Effective methods to detect viable Mycobacterium tuberculosis, the main causative agent of tuberculosis (TB), are urgently needed. To date, cultivation of M. tuberculosis is the gold standard, which depends on initial sample processing with N-acetyl-l-cysteine–sodium hydroxide (NALC-NaOH), chemicals that compromise M. tuberculosis viability and, consequently, the performance of downstream tests.
KEYWORDS: culture, decontamination, molecular bacterial load assay, Mycobacterium tuberculosis, NALC-NaOH, sputum
ABSTRACT
Effective methods to detect viable Mycobacterium tuberculosis, the main causative agent of tuberculosis (TB), are urgently needed. To date, cultivation of M. tuberculosis is the gold standard, which depends on initial sample processing with N-acetyl-l-cysteine–sodium hydroxide (NALC-NaOH), chemicals that compromise M. tuberculosis viability and, consequently, the performance of downstream tests. We applied culture and the novel molecular bacterial load assay (MBLA) to measure the loss of M. tuberculosis viability following NALC-NaOH treatment of M. tuberculosis H37Rv pure culture and clinical sputum samples from pulmonary TB patients. Compared to the bacterial loads of untreated controls, NALC-NaOH treatment of M. tuberculosis reduced the MBLA-detectable bacillary load (estimated number of CFU [eCFU] per milliliter) by 0.66 ± 0.21 log10 at 23°C (P = 0.018) and 0.72 ± 0.08 log10 at 30°C (P = 0.013). Likewise, NALC-NaOH treatment reduced the viable count on solid culture by 0.84 ± 0.02 log10 CFU/ml at 23°C (P < 0.001) and 0.85 ± 0.01 log10 CFU/ml at 30°C (P < 0.001), respectively. The reduction in the viable count was reflected by a corresponding increase in the time to positivity of the mycobacterial growth indicator tube (MGIT) liquid culture: 1.2 days at 23°C (P < 0.001) and 1.1 days at 30°C (P < 0.001). This NaOH-induced M. tuberculosis viability loss was replicated in clinical sputum samples, with the bacterial load dropping by 0.65 ± 0.17 log10 from 5.36 ± 0.24 log10 eCFU/ml to 4.71 ± 0.16 log10 eCFU/ml for untreated and treated sputa, respectively. Applying the model of Bowness et al. (R. Bowness, M. J. Boeree, R. Aarnoutse, R. Dawson, et al., J Antimicrob Chemother 70:448–455, 2015, https://doi.org/10.1093/jac/dku415) revealed that the treated MGIT time to culture positivity of 142 ± 7.02 h was equivalent to 4.86 ± 0.28 log10 CFU, consistent with the MBLA-measured bacterial load. Our study confirms the contribution of NALC-NaOH treatment to the loss of viable bacterial counts. Tests that obviate the need for decontamination may offer an alternative option for the accurate detection of viable M. tuberculosis and treatment response monitoring.
INTRODUCTION
Tuberculosis (TB) is one of the top 10 causes of death worldwide and the leading cause of death from a single infectious agent (1). In 2017, TB killed 1.7 million people, of whom 0.3 million were coinfected with HIV (1). Some of the major challenges to controlling TB are the long duration of treatment and the fact that appropriate diagnosis and monitoring of the progress of treatment require rapid methods that quantify the number of viable Mycobacterium tuberculosis bacilli in patient samples (2).
Currently, diagnosis and treatment monitoring of TB rely on less sensitive sputum smear microscopy and culture techniques that are compromised by contamination and are slow to yield results (3). Despite the low sensitivity and inability to distinguish dead from viable M. tuberculosis bacilli, sputum smear microscopy remains the most commonly used test for diagnosis and monitoring (4, 5). In 2011, the World Health Organization (WHO) rolled out the rapid, sensitive, and specific DNA-based Xpert MTB/RIF assay (Cepheid, Sunnyvale, CA, USA) for the diagnosis of TB. The Xpert MTB/RIF assay has since then improved case detection rates of TB but not treatment outcomes (1, 5). DNA is a very stable molecule which takes a long period to degrade after cell death and thus cannot be used as a marker of viability and for monitoring the bactericidal effect of anti-TB therapy (6). A DNA-positive test result in treatment follow-up specimens does not necessarily indicate the presence of viable bacilli and could mislead the assessment of treatment progress (4, 6–8). Unsuccessful attempts have been made to use propidium monoazide, a dye which penetrates and inactivates DNA from dead cells, so that tests like the Xpert/MTB RIF assay can detect viable M. tuberculosis bacilli and be used for treatment monitoring (9, 10).
Cultivation of M. tuberculosis is the reference standard for TB diagnosis and treatment monitoring. Before culture, sputum samples must be decontaminated with chemicals to reduce the growth of non-acid-fast bacilli (non-AFB) and fungi that would otherwise outgrow the slowly growing M. tuberculosis. Decontamination with N-acetyl-l-cysteine (NALC) combined with sodium hydroxide (NaOH), usually performed for 15 to 20 min, is the most recommended method (11, 12). NALC has a mucolytic property, as it breaks disulfide bonds in sputum. Once this occurs, all bacteria are exposed to NaOH, which kills fast-growing contaminants while maintaining M. tuberculosis viability. However, previous clinical studies have shown that NALC-NaOH treatment reduces the viable M. tuberculosis count on solid culture and increases the time to positivity (TTP) in liquid culture (13). Increasing the concentration of NaOH from 1% to 2% in order to eliminate all sputum contaminants resulted in a rate of negative M. tuberculosis culture results higher than that obtained with the standard concentration of 1%, confirming the detrimental effect of NaOH on M. tuberculosis viability (11).
Phenotypes of M. tuberculosis which do not grow in routine culture media without the use of resuscitation-promoting factors (rpf’s) are increasingly being recognized (14, 15). One of the major characteristics of such bacterial phenotypes is that they are dominated by fatty cells which are rich in lipids, acid fast negative, and difficult to eradicate with antibiotics (16, 17). It has recently been shown that NALC-NaOH decontamination combined with a centrifugation step is associated with a 90% loss of Mycobacterium smegmatis and that lipid-poor (LP) cells are more susceptible to this effect than lipid-rich (LR) cells (18). These emerging reports provide important evidence that detection of all subpopulations of M. tuberculosis in patient specimens may not be achieved using culture techniques or NALC-NaOH decontamination-dependent tests.
The molecular bacterial load assay (MBLA) is a molecular test for the detection of viable M. tuberculosis bacilli. It is a reverse transcriptase quantitative PCR (RT-qPCR) that quantifies the M. tuberculosis load from patient sputum using the 16S rRNA gene as a reference gene. In contrast to culture, MBLA is rapid, sensitive, and specific and does not require an NALC-NaOH decontamination step (7). Unlike mRNA, which occurs in a low copy number and is exquisitely sensitive to degradation, the higher abundance and relative stability of rRNA make MBLA a more sensitive and robust test. MBLA was recently acknowledged to be a potential biomarker for TB treatment response monitoring, replacing culture and smear microscopy, and more studies to validate the test were called for (1).
Previous studies using nondecontaminated sputa showed that MBLA has a higher sensitivity than culture (7, 19, 20). In this study, we assessed whether, like culture, NALC-NaOH decontamination has an effect on the viable M. tuberculosis count measured by MBLA and if the effect is temperature dependent, bearing in mind that the temperature of laboratories in tropical areas may be high.
MATERIALS AND METHODS
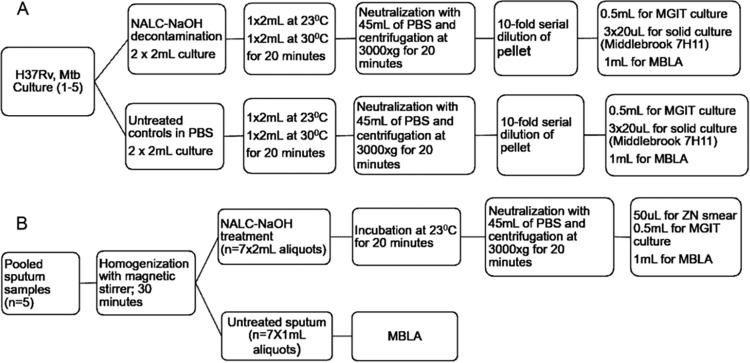
Laboratory experiments were performed using five replicates of M. tuberculosis reference strain H37Rv (ATCC 27294) and pooled sputum samples from pulmonary TB patients at the National Institute for Medical Research, Medical Research Centre (NIMR-MMRC), Mbeya, Tanzania (Fig. 1A and B).
FIG 1.
Flow diagram of laboratory experiments, conditions, and downstream tests performed for M. tuberculosis cultures and sputum specimens. (A) In vitro experimental procedure employed for each decontaminated M. tuberculosis (Mtb) culture. (B) Experimental flow for pooled patient sputum specimens. Note that 5 biological replicates of experiments were performed for in vitro M. tuberculosis cultures (culture 1 to culture 5) and 7 replicates were performed for sputum aliquots. ZN, Ziehl-Neelsen stain.
H37Rv culture.
A single colony of M. tuberculosis H37Rv (ATCC 27294) from Lowenstein-Jensen medium (LJ) was inoculated into mycobacterial growth indicator tubes (MGIT; BD Bactec MGIT; Becton, Dickinson and Company, MD, USA) supplemented with oleic acid, albumin, dextrose, and catalase (OADC; Oxoid, United Kingdom). The culture was incubated in a Bactec MGIT 960 culture system (Becton, Dickinson and Company, MD, USA). After 14 days, the culture was mixed by vortexing and 100 μl was subcultured into fresh MGIT cultures, incubated for another 14 days, and then used for the NALC-NaOH decontamination experiment and as a control (Fig. 1A).
NALC-NaOH decontamination of H37Rv cultures.
The 2-ml H37Rv culture at a concentration of ∼107 CFU/ml was processed with an equal volume of NALC-NaOH (1% final concentration of NaOH) at 23°C and 30°C for 20 min. For controls, another 2 ml of culture aliquots was treated with an equal volume of phosphate-buffered saline (PBS), pH 6.8, instead of NALC-NaOH (Fig. 1A). Following exposure to NALC-NaOH at 23°C and 30°C, the cell pellets were harvested by centrifugation at 3,000 × g for 20 min at 4°C. The pellets were serially diluted (10-fold dilutions) in PBS to determine the limit of detection (LoD) of each method. Each dilution was inoculated in an MGIT culture and incubated for a maximum of 42 days to determine the TTP. Quadruplicates of each dilution were inoculated on solid medium (Middlebrook 7H11; Becton, Dickinson and Company, MD, USA) using the method of Miles et al. (21) and incubated at 37°C for determination of colony counts (Fig. 1A). The 7H11 medium was supplemented with OADC (Oxoid, United Kingdom), and the plates were observed weekly for any growth of M. tuberculosis colonies for up to 6 weeks. All culture media (7H11 and MGIT) used for the in vitro M. tuberculosis experiments were free from selective antibiotics.
Patient sputum sample collection and processing.
We nested this study into the project Evaluation of Implementability of Rapid Molecular Monitoring Assay of Tuberculosis Treatment (EIRMMA-TBT). Sputum samples collected for the screening visit were tested for M. tuberculosis with the Xpert MTB/RIF assay. Five M. tuberculosis-positive sputum samples were pooled and homogenized with a sterile magnetic stirrer for 30 min at room temperature. Thereafter, 1-ml aliquots of pooled sputum (7 replicates) were sampled and processed for MBLA as controls. Second aliquots of 2 ml (7 replicates) were decontaminated with NALC-NaOH prior to MBLA and liquid culture (Fig. 1B).
Confirmation of M. tuberculosis in liquid culture.
To confirm the presence of M. tuberculosis in culture, a rapid culture identification test (MPT64; Becton, Dickinson and Company, MD, USA) was performed following the manufacturer’s instructions. An assay with blood agar (BA) was performed to exclude contaminations and for validation of the TTP in the MGIT culture. A drop of the MGIT culture (∼20 μl) was inoculated on BA and incubated for 48 h at 37°C.
Molecular bacterial load assay (MBLA).
(i) RNA extraction. Extraction of RNA was performed as previously described (20, 22). Briefly, 100 μl of an extraction control (Vitalbacteria; SOI Group, UK) was added to each tube prior to RNA extraction. The mixture was centrifuged at 3,000 × g for 30 min at room temperature, and cell pellets were suspended in 950 μl of RNA Pro Blue solution (MP Biomedicals). Homogenization was performed for 40 s at 6,000 rpm using a FastPrep instrument (MP Biomedicals), and RNA was extracted using a FastRNA Pro kit (MP Biomedicals) following the manufacturer’s instructions. Removal of the genomic DNA was achieved by DNase treatment for 1 h at 37°C using an Ambion Turbo DNA-free kit (Life Technologies).
(ii) RT-PCR. Reverse transcriptase PCR (RT-PCR) was performed in a Rotor-Gene 5plex platform (Corbett research) using a QuantiTect multiplex NoROX PCR mix (Qiagen, UK). Sequence-specific primers and TaqMan probes dually labeled for M. tuberculosis 16S rRNA and for the extraction control (EC) target were procured from MWG Eurofins, Germany. Master mix preparation, PCR test conditions, and amplification were set and performed as previously demonstrated (19, 20, 22). The sensitivity and specificity of the primers and probes for MBLA were previously tested against nontuberculosis mycobacteria, including a wide range of respiratory pathogens, and none of them was found to be amplified (19).
Statistical methods.
The bacterial load (BL; the number of estimated CFU [eCFU] per milliliter) determined by MBLA and the actual count of the number of CFU per milliliter on solid culture were normalized by log transformation. Then, the average, standard deviation (SD), and percent positivity for the controls and the NALC-NaOH-treated cultures were calculated for each test using Microsoft Excel (version 1810). Two-way analysis of variance (ANOVA) and then Sidak's multiple-comparison test were performed using GraphPad Prism (version 7.04) software (GraphPad Software, La Jolla, CA, USA) to determine the difference in M. tuberculosis viability loss among NALC-NaOH-treated cultures versus the controls and the different temperatures of treatment. An independent t test was used to estimate the difference in the M. tuberculosis bacterial load measured by MBLA between untreated and treated sputum. The MGIT TTP from sputum culture was converted into the number of CFU as previously published (23). Statistical significance was accepted at a P value of <0.05.
Ethical approval.
The EIRMMA-TBT study in which this analysis was nested received approval from the Mbeya Medical Research Ethics Committee (MRH/R.10/18VOLL.VII/12), the National Health Research Ethics Committee (NatHREC) of the National Institute for Medical Research in Tanzania (NIMR/HQ/R.8a/V01.IX), and the University of St. Andrews Teaching and Research Ethics Committee (MD 12678).
RESULTS
A total of five experimental repeats using five M. tuberculosis H37Rv cultures were performed between April 2017 and December 2018 (Fig. 1A). For clinical sputum samples, 22 ml of sputum was obtained after pooling five sputum samples from pulmonary TB patients. From the pooled sample, 21 1-ml aliquots were made, and of these, 7 untreated aliquots (controls) were tested by MBLA (7 aliquots at 1 ml each) and the remaining 14 were treated with NALC-NaOH (7 aliquots at 2 ml each). Seven of the NALC-NaOH-treated aliquots were tested by MBLA, MGIT liquid culture, and indirect smear microscopy (Fig. 1B).
Bacterial load estimated by MBLA.
The average bacterial load ± SD of the untreated culture was 7.79 ± 0.37 log10 eCFU/ml and 7.74 ± 0.041 log10 eCFU/ml at 23°C and 30°C respectively. In contrast the bacterial load of the NALC-NaOH-treated cultures was 7.23 ± 0.15 log10 eCFU/ml at 23°C and 7.15 ± 0.16 log10 eCFU/ml at 30°C. Consequently, the NALC-NaOH treatment caused a viable bacterial load count reduction by 0.66 ± 0.21 log10 eCFU/ml at 23°C (P = 0.0178) and 0.72 ± 0.08 log10 eCFU/ml at 30°C (P = 0.0134) compared to the counts for the controls in PBS (Fig. 2A). This reduction was equivalent to 78.42% at 23°C and 80.34% at 30°C.
FIG 2.
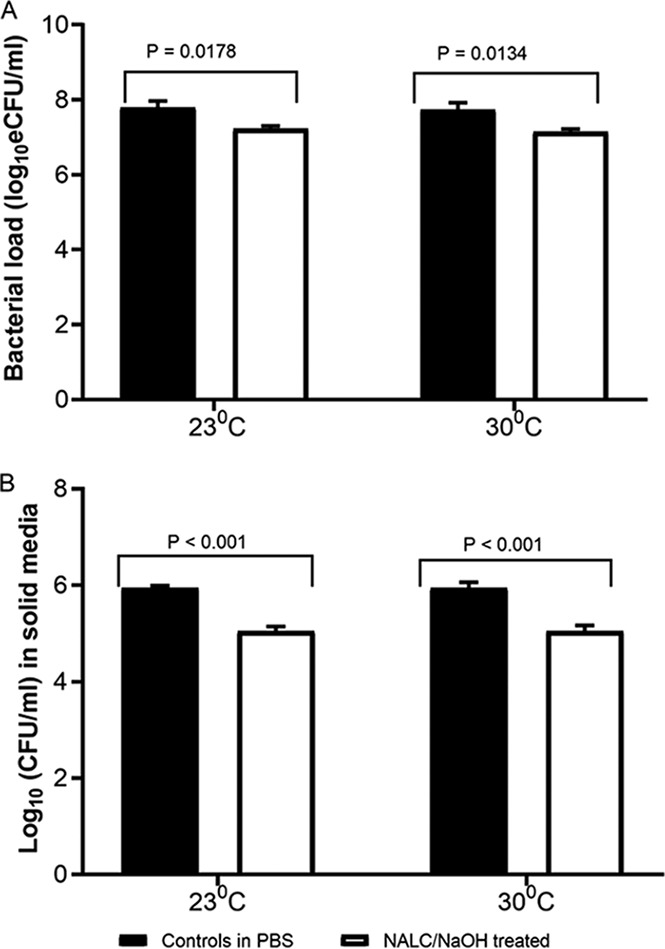
NALC-NaOH decontamination reduces the viable M. tuberculosis counts measured by MBLA and on solid culture. The reduction in the number of M. tuberculosis eCFU per milliliter measured by MBLA (A) and the reduction in the number of CFU per milliliter on Middlebrook (7H11) (B) at 23°C and 30°C. The bars and the error bars represent the mean value with the standard error of the mean (SEM). Data are for 5 independent biological replicates on different M. tuberculosis cultures.
Bacterial load by colony counts on solid culture.
The colony count of the untreated culture (average ± SD) was 5.98 ± 0.13 log10 CFU/ml and 5.95 ± 0.26 log10 CFU/ml at 23°C and 30°C, respectively. In contrast, the colony count of the NALC-NaOH-treated culture was 5.07 ± 0.19 log10 CFU/ml at 23°C and 5.04 ± 0.23 log10 CFU/ml at 30°C. Compared to the colony count in the untreated culture, NALC-NaOH treatment reduced the colony count by 0.84 ± 0.02 log10 CFU/ml at 23°C (P < 0.001) and 0.85 ± 0.01 log10 CFU/ml at 30°C (P < 0.001) (Fig. 2B).
TTP in MGIT liquid culture.
The median time to positivity (TTP) of the untreated MGIT culture was 3.0 days (range, 2.2 to 3.4 days) and 3.1 days (range, 2.1 to 3.3 days) at 23°C and 30°C, respectively, whereas it was 4.2 days (range, 3.7 to 4.4 days) and 4.2 days (range, 3.9 to 4.3) for the NALC-NaOH-treated culture at 23°C and 30°C, respectively. NALC-NaOH treatment increased the TTP by 1.2 days (P < 0.001) at 23°C and 1.1 days (P < 0.001) at 30°C.The effect was independent of PBS and the temperature of treatment (P > 0.05).
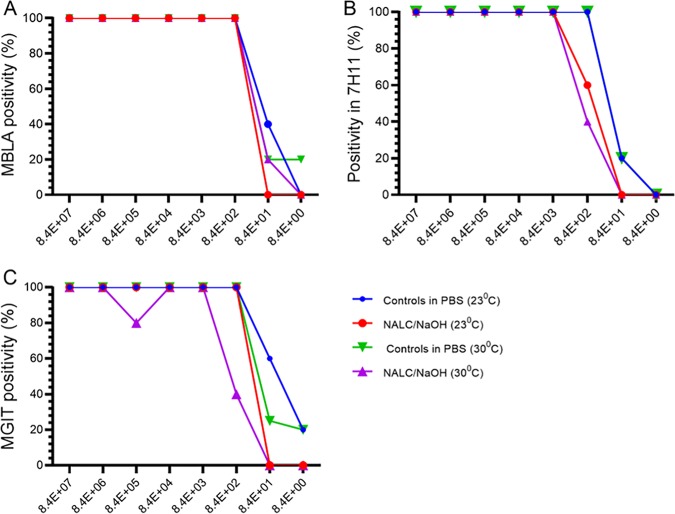
Assay positivity and LoD.
For untreated and treated cultures, the lowest bacterial load estimated by MBLA was 84 eCFU/ml (20% positivity) and 840 eCFU/ml (100% positivity), respectively, at 23°C and 8 eCFU/ml (20% positivity) and 84 eCFU/ml (20% positivity), respectively, at 30°C (Fig. 3A). On solid culture, the limit of detection (LoD) was 84 CFU/ml (20% positivity) for untreated controls and increased to 840 CFU/ml (60% positivity) at 23°C and (40% positivity) at 30°C after NALC-NaOH treatment (Fig. 3B). The LoD of MGIT culture for untreated controls was 8 eCFU/ml (20% positivity) and 84 CFU/ml (20% positivity) at 23°C and 30°C, respectively, which increased to ≥840 CFU/ml after NALC-NaOH treatment (Fig. 3C).
FIG 3.
NALC-NaOH decontamination compromises test positivity and the detection limit. The effect of NALC-NaOH decontamination on MBLA positivity (A), positivity on solid culture (Middlebrook 7H11) (B), and the positivity of MGIT liquid culture (C) is shown. Data are for 5 independent biological replicates on different M. tuberculosis cultures.
Bacterial load estimated by MBLA on sputum samples.
The average BL ± SD of untreated fresh sputum was 5.36 ± 0.24 log10 eCFU/ml and declined to 4.71 ± 0.16 log10 eCFU/ml after NALC-NaOH decontamination. The decline was 0.65 ± 0.17 log10 eCFU/ml, equivalent to a 78.74% loss of M. tuberculosis viability (Fig. 4).
FIG 4.
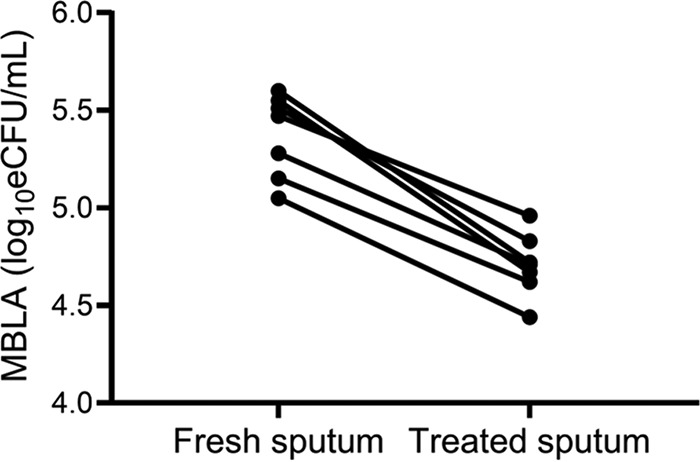
NALC-NaOH decontamination of sputum reduces the viable M. tuberculosis count measured by MBLA. P was <0.0001 between fresh sputum and treated pellet, as determined by an independent t test. Each dot represents the value of each sputum aliquot (n = 7 aliquots).
Comparison of MGIT culture with MBLA for M. tuberculosis detection after NALC-NaOH treatment of sputum.
All the NALC-NaOH-treated sputum aliquots yielded a positive MGIT culture result for M. tuberculosis, which was confirmed with the MPT64 antigen test. The true time to culture positivity (no contamination) was confirmed by a negative result on blood agar. The average TTP of the 7 cultures was 142 ± 7.02 h (5.97 ± 0.29 days). Using the TTP-to-CFU conversion model of Bowness et al. (23), we found that the average TTP was equivalent to 4.86 ± 0.28 log10 CFU, consistent with 4.71 ± 0.16 log10 eCFU/ml measured by MBLA of the same treated sputum. The higher that the TTP is, the lower that the number of CFU is, a relationship replicated by TTP and the MBLA-measured bacterial load (Table 1).
TABLE 1.
Number of CFU by MGIT culture matches that by MBLA after NALC-NaOH decontamination
| Sputum aliquot | No. of log10 eCFU/ml by MBLA of: |
MGIT culture of treated pellet |
||
|---|---|---|---|---|
| Untreated sputuma | Treated pellet | TTP (h) | No. of log10 CFUb | |
| 1 | 5.47 | 4.96 | 135 | 5.14 |
| 2 | 5.55 | 4.67 | 145 | 4.74 |
| 3 | 5.28 | 4.71 | 138 | 5.02 |
| 4 | 5.51 | 4.83 | 145 | 4.74 |
| 5 | 5.60 | 4.72 | 149 | 4.58 |
| 6 | 4.94 | 4.44 | 132 | 5.26 |
| 7 | 5.15 | 4.62 | 150 | 4.54 |
| Avg | 5.36 | 4.71 | 142 | 4.86 |
| SD | 0.24 | 0.16 | 7.02 | 0.28 |
MBLA of untreated sputum is a reference control to indicate the effect of NALC-NaOH treatment. P was 0.24 between the number of CFU by MGIT and MBLA of treated pellet and P was <0.01 between the number of CFU by MGIT and MBLA of untreated sputum, as determined by an independent t test. Data are for 7 replicates of sputum aliquots tested.
After TTP conversion.
DISCUSSION
A rapid, sensitive, and specific test with the ability to discriminate viable from dead M. tuberculosis cells is crucial for the accurate diagnosis and monitoring of TB treatment. To date, the contamination-sensitive culture-based methods remain the reference standard for M. tuberculosis viability detection and treatment monitoring (1). The NALC-NaOH decontamination step performed before sputum culture has a negative effect on viable M. tuberculosis bacilli and compromises the final culture results (24, 25). In this study, we evaluated how the NALC-NaOH decontamination process affects the viable M. tuberculosis bacterial load count quantified by MBLA compared to culture methods in order to explore the benefits of this decontamination step-free test.
Our findings concur with those of previous studies that have implicated NALC-NaOH treatment as a cause of viable M. tuberculosis loss (11, 13). We show that this loss compromises the LoD of both culture and the MBLA. While previous studies used only culture to measure the NALC-NaOH-induced M. tuberculosis viability loss, our study deployed the novel MBLA to verify and confirm these findings. We demonstrate that NALC-NaOH treatment reduces the amount of viable M. tuberculosis bacilli by 0.66 ± 0.21 log10 eCFU/ml in pure cultures and 0.65 ± 0.17 log10 eCFU/ml in patient sputa. The reduction is consistently less than 1 log in both matrix types, which are understandably different in thickness, viscosity, and sedimentation rate. Since 1% NaOH was used in both treatments, we hypothesize that the rate of loss is NaOH concentration dependent and independent of the matrix type. Whereas this degree of loss is less likely to have a negative impact on the test positivity of patients with high M. tuberculosis bacillus burdens, it may increase the likelihood of false-negative test results for patients with low burdens.
In addition, our results concur with those of the most recent in vitro work showing that NALC-NaOH decontamination is associated with a 90% loss of M. smegmatis bacteria in culture (18). With MBLA we observed an M. tuberculosis viability loss of 78.42% and 80.34% in the in vitro M. tuberculosis experiments at 23°C and 30°C respectively, whereas the loss was 78.74% in real patient sputum samples at 23°C. It is of note that culture-based estimates of M. tuberculosis viability may fail to detect viable but nonculturable bacteria (15).
The effect of nonculturable M. tuberculosis bacilli on culture positivity is more pronounced in solid culture than in liquid culture (23, 26). Our study recapitulates this, showing that MBLA detected 2 log10 eCFU/ml more in the control samples and 1.5 log10 eCFU/ml more in the NALC-NaOH-treated M. tuberculosis cultures than in the solid culture (Fig. 2A and B). Counts by solid culture are further complicated by the tendency of M. tuberculosis to clump, which means that each visible colony may not represent one cell, resulting in underestimation of the total viable bacterial count present in clinical samples (13). Therefore, the decontamination step for solid culture increases the difficulty of interpreting the result of viable counting by culture. By using MBLA, we were able to estimate the effect of NALC-NaOH-based decontamination on the total viable M. tuberculosis count, reflecting both culturable and nonculturable bacilli.
Unlike solid culture, the LoD of MGIT culture was consistent with that of MBLA, detecting counts as low as 8 and 840 eCFU/ml in untreated and treated M. tuberculosis cultures, respectively (Fig. 3A and C). Likewise, all sputum aliquots were M. tuberculosis positive by MGIT culture and MBLA after NALC-NaOH treatment, and there was no difference in the MBLA number of log10 eCFU per milliliter and the number of log10 CFU from the converted MGIT culture TTP (Table 1). It is important to note that MGIT culture requires days or weeks to detect a bacterial load similar to that which MBLA would detect and quantify within a matter of hours. The time to result for MBLA is independent of the bacterial load and is not affected by contamination (7). However, in samples with very low bacterial burdens, it is possible for MGIT culture to yield a positive result, as it depends on multiplication of M. tuberculosis cells over time, whereas MBLA quantifies the bacteria present in the sample at the time of RNA extraction.
Culture contamination rates are unacceptably high in tropical settings (27–30). We thus hypothesized that the high tropical temperatures in the range of 30°C compromise the activity of NALC-NaOH, leading to high levels of growth of contaminants. However, we found no difference in the M. tuberculosis viability loss for the NALC-NaOH decontamination of M. tuberculosis cultures at 30°C and 23°C (Fig. 2). This result suggests the same activity of NALC-NaOH at 23°C and 30°C and the possibility that contamination of an M. tuberculosis culture may not be related to the inefficiency of NALC-NaOH. It is possible that in the absence of a cold chain during transport of samples and storage, a higher-temperature environment may support the growth of fast-growing contaminants to a concentration which may not be eliminated by NALC-NaOH (25, 31).
We note that NALC-NaOH treatment is not the only cause of M. tuberculosis viable count loss. Processes such as homogenization and centrifugation have been implicated as causes of viable count loss (18). By applying similar dilution, homogenization, and centrifugation processes to untreated and treated samples, we normalized any viable count loss that would have occurred due to these factors across the two arms.
The limitation of our study is that the experiments were performed using pure cultures and pooled clinical sputum samples with a relatively high bacterial load. Therefore, we were not able to show the impact of NALC-NaOH-induced reduction of the viable M. tuberculosis count in low-burden samples. A sample size consisting of pooled sputum samples from 5 patients is not large enough to represent the diversity of M. tuberculosis strains to confirm that the NALC-NaOH-induced loss of the viable count in pure culture is indeed the same as that in clinical sputum samples. Furthermore, selective antibiotics which may reduce the viable M. tuberculosis count were not included in the culture media for the in vitro experiments but were included in MGIT cultures of clinical sputum aliquots. Addition of antibiotics may provide different results in culture (19). Nevertheless, our in vitro study design provided an opportunity to investigate the impact of NALC-NaOH on its own in the absence of other stresses, like antibiotics, and the use of pure culture was crucial to have untreated controls free of contaminants that would otherwise compromise our results (31).
Future studies will explore the impact of NALC-NaOH treatment in a variety of sputum samples from patients with different levels of M. tuberculosis burden to verify the impact on the test results for low-burden patients. We will also attempt to distinguish the viable count loss caused by chemical treatment from that stemming from centrifugation. Going forward, tests like MBLA which obviate the NALC-NaOH decontamination step could be a potential alternative to culture detection of viable M. tuberculosis and for monitoring the antituberculosis treatment response.
ACKNOWLEDGMENTS
This work was supported by a Commonwealth Studentship Award for Bariki Mtafya at the University of St. Andrews in the United Kingdom and the European and Developing Countries Clinical Trials Partnership (EDCTP) through PanACEA II grant 97118 and TWENDE grant CSA 2014-283.
We have no conflict of interest to declare.
REFERENCES
- 1.World Health Organization. 2018. Global tuberculosis report. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Mdivani N, Li H, Akhalaia M, Gegia M, Goginashvili L, Kernodle DS, Khechinashvili G, Tang YW. 2009. Monitoring therapeutic efficacy by real-time detection of Mycobacterium tuberculosis mRNA in sputum. Clin Chem 55:1694–1700. doi: 10.1373/clinchem.2009.124396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forbes BA, Hall GS, Miller MB, Novak SM, Rowlinson M, Salfinger M, Somoskövi A, Warshauer DM, Wilson L. 2018. Practice guidelines for clinical microbiology laboratories: mycobacteria. Clin Microbiol Rev 31:e00038-17. doi: 10.1128/CMR.00038-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomsen VØ, Kok-Jensen A, Philippi-Schulz S, Burkardt H, Buser M. 1999. Monitoring treatment of patients with pulmonary tuberculosis: can PCR be applied? J Clin Microbiol 37:3601–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryu YJ. 2015. Diagnosis of pulmonary tuberculosis: recent advances and diagnostic algorithms. Tuberc Respir Dis (Seoul) 78:64–71. doi: 10.4046/trd.2015.78.2.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedrich SO, Rachow A, Saathoff E, Singh K, Mangu CD, Dawson R, Phillips PPJ, Venter A, Bateson A, Boehme CC, Heinrich N, Hunt RD, Boeree MJ, Zumla A, McHugh TD, Gillespie SH, Diacon AH, Hoelscher M. 2013. Assessment of the sensitivity and specificity of Xpert MTB/RIF assay as an early sputum biomarker of response to tuberculosis treatment. Lancet Respir Med 1:462–470. doi: 10.1016/S2213-2600(13)70119-X. [DOI] [PubMed] [Google Scholar]
- 7.Sabiiti W, Mtafya B, Kuchaka D, Azam K, Viegas S, Mdolo A, Farmer ECW, Khonga M, Evangelopoulos D, Honeyborne I, Rachow A, Heinrich N, Ntinginya NE, Bhatt N, Davies GR, Jani IV, McHugh TD, Kibiki G, Hoelscher M, Gillespie SH. 2016. Optimising molecular diagnostic capacity for effective control of tuberculosis in high-burden settings. Int J Tuberc Lung Dis 20:1004–1009. doi: 10.5588/ijtld.15.0951. [DOI] [PubMed] [Google Scholar]
- 8.Pai SR, Actor JK, Sepulveda E, Hunter RL, Jagannath C. 2000. Identification of viable and non-viable Mycobacterium tuberculosis in mouse organs by directed RT-PCR for antigen 85B mRNA. Microb Pathog 28:335–342. doi: 10.1006/mpat.2000.0353. [DOI] [PubMed] [Google Scholar]
- 9.Nikolayevskyy V, Miotto P, Pimkina E, Balabanova Y, Kontsevaya I, Ignatyeva O, Ambrosi A, Skenders G, Ambrozaitis A, Kovalyov A, Sadykhova A, Simak T, Kritsky A, Mironova S, Tikhonova O, Dubrovskaya Y, Rodionova Y, Cirillo D, Drobniewski F. 2015. Utility of propidium monoazide viability assay as a biomarker for a tuberculosis disease. Tuberculosis (Edinb) 95:179–185. doi: 10.1016/j.tube.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Kayigire XA, Friedrich SO, Karinja MN, Van Der Merwe L, Martinson NA, Diacon AH. 2016. Propidium monoazide and Xpert MTB/RIF to quantify Mycobacterium tuberculosis cells. Tuberculosis 101:79–84. doi: 10.1016/j.tube.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Peres RL, Maciel ELN, Morais CG, Ribeiro FCK, Vinhas SA, Pinheiro C, Dietze R, Johnson JL, Eisenach K, Palaci M. 2009. Comparison of two concentrations of NALC-NaOH for decontamination of sputum for mycobacterial culture. Int J Tuberc Lung Dis 13:1572–1575. [PubMed] [Google Scholar]
- 12.Ratnam S, Stead FA, Howes M. 1987. Simplified acetylcysteine-alkali digestion-decontamination procedure for isolation of mycobacteria from clinical specimens. J Clin Microbiol 25:1428–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pheiffer C, Carroll NM, Beyers N, Donald P, Duncan K, Uys P, Van Helden P. 2008. Time to detection of Mycobacterium tuberculosis in BACTEC systems as a viable alternative to colony counting. Int J Tuberc Lung Dis 12:792–798. [PubMed] [Google Scholar]
- 14.Mukamolova GV, Turapov O, Malkin J, Woltmann G, Barer MR. 2010. Resuscitation-promoting factors reveal an occult population of tubercle bacilli in sputum. Am J Respir Crit Care Med 181:174–180. doi: 10.1164/rccm.200905-0661OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chengalroyen MD, Beukes GM, Gordhan BG, Streicher EM, Churchyard G, Hafner R, Warren R, Otwombe K, Martinson N, Kana BD. 2016. Detection and quantification of differentially culturable tubercle bacteria in sputum from patients with tuberculosis. Am J Respir Crit Care Med 194:1532–1540. doi: 10.1164/rccm.201604-0769OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammond RJH, Baron VO, Oravcova K, Lipworth S, Gillespie SH. 2015. Phenotypic resistance in mycobacteria: is it because I am old or fat that I resist you? J Antimicrob Chemother 70:2823–2827. doi: 10.1093/jac/dkv178. [DOI] [PubMed] [Google Scholar]
- 17.Sloan DJ, Mwandumba HC, Garton NJ, Khoo SH, Butterworth AE, Allain TJ, Heyderman RS, Corbett EL, Barer MR, Davies GR. 2015. Pharmacodynamic modeling of bacillary elimination rates and detection of bacterial lipid bodies in sputum to predict and understand outcomes in treatment of pulmonary tuberculosis. Clin Infect Dis 61:1–8. doi: 10.1093/cid/civ195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennedy JA, Baron VO, Hammond RJH, Sloan DJ, Gillespie SH. 2018. Centrifugation and decontamination procedures selectively impair recovery of important populations in Mycobacterium smegmatis. Tuberculosis (Edinb) 112:79–82. doi: 10.1016/j.tube.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Honeyborne I, McHugh TD, Phillips PPJ, Bannoo S, Bateson A, Carroll N, Perrin FM, Ronacher K, Wright L, Van Helden PD, Walzl G, Gillespie SH. 2011. Molecular bacterial load assay, a culture-free biomarker for rapid and accurate quantification of sputum Mycobacterium tuberculosis bacillary load during treatment. J Clin Microbiol 49:3905–3911. doi: 10.1128/JCM.00547-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honeyborne I, Mtafya B, Phillips PPJ, Hoelscher M, Ntinginya EN, Kohlenberg A, Rachow A, Rojas-Ponce G, McHugh TD, Heinrich N. 2014. The molecular bacterial load assay replaces solid culture for measuring early bactericidal response to antituberculosis treatment. J Clin Microbiol 52:3064–3067. doi: 10.1128/JCM.01128-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miles AA, Misra SS, Irwin JO. 1938. The estimation of the bactericidal power of the blood. J Hyg (Lond) 38:732–749. doi: 10.1017/S002217240001158X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sabiiti W, Azam K, Esmeraldo E, Bhatt N, Rachow A, Gillespie SH, Andrews S, Munich PS. 2019. Heat inactivation renders sputum safe and preserves Mycobacterium tuberculosis RNA for downstream molecular tests. J Clin Microbiol 57:e01778-18. doi: 10.1128/JCM.01778-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bowness R, Boeree MJ, Aarnoutse R, Dawson R, Diacon A, Mangu C, Heinrich N, Ntinginya NE, Kohlenberg A, Mtafya B, Phillips PPJ, Rachow A, Plemper van Balen G, Gillespie SH. 2015. The relationship between Mycobacterium tuberculosis MGIT time to positivity and CFU in sputum samples demonstrates changing bacterial phenotypes potentially reflecting the impact of chemotherapy on critical sub-populations. J Antimicrob Chemother 70:448–455. doi: 10.1093/jac/dku415. [DOI] [PubMed] [Google Scholar]
- 24.Horne DJ, Royce SE, Gooze L, Narita M, Hopewell PC, Nahid P, Steingart KR. 2010. Sputum monitoring during tuberculosis treatment for predicting outcome: systematic review and meta-analysis. Lancet Infect Dis 10:387–394. doi: 10.1016/S1473-3099(10)70071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parsons LM, Somoskovi A, Gutierrez C, Lee E, Paramasivan CN, Abimiku A, Spector S, Roscigno G, Nkengasong J. 2011. Laboratory diagnosis of tuberculosis in resource-poor countries: challenges and opportunities. Clin Microbiol Rev 24:314–350. doi: 10.1128/CMR.00059-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saito K, Warrier T, Somersan-Karakaya S, Kaminski L, Mi J, Jiang X, Park S, Shigyo K, Gold B, Roberts J, Weber E, Jacobs WR, Nathan CF. 2017. Rifamycin action on RNA polymerase in antibiotic-tolerant Mycobacterium tuberculosis results in differentially detectable populations. Proc Natl Acad Sci U S A 114:E4832–E4840. doi: 10.1073/pnas.1705385114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rojas-Ponce G, Rachow A, Guerra H, Mapamba D, Joseph J, Mlundi R, Marimoto S, Ntinginya NE, Mangu C, Framhein A, Butler A, Kohlenberg A, Ngatemelela D, Froeschl G, Maboko L, Hoelscher M, Heinrich N. 2013. A continuously monitored colorimetric method for detection of Mycobacterium tuberculosis complex in sputum. Int J Tuberc Lung Dis 17:1607–1612. doi: 10.5588/ijtld.13.0317. [DOI] [PubMed] [Google Scholar]
- 28.Chihota VN, Grant AD, Fielding K, Ndibongo B, Van Zyl A, Muirhead D, Churchyard GJ. 2010. Liquid vs. solid culture for tuberculosis: Performance and cost in a resource-constrained setting. Int J Tuberc Lung Dis 14:1024–1031. [PubMed] [Google Scholar]
- 29.Muyoyeta M, Schaap JA, De Haas P, Mwanza W, Muvwimi MW, Godfrey-Faussett P, Ayles H. 2009. Comparison of four culture systems for Mycobacterium tuberculosis in the Zambian National Reference Laboratory. Int J Tuberc Lung Dis 13:460–465. [PubMed] [Google Scholar]
- 30.Otu J, Antonio M, Cheung YB, Donkor S, Jong BD, Corrah T, Adegbola RA. 2008. Comparative evaluation of BACTEC MGIT 960 with BACTEC 9000 MB and LJ for isolation of mycobacteria in The Gambia. J Infect Dev Ctries 2:200–205. [DOI] [PubMed] [Google Scholar]
- 31.McClean M, Stanley T, Stanley S, Maeda Y, Goldsmith CE, Shepherd R, Millar BC, Dooley JSG, Moore JE, Moore JE. 2011. Identification and characterization of breakthrough contaminants associated with the conventional isolation of Mycobacterium tuberculosis. J Med Microbiol 60:1292–1298. doi: 10.1099/jmm.0.030619-0. [DOI] [PubMed] [Google Scholar]