Abstract
Purpose:
Our primary aim was to prospectively validate retrospective dose-response models of chronic radiation-associated dysphagia (RAD) after intensity modulated radiotherapy (IMRT) for oropharyngeal cancer (OPC). The secondary aim was to validate grade a ≥2 cut-point of the published videofluoroscopic dysphagia severity (Dynamic Imaging Grade for Swallowing Toxicity, DIGEST) as radiation dose-dependent.
Material and Methods:
Ninety seven patients enrolled on an IRB-approved prospective registry protocol with stage I-IV OPC underwent pre-and 3–6 month post-RT videofluoroscopy. Dose volume histograms (DVH) for swallowing regions of interest (ROI) were calculated. Dysphagia severity was graded per DIGEST criteria (dichotomized grade ≥2 as moderate/severe RAD). Recursive partitioning analysis (RPA) and Bayesian Information Criteria (BIC) were used to identify dose-volume effects associated with moderate/severe RAD.
Results:
31% developed moderate/severe RAD (i.e. DIGEST grade ≥2) at 3–6 months after RT. RPA found DVH-derived dosimetric parameters of geniohyoid/mylohyoid (GHM), superior pharyngeal constrictor (SPC), and supraglottic region were associated with DIGEST grade ≥2 RAD. V61 ≥ 18.57% of GHM had optimal model performance for prediction of DIGEST grade ≥2.
Conclusion:
The findings from this prospective longitudinal study validate prior observations that dose to submental musculature predicts for increased burden of dysphagia after oropharyngeal IMRT. Findings also support dichotomization of DIGEST grade ≥2 as a dose-dependent split for use as an endpoint in trials or predictive dose-response analysis of videofluoroscopy results.
Keywords: Radiotherapy, Dose-Volume, Dysphagia, Intensity-modulated radiation therapy, Oropharyngeal cancer, DIGEST, Videofluoroscopy
Introduction
Intensity modulated radiotherapy therapy (IMRT) is widely used and associated with high cure rates for oropharyngeal cancer (OPC) [1–4]. Despite the dosimetric advantages of this technique, IMRT with concurrent chemotherapy is associated with toxicities sufficient for up to 60% of OPC patients to require a therapeutic feeding tube [5]. In the era of the human papillomavirus (HPV) epidemic, a rapidly growing number of young oropharyngeal cancer (OPC) survivors, many of whom are non-smokers, are expected to bear the potential consequences of long-term treatment-related toxicities. Thus, functional outcomes are now a key metric of true therapeutic success [6–8].
Radiation-associated dysphagia (RAD) has detrimental effects on quality of life (QOL) and can also lead to life-threatening complications such as aspiration pneumonia [9]. Chronic aspiration rates are estimated to impact 8% to 31% of OPC survivors [10, 11], and population-level analyses report an excess mortality risk of 42% among survivors who develop aspiration pneumonia relative to those who do not [12]. There is no treatment to truly reverse chronic RAD, such that predictive toxicity models with standardized outcome measurement tools are used to refine RT technique.
Measurement of RAD is complex and highly varied. Frequently used methods include patient-reported outcome (PRO) questionnaires [8, 13–16], feeding tube rates [17], rates of aspiration pneumonia or pharyngoesophageal stricture on modified barium swallow (MBS) [18], or rates of dilation on endoscopy [18, 19]. Instrumental assessments, such as videofluoroscopy (commonly referred to as the modified barium swallow [MBS] study) are the clinical standard, increasingly incorporated into dysphagia studies. MBS and instrumentals are recognized for their ability to offer sensitivity to subclinical dysfunction such as silent aspiration and specificity regarding the pathophysiology of dysphagia [9]. Nonetheless, interpretations of such procedures (MBS) are vary widely. The Dynamic Imaging Grade of Swallowing Toxicity (DIGEST) was internally developed and validated to grade the results of the MBS study in a manner compatible with the Common Terminology Criteria for Adverse Events (CTCAE) to facilitate interdisciplinary interpretation and easily dichotomized for predictive modeling [20].
Dysphagia is a volume and dose-dependent toxicity of RT. That is, the probability of dysphagia depends on radiation dose delivered to swallowing-related structures and is enhanced by concurrent chemotherapy. Previous research supports that sparing swallowing-related structures reduces the likelihood of RAD. The authors’ previous retrospective analysis to identify RAD-candidate structures among patients treated with oropharyngeal IMRT used a soft chart abstraction method for dysphagia. Among 300 patients treated from 2002 to 2011, results of multivariate models suggested that older OPC patients (age > 62 years) who are planned to receive high volumetric RT dose to the floor of mouth region (mylohyoid/geniohyoid muscles V69 > 79.5%) had higher odds of clinically-detected chronic RAD after IMRT [21]. However, prospective tracking of RAD using validated assessment tools with comprehensive characterization of other cofounders that affect the swallowing dysfunction after RT remains an unmet need identified by recent systematic reviews within this area [22, 23]. Thus, using a prospective registry, we aimed to:
-
1)
Validate prior dose-dysphagia models after IMRT for OPC in a more robust dataset (i.e., prospective longitudinal cohort) with a better outcome measure (DIGEST). We hypothesized to detect a significant submental/floor of mouth muscle dose/dysphagia relationship.
-
2)
Determine the performance of DIGEST as a dose-dependent outcome measure of MBS, and validate the dichotomization at grade ≥2 as a radiation dose-sensitive cutoff for RAD.
Materials and Methods
Patients
This is a secondary analysis of prospectively acquired data from the MD Anderson Oropharynx Cancer Registry Patient-Reported Outcomes and Functional (PROF) Core (PA14–0947). The sample comprised all patients with OPC enrolled on PA14–0947 and treated with IMRT between April, 2015 and November, 2016 at The University of Texas MD Anderson Cancer Center (MDACC) and were analyzed under approval of the Institutional Review Board. Eligibility criteria were: (1) pathologically confirmed diagnosis of previously untreated squamous cell OPC at registry enrollment, (2) IMRT as a definitive or postoperative treatment modality, (3) availability of the RT treatment plan in the MDACC archive and (4) cancer-free at time of RAD follow-up assessment time (i.e., 3–6 months post-RT). Of the 103 eligible patients, 6 were excluded because RT treatment plans could not be restored, leaving a total of 97 patients for analysis.
Radiation treatment
Treatment planning was conducted using Pinnacle 14 software in all patients (Philips Medical Systems, Andover, MA). Target volume delineation was previously described by Garden et al [24]. IMRT was delivered through Varian 6-MV photons linear accelerators (Varian Medical Systems, Palo Alto, CA). For “split-field” IMRT technique, a larynx block was used for larynx and esophageal inlet shielding, as previously detailed [25]. Conversely, the “whole-field” technique was used if concern existed about target volume coverage with the split-field approach. During the study period, however, availability of advanced “whole-field” techniques (i.e., whole neck-field volumetric-modulated arc therapy (WF-VMAT) and WF-integrated boost) for plan optimization in our institution resulted in less frequent utilization of “split-field” IMRT relative to earlier IMRT OPC series from this institution. Such advanced techniques allow for sparing the normal tissue without compromising the tumor coverage [26]. Postoperative RT dose of 60–66 Gy was delivered per standard National Comprehensive Cancer Network (NCCN) guidelines [27] for positive margins and extracapsular extension, in conjunction with systemic therapy, and as a single modality for close margins (< 3 mm), multiple positive nodes, vascular/lymphatic/perineural invasion, pT3/4 or positive level 4/5 nodes.
Swallowing assessment
Dysphagia severity was graded according to the published Dynamic Imaging Grade for Swallowing Toxicity (DIGEST) criteria [20]. DIGEST is a validated, videofluoroscopy-based grading tool for pharyngeal phase dysphagia. The summary DIGEST rating aligns with NCI’s Common Terminology Criteria for Adverse Events framework for toxicity reporting in oncology trials [28]. DIGEST assigns a global rating of pharyngeal swallow safety and efficiency according to the interaction of the safety and efficiency profile scores (grade 0 = no pharyngeal dysphagia, 1 = mild, 2 = moderate, 3 157= severe, 4 = life threatening). Per the MD Anderson Radiation Swallowing Pathway, MBS studies are scheduled routinely pre-, and 3–6 months, and 18–24 months after RT in all patients regardless of symptoms. The post-treatment interval in this analysis is the 3–6 month time point, which can be viewed as representing the subacute period. According to the Radiation Morbidity Scoring Criteria for radiation-related toxicity, acute and late toxicities are defined as any toxicities within 90 days and > 180 days post-RT, respectively. Thus, the term subacute is an adapted term to refer to the period between 90– 180 days after RT. MBS followed a standard bolus protocol (2 trials each: 5-mL, 10-mL, and cup sips of Varibar thin liquid, Varibar pudding, and ¼ barium-coated cracker) and were recorded at 30 frames per second [28]. Digital videos from MBS were then scored by a trained, blinded speech pathologist who met reliability standards to derive the DIGEST rating. DIGEST is reliable (intra-and inter-rater weighted k 0.82–0.84 and 0.67–0.81, respectively) and discriminates pharyngeal pathophysiology (r = 0.77, p<0.001), perceived dysphagia (r = −0.41, p<0.001), and oral intake (r = −0.49, p<0.001) in HNC survivors [20]. For the current analysis, we dichotomized groups with DIGEST cut-off grade ≥2 as moderate/severe RAD based on published data indicating this as a functionally-relevant split in that patients with DIGEST grade ≥2 dysphagia have lower QOL (per MDADI) and higher restriction of oral intake [28]. DIGEST grade ≥2 indicates at least intermittent episodes (more than once but less than half of liquid trials) of silent penetration to the true vocal folds or aspiration (penetration-aspiration scale score ≥5) and/or post-swallow residue of at least 50% of a dry, solid food bolus.
Dosimetric data
Planning computed tomography (CT) Digital Imaging and Communications in Medicine (DICOM) files and associated dosimetric data were retrieved via Pinnacle 14 software (Phillips Medical System, Andover, MA). CT DICOM files were exported to a commercially available deformable image registration and segmentation software program (Velocity AI 3.0.1, Velocity Medical Solutions, Atlanta, GA). Potential RAD-related organ at risks were auto-segmented (ADMIRE, Siemens Healthcare) with a validated atlas dataset [29], and subsequently reviewed by two trained radiation oncologists (MK and SV). Dose-volume histogram (DVH) for superior/ middle/inferior pharyngeal constrictors (S/M/IPC), intrinsic tongue muscle (ITM), geniohyoid muscle (GHM), genioglossus muscle (GGM), mylohyoid muscle (MHM), anterior belly of digastric muscle (ADM), glottic, and supraglottic areas were calculated. While pharyngeal constrictors are delineated as one structure in the international guidelines [30], we elected to delineate the individual constrictors as has been defined for prior constraints in the dysphagia-optimized IMRT era [21, 31–34]. For the floor of mouth delineation, we followed the previously published guidelines from Johns Hopkins University [35].
Statistical analysis
All statistical analysis was performed using commercial statistical analysis software programs (MATLABR2014b, Mathworks, Natick, MA, and JMP v12 Pro, SAS Institute, Cary, NC, USA). RT dose distributions were interrogated via bivariate plots of cumulative group binned DVH according to DIGEST (grade <2 versus ≥2), with subsequent Wilcoxon rank sum tests and p-values plotted via heat map analysis. Bonferroni correction was performed with p< 0.00005 (i.e. accounting for n = 75 bins and 13 ROIs) deemed statistically significant. To determine whether a dose-DIGEST effect might be observed and to identify the best region of interest (ROI) candidate predictor for RAD, logistic regression with the calculation of resultant receiver operator characteristic curves (ROCs) and area-under-the-curves (AUCs) was performed.
Recursive partitioning analysis (RPA) was then conducted to assess the relative contribution of multiple ROIs to DIGEST group and to drive exploratory non-model-dependent dose-volume constraints. RPA was also used to select a dose-threshold candidate from continuous dose distributions for DIGEST grade ≥2. First, using RAD severity as the discriminant variable, candidate ROI dose-volume parameters were selected from V1-V75 (volumes receiving 1–75 Gy) and Dmean for each patient. Next, we defined dose-volume thresholds for moderate/severe RAD within the “best” dose-volume ROI candidates using 10 K-fold cross validation. Training and validation sets (80:20) were created to randomly assign patients into either set as a “pre-processing” step. The dominate column contributors were then selected for each ROI, and iterative partitions, with a minimum grouping of 20 patients per split/partition were performed until a split demonstrated a Logworth value greater than the equivalent Bonferroni-corrected p-value. After this, a multivariate predictive model for DIGEST grade ≥2 was identified by testing clinical variables with the best dose-volume candidates via a stepwise regression model with Bayesian Information Criteria (BIC) minimization optimization for model selection and comparison to determine the effect size of each RPA-driven dosimetric threshold. Then, we constructed a multivariate nominal logistic regression model, that included the resultant significant clinical variables and RPA derived dosimetric thresholds. Baseline DIGEST was retained in final models regardless of statistical significance. In the nominal model, we calculated the false discovery rate (FDR)-adjusted p-and LogWorth values to control for a Type 1 error and account for multiple comparisons.
Results
Patient characteristics
Among the 97 patients eligible for analysis, 88 were male (91%) and median age was 61 (range: 35–86) years. The majority of patients had an AJCC 7th edition N2b disease (48%). Of the 95 tumors tested, 92 (97%) were positive for either p16 or HPV and 3 (3%) were negative. Twenty-four patients (25%) received induction chemotherapy, while concurrent chemotherapy was administered to 80 patients (82%). Of these, 23 patients (24%) also received induction. Median IMRT dose was 69.96 (range: 50–70) Gy delivered using standard fractionation (80%) and whole-field technique (59%) in the majority of patients. Dose range for definitive IMRT was 64.96 to 70 Gy, and for postoperative treatment was 50 to 66 Gy; patients treated under 60 Gy were per de-escalation trial. Patient, disease, and treatment characteristics are available in Table 1.
Table 1.
Patient and tumor characteristics.
| Moderate/Severe | Univariate | |||
|---|---|---|---|---|
| All Patients N=97 (%) |
No/Mild Dysphagia (DIGEST* <2) N=67 (%) |
Dysphagia (DIGEST ≥2) N=30 (%) |
Analysis P-value |
|
| Age, median | 61 | 60 | 62 | 0.296 |
| (range), (95% CI) | (35–86),(59-62) | (35–82),(58–62) | (40–86),(59–66) | |
| Sex | 0.142 | |||
| Male | 88(91) | 59(67) | 29(33) | |
| Female | 9(9) | 8(89) | 1(11) | |
| Subsite | 0.667 | |||
| Base of Tongue | 45(46) | 30(67) | 15(33) | |
| Tonsil | 38(39) | 27(71) | 11(29) | |
| Others | 14(15) | 10(71) | 4(29) | |
| T Stage*** | <0.0001** | |||
| 0 | 7(7) | 7(100) | 0 | |
| 1 | 31(32) | 27(87) | 4(13) | |
| 2 | 32(33) | 22(69) | 10(31) | |
| 3 | 13(13) | 8(61) | 5(39) | |
| 4 | 14(14) | 3(21) | 11(79) | |
| N Stage*** | 0.286 | |||
| x-1 | 19(20) | 15(79) | 4(21) | |
| 2–3 | 78(80) | 52(67) | 26(33) | |
| Treatment Modalities | 0.0006** | |||
| Radiotherapy alone | 16 (16) | 14(87) | 2(13) | |
| CCRT Only | 57(59) | 44(77) | 13(23) | |
| Combined IC+CCRT |
23(23) | 9(39) | 14(61) | |
| Radiotherapy | 0.542 | |||
| Definitive | 88(91) | 60(68) | 28(32) | |
| Post-op | 9(9) | 7(78) | 2(22) | |
| Smoking Status | 0.163 | |||
| Former Smoker | 40(41) | 31(77) | 9(23) | |
| Current Smoker | 6(6) | 5(83) | 1(17) | |
| Never Smoker | 51(53) | 31(60) | 20(40) | |
| HPV/P16 Status | 0.935 | |||
| Positive | 90(93) | 62(69) | 28(31) | |
| Negative | 3(3) | 2(67) | 1(33) | |
| Unknown | 4(4) | 3(75) | 1(25) | |
| Baseline Dysphagia Grade (per DIGEST) | 0.033** | |||
| 0 | 65(67) | 50(77) | 15(23) | |
| 1 | 25(26) | 14(56) | 11(44) | |
| 2 | 5(5) | 3(60) | 2(40) | |
| 3 | 2(2) | 0 | 2(100) | |
|
Total RT Dose
(Gy) |
70(70–50) | 68(67–69) | 69(68–70) | 0.041** |
DIGEST per 3–6 months post-RT modified barium swallowing study
Statistically significant P-value < 0.05
TNM classification per AJCC staging 7th edition
Abbreviations: DIGEST; Dynamic Imaging Grade of Swallowing Toxicity, CCRT; concurrent chemo/radiotherapy, IC; induction chemotherapy, RT; radiotherapy.
Dysphagia classification
Pre-RT, moderate-severe dysphagia (DIGEST grade ≥2) was extremely rare with distribution of baseline DIGEST: 65 patients (67%) grade 0, 25 (26%) grade 1 “mild”, and 5 (5%) grade 2 “moderate”. Only two patients had tumor-associated severe dysphagia (DIGEST grade ≥3) on baseline videofluoroscopy, both with T3–4 disease. All patients returned for follow-up videofluoroscopy 3–6 months after RT (median 7.2, range: (4.56–10.1) months post-RT) with DIGEST distribution: 29 patients (30%) grade 0, 38 (39%) grade 1 “mild”, 18 (19%) grade 2 “moderate”, and 12 (12%) grade 3 “severe”. Thus, a total of 68 patients (70%) demonstrated MBS-evident RAD (DIGEST grade ≥1) at follow-up with 30 patients (31%) having moderate/severe RAD (DIGEST grade ≥2). Relative to baseline MBS, 74 patients (76%) demonstrated progressive pharyngeal dysfunction 3–6 months after IMRT; 54 (56%), 15 (15%) and 5 (5%) patients increased by 1, 2 and 3 DIGEST grades in severity, respectively. Distributions of dysphagia grades pre-and post-RT are depicted in Figure 1.
Figure 1.
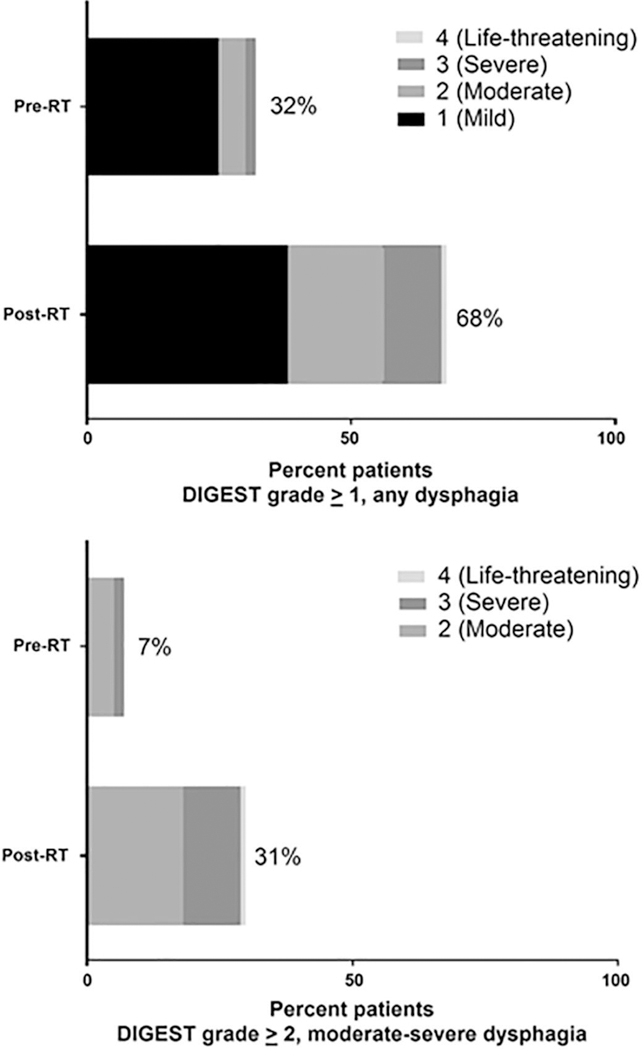
Distribution of videofluoroscopic dysphagia grade pre and post-RT (per DIGEST) (n=97).
Abbreviations: DIGEST; Dynamic Imaging Grade for Swallowing Toxicity, RT; Radiotherapy.
Clinical correlates of moderate/severe dysphagia (DIGEST grade ≥2)
Advanced T-classification (i.e., T3–4) (p = 0.0002), intensified treatment modalities (i.e., induction followed by concurrent chemoradiation, IC±CCRT) (p = 0.0006), higher total RT dose (p = 0.04), and baseline function (DIGEST ≥1) (P =0.019) were significantly associated with the DIGEST grade ≥2 post-IMRT, while age, gender, subsite, smoking status, N-category, HPV/P16 status, RT technique, and number of RT fractions failed to demonstrate an association with DIGEST group at 3–6-months follow-up. In the multivariate analysis, that include the clinical variables, intensified treatment modalities (IC+CCRT) and advanced T stage were statistically significant associated with moderate/severe RAD, (p=0.004 and 0.0089), respectively, Table 2.
Table 2.
Univariate and multivariate analysis of correlates of radiation-associated dysphagia.
| Characteristic | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P-value* | HR (95%CI) | P-value* | ||
| Age (years) | Continuous | 0.296 | |||
| Smoking Status | Smoker | 1 | |||
| Never | 2.3(0.96–5.9) | 0.061 | |||
| Gender | Male | 1 | |||
| Female | 0.25(0.01–1.5) | 0.142 | |||
| Subsite | Tonsil | 1 | |||
| Base of Tongue | 1.2(0.48–3.18) | 0.667 | |||
| HPV/P16 Status | Positive | 1 | |||
| Negative | 1.1(0.05–12) | 0.935 | |||
| T Stage | 0–2 | 1 | 1 | ||
| 3–4 | 3.8(2.25–15.7) | 0.0002** | 4.56(1.46–15.31) | 0.0089** | |
| Nodal Stage | Nx-1 | 1 | |||
| N2–3 | 1.88(0.61–7) | 0.286 | |||
| Treatment Modalities | |||||
| CCRT Only | 1 | 0.0006** | 1 | 0.004** | |
| IC+CCRT | 5.6(2.6–16.46) | 6.12(2.01–20.44) | |||
| Radiotherapy alone | 0.48(0.07–2.04) | 0.72(0.06–4.82) | |||
| Radiotherapy Technique | Split | 1 | |||
| Whole Field | 1.0(0.67–4) | 0.286 | |||
| Total Radiotherapy Dose | Continuous | 0.041** | 0.696 | ||
| Number of Radiotherapy Fractions | Continuous | 0.053 | |||
| Baseline RAD, Per | No (Score 0) | 1 | 0.019** | 1 | 0.265 |
| DIGEST | |||||
| Yes (Score 1-4) | 2.0(1.197-7.36) | 1.84(0.62-5.42) | |||
P-values were calculated by the Chi-square test.
Statistically significant p-value < 0.05.
Abbreviations: CCRT; concurrent chemo/radiotherapy, IC; induction chemotherapy, RAD; radiation-associated dysphagia, DIGEST; Dynamic Imaging Grade of Swallowing Toxicity.
Dosimetric correlates of moderate/severe dysphagia (DIGEST grade ≥2)
For all tested ROIs, except the cricopharyngeal muscle, glottic area, and esophagus, mean dose to RAD-ROIs was uniformly higher for patients with DIGEST grade ≥2 at 3–6 months post-IMRT (Figure 2), and all remained statistically significant after Bonferroni correction for multiple comparisons (Supplementary Table 1). Composite DVHs graphically demonstrate that patients with DIGEST grade ≥2 at 3–6 months post-IMRT had higher dose delivery with some variability of magnitude across ROIs, especially in the intermediate dose region of the DVHs (Figure 2). After Bonferroni correction, significant pairwise dose-volume differences were observed for submental and lingual muscles (ADM, GGM, MHM, GHM, and ITM) and the pharyngeal constrictors (SPC, MPC, IPC) as well as the supraglottic ROI (denoted in red in the heat map for each ROI in Figure 3). Likewise, logistic regression models with the calculation of AUC of ROCs show the probability of DIGEST grade ≥2 increases in the Dmean for each ROI. Specifically, the GHM displayed the highest AUC, among all tested ROIs; GHMs the best ROI candidate predictor for RAD, Table 3.
Figure 2.
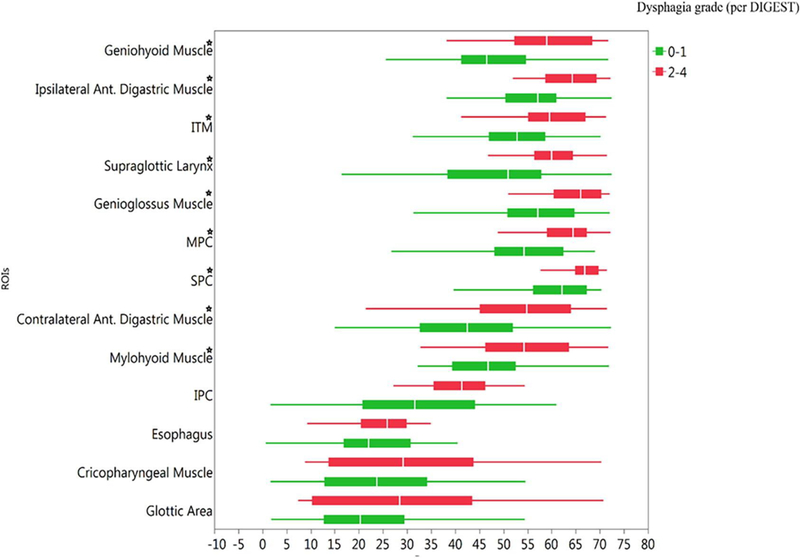
Mean dose for all ROIs by dysphagia status (per DIGEST grade) 3–6 months post-RT (n=97).
★Statistically Significant after Bonferroni correction.
Abbreviations: ROIs; region of interests, (S/M/IPC); Superior/ middle/inferior pharyngeal constrictors, ITM; intrinsic tongue muscle, Ant; anterior.
Figure 3.
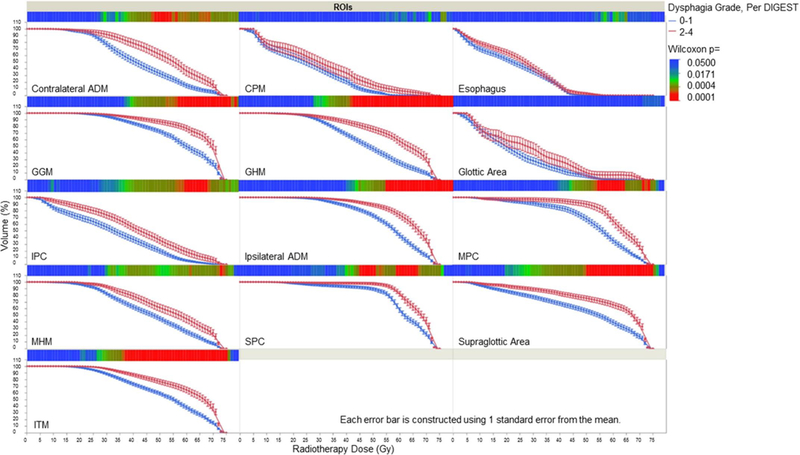
Dose volume histograms stratified by dysphagia classification (per DIGEST) 3–6 months after RT (n=97).
Abbreviations: ROIs; region of interests, ADM; anterior digastrics muscle, CPM; cricopharyngeal muscle, GGM genioglossus muscle, GHM geniohyoid muscle, (S/M/IPC); Superior/ middle/inferior pharyngeal constrictors, MHM; mylohyoid, ITM; intrinsic tongue muscle. Heatmap of statistical significance for utilized analyses, is displayed below each ROI DVH to quantify the magnitude of p-values for each 1-Gy bin, allowing visual representation of Bonferroni correction. Solid blue squares denote lack of statistical significance, while solid red squares indicate statistical significance under strict multiple-comparison correction.
Table 3.
Ordinal logistic analysis of Dmean for all ROIs contribute to RAD, (per DIGEST score) at 3–6 months.
| ROIs | BIC | P-value | AUC |
|---|---|---|---|
| GHM | 107 | 0.0000* | 0.774 |
| ITM | 105 | 0.0000* | 0.771 |
| Ipsilateral ADM | 108 | 0.0000* | 0.772 |
| Supraglottic larynx | 110 | 0.0009* | 0.757 |
| GGM | 113 | 0.0001* | 0.756 |
| SPC | 115 | 0.0002* | 0.746 |
| MHM | 115 | 0.0005* | 0.711 |
| MPC | 115 | 0.0002* | 0.745 |
| Contralateral ADM | 116 | 0.0003* | 0.730 |
| IPC | 118 | 0.0012* | 0.693 |
| Glottic area | 119 | 0.0739 | 0.585 |
| CPM | 124 | 0.0657 | 0.592 |
| Esophagus | 125 | 0.0777 | 0.602 |
Significant p-value < 0.05.
Abbreviations: Dmean; mean radiotherapy dose, ROIs; regions of interest, RAD; radiation-associated dysphagia, DIGEST; Dynamic Imaging Grade of Swallowing Toxicity, BIC; Bayesian Information Criteria, AUC; area under the curve, GHM; geniohyoid muscle, ITM; intrinsic tongue muscle, ADM; anterior digastrics muscle, GGM; genioglossus muscle, MHM; mylohyoid muscle, (S/M/IPC); superior/ middle/inferior pharyngeal constrictors, CPM; cricopharyngeal muscle.
Dosimetric thresholds of moderate/severe dysphagia (DIGEST grade ≥2)
Univariate RPA analysis led to three RPA-derived candidate OAR dose-volume thresholds for multivariate assessment of PRA-driven dosimetric thresholds, using DIGEST grade ≥2 as the discriminant criteria: GHM V61 ≥ 18.57% (LogWorth 7.3, p<0.0001), SPC V55 ≥ 97.46% (LogWorth 3.8, p = 0.0001) and supraglottic V23 ≥92.54% (LogWorth 2.8, p = 0.001); with AUC 0.9 and 0.7 across training and validation sets, respectively. The resultant statistically significant binary cut points were interrogated by confirmatory regression model to establish effect size, determine relative risk ratios. GHM, supraglottic area and SPC dose-volume parameters (specifically GHM V61and SPC V55) showed substantively lower (superior) BIC values than the other ROIs (“Very Strong” evidence grade, consistent with a >99% posterior probability of improved model performance); GHM V61 was only slightly more informative when compared to SPC V55. Finally, a multivariate nominal regression model including significant clinical variables (T-category and treatment modalities), baseline DIGEST score, and the RPA-derived dose volume thresholds model indicated GHM V61 and SPC V55 as most predictive dosimetric covariates (AUC=0.909). Multivariate results are shown in Table 4.
Table 4.
Multivariate model regressing DIGEST grade ≥2 dysphagia after RT on clinical factors and RPA-derived dose thresholds.
| Clinical and Dosimetric | Nominal Multivariate Regression Analysis |
|||
|---|---|---|---|---|
| Characteristic | OR (95%CI) | LogWorth | FDR p-value | |
| V61 of GHM ≥ 18.57% | No | 1 | 3.932 | 0.00012* |
| Yes | 32.8 (5.69–335) | |||
| V55 of SPC ≥ 97.46% | No | 1 | 2.773 | 0.0017* |
| Yes | 10.9 (2.7–57.5) | |||
| Treatment Modalities | CCRT Only | 1 | 1.723 | 0.0189* |
| IC+CCRT | 10.64 (2.27–65.72) | |||
| RT alone | 0.79 (0.005–19.02) | |||
| T Stage | 0–2 | 1 | 1.279 | 0.0526 |
| 3–4 | 5.29 (1.16–28.58) | |||
| RT Dose | 1 (1.0002–1.009) | 1.279 | 0.0526 | |
| Baseline DIGEST | 0–1 | 1 | 0.517 | 0.3037 |
| 2–3 | 1 (0.36–42.9) | |||
BIC=101.6, AICc=81.09, AUC=0.909
Discussion
This analysis of prospective registry data validates dose-response models supporting the relevance of dose to submental musculature and dysphagia after oropharyngeal IMRT [21, 34, 36]. In a modern prospective registry study of OPC patients treated with IMRT using DIGEST as a rigorous outcome measure from videofluoroscopy that considers swallowing efficiency in addition to penetration/aspiration, this report identified individual non-target and potentially sparable normal structures in the submental region most strongly contribute to RAD. The authors propose refined dose constraints derived for these structures in the present analysis. DIGEST grade ≥2 also significantly associated with higher dose to various swallowing muscle ROIs. Thus, the second critical methodologic contribution of this work is to validate the dichotomization of DIGEST at a cut-point of grade ≥2 (i.e., moderate-severe MBS-detected dysphagia) as a radiation dose sensitive (thus, likely clinically meaningful) threshold for RT-related laryngopharyngeal toxicity.
Using the DIGEST grading method, the rate of moderate/severe dysphagia (DIGEST grade ≥2) at 3–6 months post-RT was 31%. The results of our dose-response analysis support the validity of classifying moderate-severe cases of RAD using this cut-point as a marker of radiation injury to swallowing muscles. This builds upon the authors’ published work suggesting the functional relevance of this binary split wherein DIGEST grade ≥2 was most discriminate of poor QOL (per MDADI) and diet level (per PSS-HN) in an independent sample of mixed HNC patients [28]. Thus, collectively, these data suggest that contrary to popular dichotomization of toxicity endpoints using grade ≥3 as the meaningful split, DIGEST grade ≥2, similar to xerostomia, is currently our suggested binary split for endpoint reporting and predictive modeling.
It is not surprising that the observed rate of DIGEST grade ≥2 pharyngeal dysphagia is higher than the 7% MBS-detected aspiration rate previously reported after IMRT for OPC in several clinical trials that prospectively acquired MBS (regardless of dysphagia symptoms) [21]. The difference in rates is explained because the DIGEST grade accounts for both safety and efficiency of pharyngeal bolus clearance on MBS. That is, DIGEST is derived by rating both penetration/aspiration events as a measure of swallowing safety as well as pharyngeal residue as a measure of efficiency of bolus clearance. For this reason, greater numbers of dysphagia cases are detected using DIGEST rather than focusing only on aspiration. Likewise, when compared to retrospective reports from this institution [21], we detected a higher rate of moderate/severe dysphagia in this prospective registry wherein all patients were routinely evaluated with videofluoroscopy post-RT regardless of dysphagia symptom status. In the authors’ prior large retrospective series of OPC survivors treated with IMRT, using a widely published chart abstraction method, the observed rate of chronic dysphagia was only 13% based on chart abstraction of fluoroscopic or endoscopic documentation of aspiration, stricture, or pneumonia and/or gastrostomy dependence ≥12-months. This observation highlights the need for prospective assessment of toxicity regardless of symptom status, particularly when the goal is to develop predictive models of dysphagia.
In our current analysis, we found strong associations between DIGEST-derived moderate/severe RAD and dose to the superior pharyngeal constrictor (especially, SPC V55, i.e. the volume of SPC receiving ≥55Gy). It has been published that mean doses exceeding 50 Gy to the pharyngeal axis are associated with greater swallowing toxicity in OPC [37, 38]. Prospective longitudinal studies have repeatedly confirmed this correlation. For instance, Eisbruch et al. correlated videofluoroscopy-based aspiration, worsened videofluoroscopy summary scores (institution-specific, non-validated metric) to the total dose to the pharyngeal constrictor (TD25: 56Gy, TD50: 63 Gy) [39]. Likewise, this same group subsequently reported significant associations between videofluoroscopy-based aspiration and mean dose to the pharyngeal constrictors (as well as V60 > 50%) after swallowing-optimized oropharyngeal IMRT [40]. Predictive models incorporating clinical factors maintain associations between RAD and dose to the pharyngeal constrictor [41]. From the authors’ institution, secondary analyses of a small adaptive IMRT trial found similarly that videofluoroscopy derived swallowing efficiency scores (per OPSE) significantly correlated with volumetric SPC dose (V55 > 80% and V65 > 30%) in both univariate and multivariate analyses [34].
Beyond pharyngeal constrictor dose, this analysis also supports the hypothesis that submental muscle dose significantly predicted post-IMRT dysphagia. On multivariate analyses, the effect size was largest for volumetric dose to GHM relative to all other swallow muscle ROIs, supporting their independent contributions to RAD. This result serves to validate in an independent sample two recent publications supporting submental muscle dose and dysphagia relationships after OPC IMRT, and might suggest consideration for inclusion in updates to international guidelines [30]. Specifically, the authors’ prior retrospective series reported a multivariate model including age and geniohyoid GHM V69 was the best predictor (AUC 0.835) of chronic dysphagia compared to other models [21]. Likewise, Kumar et al. reported mean dose to the floor of mouth region and minimum dose to the geniohyoid associated with increased odds of abnormal penetration-aspiration scale scores from post-IMRT videofluoroscopy [35]. However, the difference in the dosimetric parameters of the candidate predictors could be contributed to the difference in the ROIs segmentation [42] (unit volume, i.e., whole floor of mouth region versus individual structure) and the timing of RAD assessment. Nevertheless, the relationship between the delivered RT dose to the submental muscle and the severity of RAD is supported by models of swallowing physiology. According to cadaveric studies by Pearson et al. [43], the geniohyoid is the most significant contributor to anterior displacement of the hyoid, which is directly related to supraglottic airway closure and esophageal opening for swallowing. Despite this independent function, the geniohyoid region dose is highly correlated with dose to other floor of mouth structures, suggesting the need to clarify its specific vulnerability to RAD in larger, prospective studies [43]. Hence, we designed the current study to address the individual contribution of adjacent floor of mouth muscle regions, an added methodologic contribution of this work. The investigators’ effort in this area started with Schwartz et al. [34] to identify the candidate dosimetric predictors by considering the oral cavity as ROI, then moved to a more granular approach with Dale et al. [21] in which the ROI was the MGHM unit. Herein, we now add individual ROI segmentation of floor of mouth muscle subunits to identify each individual ROI contribution in the current study.
Regarding the clinical factors related to post-IMRT DIGEST groups, T-category (p = 0.0002), treatment modalities (p = 0.0006), and total RT dose (p = 0.04) and presence of RAD at baseline (0.019) were significantly associated with differentials in RAD sverity per DIGEST grade in the univariate analysis. Although T-category demonstrated a predictive potential for moderate/severe RAD, this association did not maintain in multivariate models once baseline function and doses to swallowing muscles were included. Thus supporting the notion that the typical relationship between T-classification and dysphagia likely reflects a clinical surrogate for the confounding effects of baseline dysfunction and larger target volumes and higher doses for patients with large primary tumors, resulting in the inflated incidence of swallowing dysfunction in this subgroup of patients. Lastly, despite evidence suggesting age relates to the functional recovery of OPC patients, these observations were not reflected in our analysis. This could be attributed to a young cohort with less heterogeneity in age than earlier series (median age 370 61, IQR: 35–86 years).
Intensified treatment regimens of IC followed by CCRT maintained significant correlation with moderate/severe RAD in multivariate models with large effect size. The use of induction chemotherapy in locally advanced head and neck cancer remains controversial [44] with most reports failing to demonstrate an overall survival benefit of induction chemotherapy in patients with stage III or IV disease [45, 46]. Moreover, elevated risk of acute and chronic RAD is reported after combined modalities of RT and chemotherapy, either in sequential or concurrent schedules [10, 12, 47]. In the era of HPV-driven OPC, which is a RT-sensitive tumor and tend to recur distantly, treatment intensification could be manipulated [7, 48–50] [51–55] to protect the survivors from morbidities after RT [6, 56–58] and response to induction chemotherapy is one of the strategies being used in clinical studies to de-escalate radiation dose and field with early results showing improvement in swallowing function [59, 60]. However, the de-intensified therapy approaches are currently only adopted within clinical trials. This study could be used as additional evidence toward less intensified local therapy for HPV positive OPC patients from the toxicity risk point of view. In our institution, induction treatment is only considered in patients with advanced disease stages at high risk of distant failure (multiple nodes, large-volume nodal disease, low nodes) [61] after a multidisciplinary discussion [24, 62]. In the study cohort, 24 patients underwent induction followed by CCRT and they presented with advanced disease stages (i.e., T3/4±N2/3).
Herein, we validate recent retrospective results supporting floor mouth muscle dose and dysphagia relationships after oropharyngeal IMRT using a novel metric in a prospective registry dataset of almost 100 patients. It is critical to interpret the data in context of an HPV-driven population examined largely in the subacute period after radiotherapy; it is possible that results may differ for late effect dysphagia and among those with HPV-negative disease. Future work should explore these points.
Supplementary Material
Highlights.
Dosimetric parameters of submental musculature predict for the burden of dysphagia after IMRT in oropharyngeal cancer patients.
DIGEST is a dose-dependent outcome measure for swallowing toxicity profiles and predictive modeling.
DIGEST grade ≥2 is a dose-dependent split for use as an endpoint in trials or predictive dose-toxicity analysis of videofluoroscopy results.
Acknowledgements
The authors wish to acknowledge the contributions of Rohit S Kuruvilla, Robin Claire Granberry, Benjamin Greiner, Jared Harp, Vivek N Mehta, and Clare Alvarez for their contributions to data procurement. The authors also wish to acknowledge the administrative support of Janet Hampton.
References
- [1].Garden AS, Dong L, Morrison WH, Stugis EM, Glisson BS, Frank SJ, et al. Patterns of disease recurrence following treatment of oropharyngeal cancer with intensity modulated radiation therapy.Int J Radiat Oncol Biol Phys 2013;85:941–7. [DOI] [PubMed] [Google Scholar]
- [2].Eisbruch A, Harris J, Garden AS, Chao CKS, Straube W, Harari PM, et al. Multi-institutional trial of accelerated hypofractionated intensity-modulated radiation therapy for early stage oropharyngeal Cancer (RTOG 00–22). Int J Radiat Oncol Biol Phys 2010;76:1333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Daly ME, Le Q-T, Maxim PG, Loo BW, Kaplan MJ, Fischbein NJ, et al. Intensity-modulated Radiotherapy in the treatment of oropharyngeal cancer: clinical outcomes and patterns of failure. Int J Radiat Oncol Biol Phys 2010;76:1339–46. [DOI] [PubMed] [Google Scholar]
- [4].Parsons JT, Mendenhall WM, Stringer SP, Amdur RJ, Hinerman RW, Villaret DB, et al. Squamous cell carcinoma of the oropharynx. Cancer 2002;94:2967–80. [DOI] [PubMed] [Google Scholar]
- [5].Bhayani MK, Hutcheson KA, Barringer DA, Lisec A, Alvarez CP, Roberts DB, et al. Gastrostomy tube placement in patients with oropharyngeal carcinoma treated with radiotherapy or chemoradiotherapy: factors affecting placement and dependence. Head Neck 2013;35:1634–40. [DOI] [PubMed] [Google Scholar]
- [6].Eisbruch A, Schwartz M, Rasch C, Vineberg K, Damen E, Van As CJ, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys 2004;60:1425–39. [DOI] [PubMed] [Google Scholar]
- [7].Licitra L, Perrone F, Bossi P, Suardi S, Mariani L, Artusi R, et al. High-Risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol 2006;24:5630–6. [DOI] [PubMed] [Google Scholar]
- [8].Goepfert RP, Lewin JS, Barrow MP, Gunn GB, Fuller CD, Beadle BM, et al. Long-term, Prospective performance of the MD Anderson dysphagia inventory in “low-intermediate risk” oropharyngeal carcinoma after intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys 2017;97:700–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Eisbruch A, Lyden T, Bradford CR, Dawson LA, Haxer MJ, Miller AE, et al. Objective assessment of swallowing dysfunction and aspiration after radiation concurrent with chemotherapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys 2002;53:23–8. [DOI] [PubMed] [Google Scholar]
- [10].Hutcheson KA, Lewin JS, Holsinger FC, Steinhaus G, Lisec A, Barringer DA, et al. Long-term functional and survival outcomes after induction chemotherapy and risk-based definitive therapy for locally advanced squamous cell carcinoma of the head and neck. Head Neck 2014;36:474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Feng FY, Kim HM, Lyden TH, Haxer MJ, Worden FP, Feng M, et al. Intensity-modulated chemoradiotherapy aiming to reduce dysphagia in patients with oropharyngeal cancer: clinical and functional results. J Clin Oncol 2010;28:2732–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Xu B, Boero IJ, Hwang L, Le QT, Moiseenko V, Sanghvi PR, et al. Aspiration pneumonia after concurrent chemoradiotherapy for head and neck cancer. Cancer 2015;121:1303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Peponi E, Glanzmann C, Willi B, Huber G, Studer G. Dysphagia in head and neck cancer patients following intensity modulated radiotherapy (IMRT). Radiat Oncol 2011;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].van der Molen L, Heemsbergen WD, de Jong R, van Rossum MA, Smeele LE, Rasch CRN, et al. Dysphagia and trismus after concomitant chemo-intensity-modulated radiation therapy (chemo-IMRT) in advanced head and neck cancer; dose-effect relationships for swallowing and mastication structures. Radiother Oncol.106:364–9. [DOI] [PubMed] [Google Scholar]
- [15].Goepfert RP, Lewin JS, Barrow MP, Fuller CD, Lai SY, Song J, et al. Predicting two-year longitudinal MD Anderson Dysphagia Inventory outcomes after intensity modulated radiotherapy for locoregionally advanced oropharyngeal carcinoma. Laryngoscope 2017;127:842–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hedström J, Tuomi L, Finizia C, Olsson C. Correlations between patient-reported dysphagia screening and penetration–aspiration scores in head and neck cancer patients post-oncological treatment. Dysphagia. 2017:33;206–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Caudell JJ, Schaner PE, Desmond RA, Meredith RF, Spencer SA, Bonner JA. Dosimetric factors associated with long-term dysphagia after definitive radiotherapy for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 2010;76:403–9. [DOI] [PubMed] [Google Scholar]
- [18].Caudell JJ, Schaner PE, Meredith RF, Locher JL, Nabell LM, Carroll WR, et al. Factors associated with long-term dysphagia after definitive radiotherapy for locally advanced head-and-neck cancer.Int J Radiat Oncol Biol Phys 2009;73:410–5. [DOI] [PubMed] [Google Scholar]
- [19].Jensen K, Lambertsen K, Grau C. Late swallowing dysfunction and dysphagia after radiotherapy for pharynx cancer: Frequency, intensity and correlation with dose and volume parameters. Radiother Oncol. 2007;85:74–82. [DOI] [PubMed] [Google Scholar]
- [20].Goepfert RP, Lewin JS, Barrow MP, Warneke CL, Fuller CD, Lai SY, et al. Grading dysphagia as a toxicity of head and neck cancer: differences in severity classification based on MBS DIGEST and clinical CTCAE grades. Dysphagia 2018;33:185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].MD Anderson Head and Neck Cancer Symptom Working Group. Beyond mean pharyngeal constrictor dose for beam path toxicity in non-target swallowing muscles: dose-volume correlates of chronic radiation-associated dysphagia (RAD) after oropharyngeal intensity modulated radiotherapy. Radiother Oncol 2016;118:304–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Duprez F, Madani I, De Potter B, Boterberg T, De Neve W. Systematic review of dose–volume correlates for structures related to late swallowing disturbances after radiotherapy for head and neck cancer. Dysphagia 2013;28:337–49. [DOI] [PubMed] [Google Scholar]
- [23].Paleri V, Roe JW, Strojan P, Corry J, Grégoire V, Hamoir M, et a. Strategies to reduce long-term postchemoradiation dysphagia in patients with head and neck cancer: an evidence-based review. Head Neck 2014;36:431–43. [DOI] [PubMed] [Google Scholar]
- [24].Garden AS, Dong LO, Morrison WH, Sturgis EM, Glisson BS, Frank SJ, et al. Outcomes and patterns of care of patients with locally advanced oropharyngeal carcinoma treated in the early 21st century. Radiat Oncol 2013;8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dabaja B, Salehpour MR, Rosen I, Tung S, Morrison WH, Ang KK, et al. Intensity-modulated radiation therapy (IMRT) of cancers of the head and neck: comparison of split-field and whole-field techniques. Int J Radiat Oncol Biol Phys 2005;63:1000–5. [DOI] [PubMed] [Google Scholar]
- [26].Anamalayil SJ, Teo B-KK, Lin A, Lustig RA, Ahn PH. Effects of full-neck volumetric-modulated arc therapy vs split-field intensity-modulated head and neck radiation therapy on low neck targets and structures. Br J Radiol 2016;89:20160009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Park J, Yu H, Park JH, Park Y. LCD panel characterization by measuring full Jones matrix of individual pixels using polarization-sensitive digital holographic microscopy. Opt Express 2014; 22:24304–11. [DOI] [PubMed] [Google Scholar]
- [28].Hutcheson KA, Barrow MP, Barringer DA, Knott JK, Lin HY, Weber RS, et al. Dynamic imaging Grade of Swallowing Toxicity (DIGEST): scale development and validation. Cancer 2017;123:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mohamed AS, Ruangskul MN, Awan MJ, Baron CA, Kalpathy-Cramer J, Castillo R, et al. Quality assurance assessment of diagnostic and radiation therapy-simulation CT image registration for head and neck radiation therapy: anatomic region of interest-based comparison of rigid and deformable algorithms. Radiology. 2015;274:752–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Brouwer CL, Steenbakkers RJ, Bourhis J, Budach W, Grau C, Grégoire V, et al. CT-based delineation of organs at risk in the head and neck region: DAHANCA, EORTC, GORTEC, HKNPCSG, NCIC CTG, NCRI, NRG Oncology and TROG consensus guidelines. Radiother Oncol 2015;117:83–90. [DOI] [PubMed] [Google Scholar]
- [31].Petkar I, Rooney K, Roe JW, Patterson JM, Bernstein D, Tyler JM, et al. DARS: a phase III randomised multicentre study of dysphagia-optimised intensity-modulated radiotherapy (Do-IMRT) versus standard intensity-modulated radiotherapy (S-IMRT) in head and neck cancer. BMC Cancer 2016;16:770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Christianen ME, Schilstra C, Beetz I, Muijs CT, Chouvalova O, Burlage FR, et al. Predictive modelling for swallowing dysfunction after primary (chemo)radiation: results of a prospective observational study. Radiother Oncol 2012;105:107–14. [DOI] [PubMed] [Google Scholar]
- [33].Teguh DN, Levendag PC, Sewnaik A, Hakkesteegt MM, Noever I, Voet P, et al. Results of fiberoptic endoscopic evaluation of swallowing vs. radiation dose in the swallowing muscles after radiotherapy of cancer in the oropharynx. Radiother Oncol 2008;89:57–63. [DOI] [PubMed] [Google Scholar]
- [34].Schwartz DL, Hutcheson K, Barringer D, Tucker SL, Kies M, Holsinger FC, et al. Candidate dosimetric predictors of long-term swallowing dysfunction following oropharyngeal IMRT. Int J Radiat Oncol Biol Phys 2010;78:1356–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kumar R, Madanikia S, Starmer H, Yang W, Murano E, Alcorn S, et al. Radiation dose to the floor of mouth muscles predicts swallowing complications following chemoradiation in oropharyngeal squamous cell carcinoma. Oral Oncol 2014;50:65–70. [DOI] [PubMed] [Google Scholar]
- [36].Awan MJ, Mohamed ASR, Lewin JS, Baron CA, Gunn GB, Rosenthal DI, et al. Late radiation-associated dysphagia (Late-RAD) with lower cranial neuropathy after oropharyngeal radiotherapy: a preliminary dosimetric comparison. Oral Oncol 2014;50:746–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wong ATT, Lai SY, Gunn GB, Beadle BM, Fuller CD, Barrow MP, et al. Symptom burden and dysphagia associated with osteoradionecrosis in long-term oropharynx cancer survivors: a cohort analysis. Oral Oncol 2017;66:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Tsai CJ, Jackson A, Setton J, Riaz N, McBride S, Leeman J, et al. Modeling dose response for late dysphagia in patients with head and neck cancer in the modern era of definitive chemoradiation. JCO Clinical Cancer Informatics. 2017;1:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Eisbruch A, Kim HM, Feng FY, Lyden TH, Haxer MJ, Feng M, et al. Chemo-IMRT of oropharyngeal cancer aiming to reduce dysphagia: swallowing organs late complication probabilities and dosimetric correlates. Int J Radiat Oncol Biol Phys 2011;81:e93–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Feng FY, Kim HM, Lyden TH, Haxer MJ, Feng M, Worden FP, et al. Intensity-modulated radiotherapy of head and neck cancer aiming to reduce dysphagia: early dose–effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys 2007;68:1289–98. [DOI] [PubMed] [Google Scholar]
- [41].Kim DR, Duprez F, Werbrouck J, Sabbe N, Sofie DL, Boterberg T, et al. A predictive model for dysphagia following IMRT for head and neck cancer: Introduction of the EMLasso technique. Radiother Oncol 2013;107:295–9. [DOI] [PubMed] [Google Scholar]
- [42].Brouwer CL, Steenbakkers RJHM, Gort E, Kamphuis ME, van der Laan HP, van’t Veld AA, et al. Differences in delineation guidelines for head and neck cancer result in inconsistent reported dose and corresponding NTCP. Radiother Oncol 2014;111:148–52. [DOI] [PubMed] [Google Scholar]
- [43].Pearson WG, Langmore SE, Zumwalt AC. Evaluating the structural properties of suprahyoid muscles and their potential for moving the hyoid. Dysphagia. 2011;26:345–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Haddad RI, Posner M, Hitt R, Cohen EEW, Schulten J, Lefebvre JL, et al. Induction chemotherapy in locally advanced squamous cell carcinoma of the head and neck: role, controversy, and future directions. Ann Oncol 2018;29:1130–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Haddad R, O’Neill A, Rabinowits G, Tishler R, Khuri F, Adkins D, et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomized phase 3 trial. Lancet Oncol 2013;14:257–64. [DOI] [PubMed] [Google Scholar]
- [46].Cohen EE, Karrison TG, Kocherginsky M, Mueller J, Egan R, Huang CH, et al. Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol 2014;32:2735–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Francis DO, Weymuller EA Jr, Parvathaneni U, Merati AL, Yueh B. Dysphagia, stricture, and pneumonia in head and neck cancer patients: does treatment modality matter? Ann Otol Rhinol Laryngol 2010;119:391–7. [DOI] [PubMed] [Google Scholar]
- [48].Quon H, Richmon JD. Treatment deintensification strategies for HPV-associated head and neck carcinomas. Otolaryngol Clin North Am 2012;45:845–61. [DOI] [PubMed] [Google Scholar]
- [49].Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 2008;100:261–69. [DOI] [PubMed] [Google Scholar]
- [50].Bhatia A, Burtness B. Human papillomavirus-associated oropharyngeal cancer: defining risk groups and clinical trials J Clin Oncol 2015;33:3243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Haughey BH, Sinha P. Prognostic factors and survival unique to surgically treated p16+oropharyngeal cancer. Laryngoscope 2012;122 Suppl. 2:S13–S33. [DOI] [PubMed] [Google Scholar]
- [52].Mendenhall WM, Amdur RJ, Morris CG, Kirwan JM, Li JG. Intensity-modulated radiotherapy for oropharyngeal squamous cell carcinoma. Laryngoscope 2010;120:2218–22. [DOI] [PubMed] [Google Scholar]
- [53].Sedaghat AR, Zhang Z, Begum S, Palermo R, Best S, Ulmer KM, et al. Prognostic significance of human papillomavirus in oropharyngeal squamous cell carcinomas. Laryngoscope 2009;119:1542–49. [DOI] [PubMed] [Google Scholar]
- [54].Toledano I, Graff P, Serre A, Boisselier P, Bensadoun RJ, Ortholan C, et al. Intensity-modulated radiotherapy in head and neck cancer: results of the prospective study GORTEC 2004–03. Radiother Oncol 2012;103:57–62. [DOI] [PubMed] [Google Scholar]
- [55].Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010;363:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Levendag PC, Teguh DN, Voet P, van der Est H, Noever I, de Kruijf WJ, et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle: a dose-effect relationship. Radiother Oncol 2007;85:64–73. [DOI] [PubMed] [Google Scholar]
- [57].Mortensen HR, Jensen K, Aksglæde K, Behrens M, Grau C. Late dysphagia after IMRT for head and neck cancer and correlation with dose–volume parameters. Radiother Oncol 2013;107:288–94. [DOI] [PubMed] [Google Scholar]
- [58].Wilson JA, Carding PN, Patterson JM. Dysphagia after nonsurgical head and neck cancer treatment: patients’ perspectives. Otolaryngol Head Neck Surg 2011;145:767–71. [DOI] [PubMed] [Google Scholar]
- [59].Marur S, Li S, Cmelak AJ, Gillison ML, Zhao WJ, Ferris RL, et al. 1308: E1308: Phase II trial of induction chemotherapy followed by reduced-dose radiation and weekly cetuximab in patients with HPV-associated resectable squamous cell carcinoma of the oropharynx-ECOG-ACRIN Cancer Research Group. J Clin Oncol 2017;35:490–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Melotek J, Seiwert TY, Blair EA, Karrison TG, Agrawal N, Portugal L, et al. Optima: A phase II dose and volume de-escalation trial for high-and low-risk HPV+ oropharynx cancers. J Clin Oncol 2017;35 Suppl. 15:6066. [Google Scholar]
- [61].Gunn GB, Blanchard P, Garden AS, Zhu XR, Fuller CD, Mohamed AS, et al. Clinical outcomes and patterns of disease recurrence following intensity modulated proton therapy for oropharyngeal squamous carcinoma: results from a single institution prospective study. Int J Radiat Oncol Biol Phys 2016;95:360–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Dahlstrom KR, Calzada G, Hanby JD, Garden AS, Glisson BS, Li G, et al. An evolution in demographics, treatment, and outcomes of oropharyngeal cancer at a major cancer center: a staging system in need of repair. Cancer 2013;119:81–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


