Abstract
Play is an important part of normal childhood development and seen in many mammals, including rats. To better understand the interplay between genotype and postnatal experiences, the effects of neonatal handling on play were assessed in Lewis (LEW) and Fischer 344 (F344) rats. Handled litters experienced brief periods of separation during the first two postnatal weeks. F344 rats were less likely to direct nape contacts towards an untreated Sprague-Dawley (SD) partner and less likely to rotate to a supine position in response to a nape contact. When compared to rats from control litters, handled LEW and F344 rats were more likely to respond to nape contacts with complete rotations, suggesting that handling increased playful responsiveness to a comparable extent in both strains. SD rats paired with handled inbred rats had more nape contacts than those paired with non-handled rats. While handled LEW rats also tended to direct more nape contacts to the SD partner than non-handled LEW rats there was no difference between handled and non-handled F344 rats. These results could not be readily explained by handling-induced changes in either maternal care or anxiety. These data suggest that the behavioral consequences of neonatal handling may not depend to a great extent on the genetic platform that these manipulations are acting on. These data also suggest that the ability to maintain the ebb and flow between playful solicitation and playful responsiveness may be compromised in F344 rats and may contribute to the lower levels of play in this strain.
Keywords: play, adolescent, early experience, mother-infant relations, neonatal
Play is a stable and robust behavioral phenotype observed among the young of many mammalian species and in a variety of other species such as various types of birds, reptiles, and invertebrates (Burghardt, 2005; Fagen, 1981; Pellis & Pellis, 2009). Rats are particularly playful, engaging in playful interactions prior to weaning and continuing throughout the juvenile period, peaking at around 35 days of age, and then steadily decreasing as the animals reach puberty (Panksepp, 1981). Play in the rat primarily takes the form of “rough-and-tumble” activity; rats will vigorously chase each other, pounce on each other’s dorsal surface, nuzzle the nape, and pin each other (Panksepp, Siviy, & Normansell, 1984; Pellis & Pellis, 2009; Siviy & Panksepp, 2011; Vanderschuren, Niesink, & Van Ree, 1997; Vanderschuren & Trezza, 2014). Play is under fairly tight regulatory control, being quite sensitive to variations in motivational state. For example, the amount of play observed during a discrete observation period (e.g., 5 – 15 minutes) can be readily titrated by housing rats in isolation for varied amounts of time (Panksepp & Beatty, 1980; Siviy, Baliko, & Bowers, 1997); a rat that has been isolated for 4 hours will play more than a rat that has not been isolated, and a rat that has been isolated for 24 hours will play more than the rat isolated for 4 hours. Play is also believed to have an important role in the developing organism since removing the opportunity to play in young rats can have a number of adverse consequences on later behavior and social/emotional functioning (Pellis & Pellis, 2007; Spinka, Newberry, & Bekoff, 2001; Van den Berg et al., 1999) as well as cognitive functioning (Baarendse, Counotte, O’Donnell, & Vanderschuren, 2013). Housing juvenile rats with non-playful partners after weaning leads to the same constellation of social and cognitive deficits (Burleson et al., 2016; Pellis, Williams, & Pellis, 2017; Schneider, Bindila, et al., 2016) further supporting the idea that these deficits are due to a lack of play rather than a lack of social contact per se. All of this would suggest that the young mammalian brain is programmed and motivated to engage in playful behaviors, with adverse consequences resulting when opportunities for play are prevented.
Just as play can have an impact on the later behavioral and cognitive phenotype of an animal, the playful phenotype itself is likely to be sensitive to both genetic and early postnatal influences. Rats that have been selectively bred for certain behavioral and/or physiological traits differ systematically in playfulness. For example, rats selectively bred to be more susceptible to amygdala kindling play more than those resistant to amygdala kindling (Reinhart, McIntyre, Metz, & Pellis, 2006; Reinhart, Pellis, & McIntyre, 2004) as do rats selectively bred for high rates of tickling-induced vocalizations (Webber et al., 2012). Comparing established strains of rat has also been a fruitful approach for studying the extent to which play can be modulated by genetic factors. For example, the Spontaneously Hypertensive Rat is less playful than either Wistar-Kyoto or Sprague-Dawley (SD) rats (Ferguson & Cada, 2004), Wistar rats are less playful than SD rats (Manduca et al., 2014) and work in our lab has identified the inbred Fischer 344 (F344) rat as a strain that is consistently less playful than other strains commonly used in behavioral research (Siviy et al., 1997; Siviy, Crawford, Akopian, & Walsh, 2011; Siviy, Love, DeCicco, Giordano, & Seifert, 2003).
Comparing strains with robust and stable phenotypic differences such as those described above may be a particularly useful approach for examining the extent to which early postnatal effects can act on an existing genotype to modulate playfulness. The first several weeks of a rat’s life are spent almost exclusively in the nest and it is during this time that the behavior of the mother towards her pups can have considerable impact on the eventual behavioral phenotype expressed by these pups upon weaning and beyond. For example, the amount of licking and grooming received during these first weeks has been shown to have a particularly long-lasting influence on a wide range of behaviors (Champagne & Curley, 2005; Meaney, 2001). Playfulness may also be influenced by experiences received in the nest during this early period of development as male rats receiving high levels of licking and grooming during the first few weeks after birth tend to be less playful than those receiving low levels of licking and grooming when play is assessed after weaning in the home cage (Moore & Power, 1992; Parent & Meaney, 2008). However, a recent study looking at play as a function of within-litter variation in maternal care found a significant positive correlation between the amount of licking and grooming received by male rats during the first week after birth and frequency of pinning, pouncing, and latency to initial social exploration (van Hasselt et al., 2012). In other words, male rats receiving more licking and grooming as newborns were more playful and more socially curious as juveniles. While these findings may seem incompatible on the surface, it may be relevant that playfulness in the latter study was assessed during a discrete observation period in a neutral testing chamber after an acute period of isolation, whereas those studies finding that low levels of licking/grooming lead to more play assessed play in the home cage without any prior isolation. This suggests that maternal behavior in general, and licking/grooming in particular, can impact the playfulness of the pup but how this is reflected may be modulated by motivational variables.
Another approach towards studying the effects of early postnatal experiences on behavior has been to systematically manipulate the postnatal environment. It is well established that brief daily separations of rat pups from the mother during the first several weeks of life dampens the behavioral and hormonal responses to a variety of stressors while longer periods of isolation can have the opposite effect (Caldji et al., 1998; Meaney et al., 2000; Pryce, Bettschen, Barhr, & Feldon, 2001; Raineki, Lucion, & Weinberg, 2014). Similarly, pups that have experienced brief (e.g., 1 – 15 minutes) daily periods of separation from the mother have been reported to play more than those from undisturbed litters when tested after an acute period of social isolation (Aguilar, Carames, & Espinet, 2009; Siviy & Harrison, 2008). However, when play is assessed in the home cage without any prior isolation, juvenile rats that have experienced brief daily periods of separation from the mother during the first 10 days of life tend to play less than those reared in undisturbed litters (Karkow & Lucion, 2013). This is particularly intriguing since this procedure of brief daily maternal separation, also known as “handling”, has been reported to increase the amount of licking and grooming by the mother towards her litter (Liu et al., 1997). Taken together, these studies highlight the extent to which early postnatal experiences can impact later playfulness and how this impact is manifest may depend on the motivational state of the animal.
Since early postnatal experiences occur against the backdrop of a genetic framework it is important to gain a better understanding of the potential interplay between genotype and early postnatal experiences, and how these interactions may be affecting later playfulness. Having identified the F344 strain as being consistently less playful than other strains of rat, we have begun to look at the extent to which these robust strain differences in play can be tempered by systematic alterations in the early postnatal environment. In a recent study (Siviy, Eck, McDowell, & Soroka, 2017), we used a cross-fostering design to assess the relative playfulness of F344 and Lewis (LEW) rats when either in-fostered or cross-fostered. Strain differences in overall levels of playfulness were relatively unaffected by cross-fostering, suggesting that the overall urge to play in these two inbred strains may be somewhat insensitive to variations in early rearing. However, there were subtle effects associated with cross-fostering and these were most apparent in LEW rats and were dependent upon the amount of isolation prior to testing. In particular, LEW rats that were reared by F344 mothers did not show the same isolation-induced changes in playful solicitations or responsiveness as did LEW rats reared by LEW mothers. These data suggest that the overall playfulness of an animal may be particularly sensitive to genetic variation while the extent to which play can be modulated by motivational variables may be more likely influenced by epigenetic factors.
In the present study, we sought to further assess the extent to which systematic variations in the early postnatal environment could impact strain differences in the play of F344 and LEW rats. Towards this end, the effects of brief periods of maternal separation during the first two postnatal weeks on play in both F344 and LEW rats were assessed. In keeping with suggested terminology (e.g., Pryce & Feldon, 2003; Raineki et al., 2014) this manipulation will be referred to as neonatal handling. Given the extent to which early postnatal experiences may be affecting play through modulation of motivational variables, play was assessed after both 4 and 24 hours of isolation. Given the known effect of early neonatal handling on stress and anxiety, the effects of handling on behavior in the elevated plus maze was also assessed.
Methods
Subjects and housing
Female F344 and LEW (Harlan, Indianapolis) arrived at 14 days gestation. A comparable number of female Sprague-Dawley (SD) rats also arrived at 14 days gestation in order to provide standardized play partners for the F344 and LEW rats. Rats were housed on corn-cob bedding with ample nesting material in solid-bottom cages (59 cm x 38 cm x 20 cm) with food and water freely available. The colony room was maintained at 22° C with a 12/12 light/dark cycle (lights on at 08:00). All housing and testing was done in compliance with the NIH Guide for Care and Use of Laboratory Animals using protocols approved by the Institutional Animal Care and Use Committee at Gettysburg College.
Handling procedure
Pregnant females were checked in the morning and late afternoon for births, with the day of birth designated as post-natal day (PND) 0. Litters were culled on PND1 to no more than eight pups and, when possible, culled to four males and four females. Four litters from each of the inbred strains were assigned to receive brief daily periods of maternal separation (“handling”) while the remaining 4 litters from each inbred strain were assigned to be non-handled controls. For those litters assigned to the handling condition, the handling procedure began on PND2 and continued through PND15 and occurred at approximately the same time (between 11:00 and 13:00) each day. On each of these days, the mother was removed from the litter and placed in a holding cage in the main colony room. The entire litter was then transported in the home cage to an adjacent room. The pups were removed from the home cage and placed along with their littermates in a smaller container, also containing corncob bedding, which was kept warm with a heating pad. After 15 minutes, the pups were returned to the home cage, transported back to the colony room, and the mother returned to the litter. The non-handled control litters and all of the SD litters were left undisturbed, with the exception of weekly cage maintenance, until weaning.
Maternal observations
Beginning on PND2 and continuing for the next 10 days, litters were observed at various times throughout the day within four possible time windows: 09:00 – 11:00, 15:00 – 17:00, 18:00 – 19:30, 21:00 – 23:00. Using a protocol originally developed by Myers and colleagues (Myers, Brunelli, Squire, Shindeldecker, & Hofer, 1989) and used previously in our lab (Siviy et al., 2017) a discrete observation was made every 4 minutes for each litter over the course of each 1 hour period. With 16 observations in a 1 hour observation period and 28 observation periods over 10 days, this resulted in a total of 448 discrete observations. A total of 22 hours were observed during the light phase and 6 hours during the dark phase. For each observation, the location of the mother (in or out of the nest) was noted. If the mother was in the nest any type of nursing (arch-back, blanket, or passive) was noted along with any licking and grooming of the pups.
Post-weaning behaviors
Pups were weaned at 21 days of age and re-housed in groups of 3–5 in solid-bottom cages (48 cm x 27 cm x 20 cm) with rats of the same sex and same handling condition. To minimize the risk of “litter effects” (Holson & Pearce, 1992) no more than 2 rats of each sex from each litter were used for behavioral testing. With one exception, the final sample size for each group comprised of strain, handling condition, and sex was n=8. The sample size for Lewis females from the handled condition was n=7. Rats were individually handled for 2 days after weaning and were tested for play behavior between 28 and 33 days of age. Anxiety was assessed on an elevated plus maze between 36 and 40 days of age. A subset of these rats was re-tested on the elevated plus maze as adults. All behavioral testing was done approximately midway through the light phase of the light/dark cycle.
Play was assessed in a clear Plexiglas chamber (40 × 40 × 50 cm) that was enclosed within a sound-attenuated wooden chamber illuminated by a single 25W red light bulb. The floor of the testing chamber was covered with approximately 3 cm of Aspen pine shavings. Play bouts were recorded as digital video files and scored later using behavioral observation software (Noldus Observer XT: Noldus Information Technology) by an observer unaware of the strain of the animal and the treatment condition. All of the rats were initially acclimated to the testing chamber by being placed individually in the testing chamber for 10 minutes on two separate days. Play was then assessed over 2 days with each rat tested after 4 hours of isolation and after 24 hours of isolation. At least 48 hours separated the two tests and the order of testing was counterbalanced as much as possible between isolation, strain, sex, and handling condition. Each inbred rat was paired with a same-age and same-sex unfamiliar Sprague-Dawley (SD) rat in order to provide a standardized play partner for each strain. The SD partner was isolated to the same extent as the inbred rat it was paired with and was paired with the same inbred rat for the two tests.
Play was quantified by counting the frequency of contacts directed by the inbred subject rat towards the nape of the target SD rat (nape contacts) and the likelihood that a nape contact directed by the target SD rat to the test rat resulted in that rat rotating completely to a supine position (probability of a complete rotation). Nape contacts were quantified by frequency of occurrence while complete rotations were quantified in probabilistic terms by calculating the probability of a complete rotation occurring in response to a nape contact. These two measures of playfulness have been commonly used in this lab and are also thought to be controlled by independent motivational and neural substrates (Pellis & Pellis, 1991; Pellis & Pellis, 1987; Siviy et al., 1997; Siviy et al., 2011; Siviy & Panksepp, 1987). Rats were re-housed socially after all testing was completed for that day.
Anxiety was assessed by testing rats in an elevated plus maze (Med Associates, St. Albans, VT) using Ethovision video-tracking software (Noldus Information Technology, Netherlands) to track the location of the rat. The elevated plus maze has two open arms (10 cm x 50 cm), two closed arms (10 cm x 50 cm with 40 cm high walls) and a 10 cm x 10 cm center area. Rats were placed in the center of the maze and time spent in the open arms monitored for 5 minutes.
Results
Maternal observations
Observational data from the inbred strains for time in the nest, arch-back nursing, blanket nursing, passive nursing, and licking/grooming were each analyzed by a 2 × 2 ANOVA with strain of the litter (F344, LEW) and handling (non-handled control, handled) as factors (Table 1). Time spent in the nest did not differ significantly as a function of either strain or handling condition. However, there were significant strain differences in the different types of nursing. F344 rats were more likely to be engaged in arch-back nursing, F(1,12) = 27.58, p < .001, but less likely to be engaged in either blanket nursing, F(1,12) = 5.07, p = .044, or passive nursing, F(1,12) = 45.45, p < .001. There were no significant effects associated with handling or interactions for any of the types of nursing. Time spent engaged in licking and grooming also did not differ as a function of either strain or handling condition, nor was there a significant strain x handling condition interaction for licking and grooming.
Table 1.
Percentage (± SEM) of observation time dams found to be in the nest, engaged in nursing or actively licking/grooming pups for each treatment condition.
| LEW |
F344 |
||||
|---|---|---|---|---|---|
| Behavior | SD | Control | Handled | Control | Handled |
| In nest | 68.3 ± 3.1 | 68.5 ± 3.4 | 68.8 ± 1.7 | 70.6 ± 5.5 | |
| Arch back nursing | 32.8 ± 3.0 | 36.3 ± 4.4 | 56.9 ± 3.9* | 58.7 ± 5.9* | |
| Blanket nursing | 13.4 ±1.9 | 9.7 ± 1.4 | 7.8 ±1.1* | 7.4 ± 2.4* | |
| Passive nursing | 20.8 ± 2.9 | 20.1 ± 3.4 | 3.9 ± 2.0* | 3.6 ± 1.0* | |
| Licking/grooming | 7.4 ± 1.3 | 6.0 ± 0.6 | 6.9 ± 0.3 | 6.8 ± 0.8 | |
p < .05 for main effect of strain
Play behavior
Play was assessed by the number of nape contacts directed by the inbred subject towards the SD partner and the likelihood (probability) that a nape contact directed towards the inbred subject by the SD partner resulted in a complete rotation to a supine position. The data for each measure of play were analyzed by a mixed-factors ANOVA with 2 levels of strain (F344, LEW), 2 levels of handling condition (non-handled control, handled), 2 levels of sex (male, female), and 2 levels of isolation (4 hours, 24 hours). Strain, handling condition, and sex were between-subjects factors while isolation was a within-subjects factor. Any significant interactions were pursued with separate ANOVAs and t-tests with Bonferroni corrections for multiple comparisons when necessary.
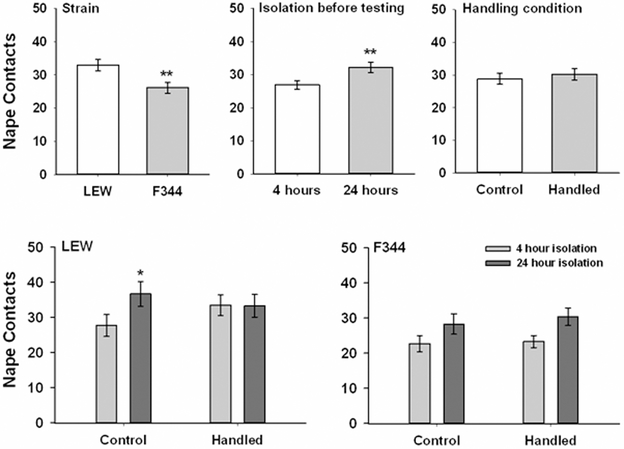
The results for nape contacts can be seen in Fig. 1. As expected, there was a significant main effect of strain, F(1,55) = 7.90, p = .007, with F344 rats having fewer nape contacts than Lewis rats. There was also an expected significant main effect of isolation, F(1,55) = 12.56, p = .01, with more nape contacts after 24 hours of isolation than after 4 hours of isolation. There was a marginal strain x isolation x handling condition interaction, F(1,55) = 3.32, p = .074. To examine this putative interaction further, separate ANOVAs with isolation and handling condition as factors were conducted separately for F344 and LEW rats. For F344 rats there was a significant main effect of isolation, F(1,30) = 10.6, p = .003, with more contacts after 24 hours of isolation than after 4 hours of isolation, suggesting that increasing isolation had the effect of increasing this measure of play to a comparable extent in both handling condition groups. For Lewis rats there was a significant isolation x handling condition interaction, F(1,29) = 4.21, p = .049. A matched-samples t-test with a Bonferroni correction comparing nape contacts between the two isolation conditions for each handling condition found a significant increase in nape contacts after 24 hours of isolation compared to 4 hours of isolation among the control group but not among the handled group.
Figure 1.
Mean (± SEM) frequency of nape contacts by control and handled F344 and LEW rats when tested after 4 and 24 hours of isolation. The top 3 panels reflect the results of the main effects associated with strain, isolation before testing and handling condition. The bottom 2 panels reflect the overall data and are collapsed across sex. * p < .05, ** p < .01
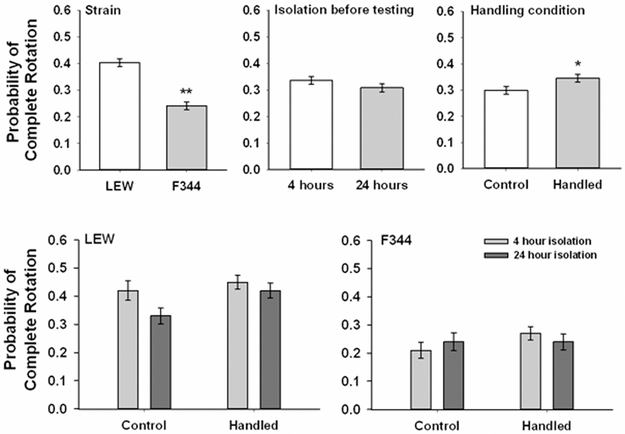
The likelihood of responding to a nape contact with a complete rotation can be seen in Fig. 2. The data were analyzed in the same way as nape contacts. There was a significant main effect of strain, F(1,55) = 61.19, p < .001, with F344 rats less likely to respond to a nape contact with a complete rotation than LEW rats. There was also a significant main effect of handling condition, F(1,55) = 4.72, p = .034, with handled rats more likely to rotate completely than control rats. No other main effects or interactions were significant.
Figure 2.
Mean (± SEM) probability of responding to a nape contact with a complete rotation to a supine position by control and handled F344 and LEW rats when tested after 4 and 24 hours of isolation. The top 3 panels reflect the results of the main effects associated with strain, isolation before testing and handling condition. The bottom 2 panels reflect the overall data and are collapsed across sex. * p < .05, ** p < .01
Elevated plus maze
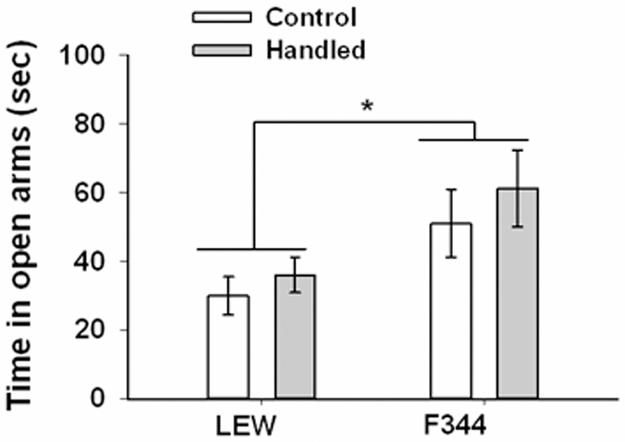
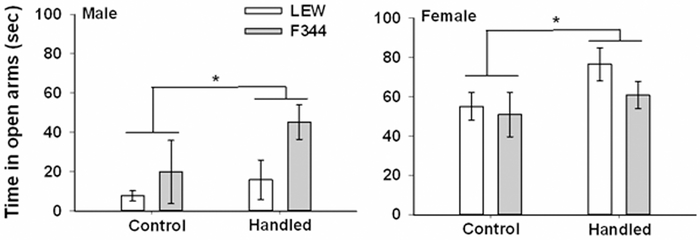
For time spent in the open arms when tested as juveniles (Fig. 3) there was a significant main effect of strain, F(1,53) = 6.98, p = .011, with F344 rats spending more time on the open arms. No other main effects or interactions were significant. A subset of these rats were tested later as adults and for this test, there was a significant main effect of handling condition, F(1,23) = 6.39, p = .019, with handled rats spending more time on the open arms than control rats (Fig. 4). There was also a significant main effect of sex, F(1,23) = 36.21, p < .001, with females spending more time on the open arms than males although this was tempered by a significant strain x sex interaction, F(1,23) = 5.65, p = .026. Further analysis of this interaction revealed that the sex difference was only apparent among LEW rats.
Figure 3.
Time (± SEM) spent in the open arms of the elevated plus maze for control and handled F344 and LEW rats when tested as juveniles. * p < .05
Figure 4.
Time (± SEM) spent in the open arms of the elevated plus maze for control and handled F344 and LEW rats when tested as adults.
Discussion
Since two landmark studies published roughly 60 years ago (Denenberg & Karas, 1959; Levine, Alpert, & Lewis, 1957) it has been well established that separating rat pups from the mother and removing them from the nest for brief (i.e., 1 – 15 minutes) periods every day for the first 2 weeks or so of life, an experimental protocol commonly known as “handling”, can have a number of behavioral, hormonal, and cognitive consequences later in life (Champagne & Curley, 2005; Meaney, 2001; Parent et al., 2005; Raineki et al., 2014). Rats that have been handled as neonates have also been reported to be more playful than non-handled control rats when tested after acute periods of isolation (Aguilar et al., 2009; Siviy & Harrison, 2008) but less playful than non-handled controls when play is observed in their home cage without any acute isolation (Karkow & Lucion, 2013). This suggests that a fairly modest manipulation to the early postnatal milieu can have an impact on the later playfulness of a rat, although the exact nature of that effect may depend on how play is assessed and the motivational state of the rat at the time of testing.
With a few exceptions (Poltyrev & Weinstock, 1999; Río-Ȧlamos et al., 2015; Río-Álamos et al., 2017; Skripuletz, Kruschinski, Pabst, von Hörsten, & Stephan, 2010; Stephan, Helfritz, Pabst, & von Horsten, 2002), most studies looking at the consequences of early postnatal manipulations have assessed these effects in a single strain of rat. In order to further examine the dynamic interplay likely to be occurring between early postnatal experiences and genetic background, the present study assessed the effects of neonatal handling on play in the inbred F344 and LEW strains. Rats of the F344 strain have been shown to be consistently less playful than rats of the LEW and other strains to which they have been compared (Siviy et al., 1997; Siviy et al., 2011; Siviy et al., 2017; Siviy et al., 2003) so comparing the impact of a well-defined manipulation such as neonatal handling in two inbred strains with well-characterized phenotypic differences may allow for a better understanding of how these types of early postnatal experiences can operate on an existing genotype to influence the behavioral phenotype.
In a recent cross-fostering study play was assessed in both cross-fostered and in-fostered F344 and LEW rats (Siviy et al., 2017) and it was found that the overall strain difference in playfulness was relatively unaffected by fostering status. In other words, F344 rats that were reared by LEW mothers were no more playful than those reared by F344 mothers. Similarly, LEW rats that were reared by F344 mothers played no less than those reared by LEW mothers. While the overall urge to play was somewhat resistant to cross-fostering and perhaps dependent to a large extent on genetic variability, how that urge was titrated by motivational factors (e.g. isolation prior to testing) was affected by cross-fostering. For example, LEW rats that were reared by F344 mothers did not show the same isolation-induced changes in playful solicitations or responsiveness as did LEW rats reared by LEW mothers. This suggests that some aspects of how play is modulated may be more sensitive to early experiences than others and this may depend to some extent on the genetic background of the rat. With this in mind, play was assessed in handled and non-handled F344 and LEW rats after 4 and 24 hours of acute social isolation.
As expected, F344 rats were less playful than LEW rats in the present study when given opportunities to play with a standard play partner. This was exhibited both in play solicitation (F344 rats having fewer nape contacts) and playful responsiveness (F344 rats less likely to respond to a nape contact with a complete rotation). Also as expected, play solicitation increased as time of isolation prior to testing increased from 4 to 24 hours while responsiveness to play solicitation was unaffected by length of isolation. Handling had subtle effects on playfulness that were dependent on the measure of play, strain, and amount of isolation prior to testing. In terms of playful responsiveness, rats that were handled during the neonatal period were more likely to respond to a nape contact with a complete rotation than rats from the non-handled control litters and this was independent of the strain of rat and isolation condition. In other words, handling increased the likelihood of responding to a nape contact with a complete rotation to the same extent in both F344 and LEW rats and to the same extent whether the rats were isolated for 4 or 24 hours prior testing. Since responding to a nape contact with a complete rotation is thought to prolong a play bout and/or signal playful intent (Pellis & Pellis, 1991) an increase in this measure of play would be consistent with other studies showing an increase in playfulness among handled rats (Aguilar et al., 2009; Siviy & Harrison, 2008).
While neonatal handling resulted in both LEW and F344 rats being more responsive to the playful overtures of their partners, this was not reflected by a parallel increase in playful solicitation as handled rats were found to be as likely overall to solicit play as non-handled controls. Despite the lack of an overall effect of handling on play solicitation, there was some indication that handling may be having a differential impact on how motivational state modulates playful solicitations of F344 and LEW rats. An expected increase in play solicitation was seen as isolation prior to testing increased from 4 to 24 hours among control LEW rats. However, this was not seen in LEW rats from the handled condition. Increasing isolation increased play solicitation to a comparable extent in both control and handled F344 rats. This pattern of results for LEW rats is remarkably similar to what we observed in a previous study looking at the effects of cross-fostering on play in LEW and F344 rats (Siviy et al., 2017). In particular, in-fostered LEW rats in that study had the expected increase in play solicitation as pre-testing isolation increased from 4 hours to 24 hours whereas cross-fostered LEW rats failed to show any motivational modulation of play solicitation. Perhaps the extent to which LEW rats respond to an increase in the motivation for play is particularly sensitive to perturbations of the early postnatal environment, such as through cross-fostering or handling. On the other hand, F344 rats may tend to be less sensitive to the effects of early postnatal influences on play solicitation. One possible interpretation of these data is that handling may have negated the extent to which increasing motivation to play can modulate play solicitation in LEW rats. It is also possible that any further increase in nape contacts among the handled LEW rats after 24 hours of prior isolation may have been prevented by a “ceiling effect”. As such, the extent to which handling affects play solicitation in LEW rats cannot be definitively ascertained entirely from these data.
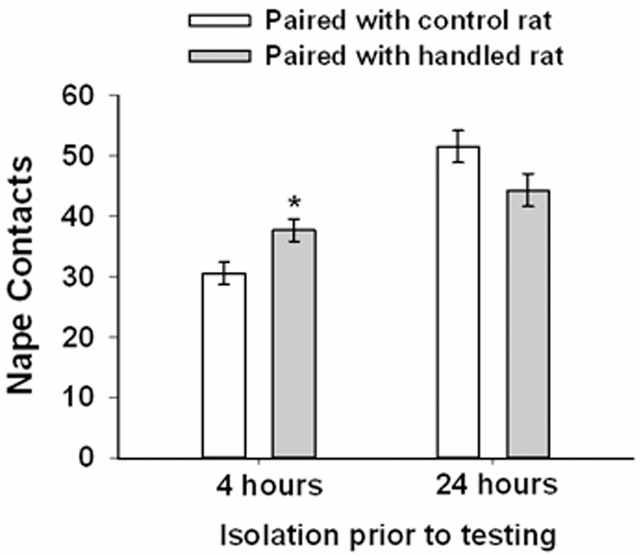
F344 and LEW rats were paired with SD rats that did not receive any postnatal manipulations beyond routine cage maintenance and that were similarly isolated prior to testing. This was done in order to provide a standardized test partner for rats of each strain and handling condition. In order to determine whether the SD rats interacted differently to their inbred partner as a function of either strain of that partner or handling condition, nape contacts directed by the SD rat towards the inbred partner were analyzed in the same way as was the data from the inbred rats. There was no significant effect associated with strain of partner, indicating that SD rats directed a comparable number of nape contacts whether partnered with a LEW or F344 rat. As expected, there was a significant effect of isolation, F(1,55) = 46.19, p < .01, with SD rats directing more nape contacts after 24 hours of isolation than after 4 hours of isolation. Interestingly, there was also a significant isolation x handling condition interaction, F(1,55) = 12.08, p = .001 (Fig. 5). Further analysis of this interaction revealed that when isolated for 4 hours prior to testing those SD rats paired with a handled partner had more nape contacts than those paired with a control rat. This was not the case when tested after 24 hours of isolation. This suggests that being paired with a handled rat changed the behavior of the target rat and likely reflects the contagious nature of play (Pellis & McKenna, 1992; Reinhart et al., 2006). These data, combined with the subtle effects of handling described above for LEW rats and the increased likelihood of rotating completely to a supine position, support previous research (Aguilar, 2010; Aguilar et al., 2009; Siviy & Harrison, 2008) showing that neonatal handling can increase playfulness in rats. With these data in mind we can also tentatively conclude that neonatal handling may be enhancing the motivation to play in LEW rats and that this is most likely to be detected when baseline levels of play are at a sub-maximal level (e.g., after 4 hours of social isolation).
Figure 5.
Mean (± SEM) frequency of nape contacts by target SD rats when paired with either a control or non-handled control inbred rat and tested after 4 and 24 hours of isolation. Data are collapsed across strain and sex of the inbred rat. * p < .05
Rats that have been handled as neonates tend to be less reactive to stress and less anxious when assessed in either the open field or elevated plus maze when tested as adults (Caldji, Francis, Sharma, Plotsky, & Meaney, 2000; Meerlo, Horvath, Nagy, Bohus, & Koolhaas, 1999; Raineki et al., 2014; Severino et al., 2004; Vallee et al., 1997). As such, we also assessed behavior in the elevated plus maze in the present study. When tested as juveniles, F344 rats spent more time on the open arms of the elevated plus maze than LEW rats and this is consistent with what we have observed previously (Siviy et al., 2017). However, handled rats spent as much time on the open arms as the non-handled rats suggesting that handling did not affect baseline levels of anxiety in either strain. Since the stress-reducing effects of handling may not be as readily apparent prior to puberty (Severino et al., 2004) a subset of rats was tested again as adults. For this test, handled rats of both strains spent more time in the open arms than non-handled rats. This shows that the handling manipulation as done in the present study was able to yield behavioral results in the elevated plus maze comparable to what would be expected based on past research. At the same time, the lack of an effect of handling when rats were tested as juveniles also suggests that the effects of handling on play cannot be easily accounted for by concomitant changes in anxiety.
The neonatal handling procedure as used in the present study has been reported to increase the amount of licking and grooming by the mother towards her litter (Liu et al., 1997) so the behavioral consequences associated with handling may be due to an increase in the amount of licking and grooming by the mother towards her litter. With this in mind, maternal behavior was observed in the present study during the first 2 postnatal weeks and no differences on any measure of maternal behavior were found between control and handled litters. While this was unexpected, other studies have also reported mixed effects of handling on licking and grooming by the mother (Pryce, Bettschen, & Feldon, 2001; Reis et al., 2014). Our observation times may also not have been optimal for detecting separation-induced alterations in maternal behavior, such as during the first hour immediately after the separation period (Skripuletz et al., 2010). Accordingly, our data do not preclude the possibility that changes in maternal behavior immediately upon reunion of mother to pups could be contributing to the effects of handling observed in the present study. Other aspects associated with the procedure, such as the actual separation from the mother or the handling of the pups at the beginning and end of each separation period also cannot be discounted as contributing factors associated with the behavioral consequences observed in the present study. With these caveats in mind, any sustained changes in maternal behavior over the course of the first 2 postnatal weeks among the handled litters cannot readily explain any effects associated with handling in the present study.
Taken together, these data suggest that the behavioral consequences of early postnatal manipulations such as neonatal handling may not depend to a great extent on the genetic platform that these manipulations are acting on. Indeed, the preponderance of data from the present study seems to indicate that LEW and F344 rats are affected to a comparable extent by neonatal handling. For example, neonatal handling decreased anxiety to a comparable extent in both adult LEW and F344 rats. In regards to play, neonatal handling increased the likelihood of responding to a nape contact with a complete rotation to a comparable extent in both LEW and F344 rats. Given that rotating completely to a supine position is thought to prolong playful interactions (Pellis & Pellis, 1991) it is likely that increases in this type of response to nape contacts among the inbred rats led to the observed increases in play solicitations among the SD partners paired with handled rats when play was at a sub-maximal level (e.g., assessed after 4 hours of isolation). Increased solicitation by the target SD rats may have then been countered with more nape contacts by the LEW rats, but not by F344 rats. This suggests that F344 rats may be particularly unresponsive to the contagious nature of play and is consistent with what has been reported by others when F344 rats are housed with rats of a more playful strain during adolescence (Schneider, Bindila, et al., 2016; Schneider, Pätz, Spanagel, & Schneider, 2016). If so, then the apparent inability of neonatal handling to impact nape contacts in F344 rats may have less to do with how rats of this strain respond to neonatal handling and more to do with an inability to adjust their behavior in response to the ebb and flow of a play bout. The ability to detect and respond to cues from a play partner may then be somewhat fragile in F344 rats and this may contribute to the lower levels of playfulness in rats of this strain.
Acknowledgements
This work was supported by NIMH grant R15MH100585 to S.M. Siviy. The technical assistance of Mary O’Mara, Josh Rubinstein, and Rose Fogliano is greatly appreciated.
References
- Aguilar R (2010). Infantile experience and play motivation. Social Neuroscience, 5, 422–440. [DOI] [PubMed] [Google Scholar]
- Aguilar R, Carames JM, & Espinet A (2009). Effects of neonatal handling on playfulness by means of reversal of the desire to play in rats (Rattus norvegicus). Journal of Comparative Psychology, 123(4), 347–356. [DOI] [PubMed] [Google Scholar]
- Baarendse PJJ, Counotte DS, O’Donnell P, & Vanderschuren LJMJ (2013). Early social experience is critical for the development of cognitive control and dopamine modulation of prefrontal cortex function. Neuropsychopharmacology, 38, 1485–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt GM (2005). The Genesis of Animal Play: Testing the Limits. Cambridge, MA: MIT Press. [Google Scholar]
- Burleson CA, Pedersen RW, Seddighi S, DeBusk LE, Burghardt GM, & Cooper MA (2016). Social play in juvenile hamsters alters dendritic morphology in the medial prefrontal cortex and attenuates effects of social stress in adulthood. Behavioral Neuroscience, 130(4), 437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldji C, Francis D, Sharma S, Plotsky PM, & Meaney MJ (2000). The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology, 22, 219–229. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, & Meaney MJ (1998). Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proceedings of the National Academy of Science (U.S.A.), 95, 5335–5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, & Curley JP (2005). How social experiences influence the brain. Current Opinion in Neurobiology, 15, 704–709. [DOI] [PubMed] [Google Scholar]
- Denenberg VH, & Karas GG (1959). Effects of infantile handling upon weight gain and mortality in the rat and mouse. Science, 130, 629–630. [DOI] [PubMed] [Google Scholar]
- Fagen R (1981). Animal Play Behavior. New York: Oxford University Press. [Google Scholar]
- Ferguson SA, & Cada AM (2004). Spatial learning/memory and social and nonsocial behaviors in the Spontaneously Hypertensive, Wistar-Kyoto and Sprague-Dawley rat strains. Pharmacology Biochemistry and Behavior, 77(3), 583–594. [DOI] [PubMed] [Google Scholar]
- Holson RR, & Pearce B (1992). Principles and pitfalls in the analysis of prenatal treatment effects in mutiparous species. Neurotoxicology and Teratology, 14, 221–228. [DOI] [PubMed] [Google Scholar]
- Karkow ARM, & Lucion AB (2013). Mild environmental intervention in mother-infant interactions reduces social play behavior in rats. Psychology and Neuroscience, 6, 39–44. [Google Scholar]
- Levine S, Alpert M, & Lewis GW (1957). Infantile experience and the maturation of the pituitary adrenal axis. Science, 126, 1347. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freeman A, Sharma S, Pearson D, Plotsky PM, & Meaney MJ (1997). Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science, 277, 1659–1662. [DOI] [PubMed] [Google Scholar]
- Manduca A, Servadio M, Campolongo P, Palmery M, Trabace L, Vanderschuren LJMJ, Cuomo V, & Trezza V (2014). Strain- and context-dependent effects of the anandamide hydrolysis inhibitor URB597 on social behavior in rats. European Neuropsychopharmacology, 24(8), 1337–1348. [DOI] [PubMed] [Google Scholar]
- Meaney MJ (2001). Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review Of Neuroscience, 24, 1161–1192. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Diorio J, Francis D, Weaver S, Yau J, Chapman K, & Seckl JR (2000). Postnatal handling increases the expression of cAMP-inducible transcription factors in the rat hippocampus: The effects of thyroid hormones and serotonin. The Journal of Neuroscience, 20, 3926–3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerlo P, Horvath KM, Nagy GM, Bohus B, & Koolhaas JM (1999). The influence of postnatal handling on adult neuroendocrine and behavioural stress reactivity. Journal of Neuroendocrinology, 11, 925–933. [DOI] [PubMed] [Google Scholar]
- Moore CL, & Power KL (1992). Variation in maternal care and individual differences in play, exploration, and grooming of juvenile Norway rat offspring. Developmental Psychobiology, 25, 165–182. [DOI] [PubMed] [Google Scholar]
- Myers MM, Brunelli SA, Squire JM, Shindeldecker RD, & Hofer MA (1989). Maternal behavior of SHR rats and its relationship to offspring blood pressures. Developmental Psychobiology, 22, 29–53. [DOI] [PubMed] [Google Scholar]
- Panksepp J (1981). The ontogeny of play in rats. Developmental Psychobiology, 14, 327–332. [DOI] [PubMed] [Google Scholar]
- Panksepp J, & Beatty WW (1980). Social deprivation and play in rats. Behavioral and Neural Biology, 30, 197–206. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Siviy SM, & Normansell L (1984). The psychobiology of play: Theoretical and methodological considerations. Neuroscience and Biobehavioral Reviews, 8, 465–492. [DOI] [PubMed] [Google Scholar]
- Parent C, Zhang T-Y, Caldji C, Bagot R, Champagne FA, Pruessner J, & Meaney MJ (2005). Maternal care and individual differences in defensive responses. Current Directions in Psychological Science, 14, 229–233. [Google Scholar]
- Parent CI, & Meaney MJ (2008). The influence of natural variations in maternal care on play fighting in the rat. Developmental Psychobiology, 50(8), 767–776. [DOI] [PubMed] [Google Scholar]
- Pellis SM, & McKenna MM (1992). Intrinsic and extrinsic influences on play fighting in rats: effects of dominance, partner’s playfulness, temperament and neonatal exposure to testosterone propionate. Behavioural Brain Research, 50, 135–145. [DOI] [PubMed] [Google Scholar]
- Pellis SM, & Pellis VC (1991). Attack and defense during play fighting appear to be motivationally independent behaviors in muroid rodents. The Psychological Record, 41, 175–184. [Google Scholar]
- Pellis SM, & Pellis VC (2007). Rough-and-tumble play and the development of the social brain. Current Directions in Psychological Science, 16, 95–98. [Google Scholar]
- Pellis SM, & Pellis VC (2009). The Playful Brain: Venturing to the Limits of Neuroscience. Oxford: Oneworld Publications. [Google Scholar]
- Pellis SM, & Pellis VM (1987). Play-fighting differs from serious fighting in both target of attack and tactics of fighting in the laboratory rat Rattus norvegicus. Aggressive Behavior, 13, 227–252. [Google Scholar]
- Pellis SM, Williams LA, & Pellis VC (2017). Adult-juvenile play fighting in rats: Insight into the experiences that facilitate the development of socio-cognitive skills. International Journal of Comparative Psychology, 30, https://escholarship.org/uc/item/30b37d05g. [Google Scholar]
- Poltyrev T, & Weinstock M (1999). Effect of gestational stress on maternal behavior in response to cage transfer and handling of pups in two strains of rat. Stress, 3, 85–95. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Bettschen D, Barhr NI, & Feldon J (2001). Comparison of the effects of infant handling, isolation, and nonhandling on acoustic startle, prepulse inhibition, locomotion, and HPA activity in the adult rat. Behavioral Neuroscience, 115, 71–83. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Bettschen D, & Feldon J (2001). Comparison of the effects of early handling and early deprivation on maternal care in the rat. Developmental Psychobiology, 38, 239–251. [DOI] [PubMed] [Google Scholar]
- Pryce CR, & Feldon J (2003). Long-term neurobehavioural impact of the postnatal environment in rats: manipulations, effects and mediating mechanisms. Neuroscience and Biobehavioral Reviews, 27, 57–71. [DOI] [PubMed] [Google Scholar]
- Raineki C, Lucion AB, & Weinberg J (2014). Neonatal handling: An overview of the positive and negative effects. Developmental Psychobiology, 56(8), 1613–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart CJ, McIntyre DC, Metz GA, & Pellis SM (2006). Play fighting between kindling-prone (FAST) and kindling-resistant (SLOW) rats. Journal of Comparative Psychology, 120, 19–30. [DOI] [PubMed] [Google Scholar]
- Reinhart CJ, Pellis SM, & McIntyre DC (2004). Development of play fighting in kindling-prone (FAST) and kindling-resistant (SLOW) rats: How does the retention of phenotypic juvenility affect the complexity of play? Developmental Psychobiology, 45(2), 83–92. [DOI] [PubMed] [Google Scholar]
- Reis AR, de Azevedo MS, de Souza MA, Lutz ML, Alves MB, Izquierdo I, Cammarota M, Silveira PP, & Lucion AB (2014). Neonatal handling alters the structure of maternal behavior and affects mother–pup bonding. Behavioural Brain Research, 265, 216–228. [DOI] [PubMed] [Google Scholar]
- Río-Ȧlamos C, Oliveras I, Cañete T, Blázquez G, Martínez-Membrives E, Tobeña A, & Fernández-Teruel A (2015). Neonatal handling decreases unconditioned anxiety, conditioned fear, and improves two-way avoidance acquisition: a study with the inbred Roman high (RHA-I)- and low-avoidance (RLA-I) rats of both sexes. Frontiers in Behavioral Neuroscience, 9, 174–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Río-Álamos C, Oliveras I, Piludu MA, Gerbolés C, Cañete T, Blázquez G, Lope-Piedrafita S, Martinez-Membrives E, Torrubia R, Tobena A, & Fernández-Teruel A (2017). Neonatal handling enduringly decreases anxiety and stress responses and reduces hippocampus and amygdala volume in a genetic model of differential anxiety: Behavioral-volumetric associations in the Roman rat strains. European Neuropsychopharmacology, 27(2), 146–158. [DOI] [PubMed] [Google Scholar]
- Schneider P, Bindila L, Schmahl C, Bohus M, Meyer-Lindenberg A, Lutz B, Spanagel R, & Schneider M (2016). Adverse Social Experiences in Adolescent Rats Result in Enduring Effects on Social Competence, Pain Sensitivity and Endocannabinoid Signaling. Frontiers in Behavioral Neuroscience, 10(203). doi: 10.3389/fnbeh.2016.00203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider P, Pätz M, Spanagel R, & Schneider M (2016). Adolescent social rejection alters pain processing in a CB1 receptor dependent manner. European Neuropsychopharmacology, 26(7), 1201–1212. [DOI] [PubMed] [Google Scholar]
- Severino GS, Fossati IAM, Padoin MJ, Gomes CM, Trevizan L, Sanvitto GL, Franci CR, Anselmo-Fraci JA, & Lucion AB (2004). Effects of neonatal handling on the behavior and prolactin stress response in male and female rats at various ages and estrous cycle phases of females. Physiology & Behavior, 81(3), 489–498. [DOI] [PubMed] [Google Scholar]
- Siviy SM, Baliko CN, & Bowers KS (1997). Rough-and-tumble play behavior in Fischer-344 and Buffalo rats: Effects of social isolation. Physiology and Behavior, 61, 597–602. [DOI] [PubMed] [Google Scholar]
- Siviy SM, Crawford CA, Akopian G, & Walsh JP (2011). Dysfunctional play and dopamine physiology in the Fischer 344 rat. Behavioural Brain Research, 220, 294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siviy SM, Eck SR, McDowell LS, & Soroka J (2017). Effects of cross-fostering on play and anxiety in juvenile Fischer 344 and Lewis rats. Phsyiology and Behavior, 169, 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siviy SM, & Harrison KA (2008). Effects of neonatal handling on play behavior and fear towards a predator odor in juvenile rats (Rattus norvegicus). Journal of Comparative Psychology, 122, 1–8. [DOI] [PubMed] [Google Scholar]
- Siviy SM, Love NJ, DeCicco BM, Giordano SB, & Seifert TL (2003). The relative playfulness of juvenile Lewis and Fischer-344 rats. Physiology and Behavior, 80, 385–394. [DOI] [PubMed] [Google Scholar]
- Siviy SM, & Panksepp J (1987). Sensory modulation of juvenile play in rats. Developmental Psychobiology, 20, 39–55. [DOI] [PubMed] [Google Scholar]
- Siviy SM, & Panksepp J (2011). In search of the neurobiological substrates for social playfulness in mammalian brains. Neuroscience and Biobehavioral Reviews, 35, 1821–1830. [DOI] [PubMed] [Google Scholar]
- Skripuletz T, Kruschinski C, Pabst R, von Hörsten S, & Stephan M (2010). Postnatal experiences influence the behavior in adult male and female Fischer and Lewis rats. International Journal of Developmental Neuroscience, 28(7), 561–571. [DOI] [PubMed] [Google Scholar]
- Spinka M, Newberry RC, & Bekoff M (2001). Mammalian play: Training for the unexpected. The Quarterly Review of Biology, 76, 141–168. [DOI] [PubMed] [Google Scholar]
- Stephan M, Helfritz F, Pabst R, & von Horsten S (2002). Postnatally induced differences in adult pain sensitivity depend on genetics, gender and specific experiences: reversal of maternal deprivation effects by additional postnatal tactile stimulation or chronic imipramine treatment. Behavioural Brain Research, 133, 149–158. [DOI] [PubMed] [Google Scholar]
- Vallee M, Mayo W, Dellu F, Le Moal M, Simon H, & Maccari S (1997). Prenatal stress induces high anxiety and postnatal handling induces low anxiety in adult offspring: Correlation with stress-induced corticosterone secretion. The Journal of Neuroscience, 17, 2626–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg CL, Hol T, Van Ree JM, Spruijt BM, Everts H, & Koolhaas JM (1999). Play is indispensable for an adequate development of coping with social challenges in the rat. Developmental Psychobiology, 34, 129–138. [PubMed] [Google Scholar]
- van Hasselt FN, Tieskens JM, Trezza V, Krugers HJ, Vanderschuren LJMJ, & Joëls M (2012). Within-litter variation in maternal care received by individual pups correlates with adolescent social play behavior in male rats. Physiology & Behavior, 106(5), 701–706. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Niesink RJM, & Van Ree JM (1997). The neurobiology of social play behavior in rats. Neuroscience and Biobehavioral Reviews, 21, 3090–3326. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, & Trezza V (2014). What the laboratory rat has taught us about social play behavior: role in behavioral development and neural mechanisms. Current Topics in Behavioral Neuroscience, 16, 189–212. [DOI] [PubMed] [Google Scholar]
- Webber ES, Harmon KM, Beckwith TJ, Peña S, Burgdorf J, Panksepp J, & Cromwell HC (2012). Selective breeding for 50 kHz ultrasonic vocalization emission produces alterations in the ontogeny and regulation of rough-and-tumble play. Behavioural Brain Research, 229(1), 138–144. [DOI] [PubMed] [Google Scholar]