Highlights
-
•
We examined neural activity to gains and losses in approximately 250 adolescent females aged 8-14 at two time points.
-
•
Assessments were separated by approximately two years
-
•
At baseline, gains were more correlated with age than losses; at follow-up, gains and losses were equally related to age.
-
•
Only reward-related neural activity increased from baseline to follow-up—and only among younger participants.
-
•
Late childhood and early adolescence appear to be characterized by specific increases in reward-related neural activity.
Keywords: Reward positivity, Reward, Adolescence, Development, Electroencephalogram, Event-related potentials
Abstract
Adolescence is frequently described as a developmental period characterized by increased sensitivity to rewards. However, previous research on age-related changes in the neural response to gains and losses have produced mixed results, with only some studies reporting potentiated neural responses during adolescence. The current study examined the ERP responses to gains and losses during a simple monetary reward (i.e., Doors) task in a large and longitudinal sample of 248 adolescent females assessed at two time points, separated by two years. At baseline, when the sample was 8- to 14-years-old, age related to larger (i.e., more positive) ERP responses to both gains and losses; moreover, age-related effects were stronger in relation to gains than losses. Overall, the amplitude of the ERP response to gains, but not losses, significantly increased from baseline to follow-up; however, this effect was moderated by age, such that reward-related ERPs only increased longitudinally among the younger participants. At the follow-up assessment, ERP responses to gains and losses were equally related to age. Collectively, these within- and between-subjects findings suggest a relatively specific developmental increase in reward-related neural activity during late childhood and early adolescence.
1. Introduction
Contingencies between actions and subsequent rewards and non-rewards are crucial in shaping behavior and for learning about the impact of actions in the environment. Electroencephalography (EEG) research has focused on a difference in the event-related potential (ERP) following feedback indicating monetary gains versus losses. Following the presentation of loss, the ERP is characterized by a relative negativity that has been referred to as the feedback-related negativity (FRN; Miltner et al., 1997), feedback error-related negativity (Holroyd and Coles, 2002), or feedback negativity (FN; Yeung et al., 2004). Many early studies interpreted the relative negativity as reflecting the evaluation of outcomes as worse than anticipated (e.g., Holroyd and Coles, 2002). More recently, however, researchers have emphasized the possibility that the difference between gains and losses is driven by variability in the response to gains. For instance, Holroyd et al. (2008) showed that the FRN has similar characteristics as the N200, and provided evidence that a reward positivity (RewP) may suppress the N200 following the presentation of reward feedback. Others have similarly suggested that reward-related ERPs are characterized by a RewP that is absent or reduced on non-reward trials (Foti et al., 2011; Weinberg et al., 2014; Kujawa et al., 2013).
The RewP has been shown to correlate with other measures of reward processing such as self-report (Bress and Hajcak, 2013), behavioral (Pizzagalli et al., 2005) and fMRI-based reward circuit activation (Carlson et al., 2011). As an index of reward processing, the RewP has been shown to be reduced in Major Depressive Disorder (MDD; Nelson et al., 2016) as well as in relation to risk for subsequent increases in depressive symptoms (Belden et al., 2016; Foti et al., 2014; Liu et al., 2014).
Adolescence is a particularly salient developmental period insofar as it has been characterized by increased reward sensitivity (Van Leijenhorst et al., 2010; Urošević et al., 2012; Galvan, 2010); paradoxically, adolescence is distinguished by rapid increases in depressive symptoms and syndromes (Lewinsohn et al., 1994). However, few studies have systematically examined developmental changes in the ERP response to gains and losses. That is, it is unclear whether developmental changes around adolescence are characterized by increases in neural response to reward specifically, or if both neural responses to gains and losses are increasing during adolescence.
Several studies in adolescents have examined the cross-sectional relationship between age and ERPs to gains and losses, as well as the relationship between age and the difference between gains and losses. Zottoli and Grose‐Fifer (2012) reported that adolescent males (14–17 years old) had larger ERPs to both gain and loss than adult males (22–26 years old). Compared to young adults (19–24 years old), another study found that early adolescents (i.e., ages 10–12) had larger ERPs to negative but not positive feedback (Eppinger et al., 2009). Conversely, Hämmerer et al. (2011), who measured the difference waveform as a ratio score, reported that younger adults (20–30 years old) showed a significantly larger ERP amplitude to losses compared to children (9–11 years old), adolescents (13–14 years old) and older adults (65–75 years old). Several other studies involving adolescents have reported no age-related effects in the ERP response to gains, losses, or their difference (Santesso et al., 2011; Yi et al., 2012; Bress et al., 2012). As one recent example, Lukie et al. (2014) assessed age-related effects on the RewP, measured as a difference waveform, in a sample of children (8–13 years old), adolescents (14–17 years old) and young adults (18–23 years old)—and found no age-related differences across the groups.
All of the aforementioned research studies were between-subject investigations that correlated age with ERP scores, or examined between-group differences in ERP scores; thus, past research has been limited by a lack of within-subject longitudinal data. An investigation by Kujawa et al. (2017) assessed developmental changes in ERPs to gains and losses in a sample of 75 children who were followed from late childhood to early and middle adolescence using a simple gambling paradigm similar to the present study. Utilizing mean activity of the ERPs from 250 to 350 ms following feedback, Kujawa and colleagues found that the ERP responses to gains and losses did not significantly increase from late childhood to early and middle adolescence1 .
Overall, studies that have examined age-related effects on ERP responses to gains and losses using cross-sectional experimental designs have yielded conflicting results. Although adolescence is frequently discussed as a developmental period characterized by increased reward sensitivity, only a subset of ERP studies supports this view—though the vast majority have not examined longitudinal changes within adolescence. This may be partially explained by methodological variation in how the ERP components were scored, as well as variation in the complexity of the paradigms used to elicit ERP components. The primary goal of the current study was to examine longitudinal changes in the ERP response to both gains and losses over a two-year period using a within-subject experimental design in a large sample of adolescent females (N = 317) that spanned the ages of 8 to 14 at baseline. We sought to determine whether ERP responses to gains and losses would change during this important period of development—and if this change would relate to age. In addition, we were able to conduct two cross-sectional analyses, correlating age with ERPs at both baseline and follow-up. Based on the mixed findings reviewed above, our working hypothesis was that both ERPs to gains and losses would relate to age cross-sectionally and increase from baseline to follow-up; we had no specific hypotheses about which ERP would show greater age-related effects or stronger developmental increases.
2. Methods
2.1. Participants
Participants in the current study were part of a large and longitudinal study of adolescent females who were assessed at baseline and again at follow-up approximately two years later. At baseline, the sample consisted of 317 adolescent females between the ages of 8 to 14 years old (M = 12.4, SD = 1.8). Of the 317 participants, three participants did not complete the doors task and nine participants were excluded due to poor EEG data quality. The final sample for analyses at baseline included 305 participants (M = 12.4, SD = 1.8). At the two-year follow-up, the sample consisted of 258 adolescent females (M = 14.4, SD = 1.8); of these, ten participants were excluded due to poor EEG data quality. The final sample for analyses at follow-up included 248 participants (M = 14.4, SD = 1.8). Participants without follow-up data were significantly older than participants with follow-up data (t = 2.43, p < .05). Participants with and without follow-up data did not differ in terms of their baseline ERP responses to gains (t = .009, p > .50) or losses (t = .41, p > .50). Analyses assessing the change in ERPs from baseline to follow-up included 240 participants with ERP data at both assessments (Baseline M = 12.3, SD = 1.8; Follow-up M = 14.3, SD = 1.8). Participants were recruited from the community through a commercial mailing list, using flyers and word of mouth. All participants and their parents provided informed consent and assent as approved by the Institutional Review Board at Stony Brook University.
2.2. Doors task
The doors task is a simple monetary reward task in which gains and losses are equiprobable (Proudfit, 2015). On each trial, participants were instructed to select between two identical doors displayed on a computer screen, using the left and right mouse buttons. The image of the doors remained on the screen until a choice was made. After making a selection, a fixation cross is presented for 1500 ms, followed by feedback stimuli that indicated whether the participant won (i.e., a green arrow pointing upward signified +$0.50) or lost (a red arrow pointing downward signified -$0.25) on that trial. The feedback stimuli remained on the screen for 2000 ms. In between each trial, text appeared on the screen that instructed participants to “Click for next round”, followed by a fixation cross presented for 1000 ms. The task contained 30 gain trials and 30 loss trials, presented in pseudo-random order. The doors task was administered using Presentation, version 17.2 (Neurobehavioral Systems, Albany, Calif.). Participants were told that they could receive between $0 to $15 dollars (rounding up) at the end of the task based on their cumulative earnings; all participants received $8 for completing the task.
2.3. EEG processing and recording
Continuous electroencephalography (EEG) was recorded while participants completed the doors task. The EEG was recorded using the ActiveTwo BioSemi system (BioSemi, Amsterdam, Netherlands) with an elastic cap containing 34-electrode sites placed according to the 10/20 system (i.e., 32 channels plus FCz and Iz). Additional electrodes were placed above and below the left eye, and near the outer canthi of the left and right eyes to monitor vertical electrooculographic (VEOG) activity and horizontal electrooculographic (HEOG) activity. Two electrodes were placed on the left and right mastoids. The EEG signal was preamplified at the electrode to improve signal-to-noise ratio, and data were digitized at a 24-bit resolution with a sampling rate of 1024 Hz using a low pass fifth order sinc filter with a half-power cutoff of 204 Hz. Active electrodes were measured online with reference to a common mode sense active electrode constructing a monopolar channel.
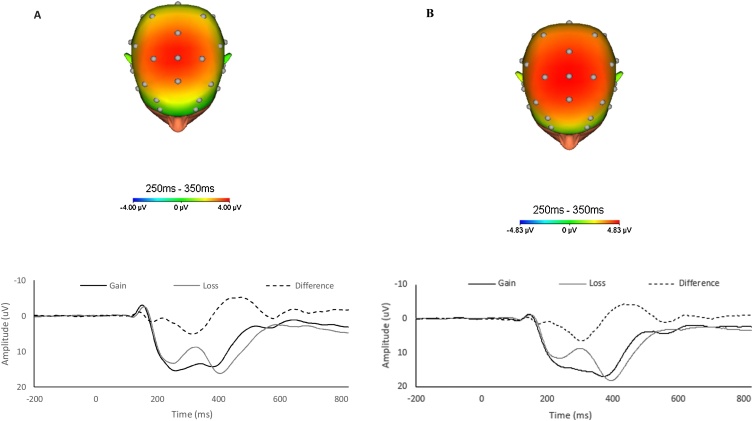
EEG data were analyzed using BrainVision Analyzer, version 2.1 (Brain Products, Gliching, Germany). The raw EEG data were re-referenced offline to the average of the left and right mastoids and band-pass filtered from 0.1 to 30 Hz. Eyeblink and ocular-movement corrections were performed using established standards described by Gratton et al. (1983). Feedback-locked epochs were extracted with a duration of 1000 ms, starting 200 ms before feedback presentation. The 200 ms segment prior to feedback onset served as the baseline. Epochs containing a voltage greater than 50 μV between sample points, a voltage difference of 300 μV within a segment, or a maximum voltage difference of less than 0.5 μV within 100 ms intervals were automatically rejected. Feedback-locked ERPs were averaged separately for gain and loss trials. For individual subjects, ERP responses to gains and losses were measured as the mean amplitude in a 100 ms time window, starting 250 following the presentation of feedback at FCz. Similar to previous studies in adolescents (Lukie et al., 2014; Eppinger et al., 2009) the ERP difference between gains and losses was maximal at FCz (Fig. 1)2 . To mimic the analyses performed by Lukie and colleagues, we also computed the area around the peak of the difference waveform by utilizing the mean activity in a 100 ms window centered around the most positive value of the gain minus loss difference between 250–350 ms for each participant.
Fig. 1.
Feedback-locked ERPs at FCz in response to monetary gains (dark) and losses (light) as well as gain-loss difference (dashed) waveform and headmap at baseline assessment (A) and at follow-up assessment (B).
2.4. Data analysis
At both the baseline and 2 year follow-up assessments, the relationships between age (in years and months) and ERPs to gains and losses were assessed using bivariate (Pearson’s r) correlations. To examine condition differences both cross-sectionally and longitudinally, we utilized repeated measures ANCOVA with age as a continuous covariate. Finally, using Pearson’s r, age at the baseline assessment was correlated with the change in ERP amplitudes to gains and losses from baseline to the follow-up assessment as well as the difference-based measures to determine whether age at baseline predicted within-subject developmental changes in ERPs.
3. Results
3.1. Baseline assessment
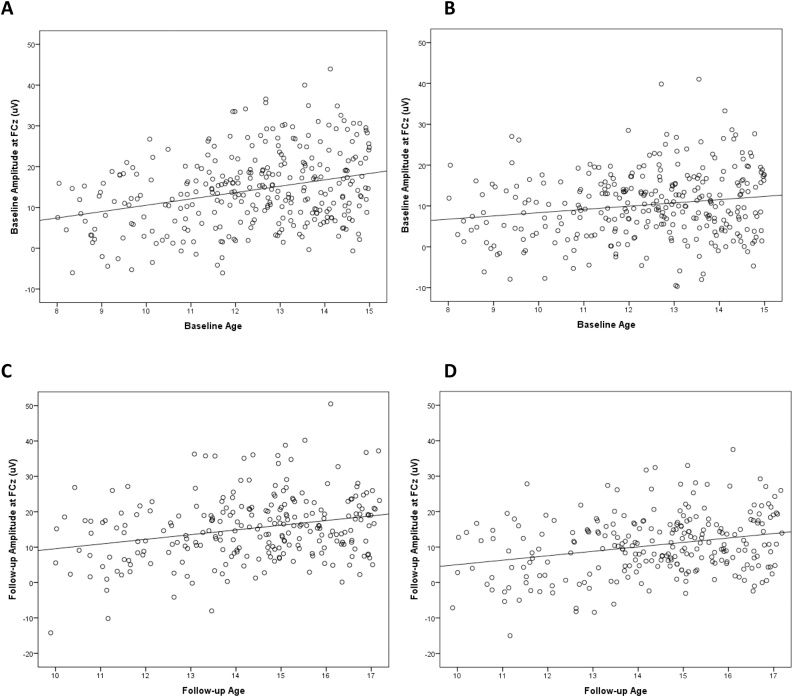
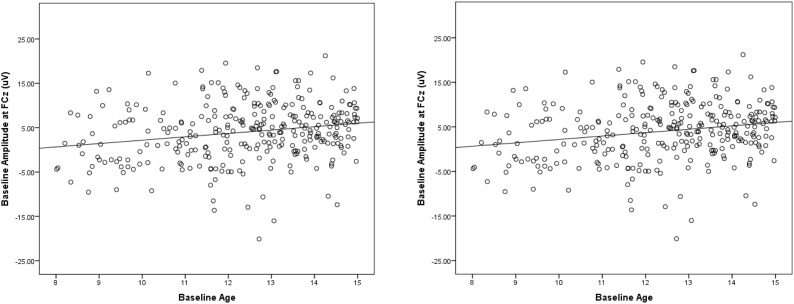
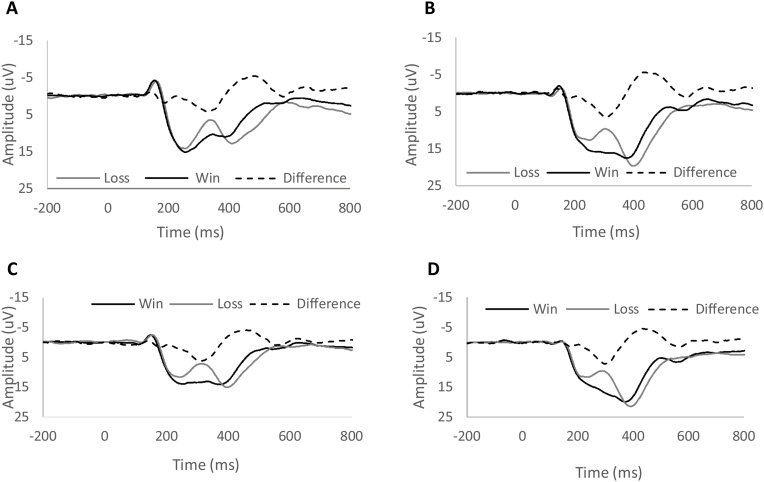
Fig. 1A presents ERPs to gains and losses at the baseline assessment, as well as the gain minus loss difference waveform and scalp distribution of the difference in the time range of the RewP. A 2 (Condition: Gain, Loss) repeated measures ANCOVA with age as a continuous covariate revealed a significant effect of Condition (F(1, 303) = 4.40, p < .05), and a significant interaction between age and condition (F(1, 303) = 13.59, p < .001). This interaction reflected the fact that although age was correlated with both the ERP response to gains (r = .30, p < .01; see Fig. 2A) and the ERP response to losses (r = .17, p < .01; see Fig. 2B), age was more correlated with the ERP response to gains than losses (z = 3.14, p < .01, two-tailed). Consistent with this, age was positively correlated with both the gain minus loss difference score (r = .21, p < .01) and the area around the peak of the difference (r = .13, p < .05; see Fig. 5). Fig. 4 (top) presents ERPs to gain and loss for younger (Fig. 4A) and older (Fig. 4B) participants (based on a mean split) at the baseline assessment. Consistent with the impressions from Fig. 2, Fig. 4, older participants at the baseline assessment were characterized by more positive ERPs to both gains and losses – though this effect was larger for gains than losses.3
Fig. 2.
Scatterplots depicting the association between age at baseline and ERP amplitude to gains (A) and ERP amplitude to losses (B) at baseline, as well as the association between age at follow-up and ERP amplitude to gains (C) and ERP amplitude to losses (D) at follow-up.
Fig. 5.
Scatterplots depicting the association between age at baseline and the gain minus loss difference waveform (left) and the area around the peak of the difference waveform (right).
Fig. 4.
Feedback-locked ERPs at FCz in response to monetary gains (dark) and losses (light) as well as gain-loss difference (dashed) at baseline for younger participants (A) and older participants (B) and at the 2-year follow-up for younger participants (C) and older participants (D). A mean split of age was performed to divide the sample.
3.2. Within-subject change from baseline to follow-up
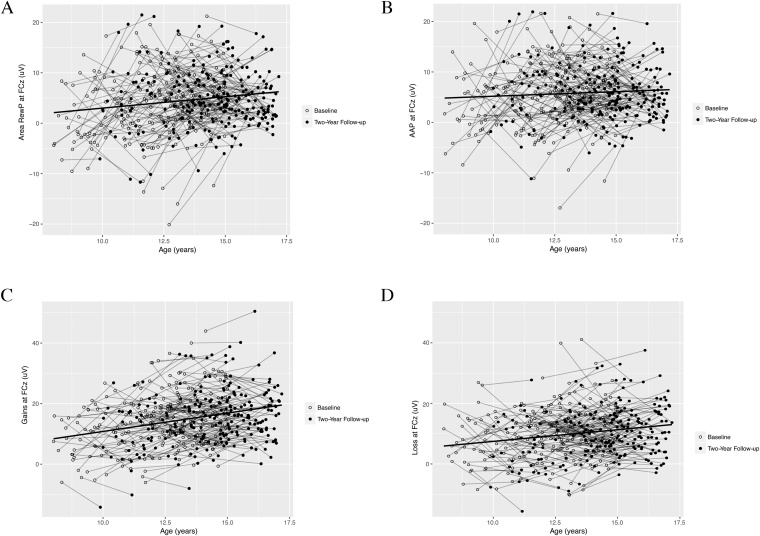
Fig. 3 presents longitudinal changes in the area around the peak of the RewP (3A), the area measure of the RewP (3B), the ERP amplitude to wins (3C) and the ERP amplitude to losses (3D). A 2 (Condition: Gain, Loss) X 2 (Time: Baseline, Follow-up) repeated measures ANCOVA with age at baseline as a continuous covariate revealed a non-significant main effect of condition (F(1, 238) = .58, p > .40), and time (F(1, 238) = .069, p > .50) and a non-significant interaction between time and age (F(1, 238) = .301, p > .50); however, there was a significant interaction between condition and age (F(1, 238) = 7.96, p < .01), a significant interaction between condition and time (F(1, 238) = 7.97,p < .01), and a significant 3-way interaction between condition, time, and age (F(1, 238) = 6.89, p < .01).
Fig. 3.
Longitudinal amplitude changes in the area around the peak (AAP) of the RewP (A), the mean area amplitude of the RewP (B), the ERP amplitude to gains (C) and the ERP amplitude to losses (D).
In terms of the Condition X Age interaction, the mean ERP response to gains across both testing sessions was more positive among older participants (r = .32, p < .001) and the mean ERP responses to losses across both testing sessions was also more positive among older participants (r = .24, p < .001); the former relationship was significantly larger than the latter (z = 2.07, p < .05, two-tailed). The Condition X Time interaction reflected the fact that the ERP response to gains increased from baseline to follow-up (F(1, 239) = 6.35, p < .05), whereas the ERP response to losses did not change (F(1, 239) = 0.76, p > .30). However, these two-way interactions were qualified by a three-way Age X Condition X Time interaction; to explore this three-way interaction, we examined the Condition by Time interaction separately among older and younger participants based on a median split. The Condition X Time interaction was significant among younger participants (F(1, 118) = 5.57, p < .05), such that the ERP response to gains increased from baseline to follow-up (F(1,118) = 4.82, p < .05) but the ERP response to losses did not change (F(1, 118) = 0.097, p > .76); however, the Condition X Time interaction was not significant among older participants (F(1, 120) = 0.20, p > .66)4,5 . A similar effect is reflected in Fig. 6, which plots the relationship between age at baseline and the change from baseline to follow-up in the gain minus loss difference—showing that there is an increased change in amplitude among younger participants from baseline to follow-up (r = -0.17, p < .01)6 .
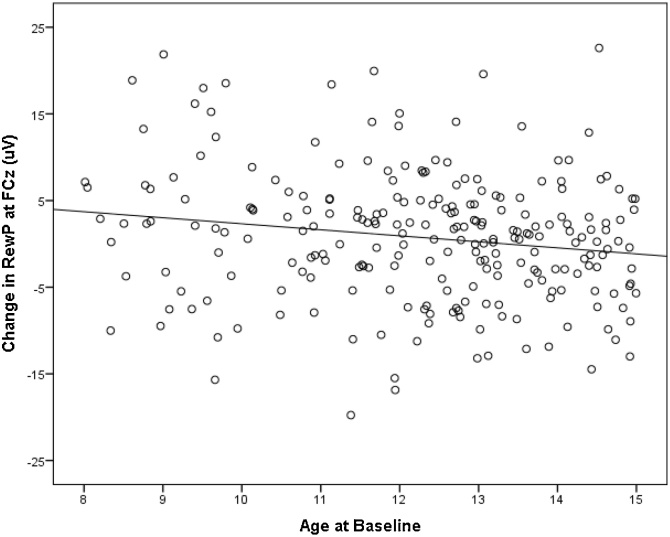
Fig. 6.
Scatterplot depicting the association between age at baseline and change in the area measure of the gain minus loss difference waveform at FCz from baseline to follow-up. The change scores were calculated by subtracting the gain minus loss difference at baseline from follow-up. Thus, the positive values on the y-axis indicate an increase from baseline to follow-up in the amplitude of the gain minus loss difference waveform. The negative correlation indicates that younger participants are showing an increase in the amplitude of the RewP from baseline to follow-up.
3.3. Two- year follow-up assessment
Fig. 1B presents ERPs for gain and loss, as well as the difference waveform and scalp distribution of the difference in the time range of the RewP, at the follow-up assessment. A 2 (Condition: Gain, Loss) repeated measures ANOVA with age at follow-up as a continuous covariate revealed a non-significant effect of Condition (F(1, 246) = 1.74, p > .15), and a non-significant interaction between age and condition (F(1, 246) = 0.12, p > .50)7 . Age was again correlated with both the ERP response to gains (Fig. 2C; r = .265, p < .001) and losses (Fig. 2D; r = .272, p < .001)—though these correlations did not differ from one another (z = -.176, p > .05, two-tailed). Fig. 4 presents ERPs from the follow-up assessment based on a mean split for younger (Fig. 4C) and older (Fig. 4D) participants. Consistent with the impressions from Fig. 2, Fig. 4, the gain minus loss difference waveform did not correlate with age (r = .022, p = 0.73); neither did the area around the peak of the difference waveform (r = -0.038, p = .55). Thus, at the 2-year follow-up assessment, age was equally related to both the ERP response to gains and losses8
4. Discussion
The present study utilized a longitudinal design to investigate developmental changes in the ERP response to gains and losses in a relatively large sample of adolescent females utilizing a simple gambling task. Age related to a larger (i.e., more positive) ERP response to gains and losses at baseline, when the sample was 8 to 14 years old; moreover, age-related effects at baseline were stronger for gains than losses such that the difference between gains and losses also related to age at baseline. Importantly, in within-subject analyses, the amplitude of the ERP response to gains, but not losses, significantly increased from baseline to follow-up—and this effect was only evident among younger participants. Younger participants were characterized by a larger increase in the gain minus loss difference waveform, the area around the peak of the difference waveform, and the residualized ERP response to rewards from baseline to follow-up. By the follow-up assessment, conducted when participants were 10 to 16, the ERP responses to both gains and losses were equally correlated with age. Thus, at follow-up assessment there was no longer evidence for specific age-related correlations with reward-related neural activity. Collectively, these within- and between-subjects findings suggest a relatively specific developmental increase in reward-related brain activity from late childhood to adolescence—an increase which may peak somewhere around age 12. These results are consistent with previous cross-sectional and longitudinal fMRI studies where ventral striatal response to reward versus loss or versus no-reward increases from late childhood through mid/late adolescence, before declining into adulthood (Braams et al., 2015; Schreuders et al., 2018; Van Leijenhorst et al., 2009; Ernst et al., 2005).
Aside from the Kujawa et al. (2017) study, the present study is the only other investigation to longitudinally examine age-related changes in ERP measures of reward processing during adolescence in the same participants. These data are contrary to Kujawa et al. (2017), who found that the mean activity of ERPs to rewards and non-rewards did not increase from childhood to adolescence within a mixed gender sample. However, the present study investigated a broader age range at each assessment (i.e. 8–14 years old at baseline; 10–16 years old at follow-up), whereas the Kujawa et al. (2017) focused on a rather narrow age range at each assessment. Additionally, the current study had many more participants, which may have provided increased power to detect age-related changes in reward specifically.
Previous cross-sectional research on the development of ERP components of reward processing across adolescence have produced mixed results. One potential issue concerns significant variation in how ERPs are scored. Of the studies that have found differences in ERPs to rewards and non-rewards during adolescence (i.e., Zottoli and Grose‐Fifer, 2012; Eppinger et al., 2009; Hämmerer et al., 2011), ERPs have been scored utilizing peak-to-peak amplitude measures. Of the studies that have not found age-related changes in the ERPs to rewards and non-rewards, ERPs were scored utilizing a peak amplitude approach (Santesso et al., 2011 and Yi et al., 2012) and mean activity of the difference waveform (Lukie et al., 2014 and Bress et al., 2012). As described by Luck (2014), peak-to-peak and peak amplitude approaches are susceptible to overlapping components and can be biased estimates of neural activity. The present study assessed developmental changes in both ERPs to rewards and losses separately, and also evaluated the difference score—as well as the area around the peak of the difference waveform. Relative to both Lukie et al. (2014) and Bress et al. (2012), an advantage of the present study was a much larger sample size and within-subject longitudinal measures of change.
Although the present sample was large, it was homogeneous insofar as only females were included. Future studies will need to determine whether these developmental results generalize to males. Future work might further determine whether the onset of puberty affects the development of reward-related neural activity, which may explain some of the discrepant findings evident in the ERP literature. Future studies might also consider decomposing the ERPs to wins and losses using time-frequency analyses, as research has shown that theta and delta activity relate to loss and win outcomes, respectively (Bernat et al., 2015; Foti et al., 2015). Investigating how theta and delta activity change during adolescence might provide additional insight into how neural responses to rewards and non-rewards develop in adolescence. Finally, trial-by-trial analyses of gains and losses might shed light on whether age modulates an individual’s ability to sustain their response to rewards and non-rewards over the course of the task.
In the context of both within- and between-subjects comparisons, the present results suggests a relatively specific developmental increase in the ERP response to rewards. These findings are consistent with the notion that early adolescence is characterized by potentiated increases in neural response to reward, an important process relevant to adaptive goal-directed behavior and individual differences (Ullsperger et al., 2014; Holroyd and Yeung, 2012) and depression (Nelson et al., 2016).
Author notes
This work was supported by the National Institutes of Health (MH097767) to GH. The authors have no conflicts of interest to report. Correspondence concerning this article should be addressed to Kreshnik Burani, Florida State University, Tallahassee, F L 32304.
Footnotes
Using Principal Component Analysis (PCA) derived factor scores, Kujawa et al. (2017) found that the RewP factor scores for both gains and losses increased from late childhood to early and middle adolescence.
A 2 (Time: Baseline, Follow-up) X 3 (Site: FCz, Fz, Cz) revealed a significant main effect of time (F(1, 239) = 4.03, p < .05) and site (F(1, 239) = 22.28, p < .001). Multiple pairwise comparisons revealed that the activity at FCz was greater than at Fz (p <. 001) and Cz (p< .005).
Age at baseline correlated with the residualized gain score (r = .26, p < .01) but not the residualized loss score (r = -.07, p > .20).
A repeated measures ANOVA to investigate the effects of the P300 on the ERP responses to gains and losses was conducted. A 2 (Time: Baseline, Follow-up) X 2 (Condition: Gains, Losses) repeated measures ANCOVA with age at baseline as a continuous covariate revealed a non-significant main effect of time (F(1, 238) = 1.39, p > .20), a non-significant time by age interaction (F(1,238) = 2.37,p > .10), non-significant time by condition interaction (F(1, 238) = 1.06, p > .31) and non-significant time X condition X age interaction (F(1, 238) = .53, p > .47). Overall, the P300 to losses was more positive compared to the P300 to gains (F(1, 238) = 5.86, p < .05). In addition, there was a significant interaction between condition and age (F(1, 238) = 6.61, p < .05). The condition by age interaction revealed the fact that the P300 to losses was significantly more positive than the P300 to gains among older participants (F(1, 121) = 5.15, p < .012) but not younger participants (F(1, 118) = .2, p > .20). The P300 was averaged separately for gain and loss trials and scored as the mean amplitude from 250 to 500 ms post feedback at Pz.
Identical effects were found using residualized gain and loss scores, and area around the peak of the difference waveform. For residualized gains, a 2 (Time: Baseline, Follow-up) repeated measures ANCOVA with age at baseline as a continuous covariate revealed a main effect of time (F(1, 238) = 7.30, p < .01) as well as a significant interaction between time and age (F(1,238) = 7.52, p < .01)—such that residualized gains increased more among younger participants. For residualized losses, a 2 (Time: Baseline, Follow-up) repeated measures ANCOVA with age at baseline as a continuous covariate revealed a significant main effect of time (F(1, 238) = 4.50, p< .05) and a significant interaction between time and age (F(1, 238) = 4.76, p < .05), such that residualized losses decreased more among younger participants. Using the area around the peak of the difference waveform, a 2(Time: Baseline, Follow-up) repeated measures ANCOVA with age at baseline as a continuous covariate revealed that there was a main effect of time (F(1, 238) = 4.4, p < .05) and a significant interaction between age and time (F(1, 238) = 4.18, p < .05), reflecting the fact that the area around the peak of the difference waveform increased more for younger participants.
Younger participants were also characterized by a larger change in the area around the peak of the difference waveform (r= -.13, p < .05), a larger change in residualized gains (r = -.18, p < .01), and a smaller change in residualized losses (r = .14, p < .05). However, age at baseline did not relate to change in the ERP response to gains (r = -.05, p = .39) or the ERP response to losses (r = .112, p > .08).
The ERP response to gains was more positive than the ERP to losses at follow-up when age was not included as a continuous covariate (F(1, 247) = 172.11, p < .001).
Age at the follow-up assessment did not correlate with residualized ERPs to gains (r = .08, p > .15) nor did it correlate with residualized ERPs to losses (r = .10, p > .11)
References
- Belden A.C., Irvin K., Hajcak G., Kappenman E.S., Kelly D., Karlow S. Neural correlates of reward processing in depressed and healthy preschool-age children. J. Am. Acad. Child Adolesc. Psychiatry. 2016;55(12):1081–1089. doi: 10.1016/j.jaac.2016.09.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernat E.M., Nelson L.D., Baskin-Sommers A.R. Time‐frequency theta and delta measures index separable components of feedback processing in a gambling task. Psychophysiology. 2015;52(5):626–637. doi: 10.1111/psyp.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braams B.R., van Duijvenvoorde A.C., Peper J.S., Crone E.A. Longitudinal changes in adolescent risk-taking: a comprehensive study of neural responses to rewards, pubertal development, and risk-taking behavior. J. Neurosci. 2015;35(18):7226–7238. doi: 10.1523/JNEUROSCI.4764-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bress J.N., Hajcak G. Self‐report and behavioral measures of reward sensitivity predict the feedback negativity. Psychophysiology. 2013;50(7):610–616. doi: 10.1111/psyp.12053. [DOI] [PubMed] [Google Scholar]
- Bress J.N., Smith E., Foti D., Klein D.N., Hajcak G. Neural response to reward and depressive symptoms in late childhood to early adolescence. Biol. Psychol. 2012;89(1):156–162. doi: 10.1016/j.biopsycho.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson J.M., Foti D., Mujica-Parodi L.R., Harmon-Jones E., Hajcak G. Ventral striatal and medial prefrontal BOLD activation is correlated with reward-related electrocortical activity: a combined ERP and fMRI study. Neuroimage. 2011;57(4):1608–1616. doi: 10.1016/j.neuroimage.2011.05.037. [DOI] [PubMed] [Google Scholar]
- Eppinger B., Mock B., Kray J. Developmental differences in learning and error processing: evidence from ERPs. Psychophysiology. 2009;46(5):1043–1053. doi: 10.1111/j.1469-8986.2009.00838.x. [DOI] [PubMed] [Google Scholar]
- Ernst M., Nelson E.E., Jazbec S., McClure E.B., Monk C.S., Leibenluft E. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25(4):1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Foti D., Weinberg A., Dien J., Hajcak G. Event‐related potential activity in the basal ganglia differentiates rewards from nonrewards: Temporospatial principal components analysis and source localization of the feedback negativity. Hum. Brain Mapp. 2011;32(12):2207–2216. doi: 10.1002/hbm.21182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D., Carlson J.M., Sauder C.L., Proudfit G.H. Reward dysfunction in major depression: multimodal neuroimaging evidence for refining the melancholic phenotype. Neuroimage. 2014;101:50–58. doi: 10.1016/j.neuroimage.2014.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D., Weinberg A., Bernat E.M., Proudfit G.H. Anterior cingulate activity to monetary loss and basal ganglia activity to monetary gain uniquely contribute to the feedback negativity. Clin. Neurophysiol. 2015;126(7):1338–1347. doi: 10.1016/j.clinph.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A. Adolescent development of the reward system. Front. Hum. Neurosci. 2010;4:6. doi: 10.3389/neuro.09.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G., Coles M.G., Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr. Clin. Neurophysiol. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hämmerer D., Li S.C., Müller V., Lindenberger U. Life span differences in electrophysiological correlates of monitoring gains and losses during probabilistic reinforcement learning. J. Cogn. Neurosci. 2011;23(3):579–592. doi: 10.1162/jocn.2010.21475. [DOI] [PubMed] [Google Scholar]
- Holroyd C.B., Coles M.G. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol. Rev. 2002;109(4):679. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd C.B., Yeung N. Motivation of extended behaviors by anterior cingulate cortex. Trends Cogn. Sci. (Regul. Ed.) 2012;16(2):122–128. doi: 10.1016/j.tics.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Holroyd C.B., Pakzad-Vaezi K.L., Krigolson O.E. The feedback correct‐related positivity: Sensitivity of the event‐related brain potential to unexpected positive feedback. Psychophysiology. 2008;45(5):688–697. doi: 10.1111/j.1469-8986.2008.00668.x. [DOI] [PubMed] [Google Scholar]
- Kujawa A., Smith E., Luhmann C., Hajcak G. The feedback negativity reflects favorable compared to nonfavorable outcomes based on global, not local, alternatives. Psychophysiology. 2013;50(2):134–138. doi: 10.1111/psyp.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A., Carroll A., Mumper E., Mukherjee D., Kessel E.M., Olino T. A longitudinal examination of event-related potentials sensitive to monetary reward and loss feedback from late childhood to middle adolescence. Int. J. Psychophysiol. 2017 doi: 10.1016/j.ijpsycho.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn P.M., Clarke G.N., Seeley J.R., Rohde P. Major depression in community adolescents: age at onset, episode duration, and time to recurrence. J. Am. Acad. Child Adolesc. Psychiatry. 1994;33(6):809–818. doi: 10.1097/00004583-199407000-00006. [DOI] [PubMed] [Google Scholar]
- Liu W.H., Wang L.Z., Shang H.R., Shen Y., Li Z., Cheung E.F., Chan R.C. The influence of anhedonia on feedback negativity in major depressive disorder. Neuropsychologia. 2014;53:213–220. doi: 10.1016/j.neuropsychologia.2013.11.023. [DOI] [PubMed] [Google Scholar]
- Luck S.J. MIT press; 2014. An Introduction to the Event-related Potential Technique. [Google Scholar]
- Lukie C.N., Montazer-Hojat S., Holroyd C.B. Developmental changes in the reward positivity: an electrophysiological trajectory of reward processing. Dev. Cogn. Neurosci. 2014;9:191–199. doi: 10.1016/j.dcn.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miltner W.H., Braun C.H., Coles M.G. Event-related brain potentials following incorrect feedback in a time-estimation task: evidence for a “generic” neural system for error detection. J. Cogn. Neurosci. 1997;9(6):788–798. doi: 10.1162/jocn.1997.9.6.788. [DOI] [PubMed] [Google Scholar]
- Nelson B.D., Perlman G., Klein D.N., Kotov R., Hajcak G. Blunted neural response to rewards as a prospective predictor of the development of depression in adolescent girls. Am. J. Psychiatry. 2016;173(12):1223–1230. doi: 10.1176/appi.ajp.2016.15121524. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D.A., Jahn A.L., O’Shea J.P. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol. Psychiatry. 2005;57(4):319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfit G.H. The gain positivity: from basic research on gain to a biomarker for depression. Psychophysiology. 2015;52(4):449–459. doi: 10.1111/psyp.12370. [DOI] [PubMed] [Google Scholar]
- Santesso D.L., Dzyundzyak A., Segalowitz S.J. Age, sex and individual differences in punishment sensitivity: Factors influencing the feedback‐related negativity. Psychophysiology. 2011;48(11):1481–1489. doi: 10.1111/j.1469-8986.2011.01229.x. [DOI] [PubMed] [Google Scholar]
- Schreuders E., Braams B.R., Blankenstein N.E., Peper J.S., Güroğlu B., Crone E.A. Contributions of reward sensitivity to ventral striatum activity across adolescence and early adulthood. Child Dev. 2018;89(3):797–810. doi: 10.1111/cdev.13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger M., Danielmeier C., Jocham G. Neurophysiology of performance monitoring and adaptive behavior. Physiol. Rev. 2014;94(1):35–79. doi: 10.1152/physrev.00041.2012. [DOI] [PubMed] [Google Scholar]
- Urošević S., Collins P., Muetzel R., Lim K., Luciana M. Longitudinal changes in behavioral approach system sensitivity and brain structures involved in reward processing during adolescence. Dev. Psychol. 2012;48(5):1488. doi: 10.1037/a0027502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leijenhorst L., Zanolie K., Van Meel C.S., Westenberg P.M., Rombouts S.A., Crone E.A. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cereb. Cortex. 2009;20(1):61–69. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L., Moor B.G., de Macks Z.A.O., Rombouts S.A., Westenberg P.M., Crone E.A. Adolescent risky decision-making: neurocognitive development of reward and control regions. Neuroimage. 2010;51(1):345–355. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Weinberg A., Riesel A., Proudfit G.H. Show me the money: the impact of actual rewards and losses on the feedback negativity. Brain Cogn. 2014;87:134–139. doi: 10.1016/j.bandc.2014.03.015. [DOI] [PubMed] [Google Scholar]
- Yeung N., Holroyd C.B., Cohen J.D. ERP correlates of feedback and reward processing in the presence and absence of response choice. Cereb. Cortex. 2004;15(5):535–544. doi: 10.1093/cercor/bhh153. [DOI] [PubMed] [Google Scholar]
- Yi F., Chen H., Wang X., Shi H., Yi J., Zhu X., Yao S. Amplitude and latency of feedback-related negativity: aging and sex differences. Neuroreport. 2012;23(16):963–969. doi: 10.1097/WNR.0b013e328359d1c4. [DOI] [PubMed] [Google Scholar]
- Zottoli T.M., Grose‐Fifer J. The feedback‐related negativity (FRN) in adolescents. Psychophysiology. 2012;49(3):413–420. doi: 10.1111/j.1469-8986.2011.01312.x. [DOI] [PubMed] [Google Scholar]