Abstract
There is growing evidence that stress-induced brain cytokines are important in the etiology of depression and anxiety. Here, we review how the neuroendocrine responses to psychological stressors affect the immediate and long-term regulation of inflammatory cytokines within the brain and highlight how the regulation changes across time with repeated stress exposure. In doing so, we report on the percentage of studies in the literature that observed increases in either IL-1β, TNF-α, or IL-6 within the hypothalamus, hippocampus, or prefrontal cortex after either acute or chronic stress exposure. The key takeaway is that catecholamines and glucocorticoids play critical roles in the regulation of brain cytokines after psychological stress exposure. Central catecholamines stimulate the release of IL-1β from microglia, which is a key factor in the further activation of microglia and recruitment of monocytes into the brain. Meanwhile, the acute elevation of glucocorticoids inhibits the production of brain cytokines via two mechanisms: the suppression of noradrenergic locus coeruleus neurons and inhibition of the NFκB signaling pathway. However, glucocorticoids and peripheral catecholamines facilitate inflammatory responses to future stimuli by stimulating monocytes to leave the bone marrow, downregulating inhibitory receptors on microglia, and priming inflammatory responses mediated by peripheral monocytes or macrophages. The activation of microglia and the elevation of peripheral glucocorticoid and catecholamine levels are both necessary during times of stress exposure for the development of psychopathologies.
Keywords: IL-1β, IL-6, TNF-α, prefrontal cortex, hypothalamus, hippocampus
Psychological stress is a major risk factor for the onset of depression and anxiety disorders [1, 2]. There is a growing amount of clinical and laboratory data indicating that the ability of stress to induce and augment the production of inflammatory cytokines is the primary mechanism by which stress exposure affects the etiology of these psychopathologies. Clinical studies demonstrate that depression is associated with elevated levels of acute-phase proteins, circulating IL-6, and increased IL-1β in cerebrospinal fluid [3–5]. Furthermore, administration of inflammatory cytokines to patients with cancer or hepatitis significantly increases the onset of depressive symptoms [6, 7]. In animal studies, central administration of the endogenous IL-1 receptor antagonist (IL-1RA) and knockout of IL-1 signaling in the brain prevents anhedonia and the onset of learned helplessness behaviors in animal models of stress-induced pathology [8–11]. That psychological stressors could result in de novo production of inflammatory responses was once thought not possible. Cells of the immune system were thought to only respond to foreign pathogens or compounds released during injury and tissue damage. Today, we know the neuroendocrine responses to stress affect immediate and long-term production and regulation of inflammatory cytokines.
This review focuses on how the neuroendocrine responses to stress affect the regulation of brain inflammatory cytokines. Our goals are to describe the key mechanisms by which neuroendocrine responses to stress affect the immediate and long-term regulation of brain inflammatory cytokines and highlight how the regulation of inflammatory cytokines change across time and with repeated stress exposure.
1. Methods
We performed a literature search using PubMed to find published articles that included the following search terms: “psychological stress, brain, cytokine, NOT review.” Because of the vast literature, we then narrowed the result to only include articles that contained IL-1β, IL-6, or TNF-α within the hippocampus, hypothalamus, or prefrontal cortex. Data were extracted from the individual articles as to stressor type, species or strain, sex, time after stress termination tissue was collected, and whether cytokines were observed to significantly increase in any of the brain areas of interest. The data were further sorted into categories on the basis of whether the stress protocol was “acute,” “subchronic,” or “chronic.” Acute stressors were defined as any stressor for which there was no break regardless of its duration. Subchronic stressors were defined as any paradigm in which animals were repeatedly exposed to stress intermittently for up to 7 days. Chronic stressors were defined as repeated stress exposure that continued for >7 days.
2. Results
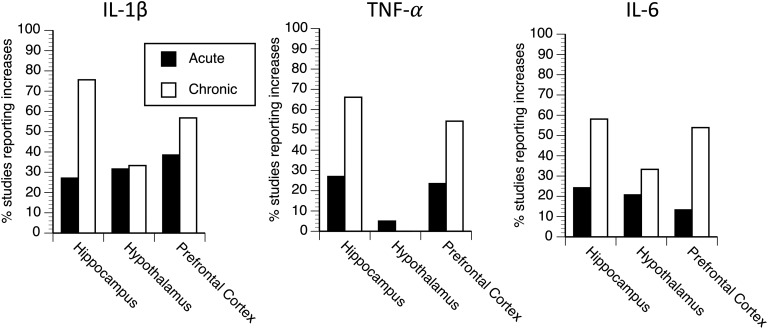
Table 1 presents the ratio of studies that report significant increases in the levels of each inflammatory cytokine within each brain area of interest after either acute or chronic stress exposure. Figure 1 presents the percentage of studies that report significant increases in the levels of IL-1β, TNF-α, or IL-6 within the hippocampus, hypothalamus, or prefrontal cortex after either acute or chronic stress. There is an important distinction between studies involving acute stress vs those involving chronic stress in regard to when brain cytokines are measured after the termination of stress exposure. After acute stress exposure, the elevations in brain cytokines were only detected within the first 4 hours after the termination of the stressor, whereas after chronic stress, the elevation in brain cytokines is almost always reported at least 12 hours after the termination of the last stressor (and most commonly 24 hours). The percentage of studies that report increases in levels of brain inflammatory cytokines after acute stress range from 5% (TNF-α in the hypothalamus) to 38.5% (IL-1β in the prefrontal cortex).
Table 1.
Ratio of the Number of Studies That Report a Significant Increas in Inflammatory Cytokines to Total Number of Studies
| Type of Stress | IL-1β | TNF-α | IL-6 |
|---|---|---|---|
| Acute stress | |||
| Hypothalamus | 18/57 | 1/20 | 6/29 |
| Hippocampus | 16/59 | 10/37 | 8/33 |
| Prefrontal cortex | 5/13 | 4/17 | 2/15 |
| Chronic stress | |||
| Hypothalamus | 6/18 | 0/10 | 2/6 |
| Hippocampus | 65/86 | 45/68 | 32/55 |
| Prefrontal cortex | 21/37 | 19/35 | 14/26 |
Figure 1.
The percentage of studies that report a significant increase in IL-1β, TNF-α, or IL-6 levels within the hippocampus, hypothalamus, or prefrontal cortex after acute or chronic stress exposure.
Several important variables have been identified that influence the chances of observing increases in brain cytokine levels after acute stress exposure; these are described in the Discussion. In terms of the percentage of studies that report elevated brain cytokine levels after chronic stress, the most common areas investigated were the hippocampus and prefrontal cortex. In these areas, the percentage of studies that report increases in levels of brain inflammatory cytokines ranged from 56.8% (IL-1β in the prefrontal cortex) to 75.6% (IL-1β in the hippocampus). The percentage of studies that report increased levels of inflammatory cytokines in the hypothalamus was substantially lower, ranging from 0% for TNF-α to 33.3% for IL-1β and IL-6. It is unclear if this is due to the fewer studies that examined cytokines in the hypothalamus compared with those that measured cytokines in the hippocampus and prefrontal cortex, or if it represents a true regional difference in cytokine production after chronic stress.
Studies investigating the effects of subchronic stressors on brain cytokines were more difficult to quantify. This was primarily because these studies fell into one of two categories that often showed opposite results. The first of these was studies that examined the sensitization of cytokine responses across time to repeated stress exposure. These studies exposed animals to 2 to 7 days of stress and measured brain cytokine responses at 0 to 4 hours after the last stressor; greater cytokine responses were often observed in animals with a history of stress exposure. The second category of studies were those that examined the duration of stress exposure necessary to result in the persistent increase in brain cytokine levels 24 hours after the last stressor. Two to 7 days of stress exposure typically were not sufficient to result in a persistent increase in brain cytokine levels, although certain models of repeated social stress consistently showed there were elevations in levels of inflammatory cytokines in CD11b+ cells within the brain after 6 days of stress. To avoid confusion regarding whether subchronic stress increases levels of brain cytokines, we elected not to include these studies in the table or figures in this article; instead, the phenomenon of stress-induced priming of brain cytokines and factors important for the persistent rise in brain cytokines are addressed in the Discussion.
3. Discussion
Stress-induced production of inflammatory cytokines is considered a form of “sterile inflammation,” a term used to describe inflammatory responses that occur in the absence of infection. One of the earliest experimental studies to examine sterile inflammation was published by J.V. Cooke and G.H. Whipple in 1918 [12], in which they demonstrated that administration of sterile toxins (e.g., turpentine, bile, proteoses) to dogs induced similar leukocytosis, fever, body weight loss, and increase in urine nitrogen levels as did injections of Staphylococcus aureus. Today, there is a growing list of endogenous danger signals known to stimulate sterile inflammation [13]. Although some danger signals are only released during times of necrotic cell death, others such as high-mobility group box 1 (HMGB1), heat-shock protein 72 (Hsp72), and ATP are actively released during times of psychological stress and initiate an inflammatory response [14–16].
Danger signals induce the release of inflammatory cytokines by acting at toll-like receptors (TLRs) and/or by inducing the activation of inflammasomes. Some danger signals such as HMGB1 and Hsp72 are endogenous ligands for TLRs that are associated with the NFκB signaling pathway, a major signaling pathway that mediates the transcription and translation of inflammatory mediators such as pro–IL-1β, IL-6, and TNF-α [14]. Alternatively, some danger signals are more specifically involved in the activation of select inflammasomes, which are intracellular multiprotein complexes that mediate the enzymatic cleavage of pro–IL-1β (and pro–IL-18) into their mature forms. One well-characterized danger signal that functions in this way is ATP, which acts at purinergic type 2X7 receptors (P2X7Rs) to induce the oligomerization and activation of the nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain protein 3 (NLRP3) inflammasomes [17], the most common inflammasome reported to become activated after psychological stress [16–20].
The NLRP3 inflammasome is unlike most inflammasomes in that it requires two signals to fully assemble and become activated. The initial “priming” signal induces the upregulation of NLRP3, a key regulatory protein for which the NLRP3 inflammasome is named. NFκB signaling upregulates NLRP3 expression (possibility mediated by HMGB1 and Hsp72 signaling during times of stress). After priming, a second “activating” signal is required for NLRP3 to interact with the rest of the inflammasome machinery, which includes an adaptor protein called apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) and the effector enzyme procaspase 1. After oligomerization of NLRP3 with ASC and procaspase1, procaspase1 is cleaved into caspase 1 that then cleaves pro–IL-1β into its active form [21]. The most likely second signal during psychological stress exposure is ATP, which is released by astrocytes in response to heightened glutaminergic activity [22] and increases in the brain during stress exposure [19]. Inhibiting NLRP3 inflammasome activation before stress exposure (e.g., P2X7R antagonists, caspase 1 inhibitors, NLRP3 knockout) protects animals from stress-induced increases in brain IL-1β levels [19, 23, 24]. More interesting is that these manipulations also block or attenuate overall levels of inflammatory cytokines, suggesting that the release of mature IL-1β is a critical initial mediator of the inflammatory cascade during times of stress. There are several excellent reviews that cover how danger signals regulate inflammatory responses [13, 25]. Here, we focus more specifically on the role stress hormones play in modulating the release of inflammatory cytokines. Although stress hormones have classically been viewed as anti-inflammatory, we now known they have pleiotropic effects that include the regulation of both pro- and anti-inflammatory responses. Figure 2 summarizes what is discussed in this review regarding the major pathways by which stress hormones regulate inflammatory cytokines during times of psychological stress.
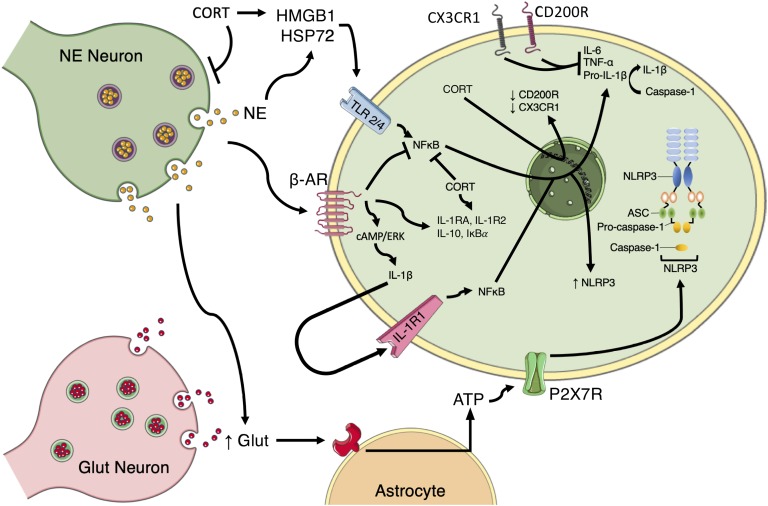
Figure 2.
Schematic of the proposed mechanisms by which norepinephrine (NE) and corticosterone/cortisol (CORT) regulate sterile inflammation. NE promotes inflammation by (i) facilitating glutamate (GLUT) release and production of ATP by astrocytes that then promote the oligomerization of the NLRP3 inflammasome; (ii) acting at β-ARs to induce IL-1β production through a cAMP/ERK-dependent pathway, which can lead to IL-1β acting in an autocrine fashion to induce NFκB signaling pathways and production of NLRP3, IL-6, TNF-α, and pro-IL-1β; and (iii) causing the release of HSP72, which acts at TLR 2/4 to stimulate NFκB signaling. CORT promotes inflammation by (i) causing the release of HMGB1, which activates TLR 2/4 signaling pathways and (ii) downregulating CD200R and CX3CR1, which are negative regulators of inflammatory responses in microglia. NE and CORT also have anti-inflammatory properties that include inhibiting NFκB signaling and upregulating IL-1RA, IL-1R2, IL-10, and IκBα. CORT also has the ability to suppress noradrenergic activity, thereby reducing NE release. ERK, extracellular signal-regulated kinase; IL-1R2, IL-1 receptor type 2; IκBα, nuclear factor of κ light polypeptide gene enhancer in β-cells inhibitor α; CD200R, CD200 receptor; CX3CR1, CX3C receptor type 1.
A. Neuroendocrine Response to Psychological Stress
Stress is often defined as the response of the body to a real or perceived threat. It is a constellation of cognitive, endocrine, and physiological responses designed to increase an organism’s chance of survival. The stress response consists of rapid central and peripheral autonomic responses that occur almost simultaneously with detection of a threatening stimulus and a slower endocrine response that develops over time. The central autonomic response to psychological stressors is primarily mediated by noradrenergic neurons located in the locus coeruleus, which releases norepinephrine in brain areas that mediate cognitive and emotional processes [26]. The central release of norepinephrine increases the general alertness of an organism and causes a shift from slow, contemplative cognitive processing to a more rapid, reflexive behavioral response [27]. At the same time, the peripheral autonomic response is mediated by the activation of the sympathetic nervous system and release of peripheral catecholamines (i.e., norepinephrine and epinephrine). The sympathetic nervous system mediates a shift in physiological processes that support fight-or-flight responses such as increasing oxygen consumption, glucose availability, and blood flow to skeletal muscles [28]. The slower endocrine response to stress is due to the activation of the hypothalamus-pituitary-adrenal axis, which results in the steady increase in circulating glucocorticoids that reach peak levels approximately 20 to 30 minutes after the onset of an acute stressor [29]. Glucocorticoids (primarily cortisol in humans and corticosterone in rodents) have many permissive and stimulatory properties that help facilitate the peripheral actions of catecholamines. For example, glucocorticoids facilitate the effects of catecholamines on vascular reactivity, inhibit the reuptake of catecholamines at neuromuscular junctions, and increase the affinity of β-adrenergic receptors (β-ARs) in arterial smooth muscle cells [30]. However, glucocorticoids also are involved in a feedback mechanism that inhibits the central autonomic response via suppressing the expression of tyrosine hydroxylase (the rate-limiting enzyme in the production of norepinephrine) in locus coeruleus neurons [31] and the release of central norepinephrine during times of stress [32, 33]. Catecholamines and glucocorticoids are the primary neuroendocrine mediators in response to stress exposure and as such, function as danger signals throughout the body to help an organism respond to and survive a threat. It is not surprising that these neuroendocrine responses have also been identified as the key mediators in the regulation of inflammatory responses within the brain after psychological stress exposure.
B. Microglia, Infiltrating Monocytes, and Brain Inflammation
Inflammatory responses within the brain after psychological stressors are primarily mediated by the activation of microglia and the eventual recruitment of circulating monocytes into the brain. Microglia are the resident macrophage population within the central nervous system. They are myeloid cells that are born in the bone marrow and migrate to the brain during early development to provide immune surveillance. Similar to other immune cells, the activation properties of microglia are influenced by their microenvironment. Within the brain, microglia receive a number of inhibitory signals (e.g., CD200, CX3CL1, CD47, and sialic acids) from neurons, astrocytes, and other cell types that suppress their activity and help prevent damage to brain tissue [34]; thus, the activation profile of microglia is significantly more restrained compared with that of peripheral macrophages [35]. However, after stress exposure, the expression of CD200R and CX3CR1 on microglia downregulates [36–39], thereby releasing microglia from the inhibitory microenvironment of the brain.
The most convincing evidence that microglia are critical mediators in the elevation of brain cytokines after stress exposure is that pharmacological suppression of microglia or depletion of microglia before stress exposure prevents the increase in brain cytokine levels after exposure to acute and chronic stressors [40–42]. In addition, many studies have reported an upregulation in microglia activation markers after stress exposure, which is nicely reviewed by Walker et al. [43], and include OX-42 [44], major histocompatibility complex class II [37], CD86 [45], CD14 [39, 45], and ionized calcium-binding adaptor molecule 1 (Iba1) [46]. There is also an increase in NLRP3 inflammasome colocalized with Iba1 expression in the prefrontal cortex after chronic stress exposure [47]. The expression of any given marker seems to vary with stress model and timing after stressor exposure [48]. It should also be noted that the cited studies were in male rodents and that significant sex differences have been reported.
Female rodents are often reported to have more microglia in an activated phenotype compared with males without any exposure to stress [49, 50], and within the hippocampus, sometimes higher basal expression of IL-1β, TNF-α, and IL-6 mRNA has been reported in female rodents [51]. Stage of the estrous cycle is critical when examining the effects of acute stress exposure on brain cytokine induction, because estrogen has anti-inflammatory effects on the induction of IL-1β after foot shock [52]. In addition, IL-6 and TNF-α production is reduced in the hippocampus of female mice after repeated cold stress, compared with male mice [53]. The largest sex difference appears to be on the effects of chronic stress on microglia themselves. Exposure to chronic restraint stress reduces Iba1 expression in the medial prefrontal cortex of females, whereas such exposure increases expression in male rodents [50]. Similar responses were observed in microglia isolated from the hippocampus of male and female rats 24 hours after tail-shock exposure, where stress exposure significantly reduced lipopolysaccharide (LPS)-evoked IL-6 release and had no effect on IL-1β production from microglia isolated from female rats, whereas significantly increased IL-6 and IL-1β production was observed from microglia isolated from male rats [54]. Although there are sex differences in the long-term effect of stress on microglia, minocycline treatment before chronic stress exposure still protected female rats against the increase of brain cytokines and onset of stress-induced depression [55].
Activation of microglia is critical for the infiltration of circulating monocytes into the brain. Depleting microglia from the brain before exposure to repeated social defeat protects animals from the infiltration of circulating monocytes and the onset of stress-associated behavioral changes [41, 56]. The infiltrating monocytes may be important because of their greater inflammatory potential compared with that of resident microglia [57, 58]. Unfortunately, administration of minocycline to animals after exposure to chronic stress does not necessarily provide therapeutic benefits, and even has been shown to worsen depressive symptoms [48]. One possible explanation for this is the differential effects minocycline has on various types of immune cells. Minocycline is commonly used to inhibit proinflammatory responses in microglia [59] but can facilitate proinflammatory responses in circulating monocytes [60, 61]. Thus, the protection minocycline provides when administered before stress exposure likely has to do with its ability to suppress the activation of microglia during the commencement of stress exposure. As stress exposure continues and circulating monocytes accumulate in the brain, the protective effects of minocycline are lost and its faciliatory effects on monocytes may make symptoms worse. Collectively, studies demonstrate that microglia mediate the initial increase in brain cytokines after stress exposure and are critical for the recruitment of circulating monocytes into the brain.
C. Catecholamine Involvement in Regulating Brain Cytokines
There is now substantial evidence that catecholamines mediate the acute increase in levels of brain cytokines after stress exposure via a β-AR–mediated pathway. We and others have shown that β-ARs mediate the production of IL-1β in the brain. This is demonstrated by (i) central administration of isoproterenol (a β-AR agonist) induces IL-1β protein production in a time-dependent fashion that is blocked by administration of propranolol (a β-AR antagonist) [62]; (ii) production of IL-1β during acute or chronic stress exposure can be blocked by administration of propranolol [40, 45, 63]; and (iii) neurotoxic lesions of noradrenergic neurons selectively prevent stress-induced IL-1β production in brain areas where norepinephrine depletion occurs [63, 64]. Similarly, catecholamines contribute to the elevation of the proinflammatory eicosanoid prostaglandin E2 in the brain after restraint stress that can be blocked by administration of propranolol [65]. The exact mechanisms by which catecholamines promote inflammatory cytokine production have not been fully elucidated.
Several cell types within the brain can produce IL-1β, including neurons [66], astrocytes [67], and microglia [68]. However, there are reasons to believe that after stress exposure, IL-1β is primarily produced by microglia. This is because isoproterenol (a β-AR agonist) induces IL-1β mRNA in cultured microglia but not in astrocytes [69], administration of minocycline prevents stress-induced increases in brain IL-1β [40, 70], and the induction of IL-1β after stress or metyrapone treatment (a corticosterone synthesis inhibitor) is observed in CD11b+ cells [45, 71].
The mechanisms by which catecholamines mediate IL-1β induction in the brain likely include direct effects of catecholamines on microglia and indirect effects on other cell types. As previously mentioned, ATP is a danger signal that is released during times of stress and can activate the NLRP3 inflammasome in microglia [22]. ATP is released by astrocytes in response to glutamate. In vivo, β-ARs enhance glutaminergic synaptic transmission [72, 73], which likely facilitates ATP release from astrocytes during times of stress. Even in the absence of glutamate signaling, β-ARs synergize with ATP to induce IL-6 [74], thus in vivo β-AR signaling likely facilitates cytokine production through augmenting the release and signaling of ATP. In addition, β-AR stimulation induces IL-1β production in cultured phagocytic cells, demonstrating that β-ARs can have direct effects on microglia and macrophages. The intracellular signaling mechanism by which β-ARs induce IL-1β is independent of NFκB and involves the activation of cAMP signaling and stimulation of extracellular signal-regulated kinase, a member of the mitogen activated protein kinase [75, 76]. It is unclear if the production of IL-1β via β-AR signaling depends on activation of an inflammasome or if it is mediated by an inflammasome-independent pathway, as is observed with other sterile inflammatory stimuli [77–79]. Clenbuterol, a β2-AR agonist, administered to rats selectively induces IL-1β and not IL-6 or TNF-α in the hypothalamus and hippocampus [80]. Figure 3 demonstrates similar responses in primary microglia incubated with various concentrations of norepinephrine. Microglia were isolated from Fischer male rats (n = 6) using a Percoll gradient that resulted in >98% CD11b/c-positive cells, as previously described [81]. Fischer rats are a more stress-responsive rat strain that has increased brain IL-1β production after stress exposure [82]. A dose-dependent increase in extracellular IL-1β is observed after incubating microglia with norepinephrine alone. Norepinephrine had no effect on LPS-induced IL-1β or TNF-α production, but significantly enhances LPS-evoked IL-6 release. It should be noted that IL-1β acts at type 1 IL-1R to stimulate the NFκB-mediated pathway that leads to the production of other cytokines, including IL-6 and TNF-α [83]. Exactly why stimulation of β-ARs does not result in the production of other cytokines in cultured microglia is unclear; perhaps production is below the sensitivity of current ELISAs or more persistent stimulation of β-ARs is necessary to drive additional cytokine responses [84]. Clearly, from the results of our literature review, IL-1β, IL-6, and TNF-α have all been observed to increase within the brain after stress exposure. Figure 2 shows the signaling pathways by which norepinephrine induces IL-1β and how IL-1β can act in an autocrine fashion to stimulate additional inflammatory responses in microglia.
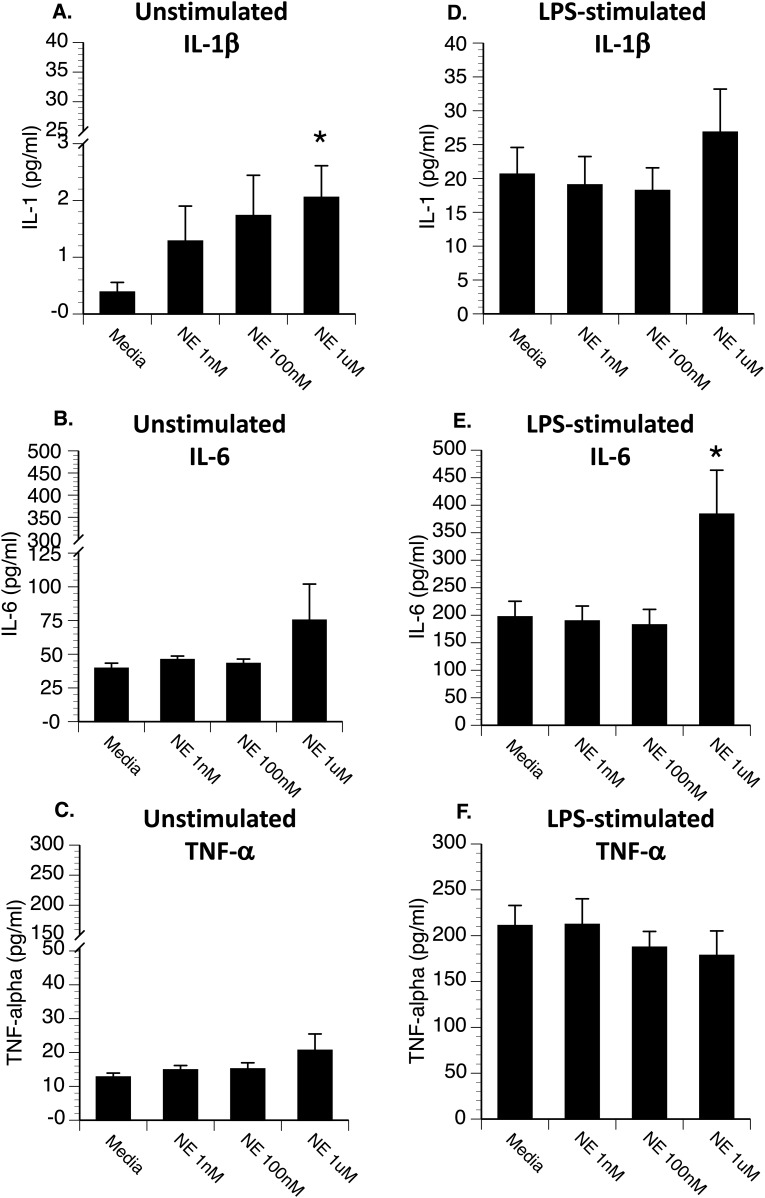
Figure 3.
Hippocampal microglia were plated in a 96-well culture plate at 10,000 cells per well and incubated with either (A–C) media (unstimulated) or (D–F) 0.5 μg LPS (stimulated), along with different concentrations of norepinephrine. After 20 hours, supernatants were collected for measurement of IL-1β, IL-6, and TNF-α by ELISA (R&D Systems). Under unstimulated conditions (A–C), norepinephrine resulted in (A) a dose-dependent increase in IL-1β (A). Under LPS-stimulated conditions (D–F), the highest concentration of norepinephrine significantly increased (E) LPS-induced IL-6 release. *P < 0.05 difference from media-treated cells.
As reported here, only approximately 20% to 30% of experiments that expose animals to acute stress report a significant increase in brain cytokines, which may have to do with the requirement of threshold levels of β-AR activation to stimulate brain IL-1β production. Evidence to support this comes from the fact that as stressor intensity increases or an animal’s stress responsiveness increases, so does the probability to observe an increase in brain cytokines.
For example, in Sprague-Dawley rats, forced swim [85], predator odor [86], and restraint [82, 87] typically fail to induce brain cytokine production, whereas exposure to tail shock [88], foot shock [39], or exposure to multiple stressors such as restraint on an orbital shaker or restraint followed by additional stressors [87, 89] is sufficient. Animal strains that show greater stress responsiveness are also more susceptible to stress-induced increases in levels of brain cytokines. For example, Fischer rats, which show greater glucocorticoid and catecholamine responses compared with Sprague-Dawley rats, have increased hypothalamic IL-1β production after restraint, whereas Sprague-Dawley rats do not unless administered a norepinephrine reuptake inhibitor [82]. Finally, prior stress exposure can sensitize locus coeruleus neurons [90] and brain IL-1β production to subsequent central administration of isoproterenol (a β-AR agonist) [91]. This might underlie why animals with prior stress history often have enhanced production of brain cytokines when re-exposed to stress [92–94]. Alternatively, another possible reason for the failure to detect inflammatory cytokine production after some stressors may simply be the detection limits of current assays. Femtomolar concentrations of IL-1β are sufficient to trigger biological responses [95], yet most assays have detection limits in the picomolar range. We predict that as the sensitivity of assays improves, the number of studies that detect increases in brain cytokine levels after acute stress will increase substantially.
Peripheral catecholamines also mediate the increase in circulating IL-1β and IL-6 during times of stress [40, 63, 96, 97]. Interestingly, both α-AR and β-AR contribute to the stress-induced increase in circulating levels of cytokines [63]. Activation of α-ARs during times of stress trigger the extracellular release of Hsp72 [98], an endogenous danger signal that can bind to TLR 2 and TLR 4 to induce inflammatory cytokines and facilitate bacterial killing [99]. Alternatively, activation of β-ARs mediate cytokine production in tissues such as the brain, pituitary, and spleen [39, 40, 45, 63, 97]. Because peripheral cytokines can induce central cytokines, there is the possibility that peripheral cytokines may contribute to the induction of central cytokines during times of stress exposure. Potentially of greater significance is the effect catecholamines have on peripheral immune cells. Prolonged epinephrine administration significantly enhances the trafficking of immune cells and significantly augments IL-6 production of macrophages at wound sites. Peripheral administration of a β-AR antagonist attenuates the epinephrine-induced enhancement in trafficking and IL-6 production at a skin wound [100]. Peripheral β-AR blockade also prevents the increase in circulating IL-6 and the number of activated splenic macrophages after repeated social defeat [97]. Thus, the increase in levels of peripheral catecholamines during times of stress plays an important role in sensitizing peripheral monocytes or macrophages. This is particularly important because the infiltration of sensitized monocytes into the brain have been proposed to be a critical factor in the increase in brain inflammation and the onset of psychopathology after chronic stress [101].
Catecholamines are not solely proinflammatory but they mediate numerous anti-inflammatory responses. For example, catecholamines induce the endogenous IL-1RA, the type 2 IL-1 “decoy” receptor, the upregulation of the anti-inflammatory cytokines IL-10 and prostaglandin 15d-PGJ2, and the increase in IκBα expression that inhibits NFκB signaling [76, 102–106]. In fact, most of the anti-inflammatory properties of catecholamines are mediated through the same β-ARs that, as we previously discussed, mediate the induction of IL-1β during times of psychological stress exposure. Administration of clenbuterol, a β2-AR agonist, to rats induces a time-dependent increase in cortical IL-1β, IL-1RA, and IL-1R2 mRNA that can be blocked by prior administration of propranolol [76]. β-AR signaling also has the ability to inhibit NFκB signaling via stimulating the intracellular rise in cAMP and activation of protein kinase A [107, 108]; thus, β-AR agonists generally attenuate inflammatory cytokine production to inflammatory agents such as LPS, Escherichia coli, and β-amyloid [62, 76, 109, 110]. McNamee et al. demonstrated that in vivo administration of a β-AR agonist induces brain IL-1β production in the absence of immune challenge but attenuates proinflammatory cytokine production after LPS administration [76], which is in agreement with our previous observation that β-ARs can mediate both pro- and anti-inflammatory effects in animals challenged with live E. coli [62]. In summary, catecholamines have both pro- and anti-inflammatory properties and their net effect on cytokine production depends on the overall signaling cascades initiated within a specific cell, such as the presence or absence of pathogen at the time of adrenergic receptor activation.
D. The Critical Role of IL-1β
Stimulation of β-ARs and the induction of IL-1β contribute to the priming of microglia. Microglia do not simply turn “on” and “off” but have many different phenotypes and activation states [35]. After psychological stress exposure, microglia can shift from a quiescent (resting) state into a primed activation state in which they do not actively release cytokines, but upon further stimulation, they release higher levels of inflammatory cytokines compared with the stimulation of quiescent microglia [43]. Central administration of isoproterenol (a β-AR agonist) is sufficient to prime microglia such that microglia isolated from the brain 24 hours after isoproterenol administration produce substantially higher levels of IL-1β and IL-6 when challenged with LPS, compared with microglia isolated from saline-treated animals [81]. Furthermore, administration of propranolol (a β-AR antagonist) prevents the priming of microglia when administered before acute foot shock [39] or repeated social defeat [45]. The most likely mechanism by which β-ARs prime microglia is by stimulating the release of IL-1β. As discussed, activation of β-ARs stimulates the release of IL-1β, and we previously demonstrated that the central administration of IL-1β is sufficient to sensitize IL-1β responses to subsequent LPS challenge [111]. Importantly, it does so without affecting circulating inflammatory cytokine responses to LPS, indicating increases in brain IL-1β selectively prime central cytokine responses. The induction of brain IL-1β during times of stress is necessary to sensitize brain cytokine responses. Central administration of the endogenous IL-1ra before tail-shock exposure prevented stress-induced priming of cytokine responses to a later LPS challenge [111]. In addition to priming central cytokine responses, IL-1β signaling is also critical for the recruitment of circulating monocytes into the brain after chronic stress [11]. This is a critical step in the onset of depression and anxiety after repeated social defeat. Regardless of stress model, blocking IL-1β signaling during stress exposure prevents the onset of anhedonia and anxiety behaviors [8–11, 19].
E. Glucocorticoid Involvement in Regulating Brain Cytokines
Glucocorticoids, particularly cortisol and corticosterone, are best known for their potent ability to inhibit inflammatory cytokine production by the suppression of the transcription factor NFκB [112, 113]. Glucocorticoid receptors directly interact with NFκB through protein-protein interactions that impair the binding of NFκB to DNA, which, in turn, results in a decrease in the transcription of proinflammatory cytokines [114–116]. Glucocorticoids also increase the expression and binding affinity of IκB that inhibits NFκB translocation to the nucleus [117]. In addition to inhibiting NFκB signaling, glucocorticoids promote the transcription and translation of anti-inflammatory regulators such as IL-10, IL-1RA, and type 2 IL-1 “decoy” receptors [118]. Thus, it should not be surprising that increased corticosterone levels during times of stress inhibit NFκB translocation [119] and that blocking the rise in circulating corticosterone by either adrenalectomy or metyrapone (a corticosterone synthesis inhibitor) results in massive and widespread increases in brain IL-1β after stress exposure, compared with controls [39, 120, 121]. However, as we have described, considerable evidence indicates that the initial stress-induced production of brain IL-1β is mediated by catecholamines through a β-AR–mediated pathway that is not dependent on NFκB activation [75].
We recently explored an alternative explanation for the suppressive effects of glucocorticoids on brain cytokines, which was based on prior findings that corticosterone inhibits tyrosine hydroxylase expression (the rate-limiting enzyme in the synthesis of catecholamines) in locus coeruleus neurons [31] and that adrenalectomized animals have substantially increased levels of norepinephrine release in the paraventricular nucleus of the hypothalamus during immobilization stress, compared with sham controls [33]. These findings suggest corticosterone has the potential to regulate brain cytokines via their ability to inhibit central noradrenergic activity. Our laboratory recently demonstrated that acute metyrapone treatment causes an increase in norepinephrine turnover and widespread increases in brain levels of IL-1β [71]. Furthermore, the increase in brain IL-1β after metyrapone was completely blocked by prior administration of propranolol (a β-AR antagonist); thus, one way glucocorticoids regulate brain IL-1β levels is by modulating norepinephrine release (Fig. 4). Interestingly, the ability of metyrapone to regulate norepinephrine turnover and brain IL-1β was only observed in male rats and not female rats, indicating there are sex differences in the regulation of locus coeruleus neurons by corticosterone that need to be explored. The ability of glucocorticoids to regulate brain cytokines via modulation of norepinephrine release does not negate the importance of the NFκB pathway in stress-induced brain cytokines. IL-1β acts at type 1 IL-1Rs to stimulate NFκB signaling pathways, which can mediate the production of additional inflammatory cytokines [83]. In fact, the induction of NFκB after stress exposure is a key mediator of depressive behavior [122, 123]. Thus, glucocorticoids can regulate both the initial catecholamine-mediated induction of brain IL-1β and the subsequent NFκB-mediated inflammatory wave that occurs after chronic stress exposure.
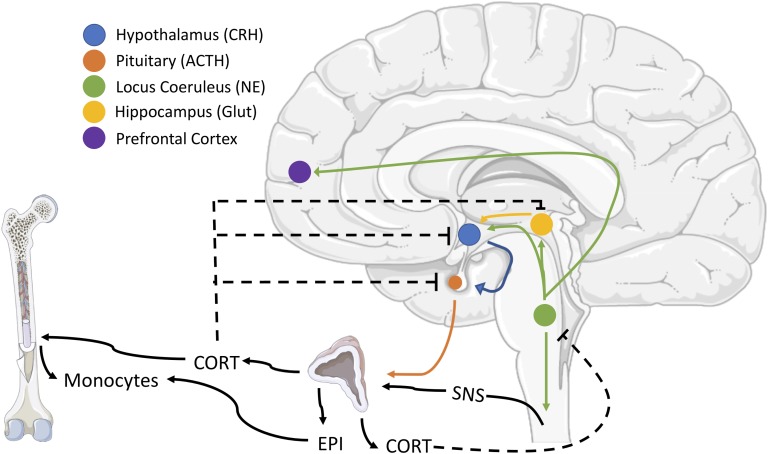
Figure 4.
Schematic of the effects of peripheral glucocorticoids and catecholamines on peripheral monocytes and feedback on locus coeruleus neurons. Brainstem noradrenergic neurons contribute to the activation of the hypothalamus-pituitary-adrenal (HPA) axis and activation of the sympathetic nervous system (SNS), which results in the collective release of circulating corticosterone or cortisol (CORT) and epinephrine (EPI), respectively. CORT causes monocytes to leave the bone marrow, whereas EPI primes their responses to future activation. CORT provides negative feedback at multiple levels of HPA regulation and inhibits central noradrenergic release. Negative feedback is indicated by dashed lines. CRH, corticotropin-releasing hormone; Glut, glutamate.
The role of glucocorticoids during times of stress is not solely to suppress inflammatory responses. As described by Sapolsky et al. [30], glucocorticoids often have preparative actions to allow an organism to be more responsive to future challenges. In terms of brain cytokines, glucocorticoids contribute to the increase in levels of brain PGE2 [65] and facilitate future inflammatory responses by contributing to the priming of microglia [124, 125] and the infiltration of circulating monocytes into the brain [126]. The priming of microglia by glucocorticoids appears to be largely because of glucocorticoids’ negative regulation of CD200R and CX3CR1 expressed by microglia [38]. Downregulation of these receptors is thought to be a key process in releasing microglia from the inhibitory microenvironment of the brain and the subsequent expression of activation markers and the release of HMGB1 [15, 36]. Central administration of ligands for CD200R or antagonists for HMGB1 before tail-shock exposure is sufficient to prevent priming of microglia cytokine responses [36]. In addition, a single injection of corticosterone (at a dose of 2.5 mg/kg that mimics the corticosterone response to inescapable tail shock) is sufficient to prime microglia cytokine responses and results in exaggerated sickness behaviors to subsequent LPS challenge [124, 127]. A summary of the pro- and anti-inflammatory effects of corticosterone is included in Fig. 2.
Glucocorticoids promote surveillance by peripheral immune cells, which is critical for the migration of circulating monocytes into the brain. Although the number of circulating monocytes can decrease during acute stress, their numbers quickly recover after the termination of the stressor [128], and greater numbers of circulating monocytes are observed 14 hours after the termination of repeated social defeat [126]. The increase in circulating monocytes after stress is likely due to the reduction in CXCL12 in the bone marrow, an important chemokine for retaining myeloid cells. Adrenalectomy and metyrapone treatment both prevent the increase in the number of circulating monocytes and the number of infiltrating monocytes into the brain after repeated social defeat. In addition, blocking stress-induced glucocorticoids during social defeat attenuates the priming effects of stress on splenic macrophage production of LPS-evoked IL-6 [126], suggesting both catecholamines and glucocorticoids aid in the priming of peripheral macrophages. Inhibiting glucocorticoids during times of stress protects animals from stress-induced depression and anxiety [129, 130] and may do so by reducing the infiltration of primed monocytes into the brain after stress exposure. Although glucocorticoids play a critical role in the eventual onset of stress-induced depression, they are unlikely to be sufficient by themselves, because chronic administration of superphysiological doses of corticosterone (i.e., >10 mg/kg) are typically necessary to induce depressive-like behaviors [131, 132].
F. Summary
As described, catecholamines and glucocorticoids have both positive and negative effects on the regulation of brain cytokines. In response to psychological stress exposure, central catecholamines stimulate the release of IL-1β from microglia through the activation of β2-ARs, and IL-1β is a key factor in the further activation of microglia and recruitment of monocytes into the brain. Meanwhile, the acute increase in glucocorticoid levels inhibits the production of brain cytokines via two mechanisms: the suppression of noradrenergic locus coeruleus neurons and inhibition of the NFκB signaling pathway. However, glucocorticoids and peripheral catecholamines also have preparative actions that facilitate inflammatory responses to future stimuli, including stimulating monocytes to leave the bone marrow, downregulating inhibitory receptors on microglia, stimulating the release of danger signals that prime the NLRP3 inflammasome, and priming peripheral monocytes/macrophages.
The induction of brain cytokines and the increase in levels of circulating glucocorticoids are both necessary for the development of stress-induced psychopathology, yet neither is likely to be sufficient by itself. There is overwhelming evidence that increases in brain IL-1β levels contribute to the etiology of stress-induced depression and anxiety; yet blocking glucocorticoids during times of stress, which results in massive increases in stress-induced brain IL-1β, protects organisms from stress-induced behavioral changes. If the elevation in brain IL-1β was solely responsible for the onset of behavioral changes, then blocking corticosterone production should make an organism more susceptible (not less) to stress-induced pathology. Likewise, a single injection of corticosterone is sufficient to alter the distribution of monocytes and prime microglia cytokine responses, but a single injection of corticosterone is not sufficient to induce symptoms of depression. This indicates that central and peripheral neuroendocrine responses to stress are necessary in the onset of stress-induced psychopathology. We propose that central catecholamines are necessary for the activation of microglia, the release of IL-1β, and the recruitment of circulating monocytes into the brain, whereas peripheral glucocorticoids and catecholamines are critical for the priming and redistribution of monocytes. Without the peripheral endocrine response, there are no primed circulating monocytes to recruit into the brain, and without the central activation of microglia, there is no signal to cause the primed circulating monocytes to migrate into the brain.
Acknowledgments
We acknowledge that stress affects a wide range of different cytokines, chemokines, and leukotrienes that could simply not be covered in this review. Likewise, because of the enormous number of studies investigating the effects of stress on brain cytokines, we could not cite every paper or discuss all the possible regulators in this mini-review.
Financial Support: This work was supported by the National Institute of Mental Health (Grants MH114049 and MH099580 to J.D.J.).
Disclosure Summary: The authors have no conflicts of interest to report.
Glossary
Abbreviations:
- β-AR, β-adrenergic receptor
HMGB1, high-mobility group box 1
- HSP72
heat-shock protein 72
- Iba1
ionized calcium-binding adaptor molecule 1
- IL-1RA
IL-1 receptor antagonist
- NLRP3
nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain protein 3
- P2X7R
purinergic type 2X7 receptor
- TLR
toll-like receptor
References and Notes
- 1. Ebert DD, Buntrock C, Mortier P, Auerbach R, Weisel KK, Kessler RC, Cuijpers P, Green JG, Kiekens G, Nock MK, Demyttenaere K, Bruffaerts R. Prediction of major depressive disorder onset in college students. Depress Anxiety. 2019;36(4):294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kessler RC. The effects of stressful life events on depression. Annu Rev Psychol. 1997;48(1):191–214. [DOI] [PubMed] [Google Scholar]
- 3. Levine J, Barak Y, Chengappa KN, Rapoport A, Rebey M, Barak V. Cerebrospinal cytokine levels in patients with acute depression. Neuropsychobiology. 1999;40(4):171–176. [DOI] [PubMed] [Google Scholar]
- 4. Köhler CA, Freitas TH, Stubbs B, Maes M, Solmi M, Veronese N, de Andrade NQ, Morris G, Fernandes BS, Brunoni AR, Herrmann N, Raison CL, Miller BJ, Lanctôt KL, Carvalho AF. Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: systematic review and meta-analysis. Mol Neurobiol. 2018;55(5):4195–4206. [DOI] [PubMed] [Google Scholar]
- 5. Maes M. Evidence for an immune response in major depression: a review and hypothesis. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19(1):11–38. [DOI] [PubMed] [Google Scholar]
- 6. Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26(5):643–652. [DOI] [PubMed] [Google Scholar]
- 7. Capuron L, Ravaud A, Miller AH, Dantzer R. Baseline mood and psychosocial characteristics of patients developing depressive symptoms during interleukin-2 and/or interferon-alpha cancer therapy. Brain Behav Immun. 2004;18(3):205–213. [DOI] [PubMed] [Google Scholar]
- 8. Maier SF, Watkins LR. Intracerebroventricular interleukin-1 receptor antagonist blocks the enhancement of fear conditioning and interference with escape produced by inescapable shock. Brain Res. 1995;695(2):279–282. [DOI] [PubMed] [Google Scholar]
- 9. Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci USA. 2008;105(2):751–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, Yirmiya R. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry. 2008;13(7):717–728. [DOI] [PubMed] [Google Scholar]
- 11. Wohleb ES, Patterson JM, Sharma V, Quan N, Godbout JP, Sheridan JF. Knockdown of interleukin-1 receptor type-1 on endothelial cells attenuated stress-induced neuroinflammation and prevented anxiety-like behavior. J Neurosci. 2014;34(7):2583–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cooke JV, Whipple GH. Proteose intoxications and injury of body protein: IV. The metabolism of dogs with sterile abscess, pancreatitis, and pleuritis. J Exp Med. 1918;28(2):223–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rock KL, Latz E, Ontiveros F, Kono H. The sterile inflammatory response. Annu Rev Immunol. 2010;28(1):321–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fleshner M, Crane CR. Exosomes, DAMPs and miRNA: features of stress physiology and immune homeostasis. Trends Immunol. 2017;38(10):768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Frank MG, Weber MD, Watkins LR, Maier SF. Stress sounds the alarmin: the role of the danger-associated molecular pattern HMGB1 in stress-induced neuroinflammatory priming. Brain Behav Immun. 2015;48:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weber MD, Frank MG, Tracey KJ, Watkins LR, Maier SF. Stress induces the danger-associated molecular pattern HMGB-1 in the hippocampus of male Sprague Dawley rats: a priming stimulus of microglia and the NLRP3 inflammasome. J Neurosci. 2015;35(1):316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iwata M, Ota KT, Duman RS. The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behav Immun. 2013;31:105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alcocer-Gómez E, Ulecia-Morón C, Marín-Aguilar F, Rybkina T, Casas-Barquero N, Ruiz-Cabello J, Ryffel B, Apetoh L, Ghiringhelli F, Bullón P, Sánchez-Alcazar JA, Carrión AM, Cordero MD. Stress-induced depressive behaviors require a functional NLRP3 inflammasome. Mol Neurobiol. 2016;53(7):4874–4882. [DOI] [PubMed] [Google Scholar]
- 19. Iwata M, Ota KT, Li XY, Sakaue F, Li N, Dutheil S, Banasr M, Duric V, Yamanashi T, Kaneko K, Rasmussen K, Glasebrook A, Koester A, Song D, Jones KA, Zorn S, Smagin G, Duman RS. Psychological stress activates the inflammasome via release of adenosine triphosphate and stimulation of the purinergic type 2X7 receptor. Biol Psychiatry. 2016;80(1):12–22. [DOI] [PubMed] [Google Scholar]
- 20. Kaufmann FN, Costa AP, Ghisleni G, Diaz AP, Rodrigues ALS, Peluffo H, Kaster MP. NLRP3 inflammasome-driven pathways in depression: Clinical and preclinical findings. Brain Behav Immun. 2017;64:367–383. [DOI] [PubMed] [Google Scholar]
- 21. Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16(7):407–420. [DOI] [PubMed] [Google Scholar]
- 22. Inoue K, Koizumi S, Tsuda M. The role of nucleotides in the neuron--glia communication responsible for the brain functions. J Neurochem. 2007;102(5):1447–1458. [DOI] [PubMed] [Google Scholar]
- 23. Su WJ, Zhang Y, Chen Y, Gong H, Lian YJ, Peng W, Liu YZ, Wang YX, You ZL, Feng SJ, Zong Y, Lu GC, Jiang CL. NLRP3 gene knockout blocks NF-κB and MAPK signaling pathway in CUMS-induced depression mouse model. Behav Brain Res. 2017;322(Pt A):1–8. [DOI] [PubMed] [Google Scholar]
- 24. Maslanik T, Mahaffey L, Tannura K, Beninson L, Greenwood BN, Fleshner M. The inflammasome and danger associated molecular patterns (DAMPs) are implicated in cytokine and chemokine responses following stressor exposure. Brain Behav Immun. 2013;28:54–62. [DOI] [PubMed] [Google Scholar]
- 25. Fleshner M, Frank M, Maier SF. Danger signals and inflammasomes: stress-evoked sterile inflammation in mood disorders. Neuropsychopharmacology. 2017;42(1):36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bangasser DA, Eck SR, Ordoñes Sanchez E. Sex differences in stress reactivity in arousal and attention systems. Neuropsychopharmacology. 2019;44(1):129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arnsten AF, Raskind MA, Taylor FB, Connor DF. The effects of stress exposure on prefrontal cortex: translating basic research into successful treatments for post-traumatic stress disorder. Neurobiol Stress. 2015;1:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Robertson D, Low PA, Polinsky RJ. Primer on the Autonomic Nervous System. 3rd ed. Amsterdam, the Netherlands: Academic Press, Elsevier; 2012. [Google Scholar]
- 29. Spencer RL, Deak T. A users guide to HPA axis research. Physiol Behav. 2017;178:43–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21(1):55–89. [DOI] [PubMed] [Google Scholar]
- 31. Makino S, Smith MA, Gold PW. Regulatory role of glucocorticoids and glucocorticoid receptor mRNA levels on tyrosine hydroxylase gene expression in the locus coeruleus during repeated immobilization stress. Brain Res. 2002;943(2):216–223. [DOI] [PubMed] [Google Scholar]
- 32. Kvetnanský R, Fukuhara K, Pacák K, Cizza G, Goldstein DS, Kopin IJ. Endogenous glucocorticoids restrain catecholamine synthesis and release at rest and during immobilization stress in rats. Endocrinology. 1993;133(3):1411–1419. [DOI] [PubMed] [Google Scholar]
- 33. Pacák K, Kvetnanský R, Palkovits M, Fukuhara K, Yadid G, Kopin IJ, Goldstein DS. Adrenalectomy augments in vivo release of norepinephrine in the paraventricular nucleus during immobilization stress. Endocrinology. 1993;133(3):1404–1410. [DOI] [PubMed] [Google Scholar]
- 34. Perry VH, Holmes C. Microglial priming in neurodegenerative disease. Nat Rev Neurol. 2014;10(4):217–224. [DOI] [PubMed] [Google Scholar]
- 35. Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27(1):119–145. [DOI] [PubMed] [Google Scholar]
- 36. Frank MG, Fonken LK, Annis JL, Watkins LR, Maier SF. Stress disinhibits microglia via down-regulation of CD200R: A mechanism of neuroinflammatory priming. Brain Behav Immun. 2018;69:62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun. 2007;21(1):47–59. [DOI] [PubMed] [Google Scholar]
- 38. Fonken LK, Weber MD, Daut RA, Kitt MM, Frank MG, Watkins LR, Maier SF. Stress-induced neuroinflammatory priming is time of day dependent. Psychoneuroendocrinology. 2016;66:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Blandino P Jr, Barnum CJ, Solomon LG, Larish Y, Lankow BS, Deak T. Gene expression changes in the hypothalamus provide evidence for regionally-selective changes in IL-1 and microglial markers after acute stress. Brain Behav Immun. 2009;23(7):958–968. [DOI] [PubMed] [Google Scholar]
- 40. Blandino P Jr, Barnum CJ, Deak T. The involvement of norepinephrine and microglia in hypothalamic and splenic IL-1beta responses to stress. J Neuroimmunol. 2006;173(1-2):87–95. [DOI] [PubMed] [Google Scholar]
- 41. McKim DB, Weber MD, Niraula A, Sawicki CM, Liu X, Jarrett BL, Ramirez-Chan K, Wang Y, Roeth RM, Sucaldito AD, Sobol CG, Quan N, Sheridan JF, Godbout JP. Microglial recruitment of IL-1β-producing monocytes to brain endothelium causes stress-induced anxiety. Mol Psychiatry. 2018;23(6):1421–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang W, Wang R, Xu J, Qin X, Jiang H, Khalid A, Liu D, Pan F, Ho CSH, Ho RCM. Minocycline attenuates stress-induced behavioral changes via its anti-inflammatory effects in an animal model of post-traumatic stress disorder. Front Psychiatry. 2018;9:558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Walker FR, Nilsson M, Jones K. Acute and chronic stress-induced disturbances of microglial plasticity, phenotype and function. Curr Drug Targets. 2013;14(11):1262–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sugama S, Fujita M, Hashimoto M, Conti B. Stress induced morphological microglial activation in the rodent brain: involvement of interleukin-18. Neuroscience. 2007;146(3):1388–1399. [DOI] [PubMed] [Google Scholar]
- 45. Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, Nelson RJ, Godbout JP, Sheridan JF. β-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 2011;31(17):6277–6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tynan RJ, Naicker S, Hinwood M, Nalivaiko E, Buller KM, Pow DV, Day TA, Walker FR. Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain Behav Immun. 2010;24(7):1058–1068. [DOI] [PubMed] [Google Scholar]
- 47. Pan Y, Chen XY, Zhang QY, Kong LD. Microglial NLRP3 inflammasome activation mediates IL-1β-related inflammation in prefrontal cortex of depressive rats. Brain Behav Immun. 2014;41:90–100. [DOI] [PubMed] [Google Scholar]
- 48. Kreisel T, Frank MG, Licht T, Reshef R, Ben-Menachem-Zidon O, Baratta MV, Maier SF, Yirmiya R. Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol Psychiatry. 2014;19(6):699–709. [DOI] [PubMed] [Google Scholar]
- 49. Schwarz JM, Sholar PW, Bilbo SD. Sex differences in microglial colonization of the developing rat brain. J Neurochem. 2012;120(6):948–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bollinger JL, Collins KE, Patel R, Wellman CL. Behavioral stress alters corticolimbic microglia in a sex- and brain region-specific manner. PLoS One. 2017;12(12):e0187631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hudson SP, Jacobson-Pick S, Anisman H. Sex differences in behavior and pro-inflammatory cytokine mRNA expression following stressor exposure and re-exposure. Neuroscience. 2014;277:239–249. [DOI] [PubMed] [Google Scholar]
- 52. Arakawa K, Arakawa H, Hueston CM, Deak T. Effects of the estrous cycle and ovarian hormones on central expression of interleukin-1 evoked by stress in female rats. Neuroendocrinology. 2014;100(2-3):162–177. [DOI] [PubMed] [Google Scholar]
- 53. Xu B, Zang SC, Li SZ, Guo JR, Wang JF, Wang D, Zhang LP, Yang HM, Lian S. HMGB1-mediated differential response on hippocampal neurotransmitter disorder and neuroinflammation in adolescent male and female mice following cold exposure. Brain Behav Immun. 2019;76:223–235. [DOI] [PubMed] [Google Scholar]
- 54. Fonken LK, Frank MG, Gaudet AD, D’Angelo HM, Daut RA, Hampson EC, Ayala MT, Watkins LR, Maier SF. Neuroinflammatory priming to stress is differentially regulated in male and female rats. Brain Behav Immun. 2018;70:257–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang C, Kalueff AV, Song C. Minocycline ameliorates anxiety-related self-grooming behaviors and alters hippocampal neuroinflammation, GABA and serum cholesterol levels in female Sprague-Dawley rats subjected to chronic unpredictable mild stress. Behav Brain Res. 2019;363:109–117. [DOI] [PubMed] [Google Scholar]
- 56. Weber MD, McKim DB, Niraula A, Witcher KG, Yin W, Sobol CG, Wang Y, Sawicki CM, Sheridan JF, Godbout JP. The influence of microglial elimination and repopulation on stress sensitization induced by repeated social defeat. Biol Psychiatry. 2019;85(8):667–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ataka K, Asakawa A, Nagaishi K, Kaimoto K, Sawada A, Hayakawa Y, Tatezawa R, Inui A, Fujimiya M. Bone marrow-derived microglia infiltrate into the paraventricular nucleus of chronic psychological stress-loaded mice. PLoS One. 2013;8(11):e81744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Weber MD, Godbout JP, Sheridan JF. Repeated social defeat, neuroinflammation, and behavior: monocytes carry the signal. Neuropsychopharmacology. 2017;42(1):46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kobayashi K, Imagama S, Ohgomori T, Hirano K, Uchimura K, Sakamoto K, Hirakawa A, Takeuchi H, Suzumura A, Ishiguro N, Kadomatsu K. Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis. 2013;4(3):e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kloppenburg M, Brinkman BM, de Rooij-Dijk HH, Miltenburg AM, Daha MR, Breedveld FC, Dijkmans BA, Verweij C. The tetracycline derivative minocycline differentially affects cytokine production by monocytes and T lymphocytes. Antimicrob Agents Chemother. 1996;40(4):934–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ingham E. Modulation of the proliferative response of murine thymocytes stimulated by IL-1, and enhancement of IL-1 beta secretion from mononuclear phagocytes by tetracyclines. J Antimicrob Chemother. 1990;26(1):61–70. [DOI] [PubMed] [Google Scholar]
- 62. Johnson JD, Cortez V, Kennedy SL, Foley TE, Hanson H III, Fleshner M. Role of central beta-adrenergic receptors in regulating proinflammatory cytokine responses to a peripheral bacterial challenge. Brain Behav Immun. 2008;22(7):1078–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Greenwood BN, Fleshner M. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience. 2005;135(4):1295–1307. [DOI] [PubMed] [Google Scholar]
- 64. Blandino P Jr, Hueston CM, Barnum CJ, Bishop C, Deak T. The impact of ventral noradrenergic bundle lesions on increased IL-1 in the PVN and hormonal responses to stress in male Sprague Dawley rats. Endocrinology. 2013;154(7):2489–2500. [DOI] [PubMed] [Google Scholar]
- 65. García-Bueno B, Madrigal JL, Pérez-Nievas BG, Leza JC. Stress mediators regulate brain prostaglandin synthesis and peroxisome proliferator-activated receptor-gamma activation after stress in rats. Endocrinology. 2008;149(4):1969–1978. [DOI] [PubMed] [Google Scholar]
- 66. Lechan RM, Toni R, Clark BD, Cannon JG, Shaw AR, Dinarello CA, Reichlin S. Immunoreactive interleukin-1 beta localization in the rat forebrain. Brain Res. 1990;514(1):135–140. [DOI] [PubMed] [Google Scholar]
- 67. Fontana A, Kristensen F, Dubs R, Gemsa D, Weber E. Production of prostaglandin E and an interleukin-1 like factor by cultured astrocytes and C6 glioma cells. J Immunol. 1982;129(6):2413–2419. [PubMed] [Google Scholar]
- 68. van Dam AM, Brouns M, Louisse S, Berkenbosch F. Appearance of interleukin-1 in macrophages and in ramified microglia in the brain of endotoxin-treated rats: a pathway for the induction of non-specific symptoms of sickness? Brain Res. 1992;588(2):291–296. [DOI] [PubMed] [Google Scholar]
- 69. Tomozawa Y, Yabuuchi K, Inoue T, Satoh M. Participation of cAMP and cAMP-dependent protein kinase in beta-adrenoceptor-mediated interleukin-1 beta mRNA induction in cultured microglia. Neurosci Res. 1995;22(4):399–409. [DOI] [PubMed] [Google Scholar]
- 70. McKim DB, Niraula A, Tarr AJ, Wohleb ES, Sheridan JF, Godbout JP. Neuroinflammatory dynamics underlie memory impairments after repeated social defeat. J Neurosci. 2016;36(9):2590–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Barnard DF, Gabella KM, Kulp AC, Parker AD, Dugan PB, Johnson JD. Sex differences in the regulation of brain IL-1β in response to chronic stress. Psychoneuroendocrinology. 2019;103:203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ji XH, Cao XH, Zhang CL, Feng ZJ, Zhang XH, Ma L, Li BM. Pre- and postsynaptic beta-adrenergic activation enhances excitatory synaptic transmission in layer V/VI pyramidal neurons of the medial prefrontal cortex of rats. Cereb Cortex. 2008;18(7):1506–1520. [DOI] [PubMed] [Google Scholar]
- 73. Otis JM, Dashew KB, Mueller D. Neurobiological dissociation of retrieval and reconsolidation of cocaine-associated memory. J Neurosci. 2013;33(3):1271–1281a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Stohl LL, Zang JB, Ding W, Manni M, Zhou XK, Granstein RD. Norepinephrine and adenosine-5′-triphosphate synergize in inducing IL-6 production by human dermal microvascular endothelial cells. Cytokine. 2013;64(2):605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tan KS, Nackley AG, Satterfield K, Maixner W, Diatchenko L, Flood PM. Beta2 adrenergic receptor activation stimulates pro-inflammatory cytokine production in macrophages via PKA- and NF-kappaB-independent mechanisms. Cell Signal. 2007;19(2):251–260. [DOI] [PubMed] [Google Scholar]
- 76. McNamee EN, Griffin EW, Ryan KM, Ryan KJ, Heffernan S, Harkin A, Connor TJ. Noradrenaline acting at beta-adrenoceptors induces expression of IL-1beta and its negative regulators IL-1ra and IL-1RII, and drives an overall anti-inflammatory phenotype in rat cortex. Neuropharmacology. 2010;59(1-2):37–48. [DOI] [PubMed] [Google Scholar]
- 77. Hegde B, Bodduluri SR, Satpathy SR, Alghsham RS, Jala VR, Uriarte SM, Chung DH, Lawrenz MB, Haribabu B. Inflammasome-independent leukotriene B4 production drives crystalline silica-induced sterile inflammation. J Immunol. 2018;200(10):3556–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Orlowski GM, Sharma S, Colbert JD, Bogyo M, Robertson SA, Kataoka H, Chan FK, Rock KL. Frontline science: multiple cathepsins promote inflammasome-independent, particle-induced cell death during NLRP3-dependent IL-1β activation. J Leukoc Biol. 2017;102(1):7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bianco F, Pravettoni E, Colombo A, Schenk U, Möller T, Matteoli M, Verderio C. Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J Immunol. 2005;174(11):7268–7277. [DOI] [PubMed] [Google Scholar]
- 80. Ryan KM, Griffin ÉW, Ryan KJ, Tanveer R, Vanattou-Saifoudine N, McNamee EN, Fallon E, Heffernan S, Harkin A, Connor TJ. Clenbuterol activates the central IL-1 system via the β2-adrenoceptor without provoking inflammatory response related behaviours in rats. Brain Behav Immun. 2016;56:114–129. [DOI] [PubMed] [Google Scholar]
- 81. Johnson JD, Zimomra ZR, Stewart LT. Beta-adrenergic receptor activation primes microglia cytokine production. J Neuroimmunol. 2013;254(1-2):161–164. [DOI] [PubMed] [Google Scholar]
- 82. Porterfield VM, Zimomra ZR, Caldwell EA, Camp RM, Gabella KM, Johnson JD. Rat strain differences in restraint stress-induced brain cytokines. Neuroscience. 2011;188:48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1(6):a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zhang X, Hartung JE, Bortsov AV, Kim S, O’Buckley SC, Kozlowski J, Nackley AG. Sustained stimulation of β 2-and β 3-adrenergic receptors leads to persistent functional pain and neuroinflammation. Brain Behav Immun. 2018;73:520–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Deak T, Bellamy C, D’Agostino LG. Exposure to forced swim stress does not alter central production of IL-1. Brain Res. 2003;972(1-2):53–63. [DOI] [PubMed] [Google Scholar]
- 86. Plata-Salamán CR, Ilyin SE, Turrin NP, Gayle D, Flynn MC, Bedard T, Merali Z, Anisman H. Neither acute nor chronic exposure to a naturalistic (predator) stressor influences the interleukin-1beta system, tumor necrosis factor-alpha, transforming growth factor-beta1, and neuropeptide mRNAs in specific brain regions. Brain Res Bull. 2000;51(2):187–193. [DOI] [PubMed] [Google Scholar]
- 87. Deak T, Bordner KA, McElderry NK, Barnum CJ, Blandino P Jr, Deak MM, Tammariello SP. Stress-induced increases in hypothalamic IL-1: a systematic analysis of multiple stressor paradigms. Brain Res Bull. 2005;64(6):541–556. [DOI] [PubMed] [Google Scholar]
- 88. O’Connor KA, Johnson JD, Hansen MK, Wieseler Frank JL, Maksimova E, Watkins LR, Maier SF. Peripheral and central proinflammatory cytokine response to a severe acute stressor. Brain Res. 2003;991(1-2):123–132. [DOI] [PubMed] [Google Scholar]
- 89. Lee B, Sur B, Yeom M, Shim I, Lee H, Hahm D-H. Effects of systemic administration of ibuprofen on stress response in a rat model of post-traumatic stress disorder. Korean J Physiol Pharmacol. 2016;20(4):357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. George SA, Knox D, Curtis AL, Aldridge JW, Valentino RJ, Liberzon I. Altered locus coeruleus-norepinephrine function following single prolonged stress. Eur J Neurosci. 2013;37(6):901–909. [DOI] [PubMed] [Google Scholar]
- 91. Porterfield VM, Gabella KM, Simmons MA, Johnson JD. Repeated stressor exposure regionally enhances beta-adrenergic receptor-mediated brain IL-1β production. Brain Behav Immun. 2012;26(8):1249–1255. [DOI] [PubMed] [Google Scholar]
- 92. Audet MC, Jacobson-Pick S, Wann BP, Anisman H. Social defeat promotes specific cytokine variations within the prefrontal cortex upon subsequent aggressive or endotoxin challenges. Brain Behav Immun. 2011;25(6):1197–1205. [DOI] [PubMed] [Google Scholar]
- 93. Hueston CM, Barnum CJ, Eberle JA, Ferraioli FJ, Buck HM, Deak T. Stress-dependent changes in neuroinflammatory markers observed after common laboratory stressors are not seen following acute social defeat of the Sprague Dawley rat. Physiol Behav. 2011;104(2):187–198. [DOI] [PubMed] [Google Scholar]
- 94. Gądek-Michalska A, Tadeusz J, Rachwalska P, Spyrka J, Bugajski J. Effect of prior stress on interleukin-1β and HPA axis responses to acute stress. Pharmacol Rep. 2011;63(6):1393–1403. [DOI] [PubMed] [Google Scholar]
- 95. Dinarello CA. The interleukin-1 family: 10 years of discovery. FASEB J. 1994;8(15):1314–1325. [PubMed] [Google Scholar]
- 96. Powell ND, Sloan EK, Bailey MT, Arevalo JM, Miller GE, Chen E, Kobor MS, Reader BF, Sheridan JF, Cole SW. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. Proc Natl Acad Sci USA. 2013;110(41):16574–16579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hanke ML, Powell ND, Stiner LM, Bailey MT, Sheridan JF. Beta adrenergic blockade decreases the immunomodulatory effects of social disruption stress. Brain Behav Immun. 2012;26(7):1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Fleshner M. Adrenergic receptors mediate stress-induced elevations in extracellular Hsp72. J Appl Physiol (1985). 2005;99(5):1789–1795. [DOI] [PubMed] [Google Scholar]
- 99. Johnson JD, Fleshner M. Releasing signals, secretory pathways, and immune function of endogenous extracellular heat shock protein 72. J Leukoc Biol. 2006;79(3):425–434. [DOI] [PubMed] [Google Scholar]
- 100. Kim MH, Gorouhi F, Ramirez S, Granick JL, Byrne BA, Soulika AM, Simon SI, Rivkah Isseroff R. Catecholamine stress alters neutrophil trafficking and impairs wound healing by β2-adrenergic receptor-mediated upregulation of IL-6. J Invest Dermatol. 2014;134(3):809–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Wohleb ES, McKim DB, Sheridan JF, Godbout JP. Monocyte trafficking to the brain with stress and inflammation: a novel axis of immune-to-brain communication that influences mood and behavior. Front Neurosci. 2015;8:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Feinstein DL, Heneka MT, Gavrilyuk V, Dello Russo C, Weinberg G, Galea E. Noradrenergic regulation of inflammatory gene expression in brain. Neurochem Int. 2002;41(5):357–365. [DOI] [PubMed] [Google Scholar]
- 103. McNamee EN, Ryan KM, Griffin EW, González-Reyes RE, Ryan KJ, Harkin A, Connor TJ. Noradrenaline acting at central beta-adrenoceptors induces interleukin-10 and suppressor of cytokine signaling-3 expression in rat brain: implications for neurodegeneration. Brain Behav Immun. 2010;24(4):660–671. [DOI] [PubMed] [Google Scholar]
- 104. McNamee EN, Ryan KM, Kilroy D, Connor TJ. Noradrenaline induces IL-1ra and IL-1 type II receptor expression in primary glial cells and protects against IL-1beta-induced neurotoxicity. Eur J Pharmacol. 2010;626(2-3):219–228. [DOI] [PubMed] [Google Scholar]
- 105. Gavrilyuk V, Dello Russo C, Heneka MT, Pelligrino D, Weinberg G, Feinstein DL. Norepinephrine increases I kappa B alpha expression in astrocytes. J Biol Chem. 2002;277(33):29662–29668. [DOI] [PubMed] [Google Scholar]
- 106. García-Bueno B, Madrigal JL, Lizasoain I, Moro MA, Lorenzo P, Leza JC. The anti-inflammatory prostaglandin 15d-PGJ2 decreases oxidative/nitrosative mediators in brain after acute stress in rats. Psychopharmacology (Berl). 2005;180(3):513–522. [DOI] [PubMed] [Google Scholar]
- 107. Ollivier V, Parry GC, Cobb RR, de Prost D, Mackman N. Elevated cyclic AMP inhibits NF-kappaB-mediated transcription in human monocytic cells and endothelial cells. J Biol Chem. 1996;271(34):20828–20835. [DOI] [PubMed] [Google Scholar]
- 108. Parry GC, Mackman N. Role of cyclic AMP response element-binding protein in cyclic AMP inhibition of NF-kappaB-mediated transcription. J Immunol. 1997;159(11):5450–5456. [PubMed] [Google Scholar]
- 109. Heneka MT, Galea E, Gavriluyk V, Dumitrescu-Ozimek L, Daeschner J, O’Banion MK, Weinberg G, Klockgether T, Feinstein DL. Noradrenergic depletion potentiates beta -amyloid-induced cortical inflammation: implications for Alzheimer’s disease. J Neurosci. 2002;22(7):2434–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52(4):595–638. [PubMed] [Google Scholar]
- 111. Johnson JD, O’Connor KA, Watkins LR, Maier SF. The role of IL-1beta in stress-induced sensitization of proinflammatory cytokine and corticosterone responses. Neuroscience. 2004;127(3):569–577. [DOI] [PubMed] [Google Scholar]
- 112. Bereshchenko O, Bruscoli S, Riccardi C. Glucocorticoids, sex hormones, and immunity. Front Immunol. 2018;9:1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Bekhbat M, Rowson SA, Neigh GN. Checks and balances: the glucocorticoid receptor and NFĸB in good times and bad. Front Neuroendocrinol. 2017;46:15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Unlap T, Jope RS. Inhibition of NFkB DNA binding activity by glucocorticoids in rat brain. Neurosci Lett. 1995;198(1):41–44. [DOI] [PubMed] [Google Scholar]
- 115. De Bosscher K, Vanden Berghe W, Vermeulen L, Plaisance S, Boone E, Haegeman G. Glucocorticoids repress NF-kappaB-driven genes by disturbing the interaction of p65 with the basal transcription machinery, irrespective of coactivator levels in the cell. Proc Natl Acad Sci USA. 2000;97(8):3919–3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Altonsy MO, Sasse SK, Phang TL, Gerber AN. Context-dependent cooperation between nuclear factor κB (NF-κB) and the glucocorticoid receptor at a TNFAIP3 intronic enhancer: a mechanism to maintain negative feedback control of inflammation. J Biol Chem. 2014;289(12):8231–8239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Scheinman RI, Cogswell PC, Lofquist AK, Baldwin AS Jr. Role of transcriptional activation of I kappa B alpha in mediation of immunosuppression by glucocorticoids. Science. 1995;270(5234):283–286. [DOI] [PubMed] [Google Scholar]
- 118. Hayashi R, Wada H, Ito K, Adcock IM. Effects of glucocorticoids on gene transcription. Eur J Pharmacol. 2004;500(1-3):51–62. [DOI] [PubMed] [Google Scholar]
- 119. Ferreira ZS, Bothorel B, Markus RP, Simonneaux V. Plasma corticosterone elevation inhibits the activation of nuclear factor kappa B (NFKB) in the Syrian hamster pineal gland. Stress. 2012;15(3):339–347. [DOI] [PubMed] [Google Scholar]
- 120. Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Watkins LR, Maier SF. Exposure to acute stress induces brain interleukin-1beta protein in the rat. J Neurosci. 1998;18(6):2239–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Nguyen KT, Deak T, Will MJ, Hansen MK, Hunsaker BN, Fleshner M, Watkins LR, Maier SF. Timecourse and corticosterone sensitivity of the brain, pituitary, and serum interleukin-1beta protein response to acute stress. Brain Res. 2000;859(2):193–201. [DOI] [PubMed] [Google Scholar]
- 122. Gray JD, Rubin TG, Hunter RG, McEwen BS. Hippocampal gene expression changes underlying stress sensitization and recovery. Mol Psychiatry. 2014;19(11):1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci USA. 2010;107(6):2669–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Frank MG, Thompson BM, Watkins LR, Maier SF. Glucocorticoids mediate stress-induced priming of microglial pro-inflammatory responses. Brain Behav Immun. 2012;26(2):337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Liu J, Mustafa S, Barratt DT, Hutchinson MR. Corticosterone preexposure increases NF-κB translocation and sensitizes IL-1β responses in BV2 microglia-like cells. Front Immunol. 2018;9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Niraula A, Wang Y, Godbout JP, Sheridan JF. Corticosterone production during repeated social defeat causes monocyte mobilization from the bone marrow, glucocorticoid resistance, and neurovascular adhesion molecule expression. J Neurosci. 2018;38(9):2328–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Hains LE, Loram LC, Taylor FR, Strand KA, Wieseler JL, Barrientos RM, Young JJ, Frank MG, Sobesky J, Martin TJ, Eisenach JC, Maier SF, Johnson JD, Fleshner M, Watkins LR. Prior laparotomy or corticosterone potentiates lipopolysaccharide-induced fever and sickness behaviors. J Neuroimmunol. 2011;239(1-2):53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Dhabhar FS, Miller AH, McEwen BS, Spencer RL. Effects of stress on immune cell distribution. Dynamics and hormonal mechanisms. J Immunol. 1995;154(10):5511–5527. [PubMed] [Google Scholar]
- 129. Krugers HJ, Lucassen PJ, Karst H, Joëls M. Chronic stress effects on hippocampal structure and synaptic function: relevance for depression and normalization by anti-glucocorticoid treatment. Front Synaptic Neurosci. 2010;2:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. McEwen BS, Gould EA, Sakai RR. The vulnerability of the hippocampus to protective and destructive effects of glucocorticoids in relation to stress. Br J Psychiatry Suppl. 1992;160(15):18–23. [PubMed] [Google Scholar]
- 131. Sterner EY, Kalynchuk LE. Behavioral and neurobiological consequences of prolonged glucocorticoid exposure in rats: relevance to depression. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(5):777–790. [DOI] [PubMed] [Google Scholar]
- 132. Johnson SA, Fournier NM, Kalynchuk LE. Effect of different doses of corticosterone on depression-like behavior and HPA axis responses to a novel stressor. Behav Brain Res. 2006;168(2):280–288. [DOI] [PubMed] [Google Scholar]