In 2019, the Clinical and Laboratory Standards Institute revised the daptomycin breakpoints for Enterococcus spp. twice in rapid succession.
KEYWORDS: CLSI, Enterococcus, breakpoint, daptomycin
ABSTRACT
In 2019, the Clinical and Laboratory Standards Institute revised the daptomycin breakpoints for Enterococcus spp. twice in rapid succession. Analyses leading to these revisions included review of testing issues, murine and human in vivo pharmacodynamics, safety of off-label doses, and treatment outcomes. The data review brought up a dilemma that is encountered with increasing frequency: a breakpoint supported by pharmacokinetic/pharmacodynamic modeling that bisected the wild-type Enterococcus faecium MIC distribution. In such instances, not only does the probability of pharmacokinetic/pharmacodynamic targets need to be taken into account but also the probability that the laboratory can generate an accurate MIC that is reproducible within one interpretive category.
COMMENTARY
On 27 February 2019, the Clinical and Laboratory Standards Institute sent an update to laboratories detailing a revision to the M100S 29th edition Enterococcus species daptomycin breakpoints, which had been published in January (Table 1). The current (second revision) breakpoints are shown in Table 2 are available in the free online version of M100 (https://clsi.org/standards/products/free-resources/access-our-free-resources/) and will be printed in the M100S 30th edition, which will be published in January 2020. Laboratories may wonder why the daptomycin breakpoint was changed a second time, and so soon after publication of the first revision. This commentary outlines the reasons for the change and strategies laboratories may take to implement the current daptomycin breakpoints.
TABLE 1.
M100S 29th edition MIC Enterococcus breakpoints for daptomycin
| Test/report group | Microorganism | Disk content | MIC breakpoint (μg/ml) for the following interpretive category: |
Comments | |||
|---|---|---|---|---|---|---|---|
| S | SDD | I | R | ||||
| B |
Enterococcus spp. |
– | ≤1 | 2−4 | –a | ≥8 | Comment 11. Daptomycin should not be reported for isolates from the respiratory tract. |
| Comment 12. The breakpoint for SDD is based on a dosage regimen of 8 to 12 mg/kg administered every 24 h in adults and is intended for serious infections due to E. faecium. Consultation with an infectious diseases specialist is recommended. | |||||||
–, not applicable.
TABLE 2.
February 2019 update to the M100S 29th edition MIC Enterococcus breakpoints for daptomycin
| Test/report group | Microorganism | Disk content | MIC breakpoint (μg/ml) for the following interpretive category: |
Comments | |||
|---|---|---|---|---|---|---|---|
| S | SDD | I | R | ||||
| B | E. faecium only | –a | – | ≤4 | – | ≥8 | Comment 11. Daptomycin should not be reported for isolates from the respiratory tract. |
| Comment 12. The breakpoint for SDD is based on a dosage regimen of 8 to 12 mg/kg administered every 24 h in adults and is intended for serious infections due to E. faecium. Consultation with an infectious diseases specialist is recommended. | |||||||
| B | Enterococcus spp. (other than E. faecium) | – | ≤2 | – | 4 | ≥8 | Comment 13. The breakpoint for susceptible is based on a dosage regimen of 6 mg/kg administered every 24 h in adults. |
| See comment 11. | |||||||
–, not applicable.
The enterococcal daptomycin breakpoints have been under review by CLSI since June 2016, when issues regarding both daptomycin treatment failures for Enterococcus spp. and the appropriate enterococcal endocarditis dose for daptomycin were first discussed. The CLSI Antimicrobial Susceptibility Testing (AST) Subcommittee convened an ad hoc working group to address these issues, which compiled existing and generated new data to support a breakpoint revision. Analyses included review of testing issues (1), murine (2), and human (3) in vivo pharmacodynamics (PD), safety of off-label doses (4, 5) and treatment outcomes (6–8). The full extent of the data reviewed and outcomes of the analyses will be described elsewhere in a rationale document to be published by CLSI and a review article (M. J. Satlin, D. P. Nicolau, R. M. Humphries, J. L. Kuti, et al., submitted for publication). Combined, the data demonstrated the following.
-
1.
A low probability of pharmacokinetic (PK)/PD target attainment for isolates of Enterococcus with daptomycin MIC of 2 to 4 μg/ml, with maximal FDA-approved doses of daptomycin (6 mg/kg of body weight/day), was found by both nonclinical (murine) and human clinical PK/PD simulations. In contrast, ≥90% probability of PK/PD target attainment is possible for these MICs, with off-label doses of 8–12 mg/kg/day.
-
2.
Clinical outcomes are improved in adults with bloodstream infections if off-label doses of 8–12 mg/kg/day are used.
-
3.
Safety of doses of 8 to 12 mg/kg/day has been documented for adults.
The majority of these data, in particular clinical PD and outcomes, consisted of evidence for infections caused by vancomycin-resistant Enterococcus faecium. This is because many isolates of E. faecium are resistant to antimicrobial agents considered first-line therapy for enterococcal endocarditis (9) (i.e., ampicillin/penicillin, vancomycin, and an aminoglycoside), leaving daptomycin and linezolid as the remaining treatment options. Similarly, alternative treatment regimens consisting of double beta-lactam therapy, such as ampicillin plus ceftriaxone, are not indicated for E. faecium (9). In contrast, most isolates of Enterococcus faecalis remain susceptible to at least one cell wall-active agent and do not display high-level aminoglycoside resistance. Nonetheless, the CLSI AST Subcommittee was hesitant to publish a daptomycin breakpoint for E. faecium alone in 2019 largely because there was concern that not all laboratories identify Enterococcus isolates to the species level. In addition, daptomycin does not have an FDA-approved indication for treatment of infections caused by E. faecium, which led to some concern regarding the ability of commercial antimicrobial susceptibility tests (cASTs) to obtain FDA clearance for an E. faecium-only breakpoint. The only enterococcal indication for the use of daptomycin is for the treatment of skin and soft tissue infections caused by vancomycin-susceptible E. faecalis.
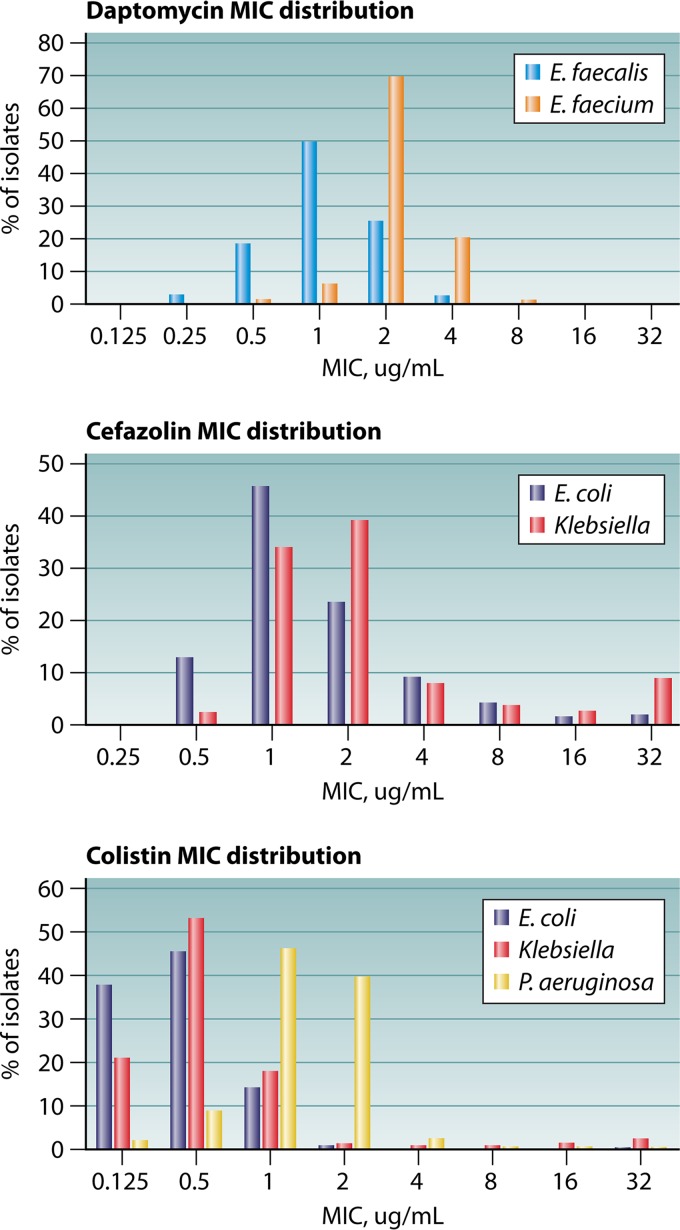
As part of the evaluations performed by CLSI, data were generated in a multicenter study of daptomycin MIC reproducibility, both by reference broth microdilution (BMD) and gradient diffusion strips (1). The study demonstrated that obtaining a reproducible daptomycin MIC for E. faecium was very difficult by both methods. These testing issues were particularly problematic the closer the isolate’s modal BMD MIC was to the breakpoint, as MICs >3 dilutions (i.e., outside the acceptable essential agreement) for the same isolate were seen in 60% of isolates tested (1). Despite these testing issues, it was agreed that physicians were in need of a daptomycin MIC and interpretation in order to best use daptomycin clinically for the treatment of serious enterococcal infections. The CLSI voted to approve a single, revised breakpoint for all enterococci (Table 1). Given that most isolates of E. faecium harbored MICs of 2 to 4 μg/ml (Fig. 1, top), the committee felt confident that most, if not all, isolates would yield an MIC in the susceptible dose dependent (SDD) category.
FIG 1.
MIC distributions for daptomycin (top), cefazolin (middle), and colistin (bottom) from the European Antimicrobial Susceptibility Testing (EUCAST) data set (14). MIC data are shown in micrograms per milliliter.
In January 2019, the issue of daptomycin testing was discussed anew. Anecdotal reports from laboratories attempting to validate their cASTs off-label with the M100S 29th edition breakpoints indicated significant challenges with poor categorical agreement, which is unsurprising given the AST study conducted by CLSI (1). Furthermore, several studies were reviewed, which indicated that isolates of E. faecium with MICs of ≤1.0 μg/ml harbor mutations to the liaFSR system (1, 10), which has been shown to be one mechanism by which E. faecium develops high-level daptomycin resistance and is associated with daptomycin tolerance in this species. While the clinical ramifications of these mutations are not fully understood, there remains concern that such isolates may fail therapy at 6 mg/kg/day daptomycin. In addition, data from proficiency testing surveys published by the College of American Pathologists indicated that nearly all laboratories perform species identification for Enterococcus isolated from blood or other sterile sites.
As such, the breakpoints were revised a second time to delete the susceptible (S) category for E. faecium (Table 2). This change was deemed to be a more appropriate breakpoint for E. faecium, the species treated most frequently with daptomycin, because of the following.
-
•
The breakpoint is the same as the M100S 28th edition breakpoint, but the interpretive categories are relabeled (i.e., SDD versus S and resistant [R] versus nonsusceptible [NS]). The 2018 breakpoint has been FDA cleared on the majority of cASTs, meaning laboratories can use the E. faecium breakpoint without validation of their cASTs off-label and adopt the breakpoint immediately.
-
•
An interpretive category of SDD with no susceptible category reduces the risk of underdosing daptomycin for E. faecium infections, both due to testing challenges and also by alerting physicians a dose increase is needed.
-
•
The breakpoint appropriately acknowledges inherent differences between E. faecium and E. faecalis susceptibility to daptomycin.
This change to interpretive criteria marks the second time CLSI standards have applied an SDD category with no susceptible category. Candida glabrata has an SDD category and R category for fluconazole with disclaimers regarding the need for maximum dosage of fluconazole and expert consultation to select a maximum dosage regimen (11). As mentioned, significant differences exist between the susceptibility of E. faecium and E. faecalis to daptomycin. First, the epidemiological cutoff (ECV, i.e., wild-type MIC) for E. faecium is ≤4 μg/ml, whereas E. faecalis isolates have ECV of ≤2 μg/ml (Fig. 1, top). In addition, in murine infection models, investigators were unable to achieve a 1-log10-unit kill of E. faecalis, regardless of the daptomycin dose or MIC (2), and very limited human PD and clinical outcome data are available for the use of daptomycin for E. faecalis infections. As such, the CLSI chose a conservative ≤2 μg/ml susceptible breakpoint for daptomycin and non-E. faecium Enterococcus species, as there were concerns that a susceptible breakpoint of ≤4 μg/ml was too high, and while it is rare, daptomycin is used occasionally to treat E. faecalis infections. An SDD category was not applied because the supportive clinical and PK/PD data for off-label doses are derived almost exclusively from E. faecium infections. In contrast, an intermediate (I) category was included to mitigate the risk of small, uncontrolled variables in the MIC test method from resulting in very major (false-susceptible) or major (false-resistant) errors (Table 2).
It is anticipated that laboratories can adopt the new daptomycin E. faecium breakpoint immediately. Several options exist, including changing S to SDD in the laboratory information system, continuing to use the S/NS interpretive categories but adding interpretive comments regarding the need for doses of 8 to 12 mg/kg/day in adults for susceptible isolates, or working with the pharmacy to ensure that E. faecium infections are treated with 8 to 12 mg/kg/day. Of importance, however, is that all laboratories identify enterococci to the species level when the enterococci are isolated from blood, if not already doing so. Implementing the Enterococcus, not the E. faecium, breakpoint is less urgent, as only rare cases of E. faecalis may be treated with daptomycin. Laboratories may opt to test and/or report daptomycin on request only for these isolates.
It is well-known that the reproducibility of the MIC test is ±1 log2 unit, or in other words, one to three MICs may be obtained for the same isolate. To date, this has not been a significant challenge, as most breakpoints fall well above the wild-type MIC distribution. However, recent reevaluation and reduction of breakpoints, based largely on PK/PD analyses, have highlighted the challenge of such inherent MIC variability. The primary reason the daptomycin Enterococcus breakpoints were reassessed a second time is that a breakpoint of ≤1 μg/ml cuts into the wild-type E. faecium MIC distribution for daptomycin (Fig. 1, top). It is not, however, the first time that PK/PD modeling has identified a breakpoint that bisects a wild-type population. Other examples include both the CLSI (≤2 μg/ml) and FDA (≤1 μg/ml) cefazolin breakpoint for Enterobacteriaceae (Fig. 1, middle), and data that suggest an appropriate Enterobacteriaceae breakpoint for colistin is ≤0.5 μg/ml (12) (Fig. 1, bottom). Of note, no cAST has attempted to obtain FDA clearance for the Enterobacteriaceae cefazolin breakpoint, even though it is technically possible to do so, using FDA breakpoints. If the bulk of isolates, including wild-type isolates, harbor MICs that fall on either side of the breakpoint, it is impossible to achieve acceptable categorical agreement, even by reference methods. Some have suggested use of triplicate testing, by both reference BMD and cASTs, as a means by which to control for this variability. However, this inevitably drives up the cost of studies aimed at demonstrating performance claims of cASTs for FDA clearance both by tripling the amount of testing and potentially increasing the amount of isolates to be tested if isolates without a mode by BMD would be excluded. At present, it is difficult enough for cAST manufacturers to prioritize, support, and complete breakpoint updates, without adding in additional testing and discrepancy analyses.
Where then, do we go from here? When establishing breakpoints, standards development organizations must remember that an MIC is not a precise measurement. Not only the probability of PK/PD targets to be achieved by MIC but also the probability of laboratory testing methods to generate an MIC that is reproducibly within a single interpretive category should be evaluated. Unfortunately, the latter analysis is almost never done. Typically, breakpoints are established using reference BMD, which may be (but often is not) performed in replicates. The variability of cASTs, which are used in clinical laboratories, are never accounted for. Similarly, when assessing disk breakpoints, use of more than one lot of disks and media is only starting to be done by CLSI. Again, performing such studies is costly, in particular for organizations like CLSI, which are volunteer based. Rather, MIC variability is accounted for by evaluating the MIC distribution of the bacterial population and avoiding a PK/PD breakpoint that cuts into this distribution.
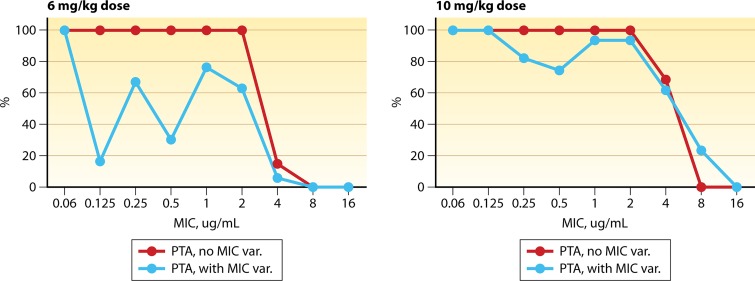
Variability in antimicrobial levels across patient populations with standard dosing is mitigated through the use of Monte Carlo simulations to predict the probability of PK/PD target attainment with clinically used doses (13). However, it should be noted that even with this step, these probabilities are an approximation. It is perhaps time that variability of MIC results be accounted for in a similar fashion. As an example, in Fig. 2, probability of target attainment is displayed, wherein MIC variability from Campeau et al. (1) is incorporated into the Monte Carlo simulation. This highlights the need for maximal dosing of antimicrobials, like daptomycin, and also the need to evaluate for MIC variability. For particularly difficult scenarios, like daptomycin and E. faecium, a categorization of S versus R may be too simplistic, but instead, a probability score could be assigned. Such a drastic change to how MIC testing is reported, however, would without a doubt require interpretation by specialists, such as infectious disease pharmacists and physicians. Without question, these challenges will continue to plague us, as antimicrobial resistance continues to emerge. It takes active participation of all parties, including the clinical microbiologist, pharmacist, physician, and industry, to address these challenges. Testing issues are central to this discussion, which requires skilled clinical microbiologist participation. Furthermore, an active role of microbiologists in patient care, in particular regarding difficult treatment scenarios such as E. faecium endocarditis is crucial to optimal patient outcomes in the era of multidrug resistance.
FIG 2.
Monte Carlo analysis for daptomycin probability of target attainment (PTA) for a 6-mg/kg/day dose versus a 10-mg/kg/day dose. Modeling was performed using a daptomycin fractional area-under the curve (fAUC) to MIC target ratio of 20. Data are presented with and without MIC variability (var) included in the analysis. MIC variability data are from reference 1.
ACKNOWLEDGMENTS
R.M.H. is an employee of Accelerate Diagnostics and a member of the CLSI AST Subcommittee.
I thank Kyle Spafford for help with the Monte Carlo analysis in Fig. 2.
The views expressed in this article do not necessarily reflect the views of the journal or of ASM.
REFERENCES
- 1.Campeau SA, Schuetz AN, Kohner P, Arias CA, Hemarajata P, Bard JD, Humphries RM. 2018. Variability of daptomycin MIC values for Enterococcus faecium when measured by reference broth microdilution and gradient diffusion tests. Antimicrob Agents Chemother 62:e00745-18. doi: 10.1128/AAC.00745-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kidd JM, Abdelraouf K, Asempa TE, Humphries RM, Nicolau DP. 2018. Pharmacodynamics of daptomycin against Enterococcus faecium and Enterococcus faecalis in the murine thigh infection model. Antimicrob Agents Chemother 62:e00506-18. doi: 10.1128/AAC.00506-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avery LM, Kuti JL, Weisser M, Egli A, Rybak MJ, Zasowski EJ, Arias CA, Contreras GA, Chong PP, Aitken SL, DiPippo AJ, Wang JT, Britt NS, Nicolau DP. 2018. Pharmacodynamic analysis of daptomycin-treated enterococcal bacteremia: it is time to change the breakpoint. Clin Infect Dis 68:1650–1657. doi: 10.1093/cid/ciy749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seaton RA, Menichetti F, Dalekos G, Beiras-Fernandez A, Nacinovich F, Pathan R, Hamed K. 2015. Evaluation of effectiveness and safety of high-dose daptomycin: results from patients included in the European Cubicin outcomes® registry and experience. Adv Ther 32:1192–1205. doi: 10.1007/s12325-015-0267-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durante-Mangoni E, Andini R, Parrella A, Mattucci I, Cavezza G, Senese A, Trojaniello C, Caprioli R, Diana MV, Utili R. 2016. Safety of treatment with high-dose daptomycin in 102 patients with infective endocarditis. Int J Antimicrob Agents 48:61–68. doi: 10.1016/j.ijantimicag.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 6.Chong PP, van Duin D, Bangdiwala A, Ivanova A, Miller WC, Weber DJ, Gilligan PH, Shea TC. 2016. Vancomycin-resistant enterococcal bloodstream infections in hematopoietic stem cell transplant recipients and patients with hematologic malignancies: impact of daptomycin MICs of 3 to 4 mg/L. Clin Ther 38:2468–2476. doi: 10.1016/j.clinthera.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Shukla BS, Shelburne S, Reyes K, Kamboj M, Lewis JD, Rincon SL, Reyes J, Carvajal LP, Panesso D, Sifri CD, Zervos MJ, Pamer EG, Tran TT, Adachi J, Munita JM, Hasbun R, Arias CA. 2016. Influence of minimum inhibitory concentration in clinical outcomes of Enterococcus faecium bacteremia treated with daptomycin: is it time to change the breakpoint? Clin Infect Dis 62:1514–1520. doi: 10.1093/cid/ciw173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moise PA, Sakoulas G, McKinnell JA, Lamp KC, DePestel DD, Yoon MJ, Reyes K, Zervos MJ. 2015. Clinical outcomes of daptomycin for vancomycin-resistant Enterococcus bacteremia. Clin Ther 37:1443–1453.e1442. doi: 10.1016/j.clinthera.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Tleyjeh IM, Rybak MJ, Barsic B, Lockhart PB, Gewitz MH, Levison ME, Bolger AF, Steckelberg JM, Baltimore RS, Fink AM, O'Gara P, Taubert KA, American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council . 2015. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 132:1435–1486. doi: 10.1161/CIR.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 10.Kebriaei R, Rice SA, Singh KV, Stamper KC, Dinh AQ, Rios R, Diaz L, Murray BE, Munita JM, Tran TT, Arias CA, Rybak MJ. 2018. Influence of inoculum effect on the efficacy of daptomycin monotherapy and in combination with beta-lactams against daptomycin-susceptible Enterococcus faecium harboring liaSR substitutions. Antimicrob Agents Chemother 62:e00315-18. doi: 10.1128/AAC.00315-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. 2017. Performance standards for antifungal susceptibility testing of yeasts, 1st ed CLSI supplement M60 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 12.Tsala M, Vourli S, Georgiou PC, Pournaras S, Tsakris A, Daikos GL, Mouton JW, Meletiadis J. 2018. Exploring colistin pharmacodynamics against Klebsiella pneumoniae: a need to revise current susceptibility breakpoints. J Antimicrob Chemother 73:953–961. doi: 10.1093/jac/dkx522. [DOI] [PubMed] [Google Scholar]
- 13.Drusano GL. 2007. Pharmacokinetics and pharmacodynamics of antimicrobials. Clin Infect Dis 45(Suppl 1):S89–S95. doi: 10.1086/518137. [DOI] [PubMed] [Google Scholar]
- 14.European Committee on Antimicrobial Susceptibility Testing. 2019. MIC and zone diameter distributions and ECOFFs. http://www.eucast.org/mic_distributions_and_ecoffs/. Accessed 4 April 2019.