillà D.; Hayes F. Uncoupling of Nucleotide Hydrolysis and Polymerization in the Para Protein Superfamily Disrupts DNA Segregation Dynamics. J. Biol. Chem. 2012, 287, 42545–42553. 10.1074/jbc.M112.410324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquenet E.; Richet E. Conserved Motifs Involved in ATP Hydrolysis by Malt, a Signal Transduction ATPase with Numerous Domains from Escherichia coli. J. Bacteriol. 2010, 192, 5181–5191. 10.1128/JB.00522-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z.; Zhang H.; Shu D.; Montemagno C.; Ding B.; Li J.; Guo P. Construction of Asymmetrical Hexameric Biomimetic Motors with Continuous Single Directional Motion by Sequential Coordination. Small 2017, 13, 1601600. 10.1002/smll.201601600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. J.; Schwartz C.; Guo P. Construction of Bacteriophage Phi29 DNA Packaging Motor and Its Applications in Nanotechnology and Therapy. Ann. Biomed. Eng. 2009, 37, 2064–2081. 10.1007/s10439-009-9723-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besche H.; Tamura N.; Tamura T.; Zwickl P. Mutational Analysis of Conserved AAA+ Residues in the Archaeal Lon Protease from Thermoplasma acidophilum. FEBS Lett. 2004, 574, 161–166. 10.1016/j.febslet.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Boyer P. D. The Binding Change Mechanism for ATP Synthase—Some Probabilities and Possibilities. Biochim. Biophys. Acta, Bioenerg. 1993, 1140, 215–250. 10.1016/0005-2728(93)90063-L. [DOI] [PubMed] [Google Scholar]
- Boyer P. D. The ATP Synthase—a Splendid Molecular Machine. Annu. Rev. Biochem. 1997, 66, 717–749. 10.1146/annurev.biochem.66.1.717. [DOI] [PubMed] [Google Scholar]
- Komoriya Y.; Ariga T.; Iino R.; Imamura H.; Okuno D.; Noji H. Principal Role of the Arginine Finger in Rotary Catalysis of F1-ATPase. J. Biol. Chem. 2012, 287, 15134–15142. 10.1074/jbc.M111.328153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepers A.; Diffley J. F. Mutational Analysis of Conserved Sequence Motifs in the Budding Yeast Cdc6 Protein. J. Mol. Biol. 2001, 308, 597–608. 10.1006/jmbi.2001.4637. [DOI] [PubMed] [Google Scholar]
- Werbeck N. D.; Schlee S.; Reinstein J. Coupling and Dynamics of Subunits in the Hexameric AAA+ Chaperone ClpB. J. Mol. Biol. 2008, 378, 178–190. 10.1016/j.jmb.2008.02.026. [DOI] [PubMed] [Google Scholar]
- Chen B.; Sysoeva T. A.; Chowdhury S.; Guo L.; De Carlo S.; Hanson J. A.; Yang H.; Nixon B. T. Engagement of Arginine Finger to ATP Triggers Large Conformational Changes in NtrC1 AAA+ ATPase for Remodeling Bacterial RNA Polymerase. Structure 2010, 18, 1420–1430. 10.1016/j.str.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly N.; Buck M. Engineered Interfaces of an AAA+ ATPase Reveal a New Nucleotide-dependent Coordination Mechanism. J. Biol. Chem. 2010, 285, 15178–15186. 10.1074/jbc.M110.103150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendler P.; Ciniawsky S.; Kock M.; Kube S. Structure and Function of the AAA+ Nucleotide Binding Pocket. Biochim. Biophys. Acta, Mol. Cell Res. 2012, 1823, 2–14. 10.1016/j.bbamcr.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Iyer L. M.; Leipe D. D.; Koonin E. V.; Aravind L. Evolutionary History and Higher Order Classification of AAA+ ATPases. J. Struct. Biol. 2004, 146, 11–31. 10.1016/j.jsb.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Putnam C. D.; Clancy S. B.; Tsuruta H.; Gonzalez S.; Wetmur J. G.; Tainer J. A. Structure and Mechanism of the RuvB Holliday Junction Branch Migration Motor. J. Mol. Biol. 2001, 311, 297–310. 10.1006/jmbi.2001.4852. [DOI] [PubMed] [Google Scholar]
- Patel J. R.; Jain A.; Chou Y. y.; Baum A.; Ha T.; García Sastre A. ATPase Driven Oligomerization of RIG I on RNA Allows Optimal Activation of Type I Interferon. EMBO Rep. 2013, 14, 780–787. 10.1038/embor.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura T.; Whiteheart S. W.; Wilkinson A. J. Conserved Arginine Residues Implicated in ATP Hydrolysis, Nucleotide-Sensing, and Inter-Subunit Interactions in AAA and AAA+ ATPases. J. Struct. Biol. 2004, 146, 106–112. 10.1016/j.jsb.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Mann D.; Teuber C.; Tennigkeit S. A.; Schröter G.; Gerwert K.; Kötting C. Mechanism of the Intrinsic Arginine Finger in Heterotrimeric G Proteins. Proc. Natl. Acad. Sci. U. S. A. 2016, 113, E8041–E8050. 10.1073/pnas.1612394113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy G. N.; Suardíaz R.; Lopata A.; Ozohanics O. R.; Vékey K. R.; Brooks B. R.; Leveles I.; Tóth J.; Vértessy B. G.; Rosta E. Structural Characterization of Arginine Fingers: Identification of an Arginine Finger for the Pyrophosphatase dUTPases. J. Am. Chem. Soc. 2016, 138, 15035–15045. 10.1021/jacs.6b09012. [DOI] [PubMed] [Google Scholar]
- Burroughs A. M.; Iyer L. M.; Aravind L.. Comparative Genomics and Evolutionary Trajectories of Viral ATP Dependent DNA-Packaging Systems. In Gene and Protein Evolution; Karger Publishers: Basel, 2007; Vol. 3, pp 48–65. [DOI] [PubMed] [Google Scholar]
- Karata K.; Inagawa T.; Wilkinson A. J.; Tatsuta T.; Ogura T. Dissecting the Role of a Conserved Motif (the Second Region of Homology) in the AAA Family of ATPases Site-Directed Mutagenesis of the ATP-Dependent Protease FtsH. J. Biol. Chem. 1999, 274, 26225–26232. 10.1074/jbc.274.37.26225. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Song C.; Irizarry L.; Dai R.; Zhang X.; Li C. H. Multifunctional Roles of the Conserved Arg Residues in the Second Region of Homology of p97/Valosin-containing Protein. J. Biol. Chem. 2005, 280, 40515–40523. 10.1074/jbc.M509636200. [DOI] [PubMed] [Google Scholar]
- Yi F.; Kong R.; Ren J.; Zhu L.; Lou J.; Wu J. Y.; Feng W. Noncanonical Myo9b-RhoGAP Accelerates RhoA GTP Hydrolysis by a Dual-Arginine-Finger Mechanism. J. Mol. Biol. 2016, 428, 3043–3057. 10.1016/j.jmb.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal M.; Dvorsky R.; Amin E.; Risse S. L.; Fansa E. K.; Zhang S.-C.; Taha M. S.; Gauhar A. R.; Nakhaei-Rad S.; Kordes C.; et al. Functional Cross-Talk between Ras and Rho Pathways a Ras-Specific GTPase-Activating Protein (p120RasGAP) Competitively Inhibits the RhoGAP Activity of Deleted in Liver Cancer (DLC) Tumor Suppressor by Masking the Catalytic Arginine Finger. J. Biol. Chem. 2014, 289, 6839–6849. 10.1074/jbc.M113.527655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupzig S.; Bouyoucef-Cherchalli D.; Yarwood S.; Sessions R.; Cullen P. J. The Ability of GAP1IP4BP to Function as a Rap1 GTPase-Activating Protein (GAP) Requires Its Ras GAP-Related Domain and an Arginine Finger Rather Than an Asparagine Thumb. Mol. Cell. Biol. 2009, 29, 3929–3940. 10.1128/MCB.00427-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kötting C.; Kallenbach A.; Suveyzdis Y.; Wittinghofer A.; Gerwert K. The GAP Arginine Finger Movement into the Catalytic Site of Ras Increases the Activation Entropy. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 6260–6265. 10.1073/pnas.0712095105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Te Heesen H.; Gerwert K.; Schlitter J. Role of the Arginine Finger in Ras· RasGAP Revealed by QM/MM Calculations. FEBS Lett. 2007, 581, 5677–5684. 10.1016/j.febslet.2007.11.026. [DOI] [PubMed] [Google Scholar]
- Barillà D.; Carmelo E.; Hayes F. The Tail of the ParG DNA Segregation Protein Remodels ParF Polymers and Enhances ATP Hydrolysis via an Arginine Finger-Like Motif. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 1811–1816. 10.1073/pnas.0607216104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A.; Yao N. Y.; Bowman G. D.; Kuriyan J.; O’Donnell M. The Replication Factor C Clamp Loader Requires Arginine Finger Sensors to Drive DNA Binding and Proliferating Cell Nuclear Antigen Loading. J. Biol. Chem. 2006, 281, 35531–35543. 10.1074/jbc.M606090200. [DOI] [PubMed] [Google Scholar]
- Garrity-Ryan L.; Kazmierczak B.; Kowal R.; Comolli J.; Hauser A.; Engel J. The Arginine Finger Domain of ExoT Contributes to Actin Cytoskeleton Disruption and Inhibition of Internalization Of Pseudomonas aeruginosa by Epithelial Cells and Macrophages. Infect. Immun. 2000, 68, 7100–7113. 10.1128/IAI.68.12.7100-7113.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B.; Zhang Y.; Collins C. C.; Johnson D. I.; Zheng Y. A Built-in Arginine Finger Triggers the Self-Stimulatory GTPase-Activating Activity of Rho Family GTPases. J. Biol. Chem. 1999, 274, 2609–2612. 10.1074/jbc.274.5.2609. [DOI] [PubMed] [Google Scholar]
- Resat H.; Straatsma T.; Dixon D. A.; Miller J. H. The Arginine Finger of RasGAP Helps Gln-61 Align the Nucleophilic Water in GAP-Stimulated Hydrolysis of GTP. Proc. Natl. Acad. Sci. U. S. A. 2001, 98, 6033–6038. 10.1073/pnas.091506998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Te Biesebeke R.; Krab I. M.; Parmeggiani A. The Arginine Finger Loop of Yeast and Human GAP Is a Determinant for the Specificity toward Ras GTPase. Biochemistry 2001, 40, 7474–7479. 10.1021/bi010027a. [DOI] [PubMed] [Google Scholar]
- Ren H.; Dou S.-X.; Rigolet P.; Yang Y.; Wang P.-Y.; Amor-Gueret M.; Xi X. G. The Arginine Finger of the Bloom Syndrome Protein: Its Structural Organization and Its Role in Energy Coupling. Nucleic Acids Res. 2007, 35, 6029–6041. 10.1093/nar/gkm544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadanaciva S.; Weber J.; Wilke-Mounts S.; Senior A. E. Importance of F1-ATPase Residue Α-Arg-376 for Catalytic Transition State Stabilization. Biochemistry 1999, 38, 15493–15499. 10.1021/bi9917683. [DOI] [PubMed] [Google Scholar]
- Ammelburg M.; Frickey T.; Lupas A. N. Classification of AAA+ Proteins. J. Struct. Biol. 2006, 156, 2–11. 10.1016/j.jsb.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Zeymer C.; Fischer S.; Reinstein J. Trans-Acting Arginine Residues in the AAA+ Chaperone ClpB Allosterically Regulate the Activity through Inter-and Intra-Domain Communication. J. Biol. Chem. 2014, 289, 32965–32976. 10.1074/jbc.M114.608828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa R.; Montgomery M. G.; Braig K.; Leslie A. G. W.; Walker J. E. The Structure of Bovine F1-ATPase Inhibited by ADP and Beryllium Flouride. EMBO J. 2004, 23, 2734–2744. 10.1038/sj.emboj.7600293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biter A. B.; Lee J.; Sung N.; Tsai F. T. F.; Lee S. Functional Analysis of Conserved cis- and trans-elements in the Hsp104 Protein Disaggregating Machine. J. Struct. Biol. 2012, 179, 172–180. 10.1016/j.jsb.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hishida T.; Han Y.-W.; Fujimoto S.; Iwasaki H.; Shinagawa H. Direct Evidence That a Conserved Arginine in RuvB AAA+ ATPase Acts as an Allosteric Effector for the ATPase Activity of the Adjacent Subunit in a Hexamer. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 9573–9577. 10.1073/pnas.0403584101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki T.; Nakazaki Y.; Watanabe Y. H.; Yoshida M. Roles of Conserved Arginines in ATP-binding Domains of AAA+ ClpB from Thermus thermophilus. FEBS J. 2011, 278, 2395–2403. 10.1111/j.1742-4658.2011.08167.x. [DOI] [PubMed] [Google Scholar]
- Moreau M. J.; McGeoch A. T.; Lowe A. R.; Itzhaki L. S.; Bell S. D. ATPase Site Architecture and Helicase Mechanism of an Archaeal. Mol. Cell 2007, 28, 304–314. 10.1016/j.molcel.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Schwartz C.; De Donatis G. M.; Fang H.; Guo P. The ATPase of the Phi29 DNA Packaging Motor Is a Member of the Hexameric AAA+ Superfamily. Virology 2013, 443, 20–27. 10.1016/j.virol.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aker J.; Hesselink R.; Engel R.; Karlova R.; Borst J. W.; Visser A. J.; De Vries S. C. In vivo Hexamerization and Characterization of the Arabidopsis AAA ATPase CDC48a Complex Using Förster Resonance Energy Transfer-Fluorescence Lifetime Imaging Microscopy and Fluorescence Correlation Spectroscopy. Plant Physiol. 2007, 145, 339–350. 10.1104/pp.107.103986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey S.; Eliason W. K.; Steitz T. A. Structure of Hexameric DnaB Helicase and Its Complex with a Domain of DnaG Primase. Science 2007, 318, 459–463. 10.1126/science.1147353. [DOI] [PubMed] [Google Scholar]
- Hacker K. J.; Johnson K. A. A Hexameric Helicase Encircles One DNA Strand and Excludes the Other During DNA Unwinding. Biochemistry 1997, 36, 14080–14087. 10.1021/bi971644v. [DOI] [PubMed] [Google Scholar]
- Yu X.; Egelman E. H. The RecA Hexamer Is a Structural Homologue of Ring Helicases. Nat. Struct. Biol. 1997, 4, 101–104. 10.1038/nsb0297-101. [DOI] [PubMed] [Google Scholar]
- Itoh H.; Takahashi A.; Adachi K.; Noji H.; Yasuda R.; Yoshida M.; Kinosita K. Jr Mechanically Driven ATP Synthesis by F 1-ATPase. Nature 2004, 427, 465–468. 10.1038/nature02212. [DOI] [PubMed] [Google Scholar]
- Kinosita K.; Yasuda R.; Noji H.; Ishiwata S. i.; Yoshida M. F1-ATPase: A Rotary Motor Made of a Single Molecule. Cell 1998, 93, 21–24. 10.1016/S0092-8674(00)81142-3. [DOI] [PubMed] [Google Scholar]
- Huang L. P.; Guo P. Use of Acetone to Attain Highly Active and Soluble DNA Packaging Protein gp16 of Phi29 for ATPase Assay. Virology 2003, 312, 449–457. 10.1016/S0042-6822(03)00241-1. [DOI] [PubMed] [Google Scholar]
- Huang L. P.; Guo P. Use of PEG to Acquire Highly Soluble DNA-Packaging Enzyme gp16 of Bacterial Virus Phi29 for Stoichiometry Quantification. J. Virol. Methods 2003, 109, 235–244. 10.1016/S0166-0934(03)00077-6. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S.; Derrington I. M.; Pavlenok M.; Niederweis M.; Gundlach J. H.; Aksimentiev A. Molecular Dynamics Study of MspA Arginine Mutants Predicts Slow DNA Translocations and Ion Current Blockades Indicative of DNA Sequence. ACS Nano 2012, 6, 6960–6968. 10.1021/nn3019943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey M. J.; Indiani C.; O'Donnell M. Reconstitution of the Mcm2-7p Heterohexamer, Subunit Arrangement, and ATP Site Architecture. J. Biol. Chem. 2003, 278, 4491–4499. 10.1074/jbc.M210511200. [DOI] [PubMed] [Google Scholar]
- Scheffzek K.; Ahmadian M. R.; Kabsch W.; Wiesmüller L.; Lautwein A.; Schmitz F.; Wittinghofer A. The Ras-RasGAP Complex: Structural Basis for GTPase Activation and Its Loss in Oncogenic Ras Mutants. Science 1997, 277, 333–339. 10.1126/science.277.5324.333. [DOI] [PubMed] [Google Scholar]
- Lammens A.; Schele A.; Hopfner K.-P. Structural Biochemistry of ATP-Driven Dimerization and DNA-Stimulated Activation of SMCATPases. Curr. Biol. 2004, 14, 1778–1782. 10.1016/j.cub.2004.09.044. [DOI] [PubMed] [Google Scholar]
- Song H. K.; Hartmann C.; Ramachandran R.; Bochtler M.; Behrendt R.; Moroder L.; Huber R. Mutational Studies on HslU and Its Docking Mode with HslV. Proc. Natl. Acad. Sci. U. S. A. 2000, 97, 14103–14108. 10.1073/pnas.250491797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubimov A. Y.; Costa A.; Bleichert F.; Botchan M. R.; Berger J. M. ATP-Dependent Conformational Dynamics Underlie the Functional Asymmetry of the Replicative Helicase from a Minimalist Eukaryote. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, 11999–12004. 10.1073/pnas.1209406109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai S.; Saijo S.; Suzuki K.; Mizutani K.; Kakinuma Y.; Ishizuka-Katsura Y.; Ohsawa N.; Terada T.; Shirouzu M.; Yokoyama S.; et al. Rotation Mechanism of Enterococcus hirae 1-ATPase Based on Asymmetric Crystal Structures. Nature 2013, 493, 703–707. 10.1038/nature11778. [DOI] [PubMed] [Google Scholar]
- Su M.; Guo E. Z.; Ding X.; Li Y.; Tarrasch J. T.; Brooks C. L.; Xu Z.; Skiniotis G. Mechanism of Vps4 Hexamer Function Revealed by Cryo-EM. Sci. Adv. 2017, 3, e1700325 10.1126/sciadv.1700325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y.; Zhang S.; Wu Z.; Li X.; Wang W. L.; Zhu Y.; Stoilova-McPhie S.; Lu Y.; Finley D.; Mao Y. Cryo-EM Structures and Dynamics of Substrate-Engaged Human 26s Proteasome. Nature 2019, 565, 49–55. 10.1038/s41586-018-0736-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soultanas P.; Wigley D. B. Unwinding the ‘Gordian Knot’ of Helicase Action. Trends Biochem. Sci. 2001, 26, 47–54. 10.1016/S0968-0004(00)01734-5. [DOI] [PubMed] [Google Scholar]
- Stinson B. M.; Baytshtok V.; Schmitz K. R.; Baker T. A.; Sauer R. T. Subunit Asymmetry and Roles of Conformational Switching in the Hexameric AAA+ Ring of ClpX. Nat. Struct. Mol. Biol. 2015, 22, 411–416. 10.1038/nsmb.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchades C.; Rampello A. J.; Shin M.; Giuliano C. J.; Wiseman R. L.; Glynn S. E.; Lander G. C. Structure of the Mitochondrial Inner Membrane AAA+ Protease YME1 Gives Insight into Substrate Processing. Science 2017, 358, eaao0464 10.1126/science.aao0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S.; Li L.; Yang F.; Wang X.; Fan F.; Yang M.; Chen C.; Li X.; Wang H.-W.; Sui S.-F. Cryo-EM Structures of the ATP-Bound Vps4E233Q Hexamer and Its Complex with Vta1 at near-Atomic Resolution. Nat. Commun. 2017, 8, 16064–16076. 10.1038/ncomms16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr E.; Szyk A.; Piszczek G.; Szczesna E.; Zuo X.; Roll-Mecak A. Katanin Spiral and Ring Structures Shed Light on Power Stroke for Microtubule Severing. Nat. Struct. Mol. Biol. 2017, 24, 717–725. 10.1038/nsmb.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A.; Baker T. A.; Sauer R. T. Rebuilt AAA+ Motors Reveal Operating Principles for ATP-Fuelled Machines. Nature 2005, 437, 1115–1120. 10.1038/nature04031. [DOI] [PubMed] [Google Scholar]
- Shu D.; Guo P. Only One pRNA Hexamer but Multiple Copies of the DNA-Packaging Protein gp16 Are Needed for the Motor to Package Bacterial Virus Phi29 Genomic DNA. Virology 2003, 309, 108–113. 10.1016/S0042-6822(03)00011-4. [DOI] [PubMed] [Google Scholar]
- Cai Y.; Xiao F.; Guo P. The Effect of N-or C-Terminal Alterations of the Connector of Bacteriophage Phi29 DNA Packaging Motor on Procapsid Assembly, pRNA Binding, and DNA Packaging. Nanomedicine 2008, 4, 8–18. 10.1016/j.nano.2007.10.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ah-Seng Y.; Lopez F.; Pasta F.; Lane D.; Bouet J.-Y. Dual Role of DNA in Regulating ATP Hydrolysis by the SopA Partition Protein. J. Biol. Chem. 2009, 284, 30067–30075. 10.1074/jbc.M109.044800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafoya S.; Liu S.; Castillo J. P.; Atz R.; Morais M. C.; Grimes S.; Jardine P. J.; Bustamante C. Molecular Switch-Like Regulation Enables Global Subunit Coordination in a Viral Ring ATPase. Proc. Natl. Acad. Sci. U. S. A. 2018, 115, 7961–7966. 10.1073/pnas.1802736115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A.; Baker T. A.; Sauer R. T. Pore Loops of the AAA+ ClpX Machine Grip Substrates to Drive Translocation and Unfolding. Nat. Struct. Mol. Biol. 2008, 15, 1147–1151. 10.1038/nsmb.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadian M. R.; Stege P.; Scheffzek K.; Wittinghofer A. Confirmation of the Arginine-Finger Hypothesis for the GAP-Stimulated GTP-Hydrolysis Reaction of Ras. Nat. Struct. Biol. 1997, 4, 686–689. 10.1038/nsb0997-686. [DOI] [PubMed] [Google Scholar]
- Bourne H. R. G Proteins: The Arginine Finger Strikes Again. Nature 1997, 389, 673–674. 10.1038/39470. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Schwartz C.; De Donatis G. M.; Guo P. Push through One-Way Valve” Mechanism of Viral DNA Packaging. Adv. Virus Res. 2012, 83, 415–465. 10.1016/B978-0-12-394438-2.00009-8. [DOI] [PubMed] [Google Scholar]
- Yu X.; Jacobs S. A.; West S. C.; Ogawa T.; Egelman E. H. Domain Structure and Dynamics in the Helical Filaments Formed by RecA and Rad51 on DNA. Proc. Natl. Acad. Sci. U. S. A. 2001, 98, 8419–8424. 10.1073/pnas.111005398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enemark E. J.; Joshua-Tor L. Mechanism of DNA Translocation in a Replicative Hexameric Helicase. Nature 2006, 442, 270–275. 10.1038/nature04943. [DOI] [PubMed] [Google Scholar]
- Guo P.; Zhao Z.. Biomotors: Linear, Rotation, and Revolution Motion Mechanisms; CRC Press: Boca Raton, FL, 2017. [Google Scholar]
- Gollnick B.; Carrasco C.; Zuttion F.; Gilhooly N. S.; Dillingham M. S.; Moreno-Herrero F. Probing DNA Helicase Kinetics with Temperature-Controlled Magnetic Tweezers. Small 2015, 11, 1273–84. 10.1002/smll.201402686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R.; Grubmuller H. Phi29 Connector-DNA Interactions Govern DNA Crunching and Rotation, Supporting the Check-Valve Model. Biophys. J. 2016, 110, 455–469. 10.1016/j.bpj.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey S. C. The Scrunchworm Hypothesis: Transitions between A-DNA and B-DNA Provide the Driving Force for Genome Packaging in Double-Stranded DNA Bacteriophages. J. Struct. Biol. 2015, 189, 1–8. 10.1016/j.jsb.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W.; Shen Y.; She Q. Nanobiomotors of Archaeal DNA Repair Machineries: Current Research Status and Application Potential. Cell Biosci. 2014, 4, 32–43. 10.1186/2045-3701-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R.; Grubmuller H. Elastic Properties and Heterogeneous Stiffness of the Phi29 Motor Connector Channel. Biophys. J. 2014, 106, 1338–1348. 10.1016/j.bpj.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram M.; Sabanayagam C.; Black L. W. Modulation of the Packaging Reaction of Bacteriophage T4 Terminase by DNA Structure. J. Mol. Biol. 2008, 381, 61–72. 10.1016/j.jmb.2008.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen N. D.; Berger J. M. Running in Reverse: The Structural Basis for Translocation Polarity in Hexameric Helicases. Cell 2009, 139, 523–534. 10.1016/j.cell.2009.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo A.; Pulido-Cid M.; Chagoyen M.; Arranz R.; González-García V. A.; Garcia-Doval C.; Castón J. R.; Valpuesta J. M.; Van Raaij M. J.; Martín-Benito J.; et al. Structural Characterization of the Bacteriophage T7 Tail Machinery. J. Biol. Chem. 2013, 288, 26290–26299. 10.1074/jbc.M113.491209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang P.-A.; Wright E. T.; Weintraub S. T.; Hakala K.; Wu W.; Serwer P.; Jiang W. Visualization of Bacteriophage T3 Capsids with DNA Incompletely Packaged in Vivo. J. Mol. Biol. 2008, 384, 1384–1399. 10.1016/j.jmb.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque F.; Wang S.; Stites C.; Chen L.; Wang C.; Guo P. Single Pore Translocation of Folded, Double-Stranded, and Tetra-Stranded DNA through Channel of Bacteriophage Phi29 DNA Packaging Motor. Biomaterials 2015, 53, 744–752. 10.1016/j.biomaterials.2015.02.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castella S.; Burgin D.; Sanders C. M. Role of ATP Hydrolysis in the DNA Translocase Activity of the Bovine Papillomavirus (BPV-1) E1 Helicase. Nucleic Acids Res. 2006, 34, 3731–3741. 10.1093/nar/gkl554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S. S.; Picha K. M. Structure and Function of Hexameric Helicases. Annu. Rev. Biochem. 2000, 69, 651–697. 10.1146/annurev.biochem.69.1.651. [DOI] [PubMed] [Google Scholar]
- Enemark E. J.; Joshua-Tor L. On Helicases and Other Motor Proteins. Curr. Opin. Struct. Biol. 2008, 18, 243–257. 10.1016/j.sbi.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton M. R.; Dillingham M. S.; Wigley D. B. Structure and Mechanism of Helicases and Nucleic Acid Translocases. Annu. Rev. Biochem. 2007, 76, 23–50. 10.1146/annurev.biochem.76.052305.115300. [DOI] [PubMed] [Google Scholar]
- Bianco P. R.; Kowalczykowski S. C. Translocation Step Size and Mechanism of the RecBC DNA Helicase. Nature 2000, 405, 368–372. 10.1038/35012652. [DOI] [PubMed] [Google Scholar]
- Dittrich M.; Schulten K. PcrA Helicase, a Prototype ATP-Driven Molecular Motor. Structure 2006, 14, 1345–1353. 10.1016/j.str.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Phelps C.; Lee W.; Jose D.; Von Hippel P. H.; Marcus A. H. Single-Molecule FRET and Linear Dichroism Studies of DNA Breathing and Helicase Binding at Replication Fork Junctions. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 17320–17325. 10.1073/pnas.1314862110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei J.; Ha T. Watching DNA Breath One Molecule at a Time. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 17173–17174. 10.1073/pnas.1316493110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W.; Jose D.; Phelps C.; Marcus A. H.; Von Hippel P. H. A Single-Molecule View of the Assembly Pathway, Subunit Stoichiometry, and Unwinding Activity of the Bacteriophage T4 Primosome (Helicase–Primase) Complex. Biochemistry 2013, 52, 3157–3170. 10.1021/bi400231s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y.; Haque F.; Shu D.; Li W.; Zhu Z.; Kotb M.; Lyubchenko Y.; Guo P. Fabrication of 14 Different RNA Nanoparticles for Specific Tumor Targeting without Accumulation in Normal Organs. RNA 2013, 19, 767–777. 10.1261/rna.037002.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo P. Biophysical Studies Reveal New Evidence for One-Way Revolution Mechanism of Bacteriophage ϕ29 DNA Packaging Motor. Biophys. J. 2014, 106, 1837–1838. 10.1016/j.bpj.2014.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]