Abstract
Purpose
Smoldering multiple myeloma (SMM) is a rare asymptomatic plasma cell disorder. Even with emerging therapeutic approaches and risk stratification, the optimal time to treat SMM remains controversial. This meta-analysis aimed to compare early treatment with deferred treatment of SMM, especially high-risk SMM.
Methods
Early treatment was defined as treatment immediately after diagnosis. Deferred treatment was initiated after progression. The primary outcome was progression. Secondary outcomes were mortality, response, and safety. PubMed, EMBASE, Medline, Cochrane, and ClinicalTrials.gov databases were searched from January 1990 to March 2019. Randomized controlled trials (RCTs) comparing early treatment with deferred treatment in SMM patients were eligible. Risk ratios (RRs) with 95% confidence interval (CI) were pooled.
Results
Eight RCTs covering 885 SMM patients were included. Considering all the different treatment approaches, early treatment significantly decreased progression of SMM (RR=0.53, 95% CI 0.33–0.87, P=0.01). In subgroup analysis, melphalan plus prednisone (RR=0.22, 95% CI 0.08–0.64, P=0.005) and immuno-modulatory drugs (RR=0.43, 95% CI 0.31–0.59, P<0.00001) significantly reduced progression. However, neither mortality nor response rate was significantly affected by early treatment. In terms of high-risk SMM patients, early treatment significantly decreased both progression (RR=0.51, 95% CI 0.37–0.70, P=0.0001) and mortality (RR=0.53, 95% CI 0.29–0.96, P=0.04). Frequently seen adverse events were infection, constipation, asthenia, and second primary malignancy. A remarkably elevated risk of constipation was associated with early treatment using immuno-modulatory agents (RR=4.43, 95% CI 2.14–9.12, P<0.0001). Second primary malignancy was significantly increased with early treatment (RR=4.13, 95% CI 1.07–15.97, P=0.04). No significant difference was identified in infection or asthenia.
Conclusion
These findings suggest that early treatment could decrease progression and mortality of high-risk SMM patients with a tolerable safety profile.
Keywords: smoldering multiple myeloma, early treatment, lenalidomide, meta-analysis
Introduction
Smoldering multiple myeloma (SMM) is an asymptomatic plasma cell disorder, which is recognized in almost 15% of all multiple myeloma (MM) cases.1,2 Without end-organ damage, SMM is often incidentally discovered. Overall, SMM patients carry a risk of progression to MM of 10% per year. High-risk SMM patients are extremely vulnerable to progression, with a progression rate of 76% in 5 years.3 In 2014, the International Myeloma Working Group (IMWG) reclassified a subgroup of ultra-high-risk SLiM SMM (bone marrow plasma cells [BMPCs] ≥60%, involved:uninvolved free light chain ratio ≥100, >1 focal lesion on magnetic resonance imaging [MRI]) into MM, since it displays a progression risk of more than 80% in 2 years. Emerging studies have proposed various risk factors, including BMPC ≥10%, monoclonal protein ≥3 g/dL, involved:uninvolved light chain ratio >8, immunoparesis, high-risk fluorescence in-situ hybridization, and evolving monoclonal protein.4–10 Consequently, it is in urgent need to offer SMM patients, especially high-risk SMM patients, the optimal management.
The current standard management of SMM is watchful waiting until progression, which has probably been chosen due to failure to extend progression-free survival (PFS), overall survival (OS), and response rate as well as the wish to avoid the obvious toxicities of early treatment. Previous experience showed that early treatment using melphalan–prednisone (MP) failed to extend OS and brought about various side effects, such as granulocytopenia, thrombocytopenia, neurotoxicity, acute leukemia, and nausea.11–13 A meta-analysis including the three studies with early MP intervention proved that early treatment could slow progression, but the effect on survival and response rate was insignificant.14 However, questions have been raised on the insignificant effect of MP on survival in that meta-analysis. It included only 262 patients, but an estimated sample size of 350 was required to convincingly measure a 15% mortality difference according to the Pogue and Yusuf formula adopted. Whether the insignificance is because of the nature of MP itself or because of the insufficient sample size requires further investigation. Various innovative drugs have been developed over the years, including immunomodulatory drugs (IMiDs) and proteasome inhibitors. Huge progress has also been made in risk stratification of SMM patients. The optimal timing of treatment for SMM patients, especially high-risk SMM patients, is also controversial. It is therefore necessary to reevaluate SMM treatment in consideration of recent advances.
With the aim of investigating the optimal timing of SMM treatment, this meta-analysis investigated differences in progression, mortality, response, and safety between early treatment and deferred treatment of SMM. Additionally, subgroup analysis was conducted on high-risk patients and on different types of treatment in order to balance benefits and risks of these vulnerable patients and to identify the most appropriate therapy.
Methods
This research was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines.15
Search strategy
PubMed, EMBASE, Medline, Cochrane, and ClinicalTrials.gov databases were independently searched by two investigators from January 1990 to March 2019, using synonyms of keywords “smoldering multiple myeloma”, “asymptomatic multiple myeloma”, “early stage multiple myeloma”, “progression”, “death”, “survival”, “response”, and “safety”. Language was limited to English. There was no restriction of country, gender, or race. Reference lists of candidate studies were also screened.
Inclusion/exclusion criteria
RCTs comparing early treatment with deferred treatment of SMM were eligible for this analysis. SMM patients were included altogether with or without risk stratifications. No previous treatment had been provided for these SMM patients. Early treatment was started immediately after SMM diagnosis. Deferred treatment was initiated after progression to symptomatic MM. The primary outcome was progression. Secondary outcomes were mortality, response and safety. Mortality was examined from randomization to all-cause death. Response was assessed by objective response rate. Studies were excluded if (i) they were cohort studies, case reports, case series, cross-sectional studies, letters, reviews, short surveys, or editorials; (ii) they only compared two different dosages of the same early treatment instead of comparing early treatment with deferred treatment.
Data extraction
Two investigators independently examined titles and abstracts for promising candidate studies, screened the full texts and subsequently the reference lists. They independently extracted data according to a preliminarily designed Microsoft Excel spreadsheet. Collected data included first author, publication year, participants, sample size, definition of early and deferred treatment, follow-up, and outcome. A third investigator resolved disagreement if unsolved after discussion. Efforts were made to contact corresponding authors of candidate studies to obtain more detailed information and to gain insight into further progress on the subject.
Quality assessment
Two investigators managed quality evaluation independently of candidate studies. All included RCTs underwent evaluation with the help of Cochrane Collaboration’s tool to examine risk of bias. Any disagreement was settled by a third investigator. Studies with two high-risk aspects were considered to have moderate risk of bias. Those with four or more high-risk aspects were considered to have high risk of bias.
Statistical analysis
Review Manager 5.3 (Cochrane Collaboration) was utilized to conduct this meta-analysis. The pooled risk ratios (RRs) of dichotomous data were calculated by the Mantel-Haenszel method. If more than one effect measure was available, the most thoroughly adjusted effect measure considering potential confounders was adopted. Heterogeneity was assessed utilizing Higgin’s I2 statistic. Significant heterogeneity existed in included studies with I2>50%, and random effects models were utilized. Otherwise, fixed-effects models were utilized. Subgroup analyses and sensitivity analyses were carried out when significant heterogeneity was found. Effects were considered significant when P<0.05.
Results
Description of studies and risk of bias
A total of 258 studies were identified from database searching. Eight RCTs involving 885 SMM patients were finally included in the meta-analysis (Figure 1), including 3 studies on MP, 2 studies on bisphosphonates, 2 on IMiDs, and 1 on siltuximab.11–13,16–20 The main features of the eight studies are summarized in Table 1. Six of the eight studies were open-label, leading to a high risk of performance and detection bias. In terms of overall risk of bias, all studies had mild to moderate risk, with no study at high risk of bias (Figure S1).
Figure 1.
Study flow diagram.
Abbreviation: RCT, randomized controlled trial.
Table 1.
Description of included studies
| Author | Year | Participants | N | Age | Early treatment | Follow-up | Outcome |
|---|---|---|---|---|---|---|---|
| Brighton16 | 2019 | High-risk SMM | E 43; D 42 | E 62(21–84) D 62(21–84) |
Siltuximab | 29.2 m | PFS, mortality, safety, antibodies to siltuximab, progressive disease indicator rate |
| Mateos17 | 2016 | High-risk SMM | E 57; D 62 | E 61(39–89) D 65(36–80) |
Lenalidomide plus dexamethasone | 75 (67–85) m | TTP, mortality, ORR, safety |
| Witzig18 | 2013 | SMM | E 35; D 33 | E 63(47–84) D 63(47–80) |
Thalidomide plus zoledronic acid | 71 (18–96) m | TTP, OS, ORR, safety |
| D’Arena19 | 2011 | SMM | E 89; D 88 | E 64(22–80) D 64(36–86) |
Pamidronate | >60 m | TTP, safety, skeletal-related events at progression |
| Musto20 | 2008 | SMM | E 81; D 82 | E 66(41–82) D 67(42–84) |
Zoledronic acid | 64.7 (36–72) m | TTP, mortality, safety, skeletal-related events at progression |
| Riccardi11 | 2000 | SMM | E 75; D 70 | E 69(39–88) D 68(33–85) |
Melphalan plus prednisone | 65 (60–139) m | Progression, mortality, ORR |
| Riccardi12 | 1994 | SMM | E 38; D 40 | NA | Melphalan plus prednisone | NA | Progression, mortality |
| Hjorth13 | 1993 | SMM | E 25; D 25 | E 71(42–91) D 72(52–84) |
Melphalan plus prednisone | 60 (36-NA) m | Time to response, mortality, relapse |
Abbreviations: E, early treatment; D, deferred treatment; ORR, objective response rate; PFS, progression-free survival; SMM, smoldering multiple myeloma; TTP, time to progression; OS, overall survival; m, month; NA, not applicable.
Figure S1.

Risk of bias summary.
Progression
In progression evaluation, seven studies, 797 (early treatment, n=401; deferred treatment, n=396) patients were considered. Considering all the different treatments included in this study, progression was significantly reduced with early treatment compared with deferred treatment (RR =0.53, 95%CI 0.33–0.87, P=0.01). However, significant heterogeneity existed (I2=86%), probably due to the different types of therapy. Subgroup analysis was conducted by sorting according to treatment type. MP (RR =0.22, 95%CI 0.08–0.64, P=0.005) and IMiDs (RR =0.43, 95%CI 0.31–0.59, P<0.00001) had a significant suppressive effect on progression, while bisphosphonates and siltuximab did not significantly affect progression (Figure 2). However, although bisphosphonates did not significantly inhibit progression, they did significantly reduce skeletal-related events at progression (RR =0.61, 95%CI 0.47–0.78, P=0.0001) (Figure S2). When the two studies which only enrolled high-risk SMM patients were excluded from sensitivity analysis, progression was still significantly reduced with early treatment compared with deferred treatment for overall SMM patients (RR =0.49, 95%CI 0.26–0.95, P=0.04).
Figure 2.
Progression in overall SMM patients.
Abbreviations: MP, melphalan-prednisone; IMiD, immunomodulatory drug; SMM, smoldering multiple myeloma.
Figure S2.
Skeletal-related events of overall SMM patients at progression.
Abbreviation: SMM, smoldering multiple myeloma.
Mortality
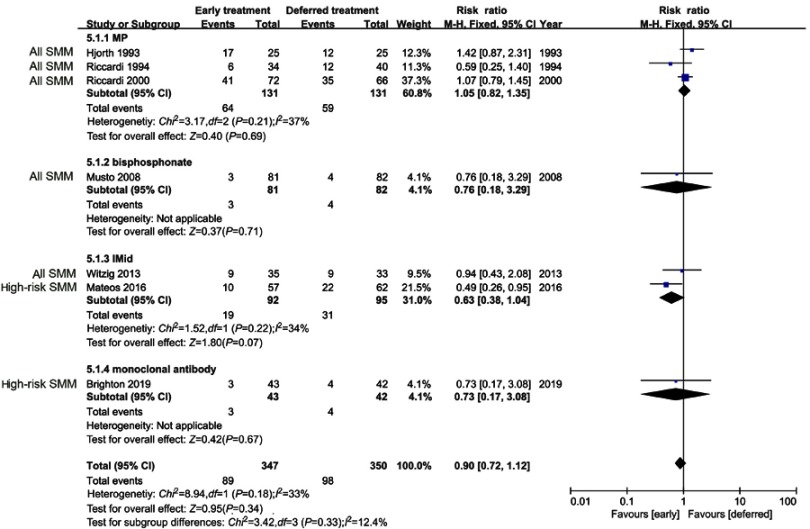
In mortality evaluation, 7 studies, 697 (early treatment, n=347; deferred treatment, n=350) patients were considered. No significant difference in mortality was revealed between early treatment and deferred treatment (RR=0.90, 95%CI 0.72–1.12, P=0.34). Although no significant heterogeneity was discovered (I2=33%), subgroup analysis was still performed in order to explore the impact of different treatments on mortality. In subgroup analysis, none of MP, bisphosphonates, IMiDs, or siltuximab significantly reduced mortality of SMM (Figure 3). In sensitivity analysis which excluded the two high-risk SMM studies, mortality of overall SMM patients was still unaffected by early treatment (RR=1.02, 95%CI 0.81–1.30, P=0.85).
Figure 3.
Mortality in overall SMM patients.
Abbreviations: MP, melphalan-prednisone; IMiD, immunomodulatory drug; SMM, smoldering multiple myeloma.
Response
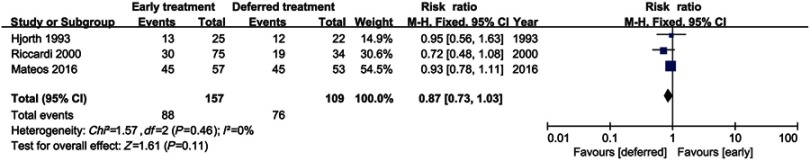
Three studies including 266 patients (early treatment, n=157; deferred treatment, n=109) were evaluable for response rate. No significant difference in response rate was found between early treatment and deferred treatment (RR=0.87, 95%CI 0.73–1.03, P=0.11), as shown in Figure 4. No heterogeneity was identified (I2=0%).
Figure 4.
Therapeutic response in overall SMM patients.
Abbreviation: SMM, smoldering multiple myeloma.
High-risk SMM
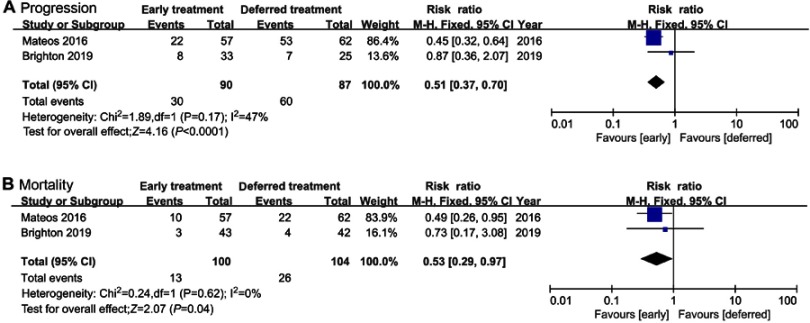
Since high-risk SMM patients are more vulnerable to progression to MM, subgroup analysis of studies enrolling high-risk SMM patients was conducted. Two studies were evaluable, involving lenalidomide and siltuximab. Both progression (RR=0.51, 95%CI 0.37–0.70, P=0.0001) and mortality (RR=0.53, 95%CI 0.29–0.97, P=0.04) were significantly suppressed by early treatment in comparison to deferred treatment. Mild heterogeneity (I2=47%) was identified in progression, while no heterogeneity (I2=0%) was discovered in mortality (Figure 5).
Figure 5.
(A) Progression and (B) mortality in high-risk SMM patients.
Abbreviation: SMM, smoldering multiple myeloma.
Adverse events
Various treatment approaches were included in our study, leading to different safety profiles. Granulocytopenia, thrombocytopenia, nausea, neurotoxicity, and acute leukemia were reported with early MP treatment. Side effects were rarely seen with bisphosphonates, which mainly included symptomatic hypocalcemia and fever. Adverse events were frequent with thalidomide, including peripheral neuropathy, skin rash, constipation, and asthenia. Lenalidomide was reported to be associated with infection, asthenia, neutropenia, skin rash, and second primary malignancies (SPMs). Infection and urinary disorders were reported with both siltuximab and placebo. Of the multiple adverse events reported in different studies, infection, constipation, asthenia, and SPMs were evaluable for meta-analysis. No significant difference in infection or asthenia was found between early treatment and deferred treatment. However, constipation was significantly more frequent with early treatment than with deferred treatment (RR=4.42, 95%CI 2.14–9.12, P<0.0001), which was reported with both thalidomide and lenalidomide. Furthermore, SPM, reported with MP and lenalidomide, was also significantly increased with early treatment compared with deferred treatment (RR=4.13, 94%CI 1.07–15.97, P=0.04). No significant heterogeneity was found (Figure S3).
Figure S3.
(A) Infection, (B) asthenia, (C) constipation, and (D) second primary malignancy in overall SMM patients.
Abbreviation: SMM, smoldering multiple myeloma.
Discussion
The current watchful waiting strategy of SMM management is probably attributable to unimproved survival and response rates as well as noticeable toxicities associated with early treatment. In the 1990s, early treatment of SMM mainly focused on MP. Three RCTs compared early MP treatment with deferred treatment of SMM patients and identified no significant difference in response rate or survival.11–13 However, it is unknown whether this lack of significance was due to the treatment itself or the relatively limited sample size and follow-up.14 In the 2000s, several studies explored the effect of bisphosphonates on progression and response rate of SMM patients. Bisphosphonates showed no significant effects on progression, except for a reduction of skeletal-related events at progression.19–21 However, bisphosphonates were reported to possibly cause osteonecrosis of the jaw, which was considered unacceptable for SMM patients. As a result, early bisphosphonate treatment only lasted for one year due to ethical consideration.19,20 IMiDs were subsequently investigated. It was reported that thalidomide triggered a mild response without significant promotion of OS.22–24 Adverse events associated with thalidomide were obvious, causing one-third of SMM patients to give up treatment early.18 The relatively high dropout rate could possibly affect the outcome. No risk stratification of SMM patients was conducted in the studies above. The first RCT which examined early treatment in high-risk patients was conducted by Mateos et al.17,25 In this study, lenalidomide significantly extended time to progression and OS of high-risk SMM patients with tolerable safety. Moreover, early siltuximab treatment of high-risk SMM patients resulted in an insignificant improvement of 1-year PFS (siltuximab group 84.5%, placebo group 74.4%). The insignificance was at least in part due to the relatively small sample size and short follow-up.
Our study indicated that early treatment could significantly slow progression of all SMM patients, and this remained significant whether the studies enrolling high-risk SMM patients were included or excluded. According to subgroup analysis, the beneficial effect on progression was mainly attributable to MP and IMiDs. Neither survival nor response rate was improved by early treatment. However, the insignificance of mortality should be interpreted with caution, because treatments after progression were not entirely comparable, mainly due to different age of patients and different time to progression. Furthermore, MM treatment has been developing over the years. The heterogeneous treatment after progression could have influenced the effect on survival. Further studies which could better unify post-progression treatment are required to confirm this phenomenon. In terms of overall SMM patients, our study was consistent with two previous meta-analyses.14,26 Since studies commonly used MP treatment at that time, He et al could only include MP as their early treatment.14 Considering the huge progress in multiple treatment options nowadays, our study was able to include more comprehensive treatment options apart from MP, such as bisphosphonates, IMiDs, and monoclonal antibodies. Moreover, we included a larger sample size of 697 SMM patients in mortality analysis, and thus could more reliably investigate any difference in mortality. Gao et al included MP or IMiDs as their early treatment.26 As for early lenalidomide treatment, only a first analysis of the QuiRedex study was published and available at that time.25 Our study not only included more treatment approaches but also updated the study on lenalidomide by including a second analysis with a longer follow-up,17 leading to a more comprehensive and reliable analysis.
In terms of high-risk SMM patients, both progression and mortality were significantly reduced by early treatment, indicating that early treatment using lenalidomide or siltuximab might slow progression and extend survival. Although the results are inspiring, they need to be interpreted with caution, because only two trials comparing early treatment with deferred treatment of high-risk SMM patients have been published and included in this meta-analysis currently. Only the study of Mateos proved significant benefit of early treatment in high-risk patients, while the impact of early treatment on high-risk patients seemed insignificant in the study of Brighton et al, whose sample size and follow-up were relatively limited. The significance of the overall effect could arise from the enlarged overall sample size, the effect of study of Mateos et al, and the nature of early treatment in high-risk patients. The number of studies on high-risk patients is limited. More RCTs on high-risk patients are needed to draw a more reliable conclusion. Moreover, they have different definitions of high risk. Mateos et al defined high risk as either BMPC ≥10% or monoclonal evidence (IgG ≥3 g/dL or IgA ≥2 g/dL or Bence Jones proteinuria >1 g/24 hrs), plus aberrant plasma cells ≥95% in the BMPC compartment with immunoparesis.17 Brighton et al defined high risk as BMPC ≥10% and serum monoclonal protein ≥3 g/dL.16 The two trials started their recruitment in 2012 and 2007, respectively, which was before the reclassification of SLiM SMM into MM by the IMWG in 2014.27 Consequently, they might have included those ultra-high-risk SLiM patients. We carefully searched the two trials for possible SLiM MM patients. In the trial by Mateos et al, serum-free light chain and MRI were not planned during the trial design. In terms of BMPC, only one patient had BMPC ≥60%. Only one SLiM MM patient was included in the study of Mateos et al. Brighton et al conducted a post-hoc analysis which removed SLiM MM patients from high-risk SMM patients when investigating progression. However, no further details were provided on mortality. So we included only truly high-risk SMM patients in progression analysis, but had to include a minority of SLiM MM patients during mortality analysis. The conclusions, especially those on mortality, should be interpreted with caution. Although only a tiny fraction of included high-risk SMM patients were actually SLiM MM patients, we were still unable to figure out whether the new classification of SLiM MM could influence our results. A number of trials investigating early treatment of SMM with the new definition are ongoing, which will provide further information on early intervention of high-risk SMM patients (Table S1).
Table S1.
Ongoing clinical trials of early treatment in SMM patients
| NCT number | Design | Arm(s) | N | Participants | Current status | Expected date |
|---|---|---|---|---|---|---|
| NCT02916771 | Phase II single-arm | Ixazomib+LD | 28 | High-risk | Recruiting | 2020-04 |
| NCT02903381 | Phase II single-arm | Nivolumab+LD | 41 | High-risk | Suspended | 2020-06 |
| NCT02279394 | Phase II single-arm | Elotuzumab+LD | 51 | High-risk | Active | 2020-01 |
| NCT03301220 | Phase III RCT | Daratumumab SC vs monitoring | 360 | High-risk | Recruiting | 2021-12 |
| NCT02415413 | Phase II single-arm | Carfilzomib+LD | 90 | High-risk | Active | 2020-05 |
| NCT02943473 | Phase II single-arm | Ibrutinib | 36 | High-risk | Recruiting | 2023-12 |
| NCT01484275 | Phase II RCT | Siltuximab vs placebo | 87 | High-risk | Active | 2019-08 |
| NCT03236428 | Phase II single-arm | Daratumumab | 40 | Low-risk | Recruiting | 2020-08 |
| NCT02697383 | Phase I single-arm | Ixazomib+D | 14 | High-risk | Active | 2019-02 |
| NCT03289299 | Phase II single-arm | Carfilzomib+Daratumumab+LD | 83 | High-risk | Recruiting | 2022-06 |
| NCT02886065 | Phase I controlled | PVX-410+Citarinostat vs PVX-410+Citarinostat+L | 20 | High-risk | Recruiting | 2021-05 |
| NCT02603887 | Phase I single-arm | Pembrolizumab | 13 | Intermediate- and high-risk | Active | 2020-07 |
| NCT01169337 | Phase II/III RCT | L vs observation | 180 | High-risk | Active | 2020-03 |
| NCT03631043 | Phase I single-arm | Personalized vaccine | 30 | Intermediate- and high-risk | Not yet recruiting | 2022-09 |
| NCT02784483 | Phase I single-arm | Atezolizumab | 20 | High-risk | Suspended | 2018-12 |
| NCT03673826 | Phase II RCT | Carfilzomib+LD vs LD | 120 | High-risk | Not yet recruiting | 2025-10 |
| NCT02960555 | Phase II single-arm | Isatuximab | 61 | High-risk | Recruiting | 2022-02 |
| NCT01572480 | Phase II single-arm | Carfilzomib+LD | 53 | High-risk | Recruiting | 2022-06 |
| NCT03591614 | Phase I single-arm | DKK1 vaccine | 18 | NA | Not yet recruiting | 2019-08 |
| NCT02492750 | Phase I/II RCT | LD+anakinra vs LD+placebo | 120 | High-risk | Suspended | 2020-07 |
Abbreviations: SMM, smoldering multiple myeloma; L, lenalidomide; D, dexamethasone; RCT, randomized controlled trial.
With regard to safety, there was no significant difference in the incidence of infection or asthenia between the two arms. However, constipation and SPM were significantly increased with early treatment. Although many trials have reported the occurrence of SPM in treating SMM patients, our study is the first meta-analysis to demonstrate the significance of this phenomenon. The trend toward increasing SPM in the early treatment group should be interpreted with caution. Further studies with longer follow-up are required to confirm this phenomenon. The studies evaluable for SPMs in our analysis adopted MP or lenalidomide as early treatment, which was consistent with previous studies investigating SPMs in MM patients. The incidence of SPM increased when MM patients received lenalidomide maintenance following high-dose melphalan, but the risk of dying from MM exceeded the risk of dying from SPMs.28 In terms of SMM patients, risks and benefits of early treatment need to be balanced.
Since progression and mortality of high-risk SMM patients were significantly suppressed by early treatment, high-risk patients could benefit from early treatment using lenalidomide with appropriate management and alert of SPMs. High-risk patients might possibly benefit from siltuximab, but trials with larger sample size and longer follow-up are required to confirm its effect. Because early treatment did not translate into benefit to survival or response rate of overall SMM patients but brought about various side effects, low-risk and intermediate-risk SMM patients might continue to receive watchful observation, conforming to the current standard care of SMM. All SMM patients are encouraged to participate in clinical trials.
This is the first meta-analysis to investigate the effects and side effects of early treatment on high-risk SMM patients. There are also some limitations in our study. First, the number of included studies, especially studies of high-risk SMM patients, is small. Treatment of MM commonly relies on proteasome inhibitors and IMiDs, but trials which investigate proteasome inhibitors for the early treatment of SMM are still ongoing. The risks and benefits of early treatment in high-risk patients will be better elucidated with a larger sample size. Second, with the various risk models identified in SMM, the definition of high risk varies from study to study. Since the published studies exploring high-risk SMM were initiated before the proposal of SLiM MM in 2014, they could have included these ultra-high-risk SLiM patients in the group of high-risk SMM patients, which could possibly affect our results of high-risk SMM. Furthermore, since the safety profiles of different treatments vary from one to another, it is difficult to meta-analyze all reported adverse events. Only infection, asthenia, constipation, and SPMs are evaluable. Future studies exploring different interventions in high-risk SMM patients are needed. It is also necessary to unify risk models in order to better manage SMM patients in the future.
Conclusion
In conclusion, our study reveals that high-risk SMM patients could benefit from early treatment in reducing progression and mortality. Lenalidomide could serve as a promising treatment choice, but treatment-related constipation and SPMs should be managed with caution. In terms of low-risk and intermediate-risk SMM patients, progression could be reduced by early treatment, but there is inadequate evidence that mortality could be reduced. The number of included RCTs is limited, and future studies which unify risk models with larger sample size are needed to confirm the conclusion.
Acknowledgments
We would like to thank previous researchers for their devoted work on management of SMM patients.
Institutional research funding was provided by Foundation for Distinguished Young Physician of Peking Union Medical College Hospital (Grant No. JQ201501), the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (Grant No. 2016-12M-1-002), the National Key Research and Development Program of China (Grant No. 2016YFC0901503), the Fundamental Research Funds for the Central Universities, the Young Scientific Research Fund of Peking Union Medical College (PUMC) (Grant No. 2017320004). The funding body had no role in study design, data collection, data analysis, or manuscript writing.
Abbreviation list
SMM, smoldering multiple myeloma; MM, multiple myeloma; RCT, randomized controlled trial; RR, risk ratio; CI, confidence interval; BMPC, bone marrow plasma cell; IMWG, International Myeloma Working Group; MRI, magnetic resonance imaging; PFS, progression-free survival; OS, overall survival; MP, melphalan-prednisone; PRISMA, Preferred Reporting Items for Systemic Reviews and Meta-analysis; IMiD, immuno-modulatory drug; SPM, second primary malignancy; SLiM, bone marrow plasma cell ≥ Sixty percent, involved: uninvolved free Light chain ratio≥100, >1 focal lesion on Magnetic resonance imaging.
Data availability
The datasets analyzed during the current study will be available from the corresponding author on reasonable request.
Supplementary materials
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Muchtar E, Kumar SK, Magen H, Gertz MA. Diagnosis and management of smoldering multiple myeloma: the razor’s edge between clonality and cancer. Leuk Lymphoma. 2018;59(2):288–299. [DOI] [PubMed] [Google Scholar]
- 2.Ravindran A, Bartley AC, Holton SJ, et al. Prevalence, incidence and survival of smoldering multiple myeloma in the United States. Blood Cancer J. 2016;6(10):e486. doi: 10.1038/bcj.2016.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dispenzieri A, Kyle RA, Katzmann JA, et al. Immunoglobulin free light chain ratio is an independent risk factor for progression of smoldering (asymptomatic) multiple myeloma. Blood. 2008;111(2):785–789. doi: 10.1182/blood-2007-08-108357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorrig R, Klausen TW, Salomo M, et al. Smoldering multiple myeloma risk factors for progression: a Danish population-based cohort study. Eur J Haematol. 2016;97(3):303–309. doi: 10.1111/ejh.12728 [DOI] [PubMed] [Google Scholar]
- 5.Waxman AJ, Mick R, Garfall AL, et al. Classifying ultra-high risk smoldering myeloma. Leukemia. 2015;29(3):751–753. doi: 10.1038/leu.2014.313 [DOI] [PubMed] [Google Scholar]
- 6.Khan R, Dhodapkar M, Rosenthal A, et al. Four genes predict high risk of progression from smoldering to symptomatic multiple myeloma (SWOG S0120). Haematologica. 2015;100(9):1214–1221. doi: 10.3324/haematol.2015.124651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hajek R, Sandecka V, Seckinger A, et al. Prediction of progression of smouldering into therapy requiring multiple myeloma by easily accessible clinical factors [in 527 patients]. Blood. 2014;124(21). ISSN:00064971 [Google Scholar]
- 8.Neben K, Jauch A, Hielscher T, et al. Progression in smoldering myeloma is independently determined by the chromosomal abnormalities del(17p), t(4;14), gain 1q, hyperdiploidy, and tumor load. J Clin Oncol. 2013;31(34):4325–4332. doi: 10.1200/JCO.2012.48.4923 [DOI] [PubMed] [Google Scholar]
- 9.Perez-Persona E, Vidriales MB, Mateo G, et al. New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood. 2007;110(7):2586–2592. doi: 10.1182/blood-2007-05-088443 [DOI] [PubMed] [Google Scholar]
- 10.Kyle RA, Remstein ED, Therneau TM, et al. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med. 2007;356(25):2582–2590. doi: 10.1056/NEJMoa070389 [DOI] [PubMed] [Google Scholar]
- 11.Riccardi A, Mora O, Tinelli C, et al. Long-term survival of stage I multiple myeloma given chemotherapy just after diagnosis or at progression of the disease: a multicentre randomized study. Cooperative group of study and treatment of multiple myeloma. Br J Cancer. 2000;82(7):1254–1260. doi: 10.1054/bjoc.1999.1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riccardi A, Ucci G, Luoni R, et al. Treatment of multiple myeloma according to the extension of the disease: a prospective, randomised study comparing a less with a more aggressive cystostatic policy. Cooperative group of study and treatment of multiple myeloma. Br J Cancer. 1994;70(6):1203–1210. doi: 10.1038/bjc.1994.474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hjorth M, Hellquist L, Holmberg E, Magnusson B, Rodjer S, Westin J. Initial versus deferred melphalan-prednisone therapy for asymptomatic multiple myeloma stage I - A randomized study. Eur J Haematol. 1993;50(2):95–102. [DOI] [PubMed] [Google Scholar]
- 14.He Y, Wheatley K, Clark O, Glasmacher A, Ross H, Djulbegovic B. Early versus deferred treatment for early stage multiple myeloma. Cochrane Database Syst Rev. 2003;(1). doi: 10.1002/14651858.CD004023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–341. doi: 10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 16.Brighton T, Khot A, Harrison SJ, et al. Randomized, double-blind, placebo-controlled, multicenter study of siltuximab in high-risk smoldering multiple myeloma. Clin Cancer Res. Epub 2019 Mar 19. [DOI] [PubMed] [Google Scholar]
- 17.Mateos MV, Hernandez MT, Giraldo P, et al. Lenalidomide plus dexamethasone versus observation in patients with high-risk smouldering multiple myeloma (QuiRedex): long-term follow-up of a randomised, controlled, phase 3 trial. Lancet Oncol. 2016;17(8):1127‐1136. doi: 10.1016/S1470-2045(16)30124-3 [DOI] [PubMed] [Google Scholar]
- 18.Witzig TE, Laumann KM, Lacy MQ, et al. A phase III randomized trial of thalidomide plus zoledronic acid versus zoledronic acid alone in patients with asymptomatic multiple myeloma. Leukemia. 2013;27(1):220–225. doi: 10.1038/leu.2012.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Arena G, Gobbi PG, Broglia C, et al. Pamidronate versus observation in asymptomatic myeloma: final results with long-term follow-up of a randomized study. Leuk Lymphoma. 2011;52(5):771‐775. doi: 10.3109/10428194.2010.535182 [DOI] [PubMed] [Google Scholar]
- 20.Musto P, Petrucci MT, Bringhen S, et al. A multicenter, randomized clinical trial comparing zoledronic acid versus observation in patients with asymptomatic myeloma. Cancer. 2008;113(7):1588‐1595. doi: 10.1002/cncr.23873 [DOI] [PubMed] [Google Scholar]
- 21.Martín A, García-Sanz R, Hernández J, et al. Pamidronate induces bone formation in patients with smouldering or indolent myeloma, with no significant anti-tumour effect. Br J Haematol. 2002;118(1):239–242. doi: 10.1046/j.1365-2141.2002.03549.x [DOI] [PubMed] [Google Scholar]
- 22.Detweiler-Short K, Hayman S, Gertz MA, et al. Long-term results of single-agent thalidomide as initial therapy for asymptomatic (smoldering or indolent) myeloma. Am J Hematol. 2010;85(10):737–740. doi: 10.1002/ajh.21821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barlogie B, van Rhee F, Shaughnessy JD Jr., et al. Seven-year median time to progression with thalidomide for smoldering myeloma: partial response identifies subset requiring earlier salvage therapy for symptomatic disease. Blood. 2008;112(8):3122–3125. doi: 10.1182/blood-2008-06-164228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weber D, Rankin K, Gavino M, Delasalle K, Alexanian R. Thalidomide alone or with dexamethasone for previously untreated multiple myeloma. J Clin Oncol. 2003;21(1):16–19. doi: 10.1200/JCO.2003.03.139 [DOI] [PubMed] [Google Scholar]
- 25.Mateos MV, Hernández MT, Giraldo P, et al. Lenalidomide plus dexamethasone for high-risk smoldering multiple myeloma. N Engl J Med. 2013;369(5):438–447. doi: 10.1056/NEJMoa1300439 [DOI] [PubMed] [Google Scholar]
- 26.Gao M, Yang G, Tompkins VS, et al. Early versus deferred treatment for smoldering multiple myeloma: a meta-analysis of randomized, controlled trials. PLoS One. 2014;9(10):e109758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kyle RA, San-Miguel JF, Mateos MV, Rajkumar SV. Monoclonal gammopathy of undetermined significance and smoldering multiple myeloma. Hematol Oncol Clin North Am. 2014;28(5):775–790. doi: 10.1016/j.hoc.2014.06.005 [DOI] [PubMed] [Google Scholar]
- 28.Musto P, Anderson KC, Attal M, et al. Second primary malignancies in multiple myeloma: an overview and IMWG consensus. Ann Oncol. 2017;28(2):228–245. doi: 10.1093/annonc/mdw606 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study will be available from the corresponding author on reasonable request.