Sepsis is a major source of mortality and morbidity globally. Accurately diagnosing sepsis remains challenging due to the heterogeneous nature of the disease, and delays in diagnosis and intervention contribute to high mortality rates.
KEYWORDS: bacteremia, host response, molecular diagnostics, sepsis, viremia
ABSTRACT
Sepsis is a major source of mortality and morbidity globally. Accurately diagnosing sepsis remains challenging due to the heterogeneous nature of the disease, and delays in diagnosis and intervention contribute to high mortality rates. Measuring the host response to infection enables more rapid diagnosis of sepsis than is possible through direct detection of the causative pathogen, and recent advances in host response diagnostics and prognostics hold promise for improving outcomes. The current review discusses recent advances in the technologies used to probe the host response to infection, particularly those based on transcriptomics. These are discussed in the context of contemporary approaches to diagnosing and prognosing sepsis, and recommendations are made for successful development and validation of host response technologies.
INTRODUCTION
Sepsis, a disorder that has evolved through three international consensus definitions over the past 3 decades, is a major source of in-hospital mortality and long-term morbidity in the United States (1). It is perhaps unsurprising that a clinical syndrome that is challenging to define is also challenging to diagnose and manage.
Following the introduction of Sepsis-3 guidelines, sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection (2). This definition may be accurate but is difficult to operationalize for research or clinical purposes. Thus, the Sepsis-3 authors suggested that organ dysfunction can be represented by an increase in the sequential organ failure assessment (SOFA) score of 2 points or more, which is associated with in-hospital mortality greater than 10%. However, few clinical tools are available for establishing that a host response is due to an infection.
The goal of this manuscript, directed toward the clinical laboratory community, is to highlight the way forward in sepsis diagnosis and management through assessment of the host response. We seek to place recent advances, particularly in transcriptomics, within the context of the other clinical diagnostics currently used in sepsis management and to address challenges faced in the application of transcriptomics to the clinical management of sepsis. Throughout, we will be guided by key questions faced by physicians encountering a patient with a potentially septic presentation, including the following: Does this patient have an infection? What is the infectious agent? What downstream diagnostics should be performed? How severe is the infection?
The historical role of the clinical laboratory in sepsis diagnosis has, naturally, been to aid in identifying a causative pathogen (3, 4) and prognosing severity (5, 6). However, methodological challenges associated with microbial culture often prevent the laboratory from providing information on a clinically relevant timescale. In practice, sepsis is often diagnosed using a presumed, rather than confirmed, source of infection.
PATHOGEN DETECTION
Blood culture is the most common laboratory method used to identify blood pathogens. Blood cultures obtained within 3 h, along with lactate measurement, early antibiotics, fluid resuscitation, and vasopressors for persistent hypotension are mandated in U.S. hospitals (SEP-1 bundle) as a core sepsis measure. While often considered a gold standard or pseudo-gold standard method for identifying the causative pathogen in a septic patient, blood culture suffers from significant analytical and clinical limitations. These limitations include the potential for contamination and false-positive results; lack of sensitivity to pathogens after initiation of antimicrobial treatment; and, most significantly, a long analysis time of approximately 24 to 48 h. Physicians presented with a potentially septic patient, especially in emergency departments, often cannot wait for the results of microbial culture before deciding what initial treatment to pursue; in fact, delayed intervention in sepsis is known to increase mortality (7, 8). Blood culture remains an important diagnostic test for sepsis, as the lowest cost and most widely available means to test for the presence of a blood pathogen. However, its long turnaround time is a severe limitation as a diagnostic and prognostic tool in emergency situations.
Over the last decade, the clinical laboratory community has recognized its potential to improve patient outcomes in sepsis by increasing the speed at which it can identify pathogens (9, 10). Matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) has accelerated the identification of isolates, but the requirement for subcultures can delay reporting of results (11, 12). Direct identification by MALDI-TOF MS on bacterial pellets from blood culture broth has shown efficacy; however, manual isolation procedures and the lack of FDA-cleared products has limited routine use. The FDA has cleared several molecular PCR-based assays that can detect a wide range of microorganisms concurrently with specific resistance genes directly from positive blood cultures, thereby decreasing the analysis time required to identify blood pathogens and determine antimicrobial susceptibility (13–16). Furthermore, fluorescence in situ hybridization combined with morphokinetic cellular analysis has been cleared by the FDA (17). A major drawback of all of the above-mentioned techniques is their requirement for a positive blood culture, which is typically obtained more than 8 h after incubation, limiting their utility as diagnostic tools in emergency situations.
A major advance has been the application of molecular technologies applied directly on whole blood without a positive blood culture. Multiplex PCR for pathogen detection was introduced in 2006 with the SeptiFast (Roche Molecular Diagnostics, Pleasanton, CA) technology (4) and with several follow-on technologies, such as the Iridica system (Abbott, Chicago, IL) (18) and T2 Biosystems (Lexington, MA) (19). This approach allows physicians to detect common bloodstream pathogens in the span of a few hours, representing a substantial improvement over the >8 h for work-up from positive blood cultures and the at least 24 h required for detection of pathogens using traditional blood culture and biochemical identification (10). Still, multiplex PCR-based technologies have not replaced blood culture-based methods for routine clinical laboratory testing, and the clinical- and cost-effectiveness of these technologies have not been established (18).
Most recently, next-generation sequencing from whole blood has been described using cell-free DNA (20, 21). A recent study demonstrated 93.7% agreement for next-generation sequencing from cell-free DNA with blood culture in patients with suspected sepsis (22). Most sequencing tests still require more than 24 h from sample collection to result report but offer hypothesis-free testing with good sensitivity compared to that of multiplex PCR.
These technologies for the identification of pathogens are often of real clinical value, and yet none have solved the sepsis problem. They suffer from common failings that can limit their utility to clinicians. As rule-in tests (when a pathogen is identified), they cannot distinguish colonization or contamination from true infection, their results come long after the 1-h treatment timeline for sepsis recommended by major societies, and even positive results (without concomitant antibiotic susceptibility testing) may not yield a change in antibiotic therapy. As rule-out tests, they are often of limited utility because the majority of patients clinically adjudicated to have sepsis do not have bacteremia (i.e., they have other nonbloodstream infections) (23). Microbiology can never “prove a negative,” and so patients must often be treated even with negative cultures.
HOST RESPONSE
Measuring the host response to infection has historically provided a more rapid but less specific means of aiding in the diagnosis of sepsis than that of direct pathogen detection. Monitoring the host response over time enables evaluation of patient response to therapy, and measurements can also provide an assessment of patient prognosis.
WHITE BLOOD CELLS
The white blood cell (WBC) count is routinely measured in the laboratory work-up of suspected sepsis but lacks sensitivity and specificity as an independent biomarker. A more nuanced assessment of white cell morphological changes in sepsis may provide greater diagnostic value. Several cell parameters have been proposed; the most-studied are the monocyte distribution width (MDW) (24) and the intensive care infection score (ICIS) (25), which measures several cell counts and parameters at once. Both have been prospectively validated as markers of disease severity but not as diagnostics of infection; alternately stated, they can discriminate between simple infections and sepsis but not between infectious and noninfectious inflammation. Further demonstration of their utility compared to standard biomarkers is needed. In a preliminary study using microfluidic techniques, granulocyte deformability distinguished patients with sepsis from healthy controls, but the clinical implications are currently unknown because healthy controls are not a clinically relevant comparator group (26). None of the described WBC metrics are FDA cleared for use in sepsis.
SINGLE-PROTEIN OR METABOLIC BIOMARKERS
Several established single biomarkers of the host response bear specific mention, including lactate, C-reactive protein (CRP), procalcitonin (PCT), and other well-studied markers.
Lactic acid, a marker of cellular hypoperfusion, is nonspecific for infection and has limited diagnostic accuracy as an independent marker. However, use of lactic acid measurements in serum to guide resuscitation has been shown to reduce mortality in patients with septic shock (27). Lactate is generally used as a prognostic marker in cases of sepsis, and its measurement is mandated as part of the SEP-1 bundle within 3 h after suspicion of sepsis. Lactate values of >2 mmol/liter identify a patient population at high risk for mortality that warrants further clinical evaluation.
C-reactive protein (CRP) is an acute phase reactant that is elevated in a variety of inflammatory states and has been studied as both a diagnostic and prognostic tool in sepsis. Several noninfectious conditions can cause an elevated CRP, so its specificity for infections is low.
Procalcitonin (PCT) is perhaps the most-studied biomarker of sepsis over the past 10 to 15 years and is available in several FDA-cleared formats. PCT is the prohormone of calcitonin; it is made constitutively and released into the blood in conditions of systemic inflammation, particularly those stemming from bacterial infection. However, PCT elevations can occur with other sources of systemic inflammation, including severe trauma and major surgery, leading to potential false-positive diagnoses of sepsis with bacterial origin. A systematic review of the literature has argued that PCT has limited diagnostic accuracy to distinguish sepsis from noninfectious systemic inflammatory response syndrome (SIRS) in the critically ill (28). Despite this limitation, several single- and multicenter studies using PCT-guided antimicrobial therapy coupled with tight adherence to clinical algorithms have shown reduced antimicrobial exposure and mortality in patients with presumed bacterial infection (29, 30). Systematic review has also demonstrated that PCT-guided therapy reduced use of antibiotics in patients with sepsis and septic shock, without an impact on patient outcomes (31). A broader review of patients with acute respiratory infection, including those with sepsis and septic shock, showed that PCT-guided therapy reduced both antibiotic use and mortality (32). Surviving sepsis campaign guidelines for sepsis management suggest that PCT measurements can shorten the duration of antimicrobial therapy and support the discontinuation of antibiotics in patients with an initial diagnosis of sepsis, although this is described as a weak recommendation with low quality of evidence (27). In standard U.S. practice, without a rigorous guideline adoption, PCT testing may make little clinical impact on antibiotic prescribing patterns (33).
PCT may play an additional role as a prognostic marker in patients with sepsis. While a single PCT value at presentation has not shown substantial prognostic value (34), multiple studies have demonstrated that PCT can aid in assessing the risk of mortality when measured serially (35–37). PCT has received FDA clearance for assessing the risk of progression to severe sepsis and septic shock in the critically ill and assessing 28-day all-cause mortality for patients diagnosed with severe sepsis or septic shock, when measured serially over several days.
Proadrenomedullin and soluble urokinase plasminogen activator receptor (suPAR) are emerging prognostic biomarkers in patients with sepsis. Proadrenomedullin has shown superior prognostic accuracy for 28-day mortality in intensive care unit (ICU) patients with sepsis or septic shock compared to that of lactic acid, CRP, procalcitonin, and severity scores, including SOFA, simplified acute physiology score II (SAPS II), and acute physiology and chronic health evaluation II (APACHE II), but is not yet approved for clinical use (38). suPAR was shown to make no difference in outcomes in a large emergency department randomized control trial (TRIAGE III) (39). It is possible that improved risk stratification alone, without separate infection diagnostic biomarkers, may be insufficient to affect clinical sepsis practice.
Single-protein (or metabolite) biomarkers may never be able to overcome their diagnostic and prognostic challenges in sepsis. These challenges stem from the fact that sepsis does not have a single, clear pathophysiology but rather constitutes a heterogeneous set of severe, inflammatory responses to infection (40). By narrowly probing the inflammatory response, single biomarkers face challenges detecting the broad spectrum of inflammatory responses associated with sepsis and distinguishing between inflammation arising from infectious versus noninfectious sources. Furthermore, a single biomarker that is elevated with uncomplicated infection and with noninfectious critical illness cannot reliably separately report on both the presence of an infection and the severity of illness (see Fig. 1).
FIG 1.
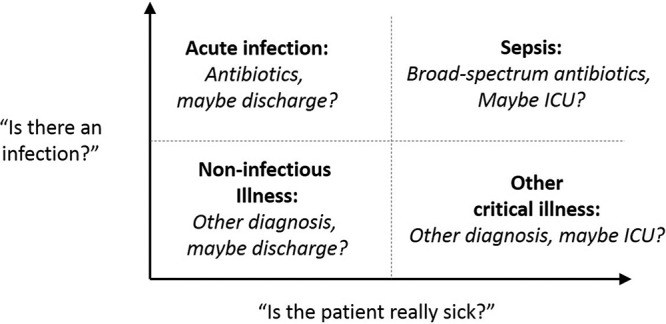
The presence of infection and severity of illness can be envisioned along orthogonal diagnostic axes. Single-protein or -metabolite sepsis biomarkers face challenges distinguishing acute uncomplicated infections (upper left quadrant) from noninfectious critical illness (lower right quadrant) since available biomarker expression can be increased in both cases.
In recent years, a new generation of host response diagnostics has been developed based on gene expression profiling, often referred to as transcriptomics. By obtaining a more global view of the host response, transcriptomics aims to both improve diagnostic accuracy for sepsis and provide an assessment of infection severity and patient prognosis.
TRANSCRIPTOMICS IN THE DIAGNOSIS OF INFECTIONS
The transcriptomics approach to diagnosis of acute infections and sepsis postulates that a broader assessment of the host response to infection, through targeted host immune gene expression (mRNA) profiling, will yield superior diagnostic and prognostic accuracy for sepsis to that achievable using targeted single biomarkers. Initial investigational transcriptomics research sought to characterize global changes in gene expression in response to acute infections, trauma, and sepsis, providing a wealth of information regarding the host response; this has been reviewed elsewhere (41). In general, any multi-mRNA panel consists of both its component mRNA variables and the mathematical algorithm that integrates those mRNAs into a diagnostic score. Only two transcriptomic sepsis scores, the SeptiScore and the Sepsis MetaScore, have been validated in independent cohorts using a locked algorithm.
The SeptiScore is the continuous output of a four-mRNA (CEACAM4, LAMP1, PLA2G7, and PLAC8) test called SeptiCyte Lab. The test is currently being commercialized (Immunexpress, Seattle, WA), and a 4- to 6-h turnaround open-pipetting version of the test has received FDA clearance as an aid to differentiate infection-positive sepsis from infection-negative systemic inflammation in patients suspected of sepsis on their first day of ICU admission. In a multicohort discovery and validation study in ICU patients, SeptiCyte Lab showed significantly higher diagnostic accuracy for discriminating sepsis from SIRS than did procalcitonin (42). A recent multicohort trial of SeptiCyte Lab sponsored by its manufacturer in 447 ICU patients showed area under the receiver operating characteristic (ROC) curve (AUC) for discriminating sepsis from SIRS ranging from 0.82 to 0.89, with negative predictive values ranging from 89% to 94% (43). It showed a head-to-head AUC of 0.85 for SeptiCyte Lab versus 0.80 for procalcitonin in patients with both measurements. However, other studies from independent groups have shown less promising results, with AUC values of 0.68 (44), 0.73 (45), and 0.75 (46) in populations with greater clinical equipoise. A comprehensive review of performance to date was conducted by Verboom et al. (47).
The Sepsis MetaScore is based on expression levels of 11 host mRNAs (CEACAM1, ZDHHC19, C9orf95/NMRK1, GNA15, BATF, C3AR1, KIAA1370, TGFBI, MTCH1, RPGRIP1, and HLA-DPB1) and was discovered across publicly available microarray data from n = 663 patients across 9 clinical cohorts (48). In the discovery data sets, the mean AUC for noninfectious SIRS versus sepsis was 0.87 (range, 0.70 to 0.98). Importantly, the sepsis infection scores described above have been compared in two independent studies. Sweeney and Khatri (46) compared the performances of SeptiCyte Lab, Sepsis MetaScore, and the FAIM3:PLAC8 gene expression ratio described by Scicluna et al. (49). Using a large number of renormalized data sets from cohorts including a total of more than 2,500 patients, the average AUC values for distinguishing sepsis from SIRS were 0.82 (MetaScore), 0.78 (FAIM3:PLAC8 ratio), and 0.73 (SeptiCyte Lab). These results were substantially upheld by Maslove et al. in an independent validation study in critically ill patients (44). For all 3 models, scores were significantly different between patients with and without sepsis. Overall accuracy for the classification of sepsis, as measured by the AUC, was highest for the Sepsis MetaScore (0.80), followed by the FAIM3:PLAC8 ratio (0.69) and the SeptiCyte Lab (0.68). In contrast, procalcitonin had a marginal utility in this population (AUC, 0.66). Results of these studies suggest that heterogeneous data may be an ideal test bed for these new metrics and that tests based on transcriptomics may outperform single-protein biomarkers such as procalcitonin.
Another major area of emphasis in transcriptomics is in differentiating bacterial and viral infections. Here again, many scores have been proposed, but few have been externally validated. Sweeney et al. discovered a 7-mRNA “bacterial/viral metascore” that distinguished bacterial from viral infections across 24 pooled validation cohorts (n = 1,040) with an overall AUC of 0.93 (48). Further, when combined with the Sepsis MetaScore (50), and taking into account noninfectious SIRS, an integrated noninfectious/bacterial/viral algorithm had a sensitivity and specificity of 94% and 60% for bacterial infections and 53% and 91% for viral infections, respectively; the negative likelihood ratio was 0.10 and translated into a negative predictive value for bacterial infection of 98.3% at a prevalence of 15%.
Other investigators have described gene sets based on peripheral blood gene expression for the diagnosis of infections (reviewed elsewhere [51]). However, most of these have used complex algorithms that required local retraining in independent cohorts and so cannot be said strictly to have been validated. For instance, a 130-probe panel was derived from microarray gene expression data from a single cohort of 273 patients with acute respiratory bacterial or viral infection or noninfectious illness (52). Based on comparison to the adjudicated clinical diagnosis, this classification system showed greater power than did procalcitonin to distinguish acute bacterial respiratory infection from noninfectious or viral illness but similar accuracy for distinguishing bacterial from viral infection. However, the authors retrained their algorithms in each “validation” cohort, risking substantial bias in reporting.
TRANSCRIPTOMICS IN RISK STRATIFICATION
Differential expression of genes related to immune function has been observed between sepsis survivors and nonsurvivors, suggesting that gene expression analysis may also hold promise as a prognostic tool for sepsis. A recent report describes three independently developed gene expression models, targeting the expression of 12 to 18 genes, that achieved similar average prognostic accuracy for 30-day mortality to that reported for proadrenomedullin and suPAR (summary AUC of roughly 0.85) (53). Of interest, the accuracy of expression-based predictors paired with clinical severity scores was significantly higher than that of clinical scores alone at the time of diagnosis. Gene expression tests for risk stratification could thus allow for better resource allocation in hospitals.
Several groups have published transcriptomic metrics to identify sepsis immune endotypes that may be useful for risk stratification or, eventually, as a companion diagnostic for immune treatments (40, 54–58). Further, transcriptomic risk stratification is not limited to sepsis; a 63-mRNA metric of blood leukocyte gene expression was recently shown to be accurate in blunt trauma patients to identify those likely to have complicated clinical trajectories (59).
In general, prospective clinical trials are needed to determine whether improved risk stratification using novel prognostic or predictive markers can improve patient outcomes in sepsis. It may be that improved risk stratification must be coupled with a concomitant improvement in infection diagnosis in order to adequately identify both noncritically ill patients with bacterial infections and critically ill patients without bacterial infections.
PITFALLS AND VALIDATION
While measuring a panel of biomarkers has the potential for better accuracy and generalizability than that of single-marker approaches, major concerns remain. The greatest pitfall is data overfitting in the discovery and consequent lack of generalizability beyond the initial study population. This can be caused by selection of the wrong mRNA targets, through overfit machine learning algorithms, or both. Validation of biomarkers using large, heterogeneous validation cohorts is one way to determine the robustness of new biomarker panels in that they can be readily compared head-to-head in the same patients. Although this method may work for comparing mRNA panels that do not require machine learning, few groups have published multimarker machine learning models that would allow outside groups to reproduce their work. In such cases, the only real estimate of real-world diagnostic performance will be the repeated validation of (i) a locked gene expression panel with (ii) a locked machine learning algorithm in independent cohorts, ideally (iii) on a platform that can give results in a clinically relevant time frame.
Transcriptomics approaches for diagnosing infections and sepsis (including distinguishing viral from bacterial etiologies) and as an aid in risk stratification have the potential to reduce sepsis morbidity, reduce unnecessary use of antibiotics, and improve resource allocation. However, further clinical validation is needed for every transcriptomic panel described above. Moreover, substantial technological advances are required for gene expression diagnostics to achieve results on a timescale relevant to emergency clinical decision-making (60). The 20- to 30-min analysis time of procalcitonin testing can serve as a benchmark for assessing progress in reducing the turnaround time of gene expression diagnostic approaches.
CONCLUSIONS
Assessment of the host response to infection using multigene transcriptomics has demonstrated potential to improve diagnostic and prognostic accuracy for sepsis over contemporary testing strategies. Continued development of accurate test solutions for sepsis based on multigene transcriptomics requires application of intelligent bioinformatics tools. Translation of multitarget mRNA measurements into routine clinical practice will require machine learning trained algorithms, instruments and assays with rapid turnaround times, and integration of result readouts into clinical algorithms. Rigorous clinical trials, spanning demographics such as ethnicity and geography, are needed to assess the impact of new tests on patient outcomes. Furthermore, careful attention to test reporting into clinically actionable cutoffs or interpretation bands will be required to clinically contextualize novel scores. Choice of appropriate comparator method for assessing the diagnostic performance of new tests may need to evolve along with methodological advances, given the limitations of contemporary tests and lack of an analytically robust gold standard. As new test solutions emerge, sepsis management guidelines will need to adapt to ensure that the maximum potential benefit of improved clinical diagnostics is transferred to patients.
ACKNOWLEDGMENTS
Timothy E. Sweeney and Oliver Liesenfeld are employees of Inflammatix. Nathan A. Ledeboer has served as an advisor to Inflammatix, Karius, and CytoVale.
REFERENCES
- 1.Liu V, Escobar GJ, Greene JD, Soule J, Whippy A, Angus DC, Iwashyna TJ. 2014. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA 312:90–92. doi: 10.1001/jama.2014.5804. [DOI] [PubMed] [Google Scholar]
- 2.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche J-D, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent J-L, Angus DC. 2016. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinha M, Jupe J, Mack H, Coleman TP, Lawrence SM, Fraley SI. 2018. Emerging technologies for molecular diagnosis of sepsis. Clin Microbiol Rev 31:e00089-17. doi: 10.1128/CMR.00089-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liesenfeld O, Lehman L, Hunfeld K-P, Kost G. 2014. Molecular diagnosis of sepsis: new aspects and recent developments. Eur J Microbiol Immunol (Bp) 4:1–25. doi: 10.1556/EuJMI.4.2014.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reinhart K, Bauer M, Riedemann NC, Hartog CS. 2012. New approaches to sepsis: molecular diagnostics and biomarkers. Clin Microbiol Rev 25:609–634. doi: 10.1128/CMR.00016-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han JE, Martin GS. 2017. Biomarkers in sepsis: on target or off the mark? Am J Med Sci 354:3–4. doi: 10.1016/j.amjms.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M. 2006. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 8.Gaieski DF, Mikkelsen ME, Band RA, Pines JM, Massone R, Furia FF, Shofer FS, Goyal M. 2010. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med 38:1045–1053. doi: 10.1097/CCM.0b013e3181cc4824. [DOI] [PubMed] [Google Scholar]
- 9.Kothari A, Morgan M, Haake DA. 2014. Emerging technologies for rapid identification of bloodstream pathogens. Clin Infect Dis 59:272–278. doi: 10.1093/cid/ciu292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Timbrook TT, Morton JB, McConeghy KW, Caffrey AR, Mylonakis E, LaPlante KL. 2017. The effect of molecular rapid diagnostic testing on clinical outcomes in bloodstream infections: a systematic review and meta-analysis. Clin Infect Dis 64:15–23. doi: 10.1093/cid/ciw649. [DOI] [PubMed] [Google Scholar]
- 11.Patel TS, Kaakeh R, Nagel JL, Newton DW, Stevenson JG. 2017. Cost analysis of implementing matrix-assisted laser desorption ionization–time of flight mass spectrometry plus real-time antimicrobial stewardship intervention for bloodstream infections. J Clin Microbiol 55:60–67. doi: 10.1128/JCM.01452-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Idelevich EA, Storck LM, Sparbier K, Drews O, Kostrzewa M, Becker K. 2018. Rapid direct susceptibility testing from positive blood cultures by the matrix-assisted laser desorption ionization–time of flight mass spectrometry-based direct-on-target microdroplet growth assay. J Clin Microbiol 56:e00913-18. doi: 10.1128/JCM.00913-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ward C, Stocker K, Begum J, Wade P, Ebrahimsa U, Goldenberg SD. 2015. Performance evaluation of the Verigene (Nanosphere) and FilmArray (BioFire) molecular assays for identification of causative organisms in bacterial bloodstream infections. Eur J Clin Microbiol Infect Dis 34:487–496. doi: 10.1007/s10096-014-2252-2. [DOI] [PubMed] [Google Scholar]
- 14.Buchan BW, Ginocchio CC, Manii R, Cavagnolo R, Pancholi P, Swyers L, Thomson RB Jr, Anderson C, Kaul K, Ledeboer NA. 2013. Multiplex identification of Gram-positive bacteria and resistance determinants directly from positive blood culture broths: evaluation of an automated microarray-based nucleic acid test. PLoS Med 10:e1001478. doi: 10.1371/journal.pmed.1001478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salimnia H, Fairfax MR, Lephart PR, Schreckenberger P, DesJarlais SM, Johnson JK, Robinson G, Carroll KC, Greer A, Morgan M, Chan R, Loeffelholz M, Valencia-Shelton F, Jenkins S, Schuetz AN, Daly JA, Barney T, Hemmert A, Kanack KJ. 2016. Evaluation of the FilmArray blood culture identification panel: results of a multicenter controlled trial. J Clin Microbiol 54:687–698. doi: 10.1128/JCM.01679-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang T-D, Melnik E, Bogaerts P, Evrard S, Glupczynski Y. 2019. Evaluation of the ePlex blood culture identification panels for detection of pathogens in bloodstream infections. J Clin Microbiol 57:e01597-18. doi: 10.1128/JCM.01597-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marschal M, Bachmaier J, Autenrieth I, Oberhettinger P, Willmann M, Peter S. 2017. Evaluation of the Accelerate Pheno system for fast identification and antimicrobial susceptibility testing from positive blood cultures in bloodstream infections caused by Gram-negative pathogens. J Clin Microbiol 55:2116–2126. doi: 10.1128/JCM.00181-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevenson M, Pandor A, Martyn-St James M, Rafia R, Uttley L, Stevens J, Sanderson J, Wong R, Perkins GD, McMullan R, Dark P. 2016. Sepsis: the LightCycler SeptiFast test MGRADE, SepsiTest and Iridica BAC BSI assay for rapidly identifying bloodstream bacteria and fungi a systematic review and economic evaluation. Health Technol Assess 20:1. doi: 10.3310/hta20460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfaller MA, Wolk DM, Lowery TJ. 2016. T2MR and T2Candida: novel technology for the rapid diagnosis of candidemia and invasive candidiasis. Future Microbiol 11:103–117. doi: 10.2217/fmb.15.111. [DOI] [PubMed] [Google Scholar]
- 20.Fung M, Zompi S, Seng H, Hollemon D, Parham A, Hong DK, Bercovici S, Dolan E, Lien K, Teraoka J, Logan AC, Chin-Hong P. 2018. Plasma cell-free DNA next-generation sequencing to diagnose and monitor infections in allogeneic hematopoietic stem cell transplant patients. Open Forum Infect Dis 5:ofy301. doi: 10.1093/ofid/ofy301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong DK, Blauwkamp TA, Kertesz M, Bercovici S, Truong C, Banaei N. 2018. Liquid biopsy for infectious diseases: sequencing of cell-free plasma to detect pathogen DNA in patients with invasive fungal disease. Diagn Microbiol Infect Dis 92:210–213. doi: 10.1016/j.diagmicrobio.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 22.Blauwkamp TA, Thair S, Rosen MJ, Blair L, Lindner MS, Vilfan ID, Kawli T, Christians FC, Venkatasubrahmanyam S, Wall GD, Cheung A, Rogers ZN, Meshulam-Simon G, Huijse L, Balakrishnan S, Quinn JV, Hollemon D, Hong DK, Vaughn ML, Kertesz M, Bercovici S, Wilber JC, Yang S. 2019. Analytical and clinical validation of a microbial cell-free DNA sequencing test for infectious disease. Nat Microbiol 4:663–674. doi: 10.1038/s41564-018-0349-6. [DOI] [PubMed] [Google Scholar]
- 23.Coburn B, Morris AM, Tomlinson G, Detsky AS. 2012. Does this adult patient with suspected bacteremia require blood cultures? JAMA 308:502–511. doi: 10.1001/jama.2012.8262. [DOI] [PubMed] [Google Scholar]
- 24.Crouser ED, Parrillo JE, Seymour C, Angus DC, Bicking K, Tejidor L, Magari R, Careaga D, Williams J, Closser DR, Samoszuk M, Herren L, Robart E, Chaves F. 2017. Improved early detection of sepsis in the ED with a novel monocyte distribution width biomarker. Chest 152:518–526. doi: 10.1016/j.chest.2017.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Geest PJ, Mohseni M, Linssen J, Duran S, de Jonge R, Groeneveld A. 2016. The intensive care infection score – a novel marker for the prediction of infection and its severity. Crit Care 20:180. doi: 10.1186/s13054-016-1366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crawford K, DeWitt A, Brierre S, Caffery T, Jagneaux T, Thomas C, Macdonald M, Tse H, Shah A, Di Carlo D, O’Neal HR. 2018. Rapid biophysical analysis of host immune cell variations associated with sepsis. Am J Respir Crit Care Med 198:280–282. doi: 10.1164/rccm.201710-2077LE. [DOI] [PubMed] [Google Scholar]
- 27.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche J-D, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, et al. 2017. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2016. Crit Care Med 45:486–552. doi: 10.1097/CCM.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 28.Tang BM, Eslick GD, Craig JC, McLean AS. 2007. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: systematic review and meta-analysis. Lancet Infect Dis 7:210–217. doi: 10.1016/S1473-3099(07)70052-X. [DOI] [PubMed] [Google Scholar]
- 29.Broyles MR. 2017. Impact of procalcitonin-guided antibiotic management on antibiotic exposure and outcomes: real-world evidence. Open Forum Infect Dis 4:ofx213. doi: 10.1093/ofid/ofx213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Jong E, van Oers JA, Beishuizen A, Vos P, Vermeijden WJ, Haas LE, Loef BG, Dormans T, van Melsen GC, Kluiters YC, Kemperman H, van den Elsen MJ, Schouten JA, Streefkerk JO, Krabbe HG, Kieft H, Kluge GH, van Dam VC, van Pelt J, Bormans L, Otten MB, Reidinga AC, Endeman H, Twisk JW, van de Garde EMW, de Smet A, Kesecioglu J, Girbes AR, Nijsten MW, de Lange DW. 2016. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis 16:819–827. doi: 10.1016/S1473-3099(16)00053-0. [DOI] [PubMed] [Google Scholar]
- 31.Prkno A, Wacker C, Brunkhorst FM, Schlattmann P. 2013. Procalcitonin-guided therapy in intensive care unit patients with severe sepsis and septic shock – a systematic review and meta-analysis. Crit Care 17:R291. doi: 10.1186/cc13157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuetz P, Wirz Y, Sager R, Christ-Crain M, Stolz D, Tamm M, Bouadma L, Luyt CE, Wolff M, Chastre J, Tubach F, Kristoffersen KB, Burkhardt O, Welte T, Schroeder S, Nobre V, Wei L, Bucher HC, Annane D, Reinhart K, Falsey AR, Branche A, Damas P, Nijsten M, de Lange DW, Deliberato RO, Oliveira CF, Maravić-Stojković V, Verduri A, Beghé B, Cao B, Shehabi Y, Jensen J-U, Corti C, van Oers JAH, Beishuizen A, Girbes ARJ, de Jong E, Briel M, Mueller B. 2018. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: a patient level meta-analysis. Lancet Infect Dis 18:95–107. doi: 10.1016/S1473-3099(17)30592-3. [DOI] [PubMed] [Google Scholar]
- 33.Huang DT, Yealy DM, Filbin MR, Brown AM, Chang C-CH, Doi Y, Donnino MW, Fine J, Fine MJ, Fischer MA, Holst JM, Hou PC, Kellum JA, Khan F, Kurz MC, Lotfipour S, LoVecchio F, Peck-Palmer OM, Pike F, Prunty H, Sherwin RL, Southerland L, Terndrup T, Weissfeld LA, Yabes J, Angus DC. 2018. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med 379:236–249. doi: 10.1056/NEJMoa1802670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu D, Su L, Han G, Yan P, Xie L. 2015. Prognostic value of procalcitonin in adult patients with sepsis: a systematic review and meta-analysis. PLoS One 10:e0129450. doi: 10.1371/journal.pone.0129450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schuetz P, Birkhahn R, Sherwin R, Jones AE, Singer A, Kline JA, Runyon MS, Self WH, Courtney DM, Nowak RM, Gaieski DF, Ebmeyer S, Johannes S, Wiemer JC, Schwabe A, Shapiro NI. 2017. Serial procalcitonin predicts mortality in severe sepsis patients: results from the multicenter procalcitonin MOnitoring SEpsis (MOSES) study. Crit Care Med 45:781–789. doi: 10.1097/CCM.0000000000002321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Azevedo JRA, Torres OJM, Beraldi RA, Ribas C, Malafaia O. 2015. Prognostic evaluation of severe sepsis and septic shock: procalcitonin clearance vs Δ sequential organ failure assessment. J Crit Care 30:219.e9. doi: 10.1016/j.jcrc.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 37.Sager R, Wirz Y, Amin D, Amin A, Hausfater P, Huber A, Haubitz S, Kutz A, Mueller B, Schuetz P. 2017. Are admission procalcitonin levels universal mortality predictors across different medical emergency patient populations? Results from the multi-national, prospective, observational TRIAGE study. Clin Chem Lab Med 55:1873–1880. doi: 10.1515/cclm-2017-0144. [DOI] [PubMed] [Google Scholar]
- 38.Elke G, Bloos F, Wilson DC, Brunkhorst FM, Briegel J, Reinhart K, Loeffler M, Kluge S, Nierhaus A, Jaschinski U, Moerer O, Weyland A, Meybohm P, SepNet Critical Care Trials Group . 2018. The use of mid-regional proadrenomedullin to identify disease severity and treatment response to sepsis - a secondary analysis of a large randomised controlled trial. Crit Care 22:79. doi: 10.1186/s13054-018-2001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schultz M, Rasmussen LJH, Andersen MH, Stefansson JS, Falkentoft AC, Alstrup M, Sandø A, Holle SLK, Meyer J, Törnkvist PBS, Høi-Hansen T, Kjøller E, Jensen BN, Lind M, Ravn L, Kallemose T, Lange T, Køber L, Rasmussen LS, Eugen-Olsen J, Iversen KK. 2018. Use of the prognostic biomarker suPAR in the emergency department improves risk stratification but has no effect on mortality: a cluster-randomized clinical trial (TRIAGE III). Scand J Trauma Resusc Emerg Med 26:69. doi: 10.1186/s13049-018-0539-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sweeney TE, Azad TD, Donato M, Haynes WA, Perumal TM, Henao R, Bermejo-Martin JF, Almansa R, Tamayo E, Howrylak JA, Choi A, Parnell GP, Tang B, Nichols M, Woods CW, Ginsburg GS, Kingsmore SF, Omberg L, Mangravite LM, Wong HR, Tsalik EL, Langley RJ, Khatri P. 2018. Unsupervised analysis of transcriptomics in bacterial sepsis across multiple datasets reveals three robust clusters. Crit Care Med 46:915–925. doi: 10.1097/CCM.0000000000003084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sweeney TE, Wong HR. 2016. Risk stratification and prognosis in sepsis: what have we learned from microarrays? Clin Chest Med 37:209–218. doi: 10.1016/j.ccm.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McHugh L, Seldon TA, Brandon RA, Kirk JT, Rapisarda A, Sutherland AJ, Presneill JJ, Venter DJ, Lipman J, Thomas MR, Klein Klouwenberg PMC, van Vught L, Scicluna B, Bonten M, Cremer OL, Schultz MJ, van der Poll T, Yager TD, Brandon RB. 2015. A molecular host response assay to discriminate between sepsis and infection-negative systemic inflammation in critically ill patients: discovery and validation in independent cohorts. PLoS Med 12:e1001916. doi: 10.1371/journal.pmed.1001916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller RR, Lopansri BK, Burke JP, Levy M, Opal S, Rothman RE, D’Alessio FR, Sidhaye VK, Aggarwal NR, Balk R, Greenberg JA, Yoder M, Patel G, Gilbert E, Afshar M, Parada JP, Martin GS, Esper AM, Kempker JA, Narasimhan M, Tsegaye A, Hahn S, Mayo P, van der Poll T, Schultz MJ, Scicluna BP, Klein Klouwenberg P, Rapisarda A, Seldon TA, McHugh LC, Yager TD, Cermelli S, Sampson D, Rothwell V, Newman R, Bhide S, Fox BA, Kirk JT, Navalkar K, Davis RF, Brandon RA, Brandon RB. 2018. Validation of a host response assay, SeptiCyte Lab, for discriminating sepsis from systemic inflammatory response syndrome in the ICU. Am J Respir Crit Care Med 198:903–913. doi: 10.1164/rccm.201712-2472OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maslove DM, Shapira T, Tyryshkin K, Veldhoen RA, Marshall JC, Muscedere J. 2019. Validation of diagnostic gene sets to identify critically ill patients with sepsis. J Crit Care 49:92–98. doi: 10.1016/j.jcrc.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 45.Koster-Brouwer ME, Verboom DM, Scicluna BP, van de Groep K, Frencken JF, Janssen D, Schuurman R, Schultz MJ, van der Poll T, Bonten MJM, Cremer OL, on behalf of the MARS Consortium . 2018. Validation of a novel molecular host response assay to diagnose infection in hospitalized patients admitted to the ICU with acute respiratory failure. Crit Care Med 46:368–374. doi: 10.1097/CCM.0000000000002735. [DOI] [PubMed] [Google Scholar]
- 46.Sweeney TE, Khatri P. 2017. Benchmarking sepsis gene expression diagnostics using public data. Crit Care Med 45:1–10. doi: 10.1097/CCM.0000000000002021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verboom DM, Koster-Brouwer ME, Varkila MRJ, Bonten MJM, Cremer OL. 2019. Profile of the SeptiCyte Lab gene expression assay to diagnose infection in critically ill patients. Expert Rev Mol Diagn 19:95–108. doi: 10.1080/14737159.2019.1567333. [DOI] [PubMed] [Google Scholar]
- 48.Sweeney TE, Wong HR, Khatri P. 2016. Robust classification of bacterial and viral infections via integrated host gene expression diagnostics. Sci Transl Med 8:346ra91. doi: 10.1126/scitranslmed.aaf7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scicluna BP, Klein Klouwenberg PMC, van Vught LA, Wiewel MA, Ong DSY, Zwinderman AH, Franitza M, Toliat MR, Nürnberg P, Hoogendijk AJ, Horn J, Cremer OL, Schultz MJ, Bonten MJ, van der Poll T. 2015. A molecular biomarker to diagnose community-acquired pneumonia on intensive care unit admission. Am J Respir Crit Care Med 192:826–835. doi: 10.1164/rccm.201502-0355OC. [DOI] [PubMed] [Google Scholar]
- 50.Sweeney TE, Shidham A, Wong HR, Khatri P. 2015. A comprehensive time-course–based multicohort analysis of sepsis and sterile inflammation reveals a robust diagnostic gene set. Sci Transl Med 7:287ra71. doi: 10.1126/scitranslmed.aaa5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holcomb ZE, Tsalik EL, Woods CW, McClain MT. 2017. Host-based peripheral blood gene expression analysis for diagnosis of infectious diseases. J Clin Microbiol 55:360–368. doi: 10.1128/JCM.01057-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsalik EL, Henao R, Nichols M, Burke T, Ko ER, McClain MT, Hudson LL, Mazur A, Freeman DH, Veldman T, Langley RJ, Quackenbush EB, Glickman SW, Cairns CB, Jaehne AK, Rivers EP, Otero RM, Zaas AK, Kingsmore SF, Lucas J, Fowler VG, Carin L, Ginsburg GS, Woods CW. 2016. Host gene expression classifiers diagnose acute respiratory illness etiology. Sci Transl Med 8:322ra11. doi: 10.1126/scitranslmed.aad6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sweeney TE, Perumal TM, Henao R, Nichols M, Howrylak JA, Choi AM, Bermejo-Martin JF, Almansa R, Tamayo E, Davenport EE, Burnham KL, Hinds CJ, Knight JC, Woods CW, Kingsmore SF, Ginsburg GS, Wong HR, Parnell GP, Tang B, Moldawer LL, Moore FE, Omberg L, Khatri P, Tsalik EL, Mangravite LM, Langley RJ. 2018. A community approach to mortality prediction in sepsis via gene expression analysis. Nat Commun 9:694. doi: 10.1038/s41467-018-03078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong HR, Cvijanovich N, Lin R, Allen GL, Thomas NJ, Willson DF, Freishtat RJ, Anas N, Meyer K, Checchia PA, Monaco M, Odom K, Shanley TP. 2009. Identification of pediatric septic shock subclasses based on genome-wide expression profiling. BMC Med 7:34. doi: 10.1186/1741-7015-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wong HR, Wheeler DS, Tegtmeyer K, Poynter SE, Kaplan JM, Chima RS, Stalets E, Basu RK, Doughty LA. 2010. Toward a clinically feasible gene expression-based sub-classification strategy for septic shock: proof of concept. Crit Care Med 38:1955–1961. doi: 10.1097/CCM.0b013e3181eb924f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong HR, Sweeney TE, Hart KW, Khatri P, Lindsell CJ. 2017. Pediatric sepsis endotypes among adults with sepsis. Crit Care Med 45:e1289–e1291. doi: 10.1097/CCM.0000000000002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wong HR, Cvijanovich NZ, Anas N, Allen GL, Thomas NJ, Bigham MT, Weiss SL, Fitzgerald JC, Checchia PA, Meyer K, Quasney M, Hall M, Gedeit R, Freishtat RJ, Nowak J, Lutfi R, Gertz S, Grunwell JR, Lindsell CJ. 2018. Endotype transitions during the acute phase of pediatric septic shock reflect changing risk and treatment response. Crit Care Med 46:e242–e249. doi: 10.1097/CCM.0000000000002932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scicluna BP, van Vught LA, Zwinderman AH, Wiewel MA, Davenport EE, Burnham KL, Nürnberg P, Schultz MJ, Horn J, Cremer OL, Bonten MJ, Hinds CJ, Wong HR, Knight JC, van der Poll T, de Beer FM, Bos LDJ, Frencken JF, Koster-Brouwer ME, van de Groep K, Verboom DM, Glas GJ, van Hooijdonk RTM, Hoogendijk AJ, Huson MA, Klouwenberg PMK, Ong DSY, Schouten LRA, Straat M, Witteveen E, Wieske L. 2017. Classification of patients with sepsis according to blood genomic endotype: a prospective cohort study. Lancet Respir Med 5:816–826. doi: 10.1016/S2213-2600(17)30294-1. [DOI] [PubMed] [Google Scholar]
- 59.Raymond SL, Hawkins RB, Wang Z, Mira JC, Stortz JA, Han F, Lanz JD, Hennessy LV, Brumback BA, Baker HV, Efron PA, Brakenridge SC, Xiao W, Tompkins RG, Cuschieri J, Moore FA, Maier RV, Moldawer LL. 1 January 2019. Prospective validation of a transcriptomic metric in severe trauma. Ann Surg doi: 10.1097/SLA.0000000000003204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mi MY, Klompas M, Evans L. 2019. Early administration of antibiotics for suspected sepsis. N Engl J Med 380:593–596. doi: 10.1056/NEJMclde1809210. [DOI] [PubMed] [Google Scholar]


