CASE
A 37-year-old immunosuppressed female presented with pain and swelling in her left lower extremity associated with a chronic nonhealing ulceration of her left lateral shin. The patient had a complex medical history most noted for systemic lupus erythematous (SLE), for which she was being treated with prednisone and cyclophosphamide. Her admission complete blood count was significant for a white blood cell count of 0.8 × 109/liter (reference range, 4.5 to 11.0/liter), hemoglobin of 7.9 g/dl (reference range, 12.0 to 16.0 g/dl), and platelet count of 81 × 109/liter (reference range, 150 to 440/liter). For her end-stage renal disease secondary to SLE, the patient was also receiving hemodialysis via a catheter in her right internal jugular vein. She reported having previous left leg skin infections caused by methicillin-resistant Staphylococcus aureus. Prior to her hospital admission, she was being treated weekly at a wound clinic for a left-lower-extremity ulcer.
On the day of presentation, the patient awoke with severe pain and erythema originating at the site of the ulcer and presented to a local hospital, where she was found to be febrile and tachycardic. After cultures were obtained, she was treated with a bolus of normal saline and intravenous vancomycin, clindamycin, and ceftazidime and transferred to our institution. In the emergency department, a biopsy sample of her left leg wound was obtained for culture. She was admitted to the intensive care unit, where cultures were obtained from her central venous line and hemodialysis catheter, and intravenous levofloxacin was added to her antibiotic regimen. The patient quickly decompensated with tachycardia and hypotension. Because of her deteriorating medical condition, the need for surgical intervention was urgent due to rapid expansion of the left leg infection. The initial 1.8- by 0.6-cm ulcer had grown into a necrotizing skin infection that measured 10 by 7 cm. This demarcated area quickly progressed to involve the left upper leg. The patient was taken to surgery on the same day as admission to our institution. During the debridement of the leg, severe necrosis was noted to extend from the left foot to the left knee and deep to the bone; therefore, the surgeons proceeded to perform an above-the-knee amputation. Left leg muscle and fascia were sampled during the procedure and sent for culture. The next morning, the patient expired after cardiopulmonary arrest.
The major findings at autopsy included marked erythematous changes involving the remaining lower extremities and trunk and multiple scattered skin bullae (Fig. 1). The adipose tissues of the upper left leg were discolored gray and appeared devitalized. Acute septal panniculitis with necrosis and skeletal muscle atrophy were also identified. Antemortem bacterial cultures obtained from multiple sites (left leg ulcer, left leg muscle and fascia, central venous catheter, and hemodialysis catheter) all grew Acinetobacter baumannii, which was identified by sequencing the first 500 bp of the 16S rRNA gene. Disk diffusion testing showed the isolate to be resistant to ampicillin-sulbactam, imipenem, cefepime, ceftazidime, trimethoprim-sulfamethoxazole, and levofloxacin but susceptible to gentamicin and intermediate to tobramycin. Postmortem cultures from the left thigh also grew multidrug-resistant A. baumannii.
FIG 1.
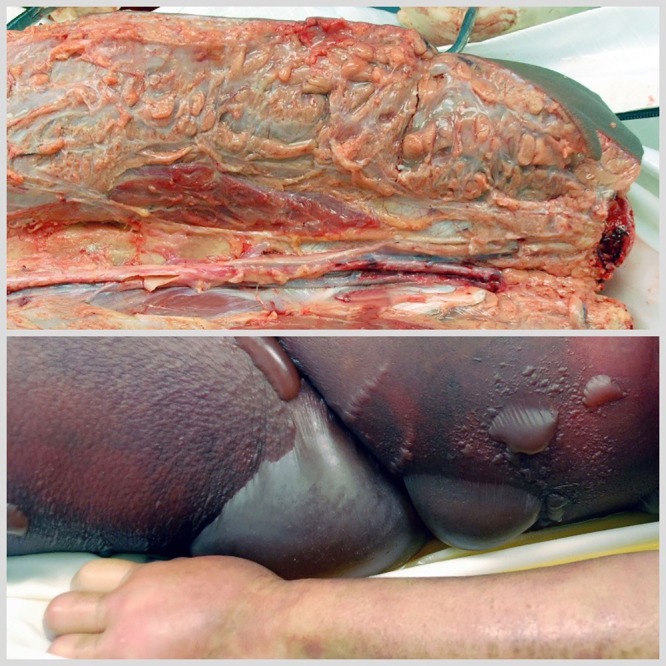
Images from the patient’s autopsy. Top, severe green/gray discoloration indicating necrosis of left thigh soft tissue after amputation above the knee. Bottom, large bullae with severe skin discoloration of abdomen with left forearm skin for comparison.
Whole-genome sequencing (WGS) of the A. baumannii isolate obtained from this case was performed using a Pacific Biosciences RSII instrument (8 single-molecule real-time [SMRT] cells at 180 min per cell). Assembly of the sequence data resulted in 3 closed circular contigs (GenBank accession numbers CP031444 to CP031446). Based on the Pasteur multilocus sequence typing (MLST) schema, the strain belongs to sequence type 2 (ST2), which is consistent with its nearest relatives as determined by WGS and the multidrug resistance observed by phenotypic testing. The smallest of the three sequences (10,879 bases) corresponds in size and content (99.9% identity) to the pAB120 plasmid previously reported in carbapenem-resistant A. baumannii isolates (1). The largest genetic element found in this isolate was 3.9 Mb in size, which was >99% identical in sequence to a number of A. baumannii genomes that have been previously sequenced (Fig. 2). The third sequence (112,216 bases) had weak homology over roughly one-quarter of its sequence to previously reported Acinetobacter plasmids (GenBank accession number CP027122) (2) but otherwise appeared to represent a novel large plasmid. Annotation using Prokka resulted in the identification of 133 open reading frames, though the vast majority of these had very little similarity to known protein families.
FIG 2.
Phylogenetic analysis of the patient’s A. baumannii isolate (circled).
DISCUSSION
The constellation of microbiological, histological, and clinical findings in the patient were compatible with necrotizing fasciitis. The patient developed septic shock secondary to her necrotizing skin and soft tissue infection. There are two main categories of necrotizing soft tissue bacterial infections, polymicrobial (type 1) and monomicrobial (type 2) (3). Type 1 polymicrobial infections are the most common type, causing up to 80% of infections. Type 1 infections are usually caused by a mixture of anaerobic and aerobic bacteria, including Clostridium species, and often show evidence of gas accumulation on radiographic imaging. Type 2 monomicrobial infections can be further characterized by the presence or absence of gas in soft tissue. Monomicrobial infections without gas are typically due to Streptococcus pyogenes, other beta-hemolytic streptococci, or Staphylococcus aureus, with S. pyogenes being the most common cause of type 2 infections. Less commonly, Vibrio vulnificus and Aeromonas hydrophila cause type 2 necrotizing fasciitis without gas and are associated with saltwater and freshwater exposure, respectively. Type 2 infections with gas are often due to Clostridium perfringens or Clostridium septicum, which are associated with traumatic and atraumatic myonecrosis, respectively. The presentation of these infections is similar. The initial presentation is severe pain at the site of infection with minimal physical findings. Edema and erythema develop shortly after. Systemic signs usually include a low-grade fever, tachycardia, and mild tachypnea. As the infection progresses, the skin will eventually show blistering, crepitus, bullae, and/or hemorrhagic blebs. Advancing systemic responses cause sepsis and shock with multiorgan system failure, which is a common end to the course of infection. Surgical exploration and debridement are critical in identifying the extent of infection and controlling its spread. As seen with this patient, amputation may be necessary. Delay of operative treatment greatly increases mortality in these cases.
Although necrotizing fasciitis can occur in healthy immunocompetent individuals without a clear portal of entry, there are factors that increase the risk of developing necrotizing fasciitis, including penetrating or blunt trauma, laceration or other skin breach (i.e., varicella, mosquito bite, and injection site), recent surgery, mucosal breach, obesity, and alcoholism. In addition, host deficiency or immunosuppressive states, such as diabetes, cirrhosis, neutropenia, and HIV infection, predispose individuals to necrotizing skin and soft tissue infections. Our patient’s risk factors included SLE, end-stage renal disease, ulceration/skin breach, and a history of lower-extremity infections.
Acinetobacter baumannii, an aerobic Gram-negative bacillus ubiquitously isolated from water, soil, sewage, and health care settings, is an important health care-associated pathogen often associated with respiratory tract, urinary tract, and bloodstream infections in immunocompromised and hospitalized patients. A. baumannii is often multidrug resistant, making it difficult to treat, and it is resistant to environmental stresses, including desiccation, allowing it to survive in the environment for a prolonged period of time. A. baumannii is an uncommon cause of community-acquired necrotizing soft tissue infections (NSTIs). However, an increase in the prevalence of A. baumannii in skin and soft tissue infections in soldiers with war trauma and in type 1and type 2 NSTI cases has been reported (4–6). Four features have been associated with NSTI with A. baumannii, which has a high mortality rate of ∼30%, as follows: (i) the host has underlying comorbidities (such as those listed above), (ii) fasciitis is often accompanied by bacteremia, (iii) treatment is complicated by multidrug resistance and the presence of copathogens (64% of cases), and (iv) surgical debridement is frequently required (84% of cases) (4). The NSTI presented here could either be a monomicrobial NSTI due to A. baumannii, or A. baumannii could be a component of a polymicrobial NSTI that was not identified by culture due to prior antibiotic exposure.
A. baumannii possesses a genomic structure that allows it to acquire multiple resistance markers and therefore become multidrug resistant. Comparative genomic analyses revealed genetic-material-carrying clusters of up to 52 distinct genes involved in encoding enzymatic and nonenzymatic resistance to several antibiotic families at once (7). The genomic plasticity of the organism for acquiring multidrug resistance and the increased prevalence of A. baumannii causing NSTI beg the question, are there characteristics of the organism or acquisition of virulence factors that lead to increased morbidity and mortality? Based on whole-genome sequencing, this patient’s A. baumannii isolate was very similar to other isolates that were not isolated from systemic disease. However, the presence of a novel large plasmid makes it tempting to suggest that the proteins it encodes might confer unique pathogenic characteristics to the isolate reported here. In the absence of additional epidemiological or functional studies, however, this link is impossible to prove, and the relative novelty of most of the potential coding sequences found within this plasmid makes further bioinformatic analysis speculative.
A portion of this work was funded under contract HSHQDC-15-C-00064 awarded by the Department of Homeland Security (DHS) Science and Technology Directorate (S&T) to the National Biodefense Analysis and Countermeasures Center (NBACC), a Department of Homeland Security (DHS) federal laboratory sponsored by the DHS Science and Technology Directorate and operated by the Battelle National Biodefense Institute.
The views and conclusions contained in this document are those of the authors and should not be interpreted as necessarily representing the official policies, either expressed or implied, of the DHS or S&T. In no event shall DHS, NBACC, S&T, or the Battelle National Biodefense Institute have any responsibility or liability for any use, misuse, inability to use, or reliance upon the information contained herein. DHS does not endorse any products or commercial services mentioned in this publication.
SELF-ASSESSMENT QUESTIONS
- What is the most common etiology of monomicrobial (type 2) necrotizing fasciitis?
-
a.Acinetobacter baumannii
-
b.Staphylococcus aureus
-
c.Streptococcus pyogenes
-
d.Vibrio vulnificus
-
a.
- What is the most important intervention in controlling the spread of acute necrotizing fasciitis?
-
a.Broad-spectrum antibiotics
-
b.Surgical debridement
-
c.Hyperbaric oxygen
-
d.Intravenous immunoglobulin
-
a.
- Which of the following contribute(s) to mortality with Acinetobacter-associated necrotizing fasciitis?
-
a.Concomitant bacteremia
-
b.Antimicrobial resistance
-
c.Comorbidities such as diabetes
-
d.All of the above
-
a.
For answers to the self-assessment questions and take-home points, see https://doi.org/10.1128/JCM.01754-18 in this issue.
REFERENCES
- 1.Povilonis J, Šeputienė V, Krasauskas R, Juškaitė R, Miškinytė M, Sužiedėlis K, Sužiedėlienė E. 2013. Spread of carbapenem-resistant Acinetobacter baumannii carrying a plasmid with two genes encoding OXA-72 carbapenemase in Lithuanian hospitals. J Antimicrob Chemother 68:1000–1006. doi: 10.1093/jac/dks499. [DOI] [PubMed] [Google Scholar]
- 2.Cafiso V, Stracquadanio S, Verde FL, Gabriele G, Mezzatesta ML, Caio C, Pigola G, Ferro A, Stefani S. 2019. Colistin resistant A. baumannii: genomic and transcriptomic traits acquired under colistin therapy. Front Microbiol 9:3195. doi: 10.3389/fmicb.2018.03195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonne SL, Kadri SS. 2017. Evaluation and management of necrotizing soft tissue infections. Infect Dis Clin North Am 31:497–511. doi: 10.1016/j.idc.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guerrero DM, Perez F, Conger NG, Solomkin JS, Adams MD, Rather PN, Bonomo RA. 2010. Acinetobacter baumannii-associated skin and soft tissue infections: recognizing a broadening spectrum of disease. Surg Infect (Larchmt) 11:49–57. doi: 10.1089/sur.2009.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sinha N, Niazi M, Lvovsky D. 2014. A fatal case of multidrug resistant Acinetobacter necrotizing fasciitis: the changing scary face of nosocomial infection. Case Rep Infect Dis 2014:705279. doi: 10.1155/2014/705279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charnot-Katsikas A, Dorafshar AH, Aycock JK, David MZ, Weber SG, Frank KM. 2009. Two cases of necrotizing fasciitis due to Acinetobacter baumannii. J Clin Microbiol 47:258–263. doi: 10.1128/JCM.01250-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fournier PE, Vallenet D, Barbe V, Audic S, Ogata H, Poirel L, Richet H, Robert C, Mangenot S, Abergel C, Nordmann P, Weissenbach J, Raoult D, Claverie JM. 2006. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genet 2:e7. doi: 10.1371/journal.pgen.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]



