Abstract
Estrogen receptor–positive early breast cancer is common and has a relatively good prognosis. It shares risk factors with cardiovascular disease, and cardiovascular disease is an important competing cause of mortality. Adjuvant endocrine therapy with aromatase inhibitors (requiring concomitant ovarian suppression in premenopausal women) or selective estrogen receptor modulators (usually tamoxifen) exert oncologic benefits by respectively inhibiting estradiol synthesis or breast estrogen receptor signaling. Aromatase inhibitors cause systemic estradiol depletion. Tamoxifen has mixed agonistic/antagonistic effects in a tissue-dependent fashion. Given that estrogens modulate cardiometabolic risk, a review of the effects of endocrine therapy on cardiometabolic outcomes is pertinent. The current, but limited, evidence suggests that tamoxifen treatment, although associated with increases in body fat, hepatic steatosis, serum triglycerides, and diabetes risk, modestly reduces low-density lipoprotein cholesterol and lipoprotein(a) and may have favorable effects on markers of subclinical atherosclerosis. Tamoxifen is associated with either no effect on, or a reduction in, cardiovascular events, and it is associated with an increase in venous thromboembolic events. Aromatase inhibitors, although fewer studies are available and often confounded by comparison with tamoxifen, have not been consistently associated with adverse changes in cardiometabolic risk factors or increases in cardiovascular events. Further clinical trials designed to evaluate cardiometabolic outcomes are needed to more accurately determine the effects of endocrine therapy on cardiovascular risks, to inform individualized decisions regarding choice and duration of endocrine therapy, and to implement evidence-based strategies to mitigate cardiometabolic risks. In the meantime, although breast cancer–specific evidence for benefit of lifestyle measures is available and recommended routinely, proactive monitoring and treatment of cardiovascular risk factors should follow general population recommendations.
Keywords: breast cancer, cardiovascular disease, aromatase inhibitor, selective estrogen receptor modulator
Breast cancer is the most common solid organ cancer diagnosed in women worldwide. In the United States, one in eight women will develop breast cancer in her lifetime, and, in 2018, there were 3.1 million breast cancer survivors living in the United States [1]. Approximately 75% of women diagnosed with early stage breast cancer have estrogen receptor–positive (ER+) disease, and virtually all of these women will be offered adjuvant endocrine therapy to inhibit ER activation and prevent recurrence [2]. This can be accomplished either with aromatase inhibitors, which require concomitant ovarian suppression in premenopausal women, or with selective estrogen receptor modulators (SERMs, usually tamoxifen), which can be used as monotherapy irrespective of menopausal status. Given that women with ER+ early breast cancer have a relatively good prognosis, with 5-year survival exceeding 90% [3], considering adverse effects of endocrine therapy is of prime importance.
Herein, we review the cardiometabolic effects of adjuvant endocrine therapy in women with ER+ early breast cancer, including effects on body composition, hepatic lipid accumulation, glucose and lipid metabolism, arterial wall, and clinical cardiovascular events.
The material discussed is based on peer-reviewed journals within the PubMed database from January 1970 to 28 February 2018. The search terms “aromatase inhibitor,” “atherosclerosis,” “breast cancer,” “body composition,” “cardiovascular disease,” “cerebrovascular disease,” “diabetes,” “hypertension,” “lipids,” “metabolic syndrome,” “selective estrogen receptor modulators,” “steatosis,” and “tamoxifen” were used. We also searched the references listed in relevant publications. Original research articles, reviews, and societal guidelines were considered.
1. Background
A. Cardiometabolic Effects of Estrogens in Women
Reflecting widespread expression of ERs, cardiometabolic effects of endogenous estrogens [mainly 17β-estradiol (estradiol), the major bioactive endogenous estrogen, and its metabolites estrone, and estriol] and of exogenous estrogens are operative in many somatic tissues [4]. These effects are complex and depend on age, comorbidities, genetic background, and, with respect to exogenous estrogens, on dose, route (oral vs transdermal), and timing (early vs late menopause) of administration, and on concomitant progestin treatment [5]. Observational studies suggest that endogenous estrogens have favorable associations with body composition, glucose and lipid metabolism, and endothelial function and atherosclerosis, especially in prematurely and early postmenopausal women [6]. Trials of estradiol treatment, administered in physiologic doses to women with premature menopause, have also reported improved cardiometabolic outcomes [7]. Effects of menopausal hormone therapy (MHT) are complex, with suggestions of some benefits in women with recent menopause (<6-10 years), but not in women who are >10 years postmenopausal [8, 9], the so-called “timing hypothesis” [10]. MHT is not recommended for prevention of cardiovascular disease [5]. Given evidence supporting cardiometabolic benefits of estrogens, especially in younger women (see further details on individual endpoints below), endocrine therapy that interferes with estrogenic signaling could increase cardiometabolic risks.
B. Effects of Endocrine Therapy on Estrogen Receptor Signaling
In premenopausal women with an intact gonadal axis, aromatase inhibitors counteract estradiol-mediated negative hypothalamic–pituitary feedback and stimulate gonadotropin-mediated ovarian estradiol production. Aromatase inhibitors require concomitant ovarian suppression, either by GnRH analogs or bilateral ovariectomy. This causes rapid and profound reductions in circulating estradiol, from premenopausal concentrations to nearly zero.
In postmenopausal women, aromatase inhibitors profoundly (>95%) inhibit aromatase, the rate-limiting enzyme in the conversion of androgens to estradiol, causing “virtually complete” circulating estradiol deficiency, with reductions of plasma estradiol to ≤3 pmol/L (the detection limit of sensitive mass spectrometry–based assays), concentrations markedly below those measured in natural menopause, typically ranging from 20 to 50 pmol/L [11]. Improved oncologic outcomes [3] provide proof-of-principle data that residual postmenopausal estrogens are of biological significance.
Tamoxifen, the most commonly used SERM, acts as a pure ER antagonist in breast tissue [12], but as a partial ER agonist in other tissues such as the skeleton, liver, uterus, and the coagulation system [13, 14]. Although rigorous human data quantifying tissue-specific ER signaling potencies of tamoxifen are lacking, tamoxifen is considered to be a less potent ER agonist than native estradiol [12]. Therefore, clinical effects of tamoxifen on tissues other than breast depend on endogenous estradiol availability. In premenopausal women, tamoxifen competes with estradiol, a more potent ER ligand, to reduce net ER signaling. In postmenopausal women who have low estradiol, the partial agonistic tamoxifen activity may increase net ER signaling. This is true for the skeleton, based on data that tamoxifen treatment accelerates bone loss in premenopausal women, but mitigates bone loss in postmenopausal women [15].
Aromatase inhibitors, owing to global estradiol deprivation, could have adverse cardiometabolic effects, whereas the effects of tamoxifen should be favorable in tissues where it acts as an ER agonist. These effects are likely modified by age, time from menopause, and the degree of preexisting cardiovascular disease, and, for tamoxifen, endogenous estradiol availability.
C. Oncologic Benefits of Endocrine Therapy
In postmenopausal women, aromatase inhibitors are superior to tamoxifen, with a modest improvement in 10-year breast cancer mortality (12.1% vs 14.2%, relative risk, 0.85; 95% CI, 0.75 to 0.96, P < 0.01) [3]. Whereas initial studies used endocrine treatment for 5 years, in women with high-risk breast cancer, in particular those with node-positive disease [16], extending endocrine therapy to 10 years increases disease-free survival, which includes a reduction in local recurrences and new primary breast cancers [17].
In premenopausal women, more aggressive estradiol deprivation has been shown to be beneficial over tamoxifen monotherapy, especially in high-risk early breast cancer. The Tamoxifen and Exemestane Trial and Suppression of Ovarian Function Trial have reported improved disease-free survival with the combined use of aromatase inhibition and ovarian suppression compared with either tamoxifen monotherapy or the combined use of tamoxifen and ovarian function suppression [18–20].
If supported by further evidence, the use of more aggressive, longer duration endocrine therapy in many women may increase, potentially exposing more women to increased risks of adverse cardiometabolic outcomes. Of note, the US Preventive Services Task Force recently recommended aromatase inhibitors for breast cancer prevention in high-risk women, which could further increase the number of women exposed to potentially adverse cardiometabolic outcomes [21].
D. Cardiometabolic Disease in Women With Early Breast Cancer
Breast cancer and cardiovascular disease share several risk factors, including postmenopausal obesity [22], hyperinsulinemia/diabetes [23], and physical inactivity [24]. In postmenopausal women with early breast cancer, cardiovascular disease risk may exceed breast cancer recurrence risk even prior to commencing endocrine therapy [25]. Women commonly receive nonendocrine therapies associated with cardiotoxicity, including radiotherapy, anthracycline-based chemotherapy, and targeted therapies such as trastuzumab [26]. Women with breast cancer have a higher risk of cardiovascular disease mortality than do women of the general population, and risk factors include older age, preexisting cardiovascular risk factors, and black ethnic origin [27]. Although breast cancer remains the most common cause of death in women with early breast cancer [28], cardiovascular death is a major cause of competing mortality. Cardiovascular disease becomes the leading cause of death in older women (≥70 years of age) [29], especially in those surviving ≥5 or years after breast cancer diagnosis [28]. Even modest adverse effects of endocrine therapy on cardiovascular outcomes may be important.
2. Effects of Endocrine Therapy on Cardiometabolic Outcomes
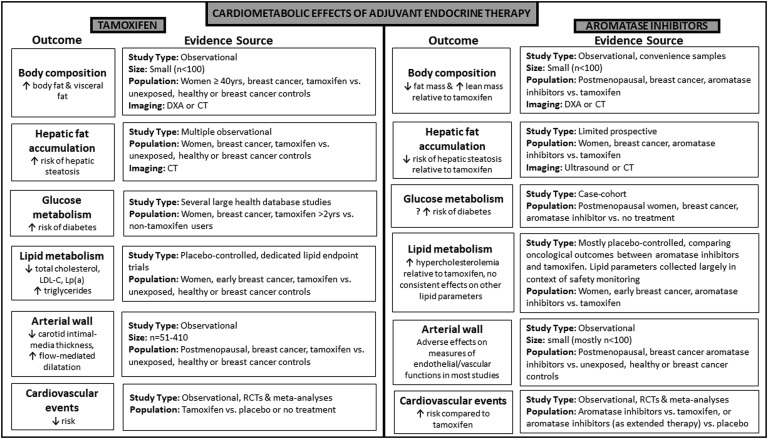
Herein, we discuss the effects of endocrine therapy on potential cardiac risk factors and clinical cardiovascular events. These are summarized in Fig. 1.
Figure 1.
Cardiometabolic effects of adjuvant endocrine therapy. Main outcomes and evidence sources for tamoxifen and aromatase inhibitors are presented. DXA, dual-energy X-ray absorptiometry; LDL, low density lipoprotein; Lp(a), lipoprotein(a); n, number; RCT, randomized control trial.
A. Body Composition
In women without breast cancer, both experimentally induced premature menopause [30] and natural menopause transition have been associated with increases in total and visceral adipose tissue [31], which, in most studies, is mitigated by estradiol add-back [30, 31]. Adiposity may be a proximate cause contributing to adverse cardiometabolic consequences of estradiol deprivation. In women with breast cancer, the postdiagnosis period is associated with weight gain, predominantly of body fat [32], and concomitant loss of muscle mass, in part due to chemotherapy-induced ovarian failure [33]. Effects of endocrine therapy on body composition are not well described. Current data are limited to observational studies of small (n < 100) convenience samples, with limited confounder adjustments. In cross-sectional, case-control studies of women with early breast cancer ≥40 years of age, using dual-energy X-ray absorptiometry or CT scanning, tamoxifen use was associated with a higher amount of body fat, visceral adipose tissue, and liver fat, compared with age- and body mass index–matched women with [34] or without [35, 36] breast cancer not receiving tamoxifen. In a longitudinal study, postmenopausal women (mean age, 61 years) with prior tamoxifen treatment of 2 to 3 years were switched to the aromatase inhibitor exemestane (n = 28) or continued on tamoxifen (n = 27). After 12 months, total fat mass (by dual-energy X-ray absorptiometry) in the exemestane group decreased by 8% but remained stable in the tamoxifen group (between-group P < 0.01), with no between-group difference in body weight, self-reported caloric intake, or physical activity [37]. In a secondary analysis of a 2-year randomized controlled trial (RCT) of 82 women with early breast cancer newly postmenopausal after chemotherapy (mean age, 51 years), women receiving aromatase inhibitors had an increase in lean mass (+1.2 kg) in conjunction with an increase in free testosterone, whereas women not receiving aromatase inhibitors (comprising women receiving SERMs or no endocrine treatment) had increased total body fat (+1%), with between-group differences being significant [38]. In one uncontrolled study of 41 premenopausal women (mean age, 44 years), fat mass increased by 3 kg and lean mass decreased by −0.8 kg 6 months after commencing tamoxifen plus ovarian suppression, and these changes were partially reversed by subsequent exercise [39]. However, given that ovarian suppression can cause changes in body composition, the extent to which reported effects were due to tamoxifen remains uncertain in this uncontrolled study [39]. Overall, the evidence suggests that tamoxifen treatment is associated with an increase in body fat. The few available studies have not demonstrated metabolically adverse changes in body composition with aromatase inhibitor treatment. More work is needed before firm conclusions can be drawn.
B. Hepatic Fat Accumulation
Hepatic steatosis is closely associated with the metabolic syndrome and may be an independent factor of cardiovascular risk [40]. Estrogens protect against hepatic steatosis, in part mediated by hepatic ERα signaling [41]. Despite acting as a partial hepatic ER agonist [42], tamoxifen treatment is associated with an increased risk of hepatic steatosis in women with breast cancer [43]. Experimental data from healthy postmenopausal women have demonstrated that this is likely due to an indirect effect of tamoxifen on reducing GH-mediated hepatic lipoprotein export, an effect not observed with estradiol treatment [44]. Although an association between tamoxifen and hepatic steatosis is well documented [36, 41, 43], effects of aromatase inhibitor treatment on hepatic fat accumulation are less well described. In an observational study of 1203 Korean women without hepatic steatosis at baseline, hepatic steatosis incidence (diagnosed by ultrasound) was higher in tamoxifen-treated than in aromatase inhibitor–treated women (128.7 vs 81.1 per 1000 person-years, P = 0.021) and associated with higher serum triglyceride and lower high-density lipoprotein (HDL) cholesterol concentrations [45]. Likewise, in a randomized 3-year prospective study of 353 Chinese women, the cumulative incidence of hepatic steatosis (diagnosed by CT) was lower in aromatase inhibitor-treated compared with tamoxifen-treated women (14.6% vs 41.1%, P < 0.0001) [46]. In summary, whereas tamoxifen is associated with an increased risk of hepatic steatosis compared with aromatase inhibitor or no endocrine treatment, aromatase inhibitor–associated risks have not been adequately investigated.
C. Glucose Metabolism
Most studies suggest that the menopausal transition [47] or premature menopause [48] is associated with increased risks of developing insulin resistance, the metabolic syndrome, and type 2 diabetes. This may be mediated by metabolically adverse changes in body composition and by the direct effect of estrogens on insulin sensitivity and pancreatic β-cells. Estrogens may also modulate glucose metabolism by effects on the central nervous system [49]. In clinical trials, MHT improves glucose homeostasis by insulin-dependent and insulin-independent mechanisms [47].
Female mice with a targeted deletion of the aromatase gene are at increased risk of diabetes, a phenotype prevented by estradiol treatment [50]. Tamoxifen promotes diabetes in female mice, and, in vitro, antagonizes estradiol-mediated protection against apoptosis in pancreatic β-cells [50]. Tamoxifen inhibits β-cell proliferation in an ERα-dependent fashion [51]. This suggests that tamoxifen has ER-antagonistic effects in pancreatic β-cells.
In an RCT of postmenopausal women at high risk of breast cancer, low-dose tamoxifen treatment (5 mg/d) decreased insulin sensitivity (estimated by the homeostasis model assessment: OR, 0.15; 95% CI, 0.03 to 0.88, P = 0.04), compared with women not receiving tamoxifen, an effect, however, derived from a post hoc analysis restricted to trial participants who were overweight or obese [52]. In a nested case-control Canadian health database study of 14,360 breast cancer survivors ≥65 years of age followed for 5.8 years, tamoxifen treatment was associated with a 24% increased risk of diabetes compared with no tamoxifen treatment (adjusted OR, 1.24; 95% CI, 1.08 to 1.42; P = 0.002) [53]. A 31% increased diabetes risk in tamoxifen users, compared with non–tamoxifen users, was also reported in a Taiwanese database study involving 22,257 women with early breast cancer ≥20 years of age followed for up to 12 years [54]. These studies included too few women receiving aromatase inhibitor treatment to reliably estimate diabetes risk [53, 54]. In a more recent case-cohort study from Israel of 2246 postmenopausal women with early breast cancer followed for 5.9 years, aromatase inhibitor treatment was associated with a higher risk of incident diabetes [hazard ratio (HR), 4.27; 95% CI, 1.42 to 12.84; P = 0.010 vs no endocrine treatment] than tamoxifen (HR, 2.25; 95% CI, 1.19 to 4.26; P = 0.013 vs no endocrine treatment) [55]. A Surveillance Epidemiology and End Results database study of postmenopausal women reported no increased diabetes risk with either tamoxifen or aromatase inhibitor treatment within 2 years of endocrine treatment initiation, suggesting that longer follow-up is required [56]. Within the limitations inherent to observational studies (e.g., residual confounding, nonrandomized treatment), the data suggest that both tamoxifen and aromatase inhibitor treatment, especially when used for >2 years, is associated with an increased risk of developing diabetes.
D. Lipid Metabolism
In postmenopausal women, low-dose oral estradiol treatment reduces serum low-density lipoprotein (LDL) cholesterol, but increases HDL cholesterol and triglycerides, with treatment-associated changes in circulating lipid concentrations ∼15% to 30% [57]. This occurs, at least in part, via hepatic ER-mediated effects on the expression of hepatic genes involved in the regulation of lipid metabolism. Compared with oral estradiol treatment, effects are less marked with transdermal administration, due to lack of hepatic first pass effect [57].
In healthy postmenopausal women, tamoxifen decreases LDL cholesterol to similar degrees [58] as oral estradiol treatment, likely due to ER agonism at the liver. In a 2-year RCT designed to evaluate the effects of tamoxifen on serum lipids in 140 postmenopausal women (44 to 64 years of age) with early breast cancer, total cholesterol decreased by 12% and LDL by 20%, with no change in HDL, whereas triglycerides increased by 20% [59]. These changes were sustained in a subgroup of 62 women followed for 5 years [60]. At 5 years, a modest decrease in lipoprotein(a) [Lp(a)] was reported [60] and subsequently confirmed by a small meta-analysis of five RCTs with 215 participants (standardized mean difference, −0.41; 95% CI, −0.68 to −0.14; P = 0.003) [61]. Similar effects of tamoxifen on lipid parameters have been reported in smaller placebo-controlled studies in women with early breast cancer [62].
There is limited RCT evidence in women with breast cancer comparing the effects of aromatase inhibitor treatment on lipid parameters with placebo. In a 2-year placebo-controlled dedicated lipid endpoint RCT in 147 postmenopausal women with early breast cancer (mean age, 60 years), treatment with the aromatase inhibitor exemestane decreased HDL by 9% compared with a 2% increase with placebo (P < 0.001), and marginally increased homocysteine (P = 0.018) [63]. Between-group differences were no longer significant 12 months after cessation of aromatase inhibitor therapy [64]. In an breast cancer prevention RCT in 4560 postmenopausal women (mean age, 62.5 years) at high risk of breast cancer, after a median follow-up of 35 months, as part of safety monitoring, no significant differences with respect to hypercholesterolemia and hypertriglyceridemia were detected between aromatase inhibitor-treated and placebo-treated groups [65].
Several studies have reported less favorable lipid profiles in women receiving aromatase inhibitors compared with tamoxifen. Beneficial effects of tamoxifen may confound interpretation of RCTs where aromatase inhibitors were compared with, or sequenced after, tamoxifen. These RCTs were not designed as lipid endpoint trials, lipid parameters were collected in the context of adverse event monitoring, and between-group differences in confounding factors (e.g. differences in baseline lipid concentrations or in use of lipid lowering-medication) cannot be excluded. In the Breast International Group 1-98 RCT [66] of 8010 postmenopausal women (mean age, 61 years) after 23 months, total cholesterol was stable in the letrozole group but decreased by 14.1% in the tamoxifen group, and hypercholesterolemia (defined as above the assay reference) was higher in the letrozole arm compared with the tamoxifen arm (43.6% vs 19.2%). Total cholesterol >7.75 mmol/L was reported in 8.5% of letrozole-treated women and in 1.9% of tamoxifen-treated women [66]. Women receiving letrozole were threefold more likely to commence cholesterol lowering medications than women receiving tamoxifen [67].
The Arimidex, Tamoxifen, Alone or in Combination (ATAC) trial [68], an RCT of 6,186 postmenopausal women (mean age, 64 years) with early stage breast cancer reported, after a median follow up of 68 months, a higher prevalence of hypercholesterolemia in the anastrozole group compared with the tamoxifen group (9.0% vs 3.5%, respectively; P < 0.0001). However, measurement of lipid levels was not protocolized, but it was left to the discretion of individual investigators [68].
Several RCTs have assessed the effects of aromatase inhibitors on serum lipids in women following primary adjuvant tamoxifen. In the Adjuvant post-Tamoxifen Exemestane Versus Nothing Applied lipid substudy [69], 411 postmenopausal women with early stage breast cancer who had received tamoxifen for at least 5 years were randomized to either an additional 5 years of exemestane or observation. In both study arms, total and LDL cholesterol increased, and triglycerides decreased. Changes were modest (<20%) compared with baseline levels, without between-group differences [69]. Likewise, in MA.17, a placebo-controlled RCT designed to assess the effects of letrozole on disease-free survival following 5 years of tamoxifen treatment, after a median follow-up of 30 months, there was no difference in the prevalence of hypercholesterolemia between letrozole-treated women (n = 2593) and placebo-treated women (n = 2594) treated women [70]. In MA.17L, an MA.17 lipid substudy involving 340 women with normal baseline serum lipid levels not receiving antilipid therapy, women treated with letrozole had marginal decreases in HDL at 6 months (P = 0.049) and marginal increases in LDL (P = 0.033) and triglycerides at 24 months (P = 0.036), compared with placebo, but not at other time points [71]. In the Tamoxifen Exemestane Adjuvant Multinational (TEAM) trial [72] 9779 postmenopausal women were given exemestane either upfront or after tamoxifen. After a median follow-up of 5.1 years, hypercholesterolemia was reported more frequently in the exemestane monotherapy arm compared with the sequential arm [230 (5%) vs 136 (3%) P < 0.0001] [72].
In summary, tamoxifen treatment is associated with modest decreases in LDL cholesterol and in Lp(a), but with increases in triglycerides that can occasionally be severe [73]. Aromatase inhibitor treatment does not appear to be associated with marked changes in lipid parameters. Changes reported with aromatase inhibitor treatment following tamoxifen appear to be due to tamoxifen washout rather than an aromatase inhibitor effect.
E. Arterial Wall
Clinical studies generally demonstrate beneficial effects of estrogens on subclinical atherosclerosis [e.g., coronary artery calcium scores, carotid intima–medial thickness (IMT)] in women with premature and early menopause, but potentially deleterious effects in late (>10 years) menopausal women [74, 75]. Several observational studies (including between 51 to 410 women) have reported associations of endocrine therapy with markers of atherosclerosis in postmenopausal women with early breast cancer. In a cross-sectional study, women (n = 67; mean age, 61 years) receiving tamoxifen for a mean duration of 2.4 years had lower carotid IMT than did controls not receiving endocrine therapy, independent of adjustment for age and cardiovascular risk factors (610 vs 660 mm, P = 0.04) [76]. In a 6-months prospective study (n = 27; mean age, 63 years), tamoxifen treatment was likewise associated with decreased carotid IMT and an increase in flow-mediated dilation compared with untreated healthy controls [77].
Three studies have assessed markers of atherosclerosis and endothelial function in postmenopausal women receiving aromatase inhibitors. In a cross-sectional study, women receiving aromatase inhibitors (n = 36; mean age, 62 years) had reduced vascular relaxation and increased endothelial dysfunction assessed by several noninvasive measures, compared with age-, body mass index–, and cardiovascular risk factor burden–matched controls (n = 25) [78]. In a prospective study, women (n = 97; mean age, 66 years) initiating aromatase inhibitors were more likely to have a deterioration in their reactive hyperemia index at 12-month follow-up compared with matched controls (29% vs 11%, P = 0.04), an effect more likely in women with at least three cardiovascular risk factors [79]. A large cross-sectional study reported no difference in carotid IMT between women treated with aromatase inhibitors (n = 410; mean age, 71 years) for a median of 53 months and age-matched controls (n = 210). However, women were older than in the other studies, and groups were not matched for cardiovascular risk [80]. Overall, the preliminary evidence, based largely on relatively small observational studies, suggests that aromatase inhibitors may have adverse effects on surrogate markers of atherosclerosis and vascular/endothelial function, whereas tamoxifen may have favorable effects. However, more definitive studies are needed.
F. Cardiovascular Events
F-1. Observational studies
Database studies including 5300 to 16,300 mostly postmenopausal women ≥60 years of age with early breast cancer from the United States [81, 82], United Kingdom [83], Denmark [84], and Taiwan [85] have assessed associations of tamoxifen with cardiovascular events, using a case-control design. These studies have reported either neutral effects or reductions in cardiovascular events with tamoxifen use. In a US database study of women with a mean age of 67 years, tamoxifen use, compared with nonusers, was not associated with risk of first stroke (OR, 1.0; 95% CI, 0.6 to 1.6) [81] or first acute myocardial infarction (AMI) (OR, 1.2; 95% CI, 0.7 to 1.9) [82], after adjustment for age and cardiovascular risk factors. Likewise, in Danish women, tamoxifen use was not associated with rates of a broad range of cardiovascular events after adjustment for age, cardiovascular risk, and nonendocrine therapy breast cancer treatments [84]. However, in studies from the United Kingdom and Taiwan, tamoxifen use was associated with a lower risk of AMI/angina (OR, 0.4; 95% CI, 0.2 to 0.7) [83], and of total cardiovascular events (HR, 0.5; 95% CI, 0.4 to 0.8) [85], after adjustment for comorbidities. Women in the Taiwanese cohort (mean age, 50 years) were younger than in other studies, and cardiovascular benefits are consistent with the timing hypothesis discussed above. Limitations include those inherent to database studies (e.g., nonrandomized treatment, variable event adjudication, and residual confounding), and that numbers of events were generally low, ranging from 100 to 200 in each study.
Larger database studies (n = 5600 to 44,000) from the United States [86–88] and Canada [89] with higher event rates (several thousands) comprising older women with breast cancer (mean age, >66 years) have compared associations of tamoxifen with aromatase inhibitors on cardiovascular events. Three studies showed no difference in AMI or stroke rates between tamoxifen and aromatase inhibitor users after variable adjustments [86–88]. One study reported a higher risk of hospitalization for acute myocardial infarction (HR, 2.02; 95% CI, 1.16 to 3.53) in aromatase inhibitor–treated women [89].
F-2. Randomized controlled clinical trials
Cardiovascular effects of adjuvant endocrine therapy have been analyzed in a large number of RCTs enrolling mostly postmenopausal women, and key RCTs [17, 66, 68, 70, 72, 90–92] are summarized in Table 1. Individual RCTs have been inconsistent, in part due to low event rates limiting statistical power. Therefore, several meta-analyses [93–100] have been conducted (Table 2). Of note, RCTs were designed to evaluate oncologic endpoints. Cardiovascular events were collected in the context of safety monitoring, with heterogeneity in definition, grading, and reporting of cardiovascular events. RCTs also differed with respect to inclusion criteria, clinical characteristics of study populations, length of follow-up, and reporting on confounders (e.g., cardiovascular risk factors, comorbid burden, use of concurrent medications), limiting interpretation of these meta-analyses. Moreover, analyses were based on study-level, investigator-reported events rather than on individual patient data.
Table 1.
Selected Trials Reporting Cardiovascular Risk Associated With Adjuvant Endocrine Therapies
| Trial | Study Population | Design | Outcomes, % or RR/HR (95% CI) | Other Cardiometabolic Outcomes |
|---|---|---|---|---|
| Scottish Cancer Trials [91] | N = 1323; tamoxifen vs none (CTx, RT status unknown) | RCT: aims to assess the effect of tamoxifen given to patients with breast cancer immediately after mastectomy (or mastectomy plus RT) (adjuvant arm) or only after the patients had had a relapse | Cardiovascular event | |
| RR, 0.67; 95% CI, 0.43–1.04 | ||||
| Cerebrovascular event | ||||
| RR, 1.23; 95% CI, 0.69–2.19 | ||||
| Rosell et al., Swedish Breast Cancer Cooperative Group [92] | N = 4150; tamoxifen 2 vs 5 y (with/without prior CTx, RT) | RCT: aims to determine the morbidity and mortality from cardiac diseases during treatment and long term after treatment with tamoxifen | Coronary heart disease | |
| HR, 0.83; 95% CI, 0.70–1.00; P = 0.048 | ||||
| Myocardial infarction | ||||
| HR, 0.75; 95% CI, 0.60–0.95; P = 0.019 | ||||
| Coronary heart disease mortality | ||||
| HR, 0.72; 95% CI, 0.53–0.97, P = 0.029 | ||||
| Myocardial infarction mortality | ||||
| HR, 0.68, 95% CI, 0.47–1.00; P = 0.051 | ||||
| ATAC Trialists’ Group [68] | N = 9366; anastrozole vs tamoxifen vs combination (with/without prior CTx, RT); median follow-up 68 mo | RCT: aims to assess the safety tolerability and risk/benefit of extended therapy beyond 5 y; lipids were not routinely assessed | Cardiovascular event | Hypercholesterolemia |
| Anastrozole 73 (2%) vs tamoxifen 66 (2%); P = 0.5 | Anastrozole 278 (9%) vs tamoxifen 108 (3.5%); P ≤ 0.0001 | |||
| Cerebrovascular event | Hypertension | |||
| Anastrozole 62 (2%) vs tamoxifen 88 (3%); P = 0.03 | Anastrozole 402 (13%) vs tamoxifen 349 (11%); P = 0.04 | |||
| The Breast International Group 1-98 Collaborative Group [66] | N = 8010; letrozole vs tamoxifen; median follow-up 26 mo (with/without prior CTx, RT) | RCT: aimed to compare | Cardiovascular event | Hypercholesterolemia |
| Letrozole with tamoxifen as adjuvant treatment of steroid hormone receptor–positive | Letrozole 162 (4.1%) vs tamoxifen 153 (3.8%); P = 0.61 | Letrozole (43.6%) vs tamoxifen (19.2%) | ||
| Breast cancer in postmenopausal women | Cerebrovascular event | |||
| 90.8% of the cholesterol values were not obtained after an overnight fast | Letrozole 39 (1%) vs tamoxifen 41 (1%); P = 0.91 | |||
| More women in the letrozole group had grade 3, 4, or 5 cardiac events (2.1% vs 1.1%, P < 0.001) | ||||
| Coombes et al., Intergroup Exemestane Study [90] | N = 4742; tamoxifen vs tamoxifen switch to exemestane; median follow-up 55 mo; prior CTx | RCT: to determine whether after 2 to 3 y of tamoxifen therapy, switching to exemestane was more effective than continuing tamoxifen therapy for the remainder of the 5 y of treatment |
Cardiovascular disease
Exemestane 984 (43%) vs tamoxifen 913 (39%); P = 0.016 |
|
| Goss et al., MA 17 [70] | N = 5187; letrozole vs placebo after initial tamoxifen; median follow-up 2.4 y (with/without prior CTx, RT) | RCT: aims to determine the effects of aromatase inhibitor letrozole on cancer outcomes after the discontinuation of 5 y of tamoxifen therapy | Cardiovascular event | Hypercholesterolemia |
| Letrozole 88 (4.1%) vs 77 (3.6%); P = 0.40 | Letrozole 257 (11.9%) vs placebo 247 (11.5%); P = 0.67 | |||
| Cerebrovascular | ||||
| RR, 1.14; 95% CI, 0.57–2.27 | ||||
| Goss et al., MA 17R [17] | N = 1918; letrozole vs placebo after AI; median follow-up of 6.3 y | RCT: aims to determine whether extending treatment with an aromatase inhibitor to 10 y may further reduce the risk of breast cancer recurrence; secondary endpoints included long-term safety | Cardiovascular events | Hypercholesterolemia |
| Letrozole 116 (12%) vs 98 (10%); P = 0.21 | Letrozole 203 (21%) vs Placebo 184 (19%); P = 0.31 | |||
| Cerebrovascular events | Hypertension | |||
| RR, 1.1; 95% CI, 0.63–2.07 | Letrozole 158 (16%) vs 145 (15%); P = 0.48 | |||
| Van de Velde et al., TEAM [72] | N = 9779; sequential = 4875 tamoxifen-exemestane vs exemestane; median follow-up 5 y (with/without prior CTx, RT); patients with substantial cardiac disease were excluded | RCT: aims to compare the long-term effects of exemestane monotherapy with sequential treatment (tamoxifen followed by exemestane) | Cardiac-related deaths | Hypertension |
| Exemestane 43 (<1%) vs sequential 28 (<1%); P = 0.11 | Exemestane 303 (6%) vs sequential 219 (5%); P = 0.0003 | |||
| Cardiac failure | Hypercholesterolemia | |||
| Exemestane 50 (1%) vs sequential 26 (<1%); P = 0.009 | Exemestane 230 (5%) vs sequential 136 (3%); P ≤ 0.0001 |
Bold text signifies statistical significance.
Abbreviations: ATAC, Arimidex, Tamoxifen Alone or in Combination; ATENA, Adjuvant Post-Tamoxifen Exemestane Versus Nothing Applied; CTx, chemotherapy; N, number; RR, relative risk; RT, radiotherapy; TEAM, Tamoxifen Exemestane Adjuvant Multinational.
Table 2.
Meta-Analyses Reporting Cardiovascular Risk Associated With Adjuvant Endocrine Therapies
| Study | Inclusion Criteria | Study Population | Comparison | Results, RR or ORa [95% CI] | Comments |
|---|---|---|---|---|---|
| Braithwaite et al., 2003 [93] | Breast cancer RCTs comparing TAM to placebo/no treatment | 32 RCTs; 52,529 women; average age 55 y | TAM vs placebo/no treatment | Stroke 1.49 [1.16–1.90] | |
| AMI 0.62 [0.41–0.93] | |||||
| AMI incidence 0.90 [0.66–1.23] | |||||
| PE 1.88 [1.17–3.01] | |||||
| DVT 1.87 [1.33–2.64] | |||||
| Cuppone et al., 2007 [94] | Phase III RCTs comparing AIs (either upfront or sequential after 2 to 3 y of TAM) with TAM | 7 RCTs; 19,818 postmenopausal women | AI vs TAM | CV events 1.31 [1.07–1.60] | AD 0.5% (NNH = 189) |
| Thromboembolic events 0.53 [0.42–0.65] | AD −1.2% (NNH = −85) | ||||
| Cerebrovascular events 0.76 [0.58–1.003] | RR combine upfront and sequential treatment | ||||
| Amir et al., 2011 [95] | Phase III RCTs >5 y in duration comparing AIs with TAM as primary endocrine therapy | 7 RCTs; 30,032 postmenopausal women | AI vs TAM | CV events 1.26a [1.10–1.43] | AD 0.8% (NNH = 132) |
| Venous thrombosis 0.55a [0.46–0.64] | AD −1.3% (NNH = −79) | ||||
| Cerebrovascular events 1.01 [0.81–1.26] | |||||
| Aydiner 2013 [96] | RCTs comparing AIs with TAM as primary, sequential, or extended therapy (after 5 y of initial adjuvant endocrine therapy) | 11 RCTs; 34,070 postmenopausal women | AI vs TAM | Total CV events 1.20a [1.02–1.42] | ORs refer to primary AI therapy; ORs for CV events were similar for sequenced therapy, but not significant for extended therapy |
| Thromboembolic events 0.61a [0.50–0.75] | |||||
| Ryden 2016 [97] | RCTs comparing AIs with TAM as primary, sequential; or extended therapy with at least 5-year follow-up | 8 RCTs; 34,489 postmenopausal women | AI vs TAM | Total CV events 1.13 [0.96–1.33] | RR refers to primary AI therapy; RRs for sequenced and extended therapy were also not significant |
| Khosrow-Khavar et al., 2016 [98] | Phase III RCTs comparing AIs with TAM, AIs to placebo/no treatment; and TAM to placebo/no treatment | 19 RCTs; 62,345 women | AI vs TAM TAM vs placebo/no treatment | Total CV events | Similar CV event results reported with sequential AI treatment; no difference in cerebrovascular events between treatments |
| Primary AI vs TAM 1.19 [1.07–1.34] | |||||
| TAM vs placebo/no treatment 0.67 [0.45–0.98] | |||||
| Goldvaser et al., 2017 [99] | Phase III RCTs comparing extended AIs with placebo/no treatment | 7 RCTs; 16,3499 postmenopausal women | AI vs placebo/no treatment | Total CV events 1.18a [1.00–1.40] | AD 0.8% (NNH =1 22) |
| No association between AIs and hypertension | |||||
| Matthews et al., 2018 [100] | Studies investigating risk of CV outcomes with use of either TAM or AI or comparing the two treatments | 15 RCTs; 11 observational studies | AI vs TAM TAM vs placebo/no treatment | Thromboembolic events | No meta-analyses conducted for other outcomes |
| Primary AI vs TAM 0.61 [0.58–0.63] | |||||
| Heart failure | |||||
| Tamoxifen vs placebo/no treatment 0.84 [0.56–1.07] |
Adapted and updated from Matthews et al. [100].
Cerebrovascular events included stroke and transient ischemic attack.
Abbreviations: AD, absolute difference; AI, aromatase inhibitor; AMI, acute myocardial infarction; CV, cardiovascular; DVT, deep venous thrombosis; NNH, number needed to harm; PE, pulmonary embolism; RR, relative risk; TAM, tamoxifen.
CV events included AMI, angina, and cardiac failure. Refers to OR being used rather than RR.
Meta-analyses comparing tamoxifen with placebo or no treatment consistently reported an increased risk of venous thromboembolism (including deep venous thrombosis and pulmonary embolism) but decreased cardiovascular events with tamoxifen [93, 98] (Table 2). Aromatase inhibitor treatment (either upfront or sequenced after tamoxifen) was consistently associated with an increased risk of cardiovascular events, but with a decreased risk of venous thromboembolism compared with tamoxifen treatment. In the extended setting (after initial endocrine therapy for 5 years), aromatase inhibitors were associated with an increased risk of cardiovascular events also when compared with placebo, although comparatively less data are available in this setting [99]. Associations with cerebrovascular events are less clear, in part due to lower event rates (Table 2). With respect to vascular mortality, a patient-level efficacy meta-analysis of 31,920 postmenopausal women [3] reported no differences in thromboembolic, cerebrovascular, or cardiac mortality between tamoxifen- and aromatase inhibitor–treated women (including upfront and sequenced therapy) within 5 years of follow-up, but vascular mortality was low [n = 394 (1.2%)] during this period.
Profound estradiol deprivation in premenopausal women could be expected to increase long-term cardiovascular risk. However, no data are available to support this hypothesis. Among Suppression of Ovarian Function Trial/Tamoxifen and Exemestane Trial participants (n = 5648, aged 40 to 46 years at randomization) with low baseline cardiovascular risk, at 8 year follow-up, <50 cardiac ischemia/infarction events (<1%) were reported [20].
In summary, tamoxifen use in postmenopausal women is associated with an increase in venous thromboembolic risk, but with either no effect on, or reduction in, cardiovascular events. Whether aromatase inhibitors have cardiovascular toxicity remains unclear, as comparisons to placebo or no treatment are limited, and increased risks relative to current or previous use of tamoxifen may be explained by a protective effect of tamoxifen. Overall, reported effect sizes for cardiovascular events were modest (relative risks <30%, absolute risks <2%). However, true risks could be underestimated because first, most observational studies excluded women with preexisting cardiovascular disease, and RCT participants were often healthier than women receiving endocrine therapy outside of clinical trials; second, follow-up was relatively short; and third, adverse event reporting in oncologic endpoint RCTs may be incomplete; for example, aromatase inhibitor treatment–associated osteoporotic fracture rates were considerably higher in a dedicated fracture endpoint trial [101] compared with rates reported as adverse events in oncologic endpoint RCTs [15]. Finally, adverse events are only collected until an efficacy outcome occurs, yet adverse cardiovascular events can continue to accumulate thereafter. In older women with early breast cancer, cardiovascular disease exceeds breast cancer as the leading cause of death 6 to 10 years after diagnosis [28, 29], emphasizing the importance of cardiovascular death as a competing cause of mortality in long-term breast cancer survivors.
3. Implications for Clinical Practice
Proactive assessment and optimization of cardiovascular risk is an important component of survivorship breast cancer care. Almost two-thirds of US women with breast cancer are overweight and obese, and obesity is a risk factor not only for adverse cardiometabolic outcomes, but also for breast cancer recurrence [102]. American Cancer Society/American Society of Clinical Oncology (ASCO) guidelines emphasize the importance of educating breast cancer survivors about lifestyle modifications [102]. ASCO recommendations for smoking cessation, nutrition (a diet high in vegetables, fruits, whole grains, and legumes but low in saturated fats), and exercise (at least 150 minutes of moderate or 75 minutes of vigorous aerobic exercise per week, including strength training exercises at least 2 days per week) are based on evidence from RCTs conducted in breast cancer survivors [102]. RCT evidence regarding efficacy of pharmacotherapy to reduce cardiometabolic risk specific to breast cancer survivors is currently not available. The ASCO guidelines recommend cardiovascular monitoring consistent with clinical standards for other high-risk populations, and pharmacological management should follow recommendations for the general population [102].
4. Conclusions
Adjuvant endocrine therapy may modulate cardiometabolic risk by several potential mechanisms (Fig. 1). Despite potential adverse effects on body and hepatic fat accumulation and on serum triglycerides, tamoxifen can reduce LDL cholesterol and Lp(a), and it may have beneficial effects on the arterial wall, effects that could contribute to tamoxifen-associated reductions in cardiovascular events. Aromatase inhibitors are associated with higher cardiovascular risks compared with tamoxifen, but whether this reflects aromatase inhibitor–related toxicity or a protective effect of tamoxifen remains uncertain.
Existing evidence regarding endocrine therapy–associated cardiometabolic risks remains incomplete owing to the scarcity of high-quality studies examining cardiometabolic risk factors, the low number of cardiovascular events reported in clinical trials, the relatively short follow-up, inconsistent adjudication of adverse events, and the predominant enrollment of women with relatively low cardiovascular risk. Even when cardiovascular data were collected in the context of an RCT, outcomes are primarily observational, given that to date no RCT has been designed to specifically evaluate cardiovascular risk. Cardiovascular disease is a major cause of mortality in older breast cancer survivors, and even minor increases in cardiovascular risks could have a significant impact. Although premenopausal women may be particularly susceptible to adverse cardiometabolic effects of estradiol depletion, their baseline risk for cardiovascular events is low, the existing studies are too small, and the follow-up is too short to estimate long-term risks. Evidence from larger studies examining the metabolic and cardiovascular consequences of endocrine therapy, including effects on body composition, glucose metabolism, and markers of atherosclerosis, are required to better define the cardiometabolic risks and to guide the clinical care of women with breast cancer to optimize long-term health outcomes. Proactive management of modifiable cardiovascular risk factors and pharmacotherapy to reduce cardiometabolic risk based on general population evidence should be an integral part of breast cancer survivorship care.
Acknowledgments
Disclosure Summary: M.G. has received research funding from Bayer, Novartis, Weight Watchers, and Lilly and speaker’s honoraria from Besins Health Care and Amgen. S.K.R. has received speaker honorarium from Counterpart (breast cancer support organization). B.Y. has received speaker honoraria from Myriad, Esai, and Novartis, consultant advisory honoraria from Novartis, Amgen, and Roche, and travel grant funding from Roche and Novartis. Y.-M.C. has nothing to disclose.
Glossary
Abbreviations:
- ASCO
American Society of Clinical Oncology
- ATAC
Arimidex Tamoxifen Alone or in Combination
- CV
cardiovascular
- DXA
dual x-ray densitometry
- ER
estrogen receptor
- ER+
estrogen receptor-positive
- estradiol
17β-estradiol
- HDL
high-density lipoprotein
- HR
hazard ratio
- IMT
intima–media thickness
- LDL
low-density lipoprotein
- Lp(a)
lipoprotein(a)
- MHT
menopausal hormone therapy
- RCT
randomized controlled trial
- SERM
selective estrogen receptor modulator
- TEAM
Tamoxifen Exemestane Adjuvant Multinational
References and Notes
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2. Nadji M, Gomez-Fernandez C, Ganjei-Azar P, Morales AR. Immunohistochemistry of estrogen and progesterone receptors reconsidered: experience with 5,993 breast cancers. Am J Clin Pathol. 2005;123(1):21–27. [DOI] [PubMed] [Google Scholar]
- 3. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386(10001):1341–1352. [DOI] [PubMed] [Google Scholar]
- 4. Santen RJ, Simpson E. History of estrogen: its purification, structure, synthesis, biologic actions, and clinical implications. Endocrinology. 2019;160(3):605–625. [DOI] [PubMed] [Google Scholar]
- 5. Stuenkel CA, Davis SR, Gompel A, Lumsden MA, Murad MH, Pinkerton JV, Santen RJ. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(11):3975–4011. [DOI] [PubMed] [Google Scholar]
- 6. Mondul AM, Rodriguez C, Jacobs EJ, Calle EE. Age at natural menopause and cause-specific mortality. Am J Epidemiol. 2005;162(11):1089–1097. [DOI] [PubMed] [Google Scholar]
- 7. Kalantaridou SN, Naka KK, Papanikolaou E, Kazakos N, Kravariti M, Calis KA, Paraskevaidis EA, Sideris DA, Tsatsoulis A, Chrousos GP, Michalis LK. Impaired endothelial function in young women with premature ovarian failure: normalization with hormone therapy. J Clin Endocrinol Metab. 2004;89(8):3907–3913. [DOI] [PubMed] [Google Scholar]
- 8. Hodis HN, Mack WJ, Henderson VW, Shoupe D, Budoff MJ, Hwang-Levine J, Li Y, Feng M, Dustin L, Kono N, Stanczyk FZ, Selzer RH, Azen SP; ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374(13):1221–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boardman HM, Hartley L, Eisinga A, Main C, Roqué i Figuls M, Bonfill Cosp X, Gabriel Sanchez R, Knight B. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst Rev. 2015; (3):CD002229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clarkson TB, Prichard RW, Morgan TM, Petrick GS, Klein KP. Remodeling of coronary arteries in human and nonhuman primates. JAMA. 1994;271(4):289–294. [PubMed] [Google Scholar]
- 11. Dowsett M, Cuzick J, Howell A, Jackson I; ATAC Trialists’ Group. Pharmacokinetics of anastrozole and tamoxifen alone, and in combination, during adjuvant endocrine therapy for early breast cancer in postmenopausal women: a sub-protocol of the “Arimidex and Tamoxifen Alone or in Combination” (ATAC) trial. Br J Cancer. 2001;85(3):317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Katzenellenbogen BS, Choi I, Delage-Mourroux R, Ediger TR, Martini PG, Montano M, Sun J, Weis K, Katzenellenbogen JA. Molecular mechanisms of estrogen action: selective ligands and receptor pharmacology. J Steroid Biochem Mol Biol. 2000;74(5):279–285. [DOI] [PubMed] [Google Scholar]
- 13. Kedar RP, Bourne TH, Powles TJ, Collins WP, Ashley SE, Cosgrove DO, Campbell S. Effects of tamoxifen on uterus and ovaries of postmenopausal women in a randomised breast cancer prevention trial. Lancet. 1994;343(8909):1318–1321. [DOI] [PubMed] [Google Scholar]
- 14. O’Regan RM, Jordan VC. The evolution of tamoxifen therapy in breast cancer: selective oestrogen-receptor modulators and downregulators. Lancet Oncol. 2002;3(4):207–214. [DOI] [PubMed] [Google Scholar]
- 15. Grossmann M, Ramchand SK, Milat F, Vincent A, Lim E, Kotowicz MA, Hicks J, Teede H. Assessment and management of bone health in women with oestrogen receptor-positive breast cancer receiving endocrine therapy: position statement of the Endocrine Society of Australia, the Australian and New Zealand Bone & Mineral Society, the Australasian Menopause Society and the Clinical Oncology Society of Australia. Clin Endocrinol (Oxf). 2018;89(3):280–296. [DOI] [PubMed] [Google Scholar]
- 16. Burstein H, Lacchetti C, Anderson H, Buchholz T, Davidson N, Gelmon K, Giordano SH, Hudis CA, Solky AJ, Stearns V, Winer EP, Griggs JJ. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: ASCO clinical practice guideline focused update. J Clin Oncol. 2019;37(5):423–438. [DOI] [PubMed] [Google Scholar]
- 17. Goss PE, Ingle JN, Pritchard KI, Robert NJ, Muss H, Gralow J, Gelmon K, Whelan T, Strasser-Weippl K, Rubin S, Sturtz K, Wolff AC, Winer E, Hudis C, Stopeck A, Beck JT, Kaur JS, Whelan K, Tu D, Parulekar WR. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med. 2016;375(3):209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pagani O, Regan MM, Walley BA, Fleming GF, Colleoni M, Láng I, Gomez HL, Tondini C, Burstein HJ, Perez EA, Ciruelos E, Stearns V, Bonnefoi HR, Martino S, Geyer CE Jr, Pinotti G, Puglisi F, Crivellari D, Ruhstaller T, Winer EP, Rabaglio-Poretti M, Maibach R, Ruepp B, Giobbie-Hurder A, Price KN, Bernhard J, Luo W, Ribi K, Viale G, Coates AS, Gelber RD, Goldhirsch A, Francis PA; TEXT and SOFT Investigators; International Breast Cancer Study Group. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371(2):107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Francis PA, Regan MM, Fleming GF, Láng I, Ciruelos E, Bellet M, Bonnefoi HR, Climent MA, Da Prada GA, Burstein HJ, Martino S, Davidson NE, Geyer CE Jr, Walley BA, Coleman R, Kerbrat P, Buchholz S, Ingle JN, Winer EP, Rabaglio-Poretti M, Maibach R, Ruepp B, Giobbie-Hurder A, Price KN, Colleoni M, Viale G, Coates AS, Goldhirsch A, Gelber RD; SOFT Investigators; International Breast Cancer Study Group. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372(5):436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Francis PA, Pagani O, Fleming GF, Walley BA, Colleoni M, Láng I, Gómez HL, Tondini C, Ciruelos E, Burstein HJ, Bonnefoi HR, Bellet M, Martino S, Geyer CE Jr, Goetz MP, Stearns V, Pinotti G, Puglisi F, Spazzapan S, Climent MA, Pavesi L, Ruhstaller T, Davidson NE, Coleman R, Debled M, Buchholz S, Ingle JN, Winer EP, Maibach R, Rabaglio-Poretti M, Ruepp B, Di Leo A, Coates AS, Gelber RD, Goldhirsch A, Regan MM; SOFT and TEXT Investigators and the International Breast Cancer Study Group. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med. 2018;379(2):122–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. The US Preventative Services Task Force AIs recommended for breast cancer prevention. Cancer Discov. 2019;9(3):311. [DOI] [PubMed] [Google Scholar]
- 22. Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K; International Agency for Research on Cancer Handbook Working Group. Body fatness and cancer—viewpoint of the IARC Working Group. N Engl J Med. 2016;375(8):794–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hardefeldt PJ, Edirimanne S, Eslick GD. Diabetes increases the risk of breast cancer: a meta-analysis. Endocr Relat Cancer. 2012;19(6):793–803. [DOI] [PubMed] [Google Scholar]
- 24. Pizot C, Boniol M, Mullie P, Koechlin A, Boniol M, Boyle P, Autier P. Physical activity, hormone replacement therapy and breast cancer risk: a meta-analysis of prospective studies. Eur J Cancer. 2016;52:138–154. [DOI] [PubMed] [Google Scholar]
- 25. Bardia A, Arieas ET, Zhang Z, Defilippis A, Tarpinian K, Jeter S, Nguyen A, Henry NL, Flockhart DA, Hayes DF, Hayden J, Storniolo AM, Armstrong DK, Davidson NE, Fetting J, Ouyang P, Wolff AC, Blumenthal RS, Ashen MD, Stearns V. Comparison of breast cancer recurrence risk and cardiovascular disease incidence risk among postmenopausal women with breast cancer. Breast Cancer Res Treat. 2012;131(3):907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yeh ET, Chang HM. Oncocardiology—past, present, and future: a review. JAMA Cardiol. 2016;1(9):1066–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gernaat SA, Ho PJ, Rijnberg N, Emaus MJ, Baak LM, Hartman M, Grobbee DE, Verkooijen HM. Risk of death from cardiovascular disease following breast cancer: a systematic review. Breast Cancer Res Treat. 2017;164(3):537–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abdel-Qadir H, Austin PC, Lee DS, Amir E, Tu JV, Thavendiranathan P, Fung K, Anderson GM. A population-based study of cardiovascular mortality following early-stage breast cancer. JAMA Cardiol. 2017;2(1):88–93. [DOI] [PubMed] [Google Scholar]
- 29. Park NJ, Chang Y, Bender C, Conley Y, Chlebowski RT, van Londen GJ, Foraker R, Wassertheil-Smoller S, Stefanick ML, Kuller LH. Cardiovascular disease and mortality after breast cancer in postmenopausal women: results from the Women’s Health Initiative. PLoS One. 2017;12(9):e0184174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shea KL, Gavin KM, Melanson EL, Gibbons E, Stavros A, Wolfe P, Kittelson JM, Vondracek SF, Schwartz RS, Wierman ME, Kohrt WM. Body composition and bone mineral density after ovarian hormone suppression with or without estradiol treatment. Menopause. 2015;22(10):1045–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Papadakis GE, Hans D, Rodriguez EG, Vollenweider P, Waeber G, Marques-Vidal P, Lamy O. Menopausal hormone therapy is associated with reduced total and visceral adiposity: the OsteoLaus Cohort. J Clin Endocrinol Metab. 2018;103(5):1948–1957. [DOI] [PubMed] [Google Scholar]
- 32. Irwin ML, McTiernan A, Baumgartner RN, Baumgartner KB, Bernstein L, Gilliland FD, Ballard-Barbash R. Changes in body fat and weight after a breast cancer diagnosis: influence of demographic, prognostic, and lifestyle factors. J Clin Oncol. 2005;23(4):774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Demark-Wahnefried W, Peterson BL, Winer EP, Marks L, Aziz N, Marcom PK, Blackwell K, Rimer BK. Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol. 2001;19(9):2381–2389. [DOI] [PubMed] [Google Scholar]
- 34. Nissen MJ, Shapiro A, Swenson KK. Changes in weight and body composition in women receiving chemotherapy for breast cancer. Clin Breast Cancer. 2011;11(1):52–60. [DOI] [PubMed] [Google Scholar]
- 35. Ali PA, al-Ghorabie FH, Evans CJ, el-Sharkawi AM, Hancock DA. Body composition measurements using DXA and other techniques in tamoxifen-treated patients. Appl Radiat Isot. 1998;49(5–6):643–645. [DOI] [PubMed] [Google Scholar]
- 36. Nguyen MC, Stewart RB, Banerji MA, Gordon DH, Kral JG. Relationships between tamoxifen use, liver fat and body fat distribution in women with breast cancer. Int J Obes Relat Metab Disord. 2001;25(2):296–298. [DOI] [PubMed] [Google Scholar]
- 37. Francini G, Petrioli R, Montagnani A, Cadirni A, Campagna S, Francini E, Gonnelli S. Exemestane after tamoxifen as adjuvant hormonal therapy in postmenopausal women with breast cancer: effects on body composition and lipids. Br J Cancer. 2006;95(2):153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van Londen GJ, Perera S, Vujevich K, Rastogi P, Lembersky B, Brufsky A, Vogel V, Greenspan SL. The impact of an aromatase inhibitor on body composition and gonadal hormone levels in women with breast cancer. Breast Cancer Res Treat. 2011;125(2):441–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hojan K, Molińska-Glura M, Milecki P. Physical activity and body composition, body physique, and quality of life in premenopausal breast cancer patients during endocrine therapy—a feasibility study. Acta Oncol. 2013;52(2):319–326. [DOI] [PubMed] [Google Scholar]
- 40. Adams LA, Anstee QM, Tilg H, Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66(6):1138–1153. [DOI] [PubMed] [Google Scholar]
- 41. Grossmann M, Wierman ME, Angus P, Handelsman DJ. Reproductive endocrinology of nonalcoholic fatty liver disease. Endocr Rev. 2019;40(2):417–446. [DOI] [PubMed] [Google Scholar]
- 42. Löfgren L, Wallberg B, Wilking N, Fornander T, Rutqvist LE, Carlström K, von Schoultz B, von Schoultz E. Tamoxifen and megestrol acetate for postmenopausal breast cancer: diverging effects on liver proteins, androgens, and glucocorticoids. Med Oncol. 2004;21(4):309–318. [DOI] [PubMed] [Google Scholar]
- 43. Ogawa Y, Murata Y, Nishioka A, Inomata T, Yoshida S. Tamoxifen-induced fatty liver in patients with breast cancer. Lancet. 1998;351(9104):725. [DOI] [PubMed] [Google Scholar]
- 44. Birzniece V, Barrett PHR, Ho KKY. Tamoxifen reduces hepatic VLDL production and GH secretion in women: a possible mechanism for steatosis development. Eur J Endocrinol. 2017;177(2):137–143. [DOI] [PubMed] [Google Scholar]
- 45. Hong N, Yoon HG, Seo DH, Park S, Kim SI, Sohn JH, Rhee Y. Different patterns in the risk of newly developed fatty liver and lipid changes with tamoxifen versus aromatase inhibitors in postmenopausal women with early breast cancer: a propensity score–matched cohort study. Eur J Cancer. 2017;82:103–114. [DOI] [PubMed] [Google Scholar]
- 46. Lin Y, Liu J, Zhang X, Li L, Hu R, Liu J, Deng Y, Chen D, Zhao Y, Sun S, Ma R, Zhao Y, Liu J, Zhang Y, Wang X, Li Y, He P, Li E, Xu Z, Wu Y, Tong Z, Wang X, Huang T, Liang Z, Wang S, Su F, Lu Y, Zhang H, Feng G, Wang S. A prospective, randomized study on hepatotoxicity of anastrozole compared with tamoxifen in women with breast cancer. Cancer Sci. 2014;105(9):1182–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mauvais-Jarvis F, Manson JE, Stevenson JC, Fonseca VA. Menopausal hormone therapy and type 2 diabetes prevention: evidence, mechanisms, and clinical implications. Endocr Rev. 2017;38(3):173–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Anagnostis P, Christou K, Artzouchaltzi AM, Gkekas NK, Kosmidou N, Siolos P, Paschou SA, Potoupnis M, Kenanidis E, Tsiridis E, Lambrinoudaki I, Stevenson JC, Goulis DG. Early menopause and premature ovarian insufficiency are associated with increased risk of type 2 diabetes: a systematic review and meta-analysis. Eur J Endocrinol. 2019;180(1):41–50. [DOI] [PubMed] [Google Scholar]
- 49. Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 2013;34(3):309–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Le May C, Chu K, Hu M, Ortega CS, Simpson ER, Korach KS, Tsai MJ, Mauvais-Jarvis F. Estrogens protect pancreatic β-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc Natl Acad Sci USA. 2006;103(24):9232–9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yuchi Y, Cai Y, Legein B, De Groef S, Leuckx G, Coppens V, Van Overmeire E, Staels W, De Leu N, Martens G, Van Ginderachter JA, Heimberg H, Van de Casteele M. Estrogen receptor α regulates β-cell formation during pancreas development and following injury. Diabetes. 2015;64(9):3218–3228. [DOI] [PubMed] [Google Scholar]
- 52. Johansson H, Gandini S, Guerrieri-Gonzaga A, Iodice S, Ruscica M, Bonanni B, Gulisano M, Magni P, Formelli F, Decensi A. Effect of fenretinide and low-dose tamoxifen on insulin sensitivity in premenopausal women at high risk for breast cancer. Cancer Res. 2008;68(22):9512–9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lipscombe LL, Fischer HD, Yun L, Gruneir A, Austin P, Paszat L, Anderson GM, Rochon PA. Association between tamoxifen treatment and diabetes: a population-based study. Cancer. 2012;118(10):2615–2622. [DOI] [PubMed] [Google Scholar]
- 54. Sun LM, Chen HJ, Liang JA, Li TC, Kao CH. Association of tamoxifen use and increased diabetes among Asian women diagnosed with breast cancer. Br J Cancer. 2014;111(9):1836–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hamood R, Hamood H, Merhasin I, Keinan-Boker L. Diabetes after hormone therapy in breast cancer survivors: a case-cohort study. J Clin Oncol. 2018;36(20):2061–2069. [DOI] [PubMed] [Google Scholar]
- 56. Santorelli ML, Hirshfield KM, Steinberg MB, Rhoads GG, Lin Y, Demissie K. Hormonal therapy for breast cancer and diabetes incidence among postmenopausal women. Ann Epidemiol. 2016;26(6):436–440. [DOI] [PubMed] [Google Scholar]
- 57. Walsh BW, Schiff I, Rosner B, Greenberg L, Ravnikar V, Sacks FM. Effects of postmenopausal estrogen replacement on the concentrations and metabolism of plasma lipoproteins. N Engl J Med. 1991;325(17):1196–1204. [DOI] [PubMed] [Google Scholar]
- 58. Grey AB, Stapleton JP, Evans MC, Reid IR. The effect of the anti-estrogen tamoxifen on cardiovascular risk factors in normal postmenopausal women. J Clin Endocrinol Metab. 1995;80(11):3191–3195. [DOI] [PubMed] [Google Scholar]
- 59. Love RR, Wiebe DA, Newcomb PA, Cameron L, Leventhal H, Jordan VC, Feyzi J, DeMets DL. Effects of tamoxifen on cardiovascular risk factors in postmenopausal women. Ann Intern Med. 1991;115(11):860–864. [DOI] [PubMed] [Google Scholar]
- 60. Love RR, Wiebe DA, Feyzi JM, Newcomb PA, Chappell RJ. Effects of tamoxifen on cardiovascular risk factors in postmenopausal women after 5 years of treatment. J Natl Cancer Inst. 1994;86(20):1534–1539. [DOI] [PubMed] [Google Scholar]
- 61. Sahebkar A, Serban MC, Penson P, Gurban C, Ursoniu S, Toth PP, Jones SR, Lippi G, Kotani K, Kostner K, Rizzo M, Rysz J, Banach M; Lipid and Blood Pressure Meta-analysis Collaboration (LBPMC) Group. The effects of tamoxifen on plasma lipoprotein(a) concentrations: systematic review and meta-analysis. Drugs. 2017;77(11):1187–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Esteva FJ, Hortobagyi GN. Comparative assessment of lipid effects of endocrine therapy for breast cancer: implications for cardiovascular disease prevention in postmenopausal women. Breast. 2006;15(3):301–312. [DOI] [PubMed] [Google Scholar]
- 63. Lønning PE, Geisler J, Krag LE, Erikstein B, Bremnes Y, Hagen AI, Schlichting E, Lien EA, Ofjord ES, Paolini J, Polli A, Massimini G. Effects of exemestane administered for 2 years versus placebo on bone mineral density, bone biomarkers, and plasma lipids in patients with surgically resected early breast cancer. J Clin Oncol. 2005;23(22):5126–5137. [DOI] [PubMed] [Google Scholar]
- 64. Geisler J, Lønning PE, Krag LE, Løkkevik E, Risberg T, Hagen AI, Schlichting E, Lien EA, Ofjord ES, Eide GE, Polli A, di Salle E, Paolini J. Changes in bone and lipid metabolism in postmenopausal women with early breast cancer after terminating 2-year treatment with exemestane: a randomised, placebo-controlled study. Eur J Cancer. 2006;42(17):2968–2975. [DOI] [PubMed] [Google Scholar]
- 65. Goss PE, Ingle JN, Alés-Martínez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, McTiernan A, Robbins J, Johnson KC, Martin LW, Winquist E, Sarto GE, Garber JE, Fabian CJ, Pujol P, Maunsell E, Farmer P, Gelmon KA, Tu D, Richardson H; NCIC CTG MAP.3 Study Investigators. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364(25):2381–2391. [DOI] [PubMed] [Google Scholar]
- 66. Thürlimann B, Keshaviah A, Coates AS, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch M, Gelber RD, Rabaglio M, Smith I, Wardley A, Price KN, Goldhirsch A; Breast International Group (BIG) 1-98 Collaborative Group. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer (published correction appears in N Engl J Med. 2006;354(20):2200). N Engl J Med. 2005;353(26):2747–2757. [DOI] [PubMed] [Google Scholar]
- 67. Borgquist S, Giobbie-Hurder A, Ahern TP, Garber JE, Colleoni M, Láng I, Debled M, Ejlertsen B, von Moos R, Smith I, Coates AS, Goldhirsch A, Rabaglio M, Price KN, Gelber RD, Regan MM, Thürlimann B. Cholesterol, cholesterol-lowering medication use, and breast cancer outcome in the BIG 1-98 study. J Clin Oncol. 2017;35(11):1179–1188. [DOI] [PubMed] [Google Scholar]
- 68. Buzdar A, Howell A, Cuzick J, Wale C, Distler W, Hoctin-Boes G, Houghton J, Locker GY, Nabholtz JM; Arimidex, Tamoxifen, Alone or in Combination Trialists’ Group. Comprehensive side-effect profile of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: long-term safety analysis of the ATAC trial. Lancet Oncol. 2006;7(8):633–643. [DOI] [PubMed] [Google Scholar]
- 69. Markopoulos C, Dafni U, Misitzis J, Zobolas V, Tzoracoleftherakis E, Koukouras D, Xepapadakis G, Papadiamantis J, Venizelos B, Antonopoulou Z, Gogas H. Extended adjuvant hormonal therapy with exemestane has no detrimental effect on the lipid profile of postmenopausal breast cancer patients: final results of the ATENA lipid substudy. Breast Cancer Res. 2009;11(3):R35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, Castiglione M, Tu D, Shepherd LE, Pritchard KI, Livingston RB, Davidson NE, Norton L, Perez EA, Abrams JS, Cameron DA, Palmer MJ, Pater JL. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst. 2005;97(17):1262–1271. [DOI] [PubMed] [Google Scholar]
- 71. Wasan KM, Goss PE, Pritchard PH, Shepherd L, Palmer MJ, Liu S, Tu D, Ingle JN, Heath M, Deangelis D, Perez EA. The influence of letrozole on serum lipid concentrations in postmenopausal women with primary breast cancer who have completed 5 years of adjuvant tamoxifen (NCIC CTG MA.17L). Ann Oncol. 2005;16(5):707–715. [DOI] [PubMed] [Google Scholar]
- 72. van de Velde CJ, Rea D, Seynaeve C, Putter H, Hasenburg A, Vannetzel JM, Paridaens R, Markopoulos C, Hozumi Y, Hille ET, Kieback DG, Asmar L, Smeets J, Nortier JW, Hadji P, Bartlett JM, Jones SE. Adjuvant tamoxifen and exemestane in early breast cancer (TEAM): a randomised phase 3 trial. Lancet. 2011;377(9762):321–331. [DOI] [PubMed] [Google Scholar]
- 73. Hozumi Y, Kawano M, Saito T, Miyata M. Effect of tamoxifen on serum lipid metabolism. J Clin Endocrinol Metab. 1998;83(5):1633–1635. [DOI] [PubMed] [Google Scholar]
- 74. Cobin RH, Goodman NF; AACE Reproductive Endocrinology Scientific Committee. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on menopause—2017 Update. Endocr Pract. 2017;23(7):869–880. [DOI] [PubMed] [Google Scholar]
- 75. Santen RJ, Allred DC, Ardoin SP, Archer DF, Boyd N, Braunstein GD, Burger HG, Colditz GA, Davis SR, Gambacciani M, Gower BA, Henderson VW, Jarjour WN, Karas RH, Kleerekoper M, Lobo RA, Manson JE, Marsden J, Martin KA, Martin L, Pinkerton JV, Rubinow DR, Teede H, Thiboutot DM, Utian WH; Endocrine Society. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2010;95(7Suppl 1):s1–s66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Simon T, Boutouyrie P, Simon JM, Laloux B, Tournigand C, Tropeano AI, Laurent S, Jaillon P. Influence of tamoxifen on carotid intima-media thickness in postmenopausal women. Circulation. 2002;106(23):2925–2929. [DOI] [PubMed] [Google Scholar]
- 77. Stamatelopoulos KS, Lekakis JP, Poulakaki NA, Papamichael CM, Venetsanou K, Aznaouridis K, Protogerou AD, Papaioannou TG, Kumar S, Stamatelopoulos SF. Tamoxifen improves endothelial function and reduces carotid intima-media thickness in postmenopausal women. Am Heart J. 2004;147(6):1093–1099. [DOI] [PubMed] [Google Scholar]
- 78. Blaes A, Beckwith H, Florea N, Hebbel R, Solovey A, Potter D, Yee D, Vogel R, Luepker R, Duprez D. Vascular function in breast cancer survivors on aromatase inhibitors: a pilot study. Breast Cancer Res Treat. 2017;166(2):541–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Maor R, Sara JD, Wanous AA, Maor E, Pruthi S, Lerman A, Sandhu NP. Attenuated peripheral endothelial function among women treated with aromatase inhibitors for breast cancer. Coron Artery Dis. 2018;29(8):687–693. [DOI] [PubMed] [Google Scholar]
- 80. Blondeaux E, Musio D, Bruzzi P, Lambertini M, Gazzola V, Poggio F, Vecchio S, Levaggi A, D’Alonzo A, Perfumo MC, Bighin C, Giraudi S, Palombo D, Del Mastro L. Treatment with aromatase inhibitors and markers of cardiovascular disease. Breast Cancer Res Treat. 2016;160(2):261–267. [DOI] [PubMed] [Google Scholar]
- 81. Geiger AM, Fischberg GM, Chen W, Bernstein L. Stroke risk and tamoxifen therapy for breast cancer. J Natl Cancer Inst. 2004;96(20):1528–1536. [DOI] [PubMed] [Google Scholar]
- 82. Geiger AM, Chen W, Bernstein L. Myocardial infarction risk and tamoxifen therapy for breast cancer. Br J Cancer. 2005;92(9):1614–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bradbury BD, Lash TL, Kaye JA, Jick SS. Tamoxifen-treated breast carcinoma patients and the risk of acute myocardial infarction and newly-diagnosed angina. Cancer. 2005;103(6):1114–1121. [DOI] [PubMed] [Google Scholar]
- 84. Hernandez RK, Sørensen HT, Jacobsen J, Pedersen L, Lash TL. Tamoxifen treatment in Danish breast cancer patients and 5-year risk of arterial atherosclerotic events: a null association. Cancer Epidemiol Biomarkers Prev. 2008;17(9):2509–2511. [DOI] [PubMed] [Google Scholar]
- 85. Yang TL, Wu TC, Huang CC, Huang PH, Chung CM, Lin SJ, Chen JW, Chan WL, Chiang CH, Leu HB. Association of tamoxifen use and reduced cardiovascular events among Asian females with breast cancer. Circ J. 2014;78(1):135–140. [DOI] [PubMed] [Google Scholar]
- 86. Ligibel JA, James O’Malley A, Fisher M, Daniel GW, Winer EP, Keating NL. Risk of myocardial infarction, stroke, and fracture in a cohort of community-based breast cancer patients. Breast Cancer Res Treat. 2012;131(2):589–597. [DOI] [PubMed] [Google Scholar]
- 87. Haque R, Shi J, Schottinger JE, Chung J, Avila C, Amundsen B, Xu X, Barac A, Chlebowski RT. Cardiovascular disease after aromatase inhibitor use. JAMA Oncol. 2016;2(12):1590–1597. [DOI] [PubMed] [Google Scholar]
- 88. Kamaraju S, Shi Y, Smith E, Nattinger AB, Laud P, Neuner J. Are aromatase inhibitors associated with higher myocardial infarction risk in breast cancer patients? A Medicare population-based study. Clin Cardiol. 2019;42(1):93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Abdel-Qadir H, Amir E, Fischer HD, Fu L, Austin PC, Harvey PJ, Rochon PA, Lee DS, Anderson GM. The risk of myocardial infarction with aromatase inhibitors relative to tamoxifen in post-menopausal women with early stage breast cancer. Eur J Cancer. 2016;68:11–21. [DOI] [PubMed] [Google Scholar]
- 90. Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, Delozier T, Jones SE, Alvarez I, Bertelli G, Ortmann O, Coates AS, Bajetta E, Dodwell D, Coleman RE, Fallowfield LJ, Mickiewicz E, Andersen J, Lønning PE, Cocconi G, Stewart A, Stuart N, Snowdon CF, Carpentieri M, Massimini G, Bliss JM, van de Velde C; Intergroup Exemestane Study. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer (published corrections appears in N Engl J Med. 2004;351(23):2461 and N Engl J Med. 2006;355(16):1746). N Engl J Med. 2004;350(11):1081–1092. [DOI] [PubMed] [Google Scholar]
- 91. Adjuvant tamoxifen in the management of operable breast cancer: the Scottish Trial. Report from the Breast Cancer Trials Committee, Scottish Cancer Trials Office (MRC), Edinburgh. Lancet. 1987;2(8552):171–175. [PubMed] [Google Scholar]
- 92. Rosell J, Nordenskjöld B, Bengtsson NO, Fornander T, Hatschek T, Lindman H, Malmström PO, Wallgren A, Stål O, Carstensen J. Effects of adjuvant tamoxifen therapy on cardiac disease: results from a randomized trial with long-term follow-up. Breast Cancer Res Treat. 2013;138(2):467–473. [DOI] [PubMed] [Google Scholar]
- 93. Braithwaite RS, Chlebowski RT, Lau J, George S, Hess R, Col NF. Meta-analysis of vascular and neoplastic events associated with tamoxifen. J Gen Intern Med. 2003;18(11):937–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Cuppone F, Bria E, Verma S, Pritchard KI, Gandhi S, Carlini P, Milella M, Nisticò C, Terzoli E, Cognetti F, Giannarelli D. Do adjuvant aromatase inhibitors increase the cardiovascular risk in postmenopausal women with early breast cancer? Meta-analysis of randomized trials. Cancer. 2008;112(2):260–267. [DOI] [PubMed] [Google Scholar]
- 95. Amir E, Seruga B, Niraula S, Carlsson L, Ocaña A. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst. 2011;103(17):1299–1309. [DOI] [PubMed] [Google Scholar]
- 96. Aydiner A. Meta-analysis of breast cancer outcome and toxicity in adjuvant trials of aromatase inhibitors in postmenopausal women. Breast. 2013;22(2):121–129. [DOI] [PubMed] [Google Scholar]
- 97. Rydén L, Heibert Arnlind M, Vitols S, Höistad M, Ahlgren J. Aromatase inhibitors alone or sequentially combined with tamoxifen in postmenopausal early breast cancer compared with tamoxifen or placebo—meta-analyses on efficacy and adverse events based on randomized clinical trials. Breast. 2016;26:106–114. [DOI] [PubMed] [Google Scholar]
- 98. Khosrow-Khavar F, Filion KB, Al-Qurashi S, Torabi N, Bouganim N, Suissa S, Azoulay L. Cardiotoxicity of aromatase inhibitors and tamoxifen in postmenopausal women with breast cancer: a systematic review and meta-analysis of randomized controlled trials. Ann Oncol. 2017;28(3):487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Goldvaser H, Barnes TA, Šeruga B, Cescon DW, Ocaña A, Ribnikar D, Amir E. Toxicity of extended adjuvant therapy with aromatase inhibitors in early breast cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2018;110(1):31–39. [DOI] [PubMed] [Google Scholar]
- 100. Matthews A, Stanway S, Farmer RE, Strongman H, Thomas S, Lyon AR, Smeeth L, Bhaskaran K. Long term adjuvant endocrine therapy and risk of cardiovascular disease in female breast cancer survivors: systematic review. BMJ. 2018;363:k3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Gnant M, Pfeiler G, Dubsky PC, Hubalek M, Greil R, Jakesz R, Wette V, Balic M, Haslbauer F, Melbinger E, Bjelic-Radisic V, Artner-Matuschek S, Fitzal F, Marth C, Sevelda P, Mlineritsch B, Steger GG, Manfreda D, Exner R, Egle D, Bergh J, Kainberger F, Talbot S, Warner D, Fesl C, Singer CF; Austrian Breast and Colorectal Cancer Study Group. Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(9992):433–443. [DOI] [PubMed] [Google Scholar]
- 102. Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL, Cannady RS, Pratt-Chapman ML, Edge SB, Jacobs LA, Hurria A, Marks LB, LaMonte SJ, Warner E, Lyman GH, Ganz PA. American Cancer Society/American Society of Clinical Oncology breast cancer survivorship care guideline. J Clin Oncol. 2016;34(6):611–635. [DOI] [PubMed] [Google Scholar]