Abstract
An efficient and highly enantioselective Conia-ene-type process has been developed. Reactions are catalyzed by a combination of B(C6F5)3, an N-alkylamine and a BOX–ZnI2 complex. Specifically, through cooperative action of B(C6F5)3 and amine, ketones with poorly acidic α-C–H bonds can be converted in situ to the corresponding enolates. Subsequent enantioselective cyclization involving a BOX–ZnI2-activated alkyne leads to the formation of various cyclopentenes in up to 99% yield and 99:1 er.
Graphical Abstract
Cooperative acid/base catalysis may be used to promote an enantioselective reaction between an in situ generated, acid-activated electrophile and a base-activated nucleophile.1,2 One of the key challenges in developing such transformations is to find a way for bypassing undesirable mutual quenching. Although one possible solution is to avoid utilizing a combination that exhibits high affinity (i.e., hard–hard or soft–soft pairing),1,2 this approach has been confined to cases that involve weakly or moderately acidic and/or basic catalysts along with substrates that are acid- or base-sensitive. Development of highly efficient and unquenchable cooperative catalyst systems that are capable of promoting enantioselective reactions between relatively unactivated starting materials stands as a largely unresolved challenge.
The application of frustrated Lewis pairs (FLPs), consisting of hindered and electronically disparate Lewis acids and Lewis bases, has recently emerged as an enabling strategy for overcoming undesirable mutual quenching.3,4 Furthermore, FLPs that are comprised of Lewis acidic B(C6F5)3 and a Brønsted basic N-alkylamine catalyst have been shown to promote Mannich-type and α-amination reactions with ketone, ester or amide pro-nucleophiles (pKa ~20–30).5 An ammonium ion derived from an N-alkylamine catalyst is thought to serve as a poorly activating Brønsted acid catalyst;1,2 these methods therefore demand acid-sensitive electrophiles such as N-Boc-benzaldimines or dimethyl azodicarboxylate. We reasoned that catalyst systems comprised of B(C6F5)3, an N-alkylamine and a Lewis acid co-catalyst for electrophile activation might pave the way for development of reactions between unactivated pro-nucleophiles and electrophiles.
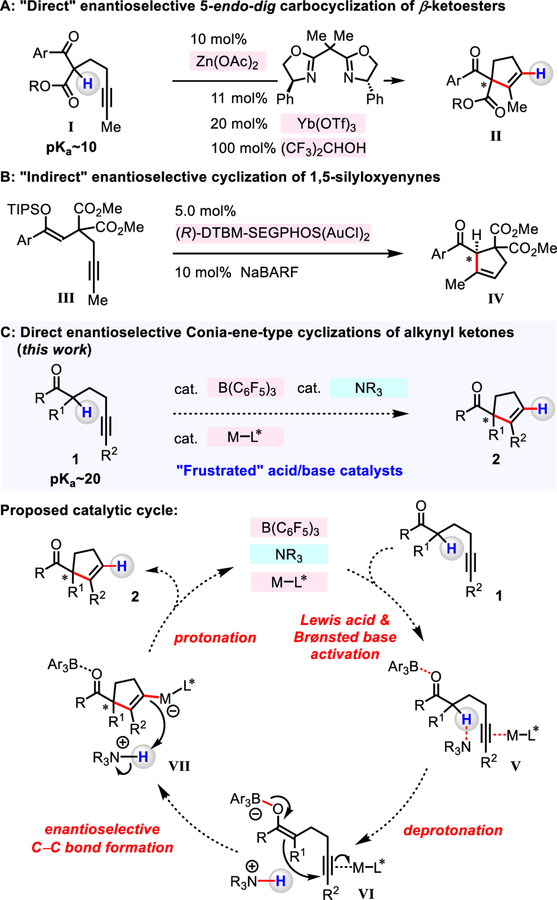
Enantioselective cycloadditions of 1,3-dicarbonyl compounds with a tethered alkyne moiety (5-exo-dig and 5-endo-dig processes) offer access to valuable five-membered ring structures that bear an exo-methylene moiety (e.g., I to II; Figure 1A) or cyclopentene derivatives.6–9 These “direct” Conia-ene-type reactions can be promoted by cooperative Lewis acid/Lewis acid catalysts (e.g., Pd/Yb-, La/Ag-, Zn/Yb-based),7 or enantiomerically pure Lewis basic amine/Lewis acid catalysts (e.g., Cu-, Ag-based).8 Such transformations allow for in situ generation of an enolate equivalent by Lewis acid or Lewis base catalyzed deprotonation, in addition to an electrophilic alkyne unit by Lewis acid activation.4 Nonetheless, the requisite weakly to moderately acidic and/or basic catalysts render the approach confined to readily enolizable 1,3-dicarbonyl compounds (e.g., I; pKa ~10).6–8 With catalysts that are more strongly acidic and/or basic and capable of deprotonating less acidic ketones (pKa ~20), mutual quenching can be an issue.1,2 Consequently, pre-formation of silicon enolate is needed for enantioeselective R3P/Au-catalyzed (5-endo-dig) cyclization of ketones (III to IV; Figure 1B).10 Synergetic catalyst systems that promote direct enantioselective Conia-ene-type processes with mono-carbonyl compounds remain unprecedented. Herein, we describe enantioselective direct Conia-ene-type reaction of ketones promoted by cooperative action of B(C6F5)3, an N-alkylamine and a chiral Lewis acid co-catalyst (Figure 1C).
Figure 1.
Enantioselective 5-endo-dig cycloadditions.
In contemplating ways to design an enantioselective method for cyclization of ketones that contain an alkyne unit (1), we envisioned a catalyst system that is comprised of a strongly Lewis acidic B(C6F5)3, a Brønsted basic N-alkylamine, and a chiral Lewis acid co-catalyst (Figure 1C). By employing structurally and electronically different organoborane and chiral Lewis acid co-catalyst that could have overlapping functions, we might be able to control the ability of B(C6F5)3 to serve as a carbonyl activator, and use the chiral Lewis acid co-catalyst to elevate alkyne reactivity (V). We surmised that N-alkylamine could deprotonate B(C6F5)3-activated ketone of 1, generating an enolate and an ammonium ion (VI). In the meantime, a chiral Lewis acid co-catalyst (ML*) would activate the alkyne unit (VI). An ensuing enantiodetermining 5-endo-dig cyclization of the enolate and the alkyne would deliver VII. Subsequent protonation of C–ML* bond by the ammonium ion would afford the desired cyclopentenyl product 2. One key advantage provided by the untethered catalyst system would be that efficiency and stereoselectivity might be optimized through rapid evaluation of readily accessible Lewis acids, chiral ligands, and N-alkylamines.
To identify an optimal catalyst combination, we probed the ability of B(C6F5)3, 1,2,2,6,6-pentamethylpiperidine (PMP), and various chiral Lewis acid/ligand complexes to catalyze the cyclization of 1-phenylnon-5-yn-1-one 1a (CH2Cl2, 12 h, 22 °C), to generate 2a (Table 1).11 The combination of 10 mol% ZnI2 and Ph–PyBOX (L1) or Ph–DBFOX (L2) afforded 2a in 20% and 7% yield, and 83:17 and 55:45 er, respectively. With Ph–BOX (L3) as the ligand, the desired product was generated in >95% yield and 89:11 er. Catalysts derived from alkyl-substituted L4 (10% yield and 75:25 er) and L5 (6% yield, 71:28 er) were less effective. Evaluation of Ph–BOX ligands with varying 2,2’-substiutents (cyclopropyl (L6), diisopropyl (L7), and dibenzyl (L8)) led us to establish that L8 is the most effective, providing 2a in >95 % yield and 97:3 er.12
Table 1.
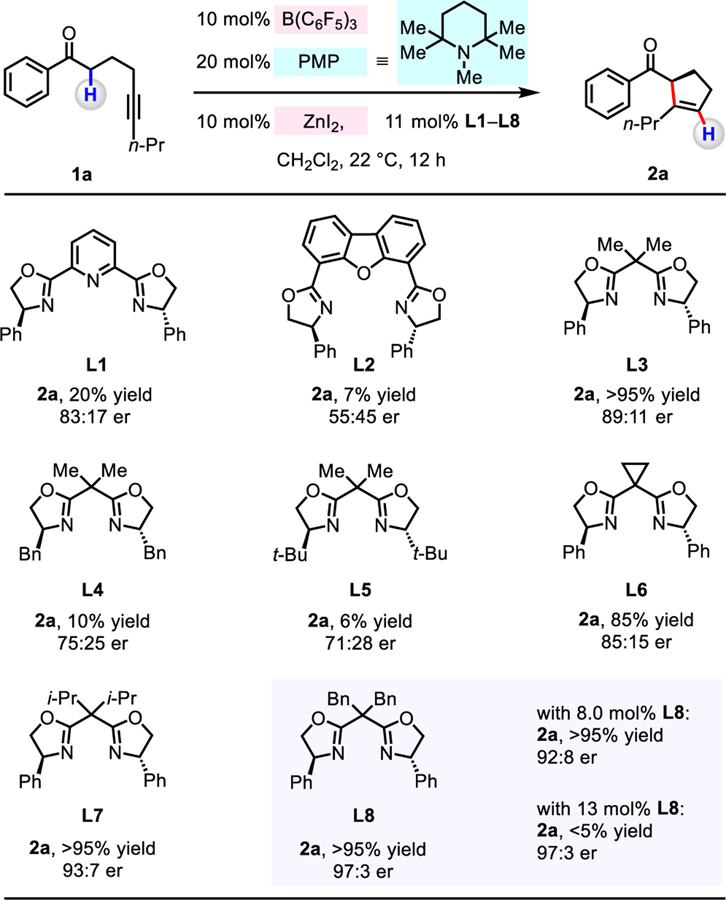 |
Conditions: 1-phenylnon-5-yn-1-one (1a, 0.2 mmol), B(C6F5)3 (10 mol%), 1,2,2,6,6-pentamethylpiperidine (20 mol%), ZnI2 (10 mol%), bis-oxazoline ligand (11 mol%), CH2Cl2 (1.0 mL), under N2, 22 °C, 12 h.
Yield was determined by 1H NMR analysis of unpurified reaction mixtures with mesitylene as the internal standard. The er values were determined by HPLC analysis of the purified product.
The amount of a bis-oxazoline ligand had a notable impact on efficiency and er. In the absence of L8, under otherwise identical conditions, rac-2a was isolated in >95% yield;13 as a consequence, there was diminution in enantioselectivity when 10 mol% of ZnI2 and 8.0 mol% of L8 was used (2a in >95% yield, 92:8 er). On the other hand, with excess L8 (13 mol%), 2a was obtained in <5% yield, suggesting that the Lewis basic oxazoline units of L8 can deactivate B(C6F5)3. Preformed and purified ZnI2/L8 complex may be used with similar effect.14
Next, we set out to identify an effective organoborane and Brønsted base catalysts, and optimize other reaction parameters (Table 2). Whereas with Et3N, 2a was obtained in >95% yield and 97:3 er (entry 1), none of the desired product could be detected with DBU as the base (entry 2). With less B(C6F5)3 (7.5 mol%), PMP (15 mol%), ZnI2/L8 (7.5 mol%), efficiency suffered but not enantioselectivity (90% yield, 97:3 er; entry 4). Efficiency improved at 40 °C (entry 5), allowing catalyst loading to be reduced to 5.0 mol% B(C6F5)3, 10 mol% PMP and 5.0 mol% ZnI2/L8; under these conditions, 2a was produced in 92% yield and 97:3 er (entry 6). No product was generated without the Brønsted base, the organoborane catalyst or the ZnI2/L8 (entries 7–9); the same was the case when the smaller BF3•OEt2 or the less acidic BPh3 were used (entries 10–11). Thus, the appropriately acidic B(C6F5)3, sizeable and electron-rich PMP, together with sterically demanding ZnI2/L8 complex emerged as the most effective combination.
Table 2.
 | |||||
|---|---|---|---|---|---|
| entry | Lewis acid (mol%) | Brønsted base (mol%) | Znl2/L8 (mol%) | yield of 2a (%) | er |
| 1 | B(C6F5)3 (10) | NET3 (20) | 10 | >95 | 97:3 |
| 2 | B(C6F5)3 (10) | DBU (20) | 10 | 0 | ND |
| 3 | B(C6F5)3 (10) | PMP (20) | 10 | >95 | 97:3 |
| 4 | B(C6F5)3 (7.5) | PMP (15) | 7.5 | 90 | 97:3 |
| 5c | B(C6F5)3 (7.5) | PMP (15) | 7.5 | >95 | 97:3 |
| 6c | B(C6F5)3 (5.0) | PMP (10) | 5.0 | 92 | 97:3 |
| 7 | B(C6F5)3 (5.0 | none | 5.0 | 0 | ND |
| 8 | none | PMP (10) | 5.0 | 0 | ND |
| 9 | B(C6F5)3 (5.0) | PMP (10) | 0 | 0 | ND |
| 10 | BF3·OEt2 (5.0) | PMP (10) | 5.0 | 0 | ND |
| 11 | BPh3 (5.0) | PMP (10) | 5.0 | 0 | ND |
Conditions: 1-phenylnon-5-yn-1-one (1a, 0.2 mmol), organoborane, Brønsted base, ZnI2/L8 complex, CH2Cl2 (1.0 mL), under N2, 22 °C, 12 h.
Yield was determined by 1H NMR analysis of unpurified reaction mixtures with mesitylene as the internal standard. The er values were determined by HPLC analysis of the purified product.
The reaction mixture was allowed to stir at 40 °C.
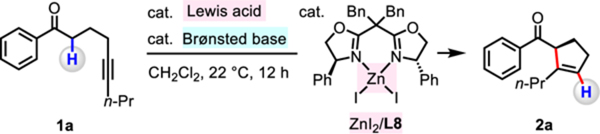
A variety of 1-phenyl ketones with different alkyne substituents proved to be suitable substrates (2a–2i; Table 3). With 1-phenylhex-5-yn-1-one, containing a terminal alkyne moiety (R = H), the transformation was inefficient and moderately enantioselective (10% yield, 70:30 er).15 In contrast, 1-phenylhept-5-yn-1-one, which bears an internal alkyne (R = Me), was converted to 2b in 96% yield and 82:18 er. Reactions involving substrates that carry a larger ethyl, n-propyl, n-butyl, and iso-butyl substituent, afforded the desired products in >95% yield and 96:4–97:3 er (2a, 2c–2e). Although benzyl-substituted substrate furnished 2f in 82% yield and 98:2 er, phenyl-substituted substrate was less efficient (2g, 50% yield, 91:9 er). As indicated by the formation of 2h (97% yield, 94:6 er) and 2i (94% yield, 97:3 er), the presence of a carboxylic ester or a monosubstituted alkene is tolerated.
Table 3.
Conia-Ene-Type Reactions with Different Alkyne Substituents a,b
aConditions: Alkynyl ketone (1a-1d, 1h, 1i; 0.2 mmol), B(C6F5)3 (7.5 mol%), 1,2,2,6,6-pentamethylpiperidine (15 mol%), ZnI2/L8 (7.5 mol%), CH2Cl2 (1.0 mL), under N2, 40 °C, 12 h. bYield of isolated and purified product. The er values were determined by HPLC analysis of the purified product. cAlkynyl ketone (1e-1g; 0.2 mmol), B(C6F5)3 (10 mol%), 1,2,2,6,6-pentamethylpiperidine (20 mol%), ZnI2/L8 (10 mol%), ClCH2CH2Cl (1.0 mL), under N2, 60 °C, 24 h.
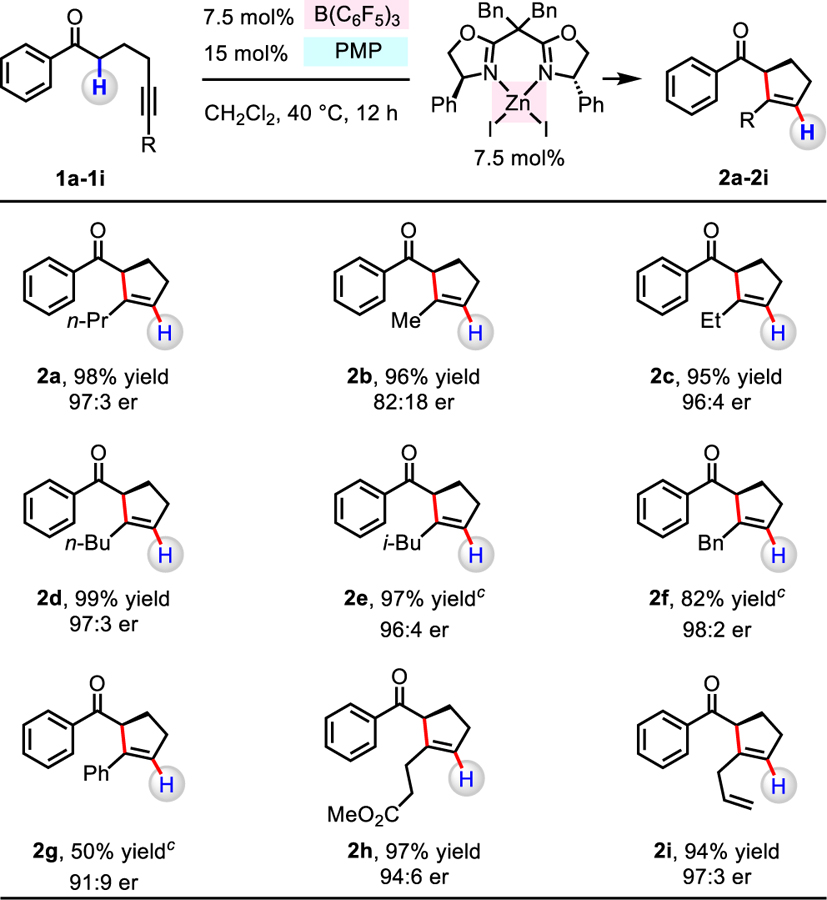 |
We then investigated reactions with different aryl- and alkyl-substituted ketones (Table 4). 2-Methoxyphenyl- and 4-bromophenyl-substituted ketones gave 2j (95% yield, 93:7 er) and 2k (97% yield, 97:3 er), respectively. The process involving indanone derivative did not afford 2l when PMP was employed as a Brønsted base catalyst, probably because it is too hindered to deprotonate a tertiary C–H bond within a B(C6F5)3-activated ketone. In the case of using less hindered 1-methylpiperidine, 2l, which possesses an α-quaternary carbon center was produced in 98% yield and 99:1 er. Tetralone-derived 2m was formed inefficiently (20% yield) but in 98:2 er, perhaps an indication of severe steric repulsion between the n-propyl substituent and the tetralone ring. Thus, we were able to convert a Me-substituted substrate to 2n in 73% yield and 91:9 er. Thiophene-substituted 2o (99% yield, 98:2 er) and furan-substituted 2p (99% yield, 97:3 er) provide further evidence regarding the approach’s notable scope. Transformations with alkyl ketones were similarly effective, as represented by 2q (91% yield, 95:5 er) and 2r (77% yield, 96:4 er). Cyclopentanone derivative 2s was generated with 1-methylpiperidine as the Brønsted base, but in diminished er (82% yield, 80:20 er).
Table 4.
Conia-Ene-Type Reactions with Different Ketones a,b
aConditions: Alkynyl ketone (1j-1l, 1o-1s; 0.2 mmol), B(C6F5)3 (7.5 mol%), 1,2,2,6,6-pentamethylpiperidine (15 mol%), ZnI2/L8 (7.5 mol%), CH2Cl2 (1.0 mL), under N2, 40 °C, 12 h. bYield of isolated and purified product. The er values were determined by HPLC analysis of the purified product. c1-Methylpiperidine was used as a Brønsted base catalyst. dAlkynyl ketone (1m, 1n; 0.2 mmol), B(C6F5)3 (10 mol%), 1-methylpiperidine (20 mol%), ZnI2/L8 (10 mol%), ClCH2CH2Cl (1.0 mL), under N2, 60 °C, 24 h.
 |
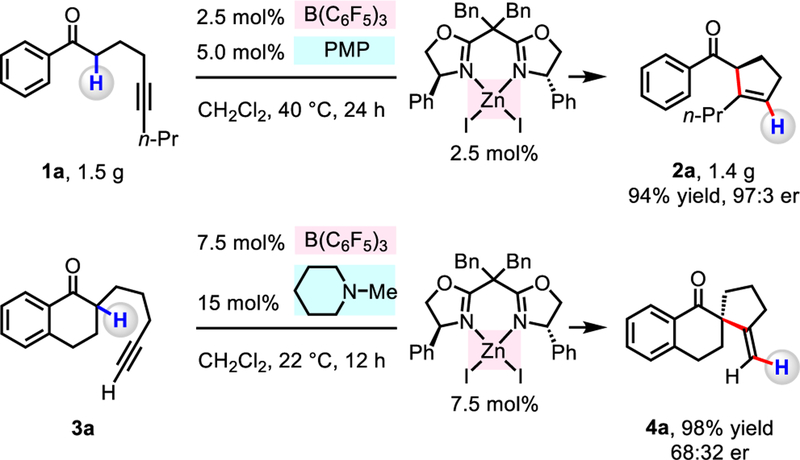
The method is readily scalable. Treatment of 1.5 g (7.0 mmol) of 1a with 2.5 mol% B(C6F5)3, 5.0 mol% PMP and 2.5 mol% ZnI2/L8 (CH2Cl2, 24 h, 40 °C) afforded 2a in 94% yield (6.6 mmol, 1.4 g) and 97:3 er (Scheme 1). Moreover, we discovered that cycloaddition of 1-phenylhept-6-yn-1-one (3a) (5-exo-dig) is facilitated by B(C6F5)3, 1-methylpiperidine and ZnI2/L8 to deliver exo-methylene-substituted cyclopentane 4a in 98% yield but just 68:32 er. Studies are underway to enhance the applicability of the approach.
Scheme 1.
Scale-up experiments and 5-exo-dig cycloaddition of 3a
To summarize, we have designed an efficient and enantioselective Conia-ene-type reaction by implementing the cooperative action of a three-component catalyst system, which consists of a pair of Lewis acids and a Brønsted basic amine. We show that by tuning of different features of Lewis acids that possess overlapping functions, it is possible to engage Lewis acid catalysts to serve as an activator of a carbonyl group or an activator of electron-rich alkyne. Accordingly, efficiency and stereoselectivity of C–C bond forming reactions between in situ generated enolate and chiral Lewis acid-activated alkyne can be conveniently enhanced without significant loss in er, which might arise due to intervention by an achiral Lewis acid component. The principles outlined herein, entailing separate and independently operational Lewis acidic co-catalysts and a Brønsted base catalyst, provide a rational framework for further development of processes involving weakly acid- and/or base-sensitive substrates. Studies aimed at achieving these objectives are in progress.
Supplementary Material
Acknowledgements.
Financial support was provided by the NIH (GM-128695) and Boston College. We thank Professor Amir H. Hoveyda (Boston College) for helpful discussions. We also thank Dr. Bo Li and Dr. Malte S. Mikus (Boston College) for X-ray crystallographic analysis.
Footnotes
The authors declare no competing financial interest.
Supporting Information Available: Experimental procedures and spectral data for all new compounds (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).For selected reviews on enantioselective cooperative catalysis, see: (a) Yamamoto H; Futatsugi K “Designer Acids”: Combined Acid Catalysis for Asymmetric Synthesis. Angew. Chem., Int. Ed 2005, 44, 1924–1942. [DOI] [PubMed] [Google Scholar]; (b) Paull DH; Abraham CJ; Scerba MT; Alden-Danforth E; Lectka T Bifunctional Asymmetric Catalysis: Cooperative Lewis Acid/Base Systems. Acc. Chem. Res 2008, 41, 655–633. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kobayashi S; Mori Y; Fossey JS; Salter MM Catalytic Enantioselective Formation of C–C Bonds by Addition to Imines and Hydrazones: A Ten-Year Update. Chem. Rev 2011, 111, 2626–2704. [DOI] [PubMed] [Google Scholar]; (d) Trost BM; Bartlett MJ ProPhenol-Catalyzed Asymmetric Additions by Spontaneously Assembled Dinuclear Main Group Metal Complexes. Acc. Chem. Res 2015, 48, 688–701. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Shibasaki M; Kumagai N in Cooperative Catalysis: Designing Efficient Catalysts for Synthesis, Peters R, Eds.; Wiley-VCH: New York, 2015; Chapter 1. [Google Scholar]
- (2).For selected reviews on enantioselective non-covalent catalysis, see: (a) Hashimoto T; Maruoka K Recent Development and Application of Chiral Phase-Transfer Catalysts. Chem. Rev 2007, 107, 5656–5682. [DOI] [PubMed] [Google Scholar]; (b) Ooi T; Maruoka K Recent Advances in Asymmetric Phase-Transfer Catalysis. Angew. Chem., Int. Ed 2007, 46, 4222–4266. [DOI] [PubMed] [Google Scholar]; (c) Adair G; Mukherjee S; List B TRIP - A powerful Brønsted acid catalyst for asymmetric synthesis. Aldrichimica Acta 2008, 41, 31–39. [Google Scholar]; (d) Zhang Z; Schreiner PR (Thio)urea organocatalysis—What can be learnt from anion recognition? Chem. Soc. Rev 2009, 38, 1187–1198. [DOI] [PubMed] [Google Scholar]; (e) Phipps RJ; Hamilton GL; Toste FD The progression of chiral anions from concepts to applications in asymmetric catalysis. Nat. Chem 2012, 4, 603–614. [DOI] [PubMed] [Google Scholar]; (f) Brak K; Jacobsen EN Asymmetric Ion-Pairing Catalysis. Angew. Chem., Int. Ed 2013, 52, 534–561. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Neel AJ; Hilton MJ; Sigman MS; Toste FD Exploiting non-covalent π interactions for catalyst design. Nature 2017, 543, 637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).For reviews of frustrated Lewis pair chemistry, see: (a) Frustrated Lewis Pairs I; Stephan DW; Erker G Eds.; Springer Press: New York, 2013; Vol. 332. [Google Scholar]; (b) Frustrated Lewis Pairs II: Expanding the Scope; Erker G; Stephan DW Eds.; Springer: Berlin, 2013; Vol. 334. [Google Scholar]; (c) Ashley AE; O’Hare D FLP-Mediated Activations and Reductions of CO2 and CO. Top. Curr. Chem 2013, 334, 191–218. [DOI] [PubMed] [Google Scholar]; (d) Feng X; Du H Metal-free asymmetric hydrogenation and hydrosilylation catalyzed by frustrated Lewis pairs. Tetrahedron Lett 2014, 55, 6959–6964. [Google Scholar]; (e) Stephan DW; Erker G Frustrated Lewis Pair Chemistry: Development and Perspectives. Angew. Chem., Int. Ed 2015, 54, 6400–6441. [DOI] [PubMed] [Google Scholar]; (f) Stephan DW Frustrated Lewis Pairs J. Am. Chem. Soc 2015, 137, 10018–10032. [DOI] [PubMed] [Google Scholar]; (g) Oestreich M; Hermeke J; Mohr J A unified survey of Si–H and H–H bond activation catalysed by electron-deficient boranes. Chem. Soc. Rev 2015, 44, 2202–2220. [DOI] [PubMed] [Google Scholar]; (h) Stephan DW The broadening reach of frustrated Lewis pair chemistry. Science 2016, 354, aaf7229. [DOI] [PubMed] [Google Scholar]
- (4).For activation of alkynes by B(C6F5)3, see: (a) Dureen MA; Brown CC; Stephan DW Addition of Enamines or Pyrroles and B(C6F5)3 “Frustrated Lewis Pairs” to Alkynes. Organometallics 2010, 29, 6422–6432. [Google Scholar]; (b) Hansmann MM; Melen RL; Rominger F; Hashmi ASK; Stephan DW Activation of Alkynes with B(C6F5)3–Boron Allylation Reagents Derived from Propargyl Esters. J. Am. Chem. Soc 2014, 136, 777–782. [DOI] [PubMed] [Google Scholar]
- (5).(a) Chan JZ; Yao W; Hastings BT; Lok CK; Wasa M Direct Mannich-Type Reactions Promoted by Frustrated Lewis Acid/Brønsted Base Catalysts. Angew. Chem., Int. Ed 2016, 55, 13877–13881. [DOI] [PubMed] [Google Scholar]; (b) Shang M; Wang X; Koo SM; Youn J; Chan JZ; Yao W; Hastings BT; Wasa M Frustrated Lewis Acid/Brønsted Base Catalysts for Direct Enantioselective α-Amination of Carbonyl Compounds. J. Am. Chem. Soc 2017, 139, 95–98. [DOI] [PubMed] [Google Scholar]; (c) Shang M; Cao M; Wang Q; Wasa M Enantioselective Direct Mannich-Type Reaction Catalyzed by Frustrated Lewis Acid/Brønsted Base Complexes. Angew. Chem., Int. Ed 2017, 56, 13338–13526. [DOI] [PubMed] [Google Scholar]
- (6).(a) Conia JM; Perchec PL The Thermal Cyclisation of Unsaturated Carbonyl Compounds. Synthesis 1975, 1, 1–19. [Google Scholar]; (b) Hack D; Blümel M; Chauhan P; Philipps AR; Enders D Catalytic Conia-ene and related reactions. Chem. Soc. Rev 2015, 44, 6059–6093. [DOI] [PubMed] [Google Scholar]
- (7).(a) Corkey BK; Toste FD Catalytic Enantioselective Conia-Ene Reaction. J. Am. Chem. Soc 2005, 127, 17168–17169. [DOI] [PubMed] [Google Scholar]; (b) Matsuzawa A; Mashiko T; Kumagai N; Shibasaki M La/Ag Heterobimetallic Cooperative Catalysis: A Catalytic Asymmetric Conia-Ene Reaction. Angew. Chem., Int. Ed 2011, 123, 7758–7761. [DOI] [PubMed] [Google Scholar]; (c) Suzuki S; Tokunaga E; Reddy DS; Matsumoto T; Shiro M; Shibata N Enantioselective 5-endo-dig Carbocyclization of β-Ketoesters with Internal Alkynes Employing a Four Component Catalyst System. Angew. Chem., Int. Ed 2012, 51, 4131–4135. [DOI] [PubMed] [Google Scholar]
- (8).(a) Yang T; Ferrali A; Sladojevich F; Campbell L; Dixon DJ Brønsted Base/Lewis Acid Cooperative Catalysis in the Enantioselective Conia-Ene reaction. J. Am. Chem. Soc 2009, 131, 9140–9141. [DOI] [PubMed] [Google Scholar]; (b) Shaw S; White JD A New Iron (III)–Salen Catalyst for Enantioselective Conia-ene Carbocyclization. J. Am. Chem. Soc 2014, 136, 13578–13581. [DOI] [PubMed] [Google Scholar]; (c) Blümel M; Hack D; Ronkartz L; Vermeeren C; Enders D Development of an enantioselective amine–silver co-catalyzed Conia-ene reaction. Chem. Commun 2017, 53, 3956–3959. [DOI] [PubMed] [Google Scholar]
- (9).For selected examples on application of Conia-ene-type reaction, see: (a) >Staben ST; Kennedy-Smith JJ; Huang D; Corkey BK; LaLonde RL; Toste FD Gold (I)-Catalyzed Cyclizations of Silyl Enol Ethers: Application to the Synthesis of (+)-Lycopladine A. Angew. Chem., Int. Ed 2006, 45, 5991–5994. [DOI] [PubMed] [Google Scholar]; (b) Tsuji H; Yamagata KI; Itoh Y; Endo K; Nakamura M; Nakamura E Indium-Catalyzed Cycloisomerization of ω-Alkynyl-β-ketoesters into Six- to Fifteen-Membered Rings. Angew. Chem., Int. Ed 2007, 46, 8060–8062. [DOI] [PubMed] [Google Scholar]; (c) Takahashi K; Midori M; Kawano K; Ishihara J; Hatakeyama S Entry to Heterocycles Based on Indium-Catalyzed Conia-Ene Reactions: Asymmetric Synthesis of (–)-Salinosporamide A. Angew. Chem., Int. Ed 2008, 47, 6244–6246. [DOI] [PubMed] [Google Scholar]; (d) Liu X; Lee CS Total synthesis of (–)-Teucvidin. Org. Lett 2012, 14, 2886–2889. [DOI] [PubMed] [Google Scholar]; (e) Huwyler N; Carreira EM Total Synthesis and Stereochemical Revision of the Chlorinated Sesquiterpene (±)-Gomerone C. Angew. Chem., Int. Ed 2012, 51, 13066–13069. [DOI] [PubMed] [Google Scholar]; (f) Persich P; Llaveria J; Lhermet R; de Haro T; Stade R; Kondoh A; Fürstner A Increasing the Structural Span of Alkyne Metathesis. Chem. Eur. J 2013, 19, 13047–13058. [DOI] [PubMed] [Google Scholar]; (g) Xiong X; Li Y; Lu Z; Wan M; Deng J; Wu S; Shao H; Li A Synthesis of the 6, 6, 5, 7-tetracyclic core of daphnilongeranin B. Chem. Commun 2014, 50, 5294–5297. [DOI] [PubMed] [Google Scholar]; (h) Hartrampf FW; Furukawa T; Trauner D A Conia-Ene-Type Cyclization under Basic Conditions Enables an Efficient Synthesis of (–)-Lycoposerramine R. Angew. Chem., Int. Ed 2017, 56, 893–896. [DOI] [PubMed] [Google Scholar]; (i) Ye Q; Qu P; Snyder SA Total Syntheses of Scaparvins B, C, and D Enabled by a Key C–H Functionalization. J. Am. Chem. Soc 2017, 139, 18428–18431. [DOI] [PubMed] [Google Scholar]; (j) Hartrampf FW; Trauner D Total Synthesis of Lycopladine A and Carinatine A via a Base-Mediated Carbocyclization. J. Org. Chem 2017, 82, 8206–8212. [DOI] [PubMed] [Google Scholar]
- (10).(a) Corkey BK; Toste FD Palladium-Catalyzed Enantioselective Cyclization of Silyloxy-1, 6-Enynes. J. Am. Chem. Soc 2007, 129, 2764–2765. [DOI] [PubMed] [Google Scholar]; (b) Brazeau JF; Zhang S; Colomer I; Corkey BK; Toste FD Enantioselective Cyclizations of Silyloxyenynes Catalyzed by Cationic Metal Phosphine Complexes. J. Am. Chem. Soc 2012, 134, 2742–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11). For a comprehensive evaluation of Fe, Cu, Ag, Mg, In, Yb, Au-based Lewis acids and their complexes with various chiral ligands, see the SI.
- (12). For the determination of absolute configuration for product 2a, see the SI. The absolute configuration for the other products was assigned in analogy.
- (13). For the data involving evaluation of achiral Lewis acid co-catalysts, see the SI.
- (14).(a) Thorhauge J; Roberson M; Hazell RG; Jørgensen KA On the Intermediates in Chiral Bis(oxazoline)copper(II)-Catalyzed Enantioselective Reactions—Experimental and Theoretical Investigations. Chem. Eur. J 2002, 8, 1888–1898. [DOI] [PubMed] [Google Scholar]; (b) Desimoni G; Faita G; Jørgensen KA C2-Symmetric Chiral Bis(oxazoline) Ligands in Asymmetric Catalysis. Chem. Rev 2011, 111, 284–437. [DOI] [PubMed] [Google Scholar]
- (15). For the experimental results involving the enantioselective cyclization of 1-phenylhex-5-yn-1-one, see the SI.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.