Abstract
Patients with high-risk renal cell carcinoma (RCC) experience high rates of recurrence despite definitive surgical resection. Recent trials of adjuvant tyrosine kinase inhibitor therapy have provided conflicting efficacy results at the cost of significant adverse events. PD-1 blockade via monoclonal antibodies has emerged as an effective disease-modifying treatment for metastatic RCC. There is emerging data across other solid tumors of the potential efficacy of neoadjuvant PD-1 blockade, and preclinical evidence supporting a neoadjuvant over adjuvant approach. PROSPER RCC is a Phase III, randomized trial evaluating whether perioperative nivolumab increases recurrence-free survival in patients with high-risk RCC undergoing nephrectomy. The neoadjuvant component, intended to prime the immune system for enhanced efficacy, distinguishes PROSPER from other purely adjuvant studies and permits highly clinically relevant translational studies.
Keywords: : clinical trial, kidney cancer, neoadjuvant therapy, nephrectomy, nivolumab, perioperative, presurgical, PROSPER RCC, renal cell carcinoma
While many patients with early stage kidney cancer can be cured by removal of the tumor and kidney (‘nephrectomy’), upward of 40% of patients can recur due to microscopic spread of the cancer prior to surgery. Adding anticancer drugs that are effective in the metastatic setting to surgery has potential to eliminate the microscopic disease and increase cure rates. The PROSPER renal cell carcinoma study is testing whether adding nivolumab, a drug that engages the immune system to better recognize, fight and eliminate the cancer, will improve disease control over surgery alone. Nivolumab will be given before and after surgery to see if it reduces the chance of the disease returning and decreases death from kidney cancer compared with patients receiving surgery only.
Due to an increase in both risk factors and incidental diagnoses, the worldwide incidence of kidney cancer rose for almost 30 years before its recent stabilization [1]. In the USA, >60,000 patients are diagnosed with kidney cancer yearly with renal cell carcinoma (RCC) representing the most common entity and 30% of patients initially presenting with clinical stage II disease (T2N0M0) or greater [2]. While many with nonmetastatic RCC can be cured with surgery, upward of 40% recur due to micrometastatic disease at the time of surgery [3]. To address this clinical need, multiple trials have investigated the addition of systemic therapy after surgery in nonmetastatic disease based on effectiveness in the metastatic setting [4]. However, despite over 13 randomized trials testing adjuvant systemic therapies in the last 40 years [5], there has been only one positive study. In this trial highlight, we discuss the recent controversy over the adjuvant use of targeted therapies and highlight the ongoing trials of perioperative immune checkpoint blockade with a focus on the PROSPER RCC Study.
Adjuvant targeted therapy
Advances in systemic therapy for RCC with targeted therapies, such as sunitinib and pazopanib, developed against the hypoxia inducible factor pathway integral in RCC development have improved outcomes for patients with metastatic disease [6]. Therefore, moving these agents earlier to the adjuvant setting with the goal of eliminating micrometastatic disease was a natural and rationale choice for patients at high risk of recurrence.
Six trials testing adjuvant tyrosine kinase inhibitors (TKI) or mTOR inhibition were undertaken and four have reported with conflicting results (Table 1) [7–10]. The first was the ASSURE trial (ECOG-ACRIN E2805), a three-arm Phase III study which randomized 1943 patients with pT1b (grade 3–4), pT2-4 or TanyN+ M0 disease to sunitinib, sorafenib or placebo for 54 weeks of treatment. Clear cell and nonclear cell diseases were permitted. There was no difference in disease-free survival (DFS) or overall survival (OS) with a median follow-up of 5.8 years [8]. In addition, 63% of patients assigned to treatment experienced grade 3 or worse adverse events, 45% required dose reduction when starting at the full dose (32% when starting at the reduced dose) and 51% discontinued therapy early compared with 29% assigned to placebo. Soon thereafter, results from S-TRAC, a Phase III study which randomized 615 M0 patients (pT3-4 or TanyN+) to sunitinib or placebo, demonstrated improved DFS (hazard ratio [HR]: 0.76 [0.59–0.98]; p = 0.03) but no difference in OS at a median follow-up of 5.4 years [10]. S-TRAC had some notable differences in eligibility and dosing compared with ASSURE, which may account for the disparate results. It included a somewhat higher risk population with clear cell only disease, started all patients on full dose sunitinib, and did not allow dose reductions as low as ASSURE. Further, S-TRAC required blinded independent central radiologic review. Despite these differences, upon post hoc retrospective analyses of the ASSURE study in patients with a phenotype similar to S-TRAC (i.e., ≥pT3, clear cell histology or patients receiving higher dose intensity), the ASSURE investigators continued to see no differences in recurrence-free survival (RFS) [11].
Table 1. . Clinical trials evaluating adjuvant tyrosine kinase and mTOR inhibition for renal cell carcinoma.
| Trial | Status | Treatments compared | Stage for inclusion | Histology | Primary outcome | Ref. |
|---|---|---|---|---|---|---|
| ASSURE | Reported | Sunitinib, sorafenib or placebo for 54 weeks | pT1b (grade 3–4), pT2-4 or TanyN1 | Clear cell or nonclear cell | DFS; no difference | [8] |
| S-TRAC | Reported | Sunitinib or placebo for 1 year | pT3-4 or TanyN1 | Clear cell only | DFS; improved (HR: 0.76 [0.59–0.98]; p = 0.03) for sunitinib | [10] |
| PROTECT | Reported | Pazopanib or placebo for 1 year | pT2 (grade 3–4), pT3-4, or TanyN1 | Clear cell or predominantly clear cell | DFS; no difference | [9] |
| ATLAS | Reported | Axitinib or placebo for 1–3 years | pT2 or TanyN1 | Clear cell or predominantly clear cell | DFS; no difference, stopped early for futility | [7] |
| SORCE | Ongoing | Sorafenib (3 years), sorafenib (1 year) or placebo | Leibovich score 3–11 | Clear cell or nonclear cell | DFS; ongoing | NCT00492258 |
| EVEREST | Ongoing | Everolimus or placebo for 54 weeks | pT1b (grade 3–4), pT2-4 or TanyN1 | Clear cell or nonclear cell | RFS; ongoing | NCT01120249 |
DFS: Disease-free survival; HR: Hazard ratio; RFS: Recurrence-free survival.
The Phase III PROTECT trial investigated 1 year of adjuvant pazopanib (800 mg) versus placebo in 1538 patients with pT2 (grade 3–4), pT3-4 or TanyN1 disease [9]. The primary end point was amended early in the trial to be DFS at a lower starting dose of 600 mg daily due to toxicity issues such as liver dysfunction. No statistically significant difference in DFS was observed at the 600 mg dosing compared with placebo (n = 1135; HR: 0.862 (0.699–1.063); p = 0.165). The PROTECT study highlighted that dose intensity may matter as a DFS benefit was observed among the 403 patients receiving 800 mg of pazopanib (HR: 0.693 [0.510–0.943]; p < 0.05) and in the overall cohort of 1538 patients (HR: 0.802 [0.675–0.954]; p < 0.05). However, no benefit in OS has yet been demonstrated among any subset or the total cohort.
Notably, a fourth Phase III trial evaluating 1 year of adjuvant axitinib (ATLAS) was recently stopped early due to futility for the DFS primary end point. Complementary to S-TRAC, subset analysis of ATLAS suggested a potential benefit in the highest-risk subpopulation [7]. Overall, ASSURE, S-TRAC, PROTECT and ATLAS provide conflicting evidence on whether adjuvant TKI therapy improves DFS for patients with high-risk, nonmetastatic RCC, but all show that any benefit would come at the cost of potentially significant side effects and without assurance of a survival benefit at 5-years. Across these trials, approximately 30–50% discontinued drug due to toxicity [8–10].
A decade after its approval for metastatic RCC, and based on the positive DFS results of S-TRAC, sunitinib was approved in November 2017 by the US FDA for the adjuvant treatment of high-risk clear cell RCC after nephrectomy. Despite the approval of sunitinib, many practitioners remain conflicted due to the potential toxicity and lack of proven OS benefit. Indeed, a panel of experts and patients advocates from the European Association of Urology (EAU) does not recommend its use believing its side effects may outweigh the potential benefit [12]. The NCCN gives it a class 2B rating citing lower level evidence [13].
Adjuvant checkpoint blockade
RCC has been long recognized as an immunotherapy sensitive cancer based on the modest successes and rare but real complete responses to therapies in the cytokine era such as IFN-α and high dose IL-2 [4,14,15]. Modern immunotherapies directed against immune checkpoints have recently transformed the treatment paradigm for RCC. PD-1 is a physiologic immune checkpoint that cancers have used to their advantage when they upregulate PD-L1 on their tumor surface. When engaged by tumor PD-L1, it can lead to T-cell anergy or apoptosis permitting tumor cells to avoid destruction [16].
Nivolumab, an antibody to PD-1, was the first checkpoint inhibitor shown to be effective in metastatic RCC. In the Phase III Checkmate 025 study for patients with antiangiogenic-refractory metastatic RCC, nivolumab significantly improved OS and objective response rates compared with everolimus [17]. It also demonstrated a better adverse event profile compared with everolimus (19 vs 37% grade 3–4 events). The landscape shifted yet again recently with the results from Checkmate 214 which demonstrated a significant objective response rates and OS benefit for the combination of ipilimumab and nivolumab over sunitinib in the first-line setting for intermediate- and poor-risk patients [18]. These results have led the EAU and FDA to declare the combination of monoclonal antibodies against CTLA-4 and PD-1, ipilimumab and nivolumab, as a new standard of care for intermediate- and poor-risk metastatic RCC [18,19]. Updated data in the favorable risk subgroup of Checkmate 214 also suggested no significant difference in outcomes between the combination immunotherapy and sunitinib making it a reasonable option in those patients as well [20].
Capitalizing on the success of PD-1 pathway blockade in the metastatic setting and its general tolerability makes moving these agents earlier to the micrometastatic setting, where disease burden is less and the immune system may be more intact, an attractive approach. The enthusiasm has resulted in at least five ongoing Phase III perioperative studies for patients at high-risk of RCC recurrence including evaluation of checkpoint inhibitor combinations such as ipilimumab and nivolumab (Checkmate 914; NCT03138512) and durvalumab with or without tremelimumab (RAMPART; NCT03288532), as well as monotherapy studies such as nivolumab (PROSPER RCC, NCT03055013), pembrolizumab (KEYNOTE- 564; NCT03142334) and atezolizumab (IMmotion010; NCT03024996). PROSPER and the latter two trials permit patients with limited metastatic burden who can be rendered NED (no evidence of disease) prior to adjuvant therapy to enroll. All but one, the PROSPER RCC study, are purely adjuvant studies.
Perioperative checkpoint blockade: PROSPER RCC
PROSPER RCC (NCT03055013) is a Phase III, randomized trial designed to evaluate the impact of perioperative nivolumab compared with observation on RFS for patients undergoing radical or partial nephrectomy for high-risk RCC. The unblinded study is being conducted by National Clinical Trials Network of the National Cancer Institute (NCI). The study drug is provided by the Bristol-Myers Squibb under a Cooperative Research and Development Agreement with the NCI.
Background & rationale
Although the pure adjuvant approach has been the general strategy for Phase III studies in RCC, a compelling argument can be made for incorporating neoadjuvant therapy, which has been utilized for other urological malignancies including urothelial carcinoma of the bladder and the upper urinary tracts [21–24]. Early data suggest the addition of neoadjuvant pembrolizumab to standard chemotherapy for high-risk breast cancer may triple the rate of pathologic complete response [25]. Similarly, in early stage non-small-cell lung cancer, two doses of preoperative nivolumab induced a significant pathologic complete response rate of 45% [26]. In muscle invasive bladder cancer, preliminary results from the PURE-01 study showed a complete response rate of 40.5% after three cycles of pembrolizumab, and ABACUS reported a 29% complete response rate after two cycles of atezolizumab among patients with urothelial carcinoma undergoing cystectomy [27,28].
Based on the mechanism of PD-1 blockade, RCC is another prime candidate for neoadjuvant PD-1 inhibition which may enhance the antitumor T cell response that is naturally induced by the primary tumor. PD-1 blockade prior to nephrectomy when the tumor (‘antigen’) is in place has greater potential to augment the immune response compared with blockade after nephrectomy alone (Figure 1). Indeed, in murine models employing an aggressive breast cancer cell line a short course of neoadjuvant immunotherapy improved survival to greater degree than adjuvant therapy, and the primary tumor was required for the expansion of the tumor specific T cells [29]. Elevated tumor-specific CD8+ T cells after neoadjuvant treatment were predictive of survival [29].
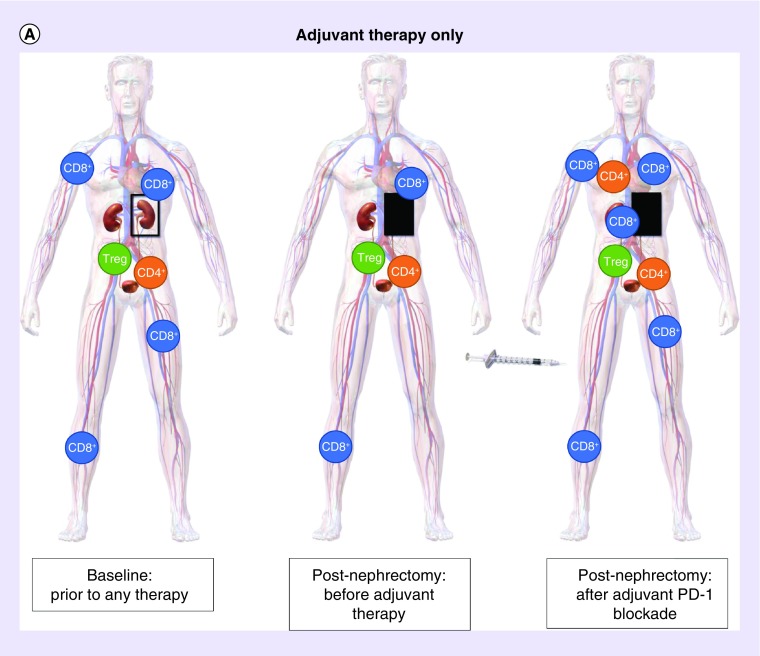
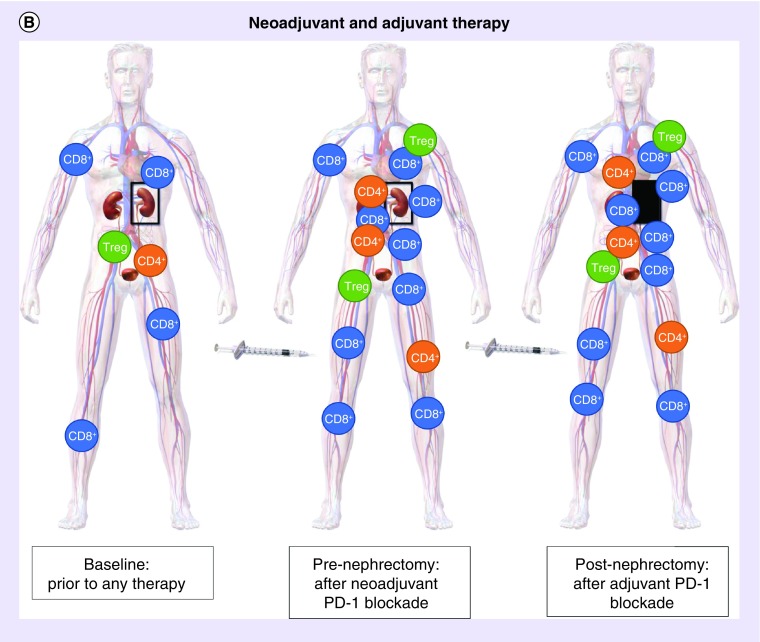
Figure 1. . Schematic theoretic representation of the potential immune system priming with neoadjuvant PD-1 blockade compared with adjuvant therapy only.
(A) Adjuvant PD-1 blockade after surgery: without neoadjuvant PD-1 blockage, nephrectomy can lead to decrease in PD-1 expression on all peripheral blood mononuclear cell types. Subsequent adjuvant administration of anti-PD-1 therapy elicits a response with antitumor T cells throughout the body including the tumor microenvironment, lymph node drainage, and distant sites. (B) Combined neoadjuvant and adjuvant PD-1 blockage with surgery: neoadjuvant PD-1 blockade primes the immune system prior to surgery when the tumor is in place resulting in proliferation of antitumor T cells prior to nephrectomy in the presence of tumor antigen. The response is augmented after nephrectomy by continued adjuvant PD-1 inhibition.
Image adapted from: WikiJournal of Medicine Medical gallery of Blausen Medical 2014.
The PROSPER RCC trial was designed to test a three-pronged approach to treating high-risk RCC by implementing neoadjuvant, surgical and adjuvant therapies [30]. A single-agent strategy was selected to minimize toxicity as patients may be cured by surgery alone. In addition, one neoadjuvant dose of 480 mg of nivolumab (formerly two doses of 240 mg 2 weeks apart) may be inadequate to eliminate all micrometastatic disease that remains after surgery. Thus, PROSPER continues to engage the immune system with 9 months of adjuvant nivolumab. If positive, PROSPER is poised to transform the current treatment paradigm for patients with high-risk RCC.
Design
Study design
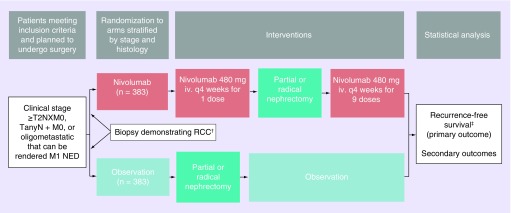
PROSPER RCC is a Phase III, randomized trial for patients with high-risk or oligometastatic RCC of any histology comparing the perioperative administration of nivolumab before and after nephrectomy to observation only after nephrectomy (Figure 2). Importantly, the study is unblinded without a placebo control. Patients meeting criteria for randomization will be assigned in a 1:1 fashion to each arm stratified by clinical T, N and M stages. The decision to pursue radical versus partial nephrectomy will be at the surgeon’s discretion accounting for patient preference, safety and feasibility.
Figure 2. . PROSPER renal cell carcinoma schema.
†Biopsy is mandated for patients assigned to the nivolumab arm. Biopsy is encouraged, but not mandated, for patients assigned to the observation arm. Management of oligometastatic disease can be done before or after nephrectomy as long as within a 12-week time frame.
‡The primary outcome is evaluated after exclusion of non-RCC surgical pathology.
iv.: Intravenous; RCC: Renal cell carcinoma.
Given the emphasis on neoadjuvant nivolumab for patients assigned to the treatment arm, inclusion criteria are based on clinical staging rather than the pathologic staging generally employed in adjuvant trials. All patients enrolled in PROSPER RCC and randomized to the neoadjuvant arm will undergo a renal mass biopsy to ensure the patient has RCC. Given the limited ability for standard imaging modalities and composite models to distinguish malignant and benign renal masses, biopsy provides a diagnostic tool with high positive predictive value for malignancy [31,32]. Although all RCC histologies are allowed, nonclear cell histology will be limited to 15% of accrual to retain sufficient power for outcomes in the clear cell RCC subset. Biopsy is encouraged, but not mandated, for patients assigned to the nephrectomy followed by observation arm.
Although RFS is the primary end point, OS will be a key secondary outcome given the negative findings from adjuvant TKI trials to date [8–10]. Surgical pathology is also of interest as 20% of clinical T2N0M0 kidney cancer is known to be upstaged to pT3a following surgery [33]. Pathologic stage distribution, T-cell infiltration and PD-L1 expression via immunohistochemistry in PROSPER RCC should provide insight into the potential impact of neoadjuvant nivolumab on the local tumor and allow exploratory identification of subsets of patients that may derive the greatest benefit.
Eligibility criteria
Key inclusion criteria include age ≥18 years, clinical stage ≥T2Nx or TanyN+ disease, good performance status, adequate end organ function and planned to undergo radical or partial nephrectomy. Patients with oligometastatic disease defined as ≤3 metastases are permitted if they are planned to undergo local treatment of the metastases within 12 weeks of nephrectomy. Patients with any liver, bone or brain metastases are excluded regardless of number of sites. Local treatments to manage oligometastatic sites include metastasectomy, thermal ablation or stereotactic radiation.
Preoperative biopsy among patients randomized to neoadjuvant nivolumab must demonstrate RCC of any histology. Sarcomatoid features or unclassified histology are permitted. A nondiagnostic biopsy is considered a good faith effort, and patients do not need to undergo a second biopsy if otherwise consistent with RCC by imaging characteristics. Box 1 provides further details on conditions and criteria leading to exclusion such as other current malignancies, certain autoimmune diseases, corticosteroid use restrictions, liver disease, serious intercurrent disease and hypersensitivity to monoclonal antibodies. Patients randomized to observation only after nephrectomy will be excluded from the primary analysis if there is evidence of non-RCC pathology.
Box 1. Key inclusion and exclusion criteria for PROSPER renal cell carcinoma.
Key inclusion criteria†
Age ≥18 years of age
Clinical stage ≥T2NX or TanyN+ disease; oligometastatic disease (≤3 sites of metastases) is permitted if planned to undergo local treatment of the metastases within 12 weeks of nephrectomy (metastasectomy, thermal ablation or stereotactic radiation)
Preoperative biopsy demonstrating RCC within 4 months of randomization (if assigned to nivolumab arm) or suspicion of RCC (if assigned to observation arm with exclusion from primary analysis if non-RCC evidence on surgical pathology)
Planned to undergo radical or partial nephrectomy; bilateral synchronous RCC is eligible only if the tumors can be resected at the same time with adequate residual renal function
ECOG 0 or 1
Serum lab values within 4 weeks of randomization
White blood cell count ≥2000/μl
Absolute granulocyte count ≥1.5/mm3
Platelet count ≥100,000/ mm3
Hemoglobin ≥0.9 g/dl
Cr ≤1.5× ULN or calculated creatinine clearance ≥40ml/min
Total bilirubin ≤1.5× ULN; <3.0× ULN for subjects with Gilbert syndrome
AST and ALT ≤2.5× ULN
Key exclusion criteria †
Concurrent or prior systemic or local anticancer therapy for RCC‡
Prior history of RCC resected with curative intent within past 5 years
Bilateral synchronous RCC requiring removal of both kidneys and subsequent hemodialysis
Any metastasis to the liver, bone or central nervous system
Most other current malignancies§
Active or known autoimmune disease¶
Ongoing condition requiring systemic corticosteroids or immunosuppressive medications#
Uncontrolled adrenal insufficiency
Known chronic active liver disease or acute/chronic hepatitis B or hepatitis C virus infection
Serious intercurrent illness
†See NCT03055013 for full listing of eligibility criteria.
‡Examples include radical or partial nephrectomy for RCC, metastasectomy for RCC, radiation to the renal bed or distant metastatic sites, antineoplastic systemic therapy for RCC, and prior treatment with any antibody or drug targeting T-cell co-stimulation or checkpoint pathways (e.g., anti-PD-1, anti-PD-L1, anti-PD-L2, anti CD-137, anti-CTLA-4).
§The following exceptions are permitted: low-risk prostate cancer on active surveillance, adequately treated basal cell or squamous cell skin cancer, in situ cervical cancer, stage I or II cancer currently in complete remission, or any other cancer from which the patient has been disease-free ≥3 years prior to registration and not receiving any current treatment are allowed. A history of Ta urothelial cancer is permitted but not ≥T1 disease.
¶Except vitiligo, T1DM, asymptomatic or residual hypothyroidism that may require hormone replacement, psoriasis not requiring systemic treatment, and conditions not expected to recur.
#Except topical/ocular/intra-articular/intranasal/inhaled steroids or adrenal replacement in absence of active autoimmune disease; brief (<3 week) course of corticosteroids for prophylaxis or treatment of nonautoimmune conditions is permitted if >14 days since last dose.
ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; Cr: Creatinine; ECOG: Eastern Cooperative Oncology Group; RCC: Renal cell carcinoma; ULN: Upper limit of normal.
Planned sample size
The planned sample size accounts for up to 5% of patients to have non-RCC pathology at time of nephrectomy leading to exclusion. Therefore, the total planned accrual is 805 patients to ensure that at least 766 patients with RCC will be randomized in a 1:1 fashion between the nivolumab and observation arms (383 per arm). Sample size calculations were based on the historical control cohort of ASSURE which had a RFS of 55.8% at 5-years and assumption that 10% of patients will either not undergo nephrectomy or not be disease-free after surgery [9]. Interim analyses and one-sided type 1 error rate of 2.5% for a stratified log-rank test are incorporated into calculations. The planned sample size will provide 84.2% power to detect an absolute difference in RFS of 14.4% or a planned increase from 55.8% in the observation arm to 70.2% in the nivolumab arm (HR = 0.70). For the clear cell RCC subset, there is 82% power to detect a similar increase in RFS (13%; HR: 0.70). Additionally, there will be 80% power in the secondary end point of 5-year OS to detect an increase from 78.7 to 85.2% (HR: 0.67).
Study procedures
PROSPER RCC opened to enrollment on 2 February 2017 and is actively recruiting. Initially, patients assigned to the nivolumab arm received nivolumab 240 mg iv. every 2 weeks for two total doses followed by planned radical or partial nephrectomy at a minimum of 7 days but no more than 21 days after the last presurgical dose. Following surgery, patients received 12 adjuvant doses – 240 mg every 2 weeks for six doses then 480 mg every 4 weeks for six doses. To enhance quality of life and facilitate surgical scheduling, the protocol has been revised to transition all dosing to the 480 mg every 4 weeks schedule with only one neoadjuvant administration and a total of nine planned adjuvant doses (Figure 2). Patients assigned to the observation arm will proceed with planned radical or partial nephrectomy within 8 weeks after randomization. Patients with evidence of non-RCC surgical pathology will be excluded from the primary analysis.
All patients will undergo routine clinical postoperative surveillance with cross-sectional imaging at 20 weeks and 40 weeks after randomization followed by every 6 months through year 3 and then annually from years 4 to 10. Imaging studies will be banked to allow for potential blinded central review if deemed necessary and for future correlative studies in association with our ACRIN colleagues.
Outcome measures/end points
The primary end point is 5-year RFS defined as the interval from randomization (excluding patients with non-RCC surgical pathology) to the detection of disease recurrence or death with censoring at last follow-up for patients who have not recurred. After randomization, patients who do not receive surgery or with any evidence of continued disease after surgery will be analyzed as recurrence events occurring on day 1. Key secondary end points include OS for the entire cohort and RFS and OS in the clear cell RCC subset.
Safety and quality of life are important end points given the potential toxicity of PD-1 blockade. Quality of life will be assessed via the NCCN/FACT Kidney Symptom Index-19, PROMIS Physical Function 10-item short form, and nine questions from the PRO-CTCAE measuring patient-reported toxicity.
Imaging, pathology and serum samples are being collected for future correlative studies including assessment of clinical and pathologic biomarkers and predictive gene expression patterns. The planned tissue collection will provide high quality data on clinical stage, biopsy pathology, surgical pathology, serologic immune changes and longitudinal outcomes. The captured data and tissue may also help clarify outstanding questions related to predicting outcomes by stage, utility of preoperative renal biopsy for risk-stratification and identifying predictive biomarkers [30,34]. The impact of priming on the primary tumor due to neoadjuvant nivolumab will be assessed by evidence of trafficking and proliferation of CD8-positive T cells. The effects of baseline and changes in PD-L1 expression on outcomes will be assessed. Banking of imaging studies may support studies aimed at characterizing the presence and severity of cancer such as with breast or prostate cancers (BI-RADS, PIRADS; now Ki-RADs) [35]. Genomic analyses will sequence the exome and transcriptome for mutational and neoantigen loads as well as pathway activation for correlation with outcomes. Both gene expression patterns and cytokine signatures will be explored. In addition, a planned substudy will generate patient RCC organoids to characterize in vitro and in vivo growth rates, histopathology and immunohistochemistry for comparison with the original RCC tumor sample.
Statistical considerations
Patients with evidence of non-RCC at the time of surgical pathology review will be excluded from the primary analysis. Baseline data on demographics and clinical stage will be tabulated by arm. Descriptive statistics will note median follow-up, adherence to frequency of follow-up imaging and the proportion of patients that deviated from the protocol in either arm (e.g., did not undergo surgery or receive the full course of nivolumab). Intent-to-treat analyses will employ a stratified log-rank test and derive comparative effect measures (HRs). Survival curves will be generated for the planned survival outcomes. Although every effort will be made to obtain scans at the scheduled time points, sensitivity analyses will be employed by carrying forward dates of events detected at nonscan visits and comparing the proportion alive and recurrence-free at the 27-month postrandomization scan.
The frequency and type of adverse events will be graded and reported by arm. A Data Safety Monitoring Committee will regularly review safety data from first enrollment through release for analysis and may recommend stopping the study for harm at 25% information if the lower bound of the 95% CI from the nivolumab/observation HR is above 1.0. Futility monitoring will begin at 44% information.
Correlative and exploratory analyses will stratify patient subsets based on pathologic variables including histology for the outcome of RFS. The primary quality of life analysis will be performed after the 40-week follow-up forms have been completed, and then a second planned analysis will occur when all patients have completed the 2-year forms.
Conclusion
Despite surgical resection, up to 45% of patients with high-risk RCC may recur and over 20% of those patients die within 5 years. There has been subdued enthusiasm for adopting adjuvant antiangiogenic therapies given the trade-off between high adverse event profiles and debatable clinical benefit. PD-1 blockade via modern monoclonal antibodies has emerged as an effective disease-modifying treatment for metastatic RCC with preclinical evidence and early stage trials in other solid tumors supporting a potential role in the locally advanced setting, especially when given neoadjuvantly. PROSPER RCC is unique among the ongoing Phase III adjuvant trials by employing a neoadjuvant component to prime the immune system with PD-1 blockade prior to surgery. The presurgical dosing will also permit highly translational correlative work capitalizing on the availability of pre- and postnivolumab treated tissue and sera with rigorous contemporary control tissue from the observation arm. The study is powered to assess clinically meaningful end points of RFS and OS and if positive, will dramatically shift the treatment paradigm for high-risk RCC.
Executive summary.
Background
Patients with high-risk renal cell carcinoma (RCC) experience high rates of recurrence despite definitive surgical resection. Recent trials of adjuvant tyrosine kinase inhibitor therapy show conflicting efficacy results and that administration comes at the cost of significant rates of toxicity and intolerability with no definite impact on overall survival. PD-1 blockade via monoclonal antibodies has emerged as an effective disease-modifying treatment for metastatic RCC with preclinical evidence supporting a potential role in the locally advanced setting.
The Trial: PROSPER RCC
A Phase III, nonplacebo controlled randomized trial designed to evaluate the impact of perioperative nivolumab compared with observation on recurrence-free survival in 805 patients undergoing nephrectomy for high-risk RCC.
Objectives
PROSPER RCC will provide the first comparative data on a three-pronged approach to improve outcomes for high-risk RCC by employing both neoadjuvant and adjuvant therapy with the PD-1 inhibitor nivolumab in addition to standard of care nephrectomy.
The primary objective is to improve recurrence free survival.
Key secondary objectives are to increase overall survival and quality of life as well as ensure safety and tolerability.
The neoadjuvant administration of nivolumab is intended to prime the immune system when the majority of the tumor antigen is in place to induce a more robust antitumor response.
A wealth of correlative science has been tightly integrated into the study given the unique availability of pre- and postnivolumab tumor tissue and sera which provides a major opportunity for biomarker discovery.
Acknowledgements
We would like to thank the patients and their families; J Manola (initial statistician); the ECOG-ACRIN Operations Team: G Ipock, J Corkery, A Bricker, S Archambault; and the many PROSPER co-investigators enrolling patients every day.
Footnotes
Supplementary data
An infographic accompanies this paper at the end of the references section. To download the infographic that accompanies this paper, please visit the journal website at: www.futuremedicine.com/doi/full/10.2217/fon-2018-0951
Financial & competing interests disclosure
The study is coordinated by the ECOG-ACRIN Cancer Research Group and supported by the National Cancer Institute. The study drug is provided by the Bristol-Myers Squibb under a Cooperative Research and Development Agreement with the National Cancer Institute. A Kapoor had research funding from Pfizer Oncology, Novartis Oncology, BMS and Roche. CG Drake is an advisor/consultant for BMS, Compugen, Roche/Genentech, Regeneron, AZ Medimmune and Merck; and a co-inventor on patents licensed from Johns Hopkins to AZ/Medimmune and to BMS. DYC Heng is an advisor for BMS, Pfizer, Novartis and Exelixis. PN Lara is a consultant for BMS. TK Choueiri has research funding from AstraZeneca, Bayer, BMS, Cerulean, Eisai, Foundation Medicine, Inc., Exelixis, Ipsen, Tracon, Genentech, Roche, Roche Products Limited, GlaxoSmithKline, Merck, Novartis, Peloton, Pfizer, Prometheus Labs, Corvus, Calithera, Analysis Group and Takeda; is a consultant/advisor to AstraZeneca, Alexion, Sanofi/Aventis, Bayer, BMS, Cerulean, Eisai, Foundation Medicine, Inc., Exelixis, Genentech, Heron Therapeutics, Roche, GlaxoSmithKline, Merck, Novartis, Peloton, Pfizer, EMD Serono, Prometheus Labs, Corvus, Ipsen, Up-to-Date, NCCN and Analysis Group; and has received honoraria from AstraZeneca, Alexion, Sanofi/Aventis, Bayer, BMS, Cerulean, Eisai, Foundation Medicine, Inc., Exelixis, Genentech, Roche, GlaxoSmithKline, Merck, Novartis, Peloton, Pfizer, EMD Serono, Prometheus Labs, Corvus, Ipsen, Up-to-Date, NCCN, Analysis Group, NCCN, Michael J. Hennessy (MJH) Associates, Inc. (Healthcare Communications Company with several brands such as OnClive and PER), L-path, Kidney Cancer Journal, Clinical Care Options, Platform Q, Navinata Healthcare, Harborside Press, American Society of Medical Oncology, NEJM, Lancet Oncology and Heron Therapeutics. EA Singer has received research support from Astellas/Medivation, grant from the National Cancer Institute (P30CA072720). WM Stadler is a consultant for Astra-Zeneca, Bayer, BMS, Caremark/CVS, Clovis, Eisai, Genentech, Pfizer, research support (to institution): AbbVie, Astra-Zeneca, Bayer, Bristol-Myers-Squibb, Boehringer-Ingelheim, Calithera, Eisai, Exelixis, Genentech (Roche), Merck, Novartis, Pfizer, Tesaro, X4Pharmaceuticals; and has held miscellaneous/editorial roles for Cancer (ACS) and Up-To-Date. S Signoretti has received research support (to institution) from Exelixis, AstraZeneca, Bristol-Myers Squibb consultant: Merck, AstraZeneca/MedImmune, Verastem, American Association for Cancer Research and National Cancer Institute; and receives royalties for CDX2 antibody from BioGenex Laboratories. RT Gupta is a consultant for Bayer Pharma AG, Invivo Corp., and Bard; and is also on the speakers bureau for Bayer Pharma AG. MD Michaelson is an advisor for Pfizer, Novartis and Exelixis. DF McDermott is a consultant for Kidney Cancer Research: BMS, Pfizer, Merck, Novartis, Eisai, Exelixis, Array BioPharm and Genentech BioOncology; and has received research support from Kidney Cancer Research: Prometheus. D Cella is a consultant for Bristol-Myers Squibb, Bayer, Pfizer, Novartis, Aveo, Exelixis and Merck; and has received research support (to institution) from Bristol-Myers Squibb, Bayer, Pfizer, Novartis and GlaxoSmithKline. LI Wagner is a consultant for Celgene Inc. and Connect Myeloma registry. NB Haas is an advisor for Exelixis and Novartis. MA Carducci is an advisor for Astellas, Pfizer, Roche/Genentech, Merck, AbbVie; and has received research support (to institution) from Astra Zeneca, EMD Serrano, Pfizer, Bristol-Myers Squibb and Gilead. LC Harshman is an advisor for Bayer, Genentech, Dendreon, Pfizer, Medivation/Astellas, Kew Group, Theragene, Corvus, Merck, Exelixis; Novartis and Jounce; and has received research support (to institution) from Bayer, Sotio, Bristol-Myers Squib, Merck, Takeda, Dendreon/Valeant, Janssen, Medivation/Astellas, Genentech and Pfizer. ME Allaf has received research support from Progenics and is NIH funded (grant number: U10CA180820). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Trial registration
This study was registered on ClinicalTrials.gov (NCT03055013) as well as SWOG (CTSU/EA8143).
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat. Rev. Urol. 7(5), 245–257 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel HD, Gupta M, Joice GA. et al. Clinical stage migration and survival for renal cell carcinoma in the United States. Eur. Urol. Oncol. 10.1016/j.euo.2018.08.023 (2018) (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 3.Chin AI, Lam JS, Figlin RA, Belldegrun AS. Surveillance strategies for renal cell carcinoma patients following nephrectomy. Rev. Urol. 8(1), 1–7 (2006). [PMC free article] [PubMed] [Google Scholar]
- 4.Patel HD, Karam JA, Allaf ME. Surgical management of advanced kidney cancer: the role of cytoreductive nephrectomy and lymphadenectomy. J. Clin. Oncol. 36(36), 3601–3607 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Harshman LC, Xie W, Moreira RB. et al. Evaluation of disease-free survival as an intermediate metric of overall survival in patients with localized renal cell carcinoma: a trial-level meta-analysis. Cancer 124(5), 925–933 (2018). [DOI] [PubMed] [Google Scholar]; • This systematic review evaluated adjuvant trials for localized renal cell carcinoma and performed a meta-analysis showing weak correlation between 5-year disease-free survival (DFS) and 5-year overall survival (OS).
- 6.Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal-cell carcinoma. N. Engl. J. Med. 376(4), 354–366 (2017). [DOI] [PubMed] [Google Scholar]; • This review article highlights the background and evolution of systemic therapy for metatastic renal cell carcinoma, which is related to the justification for moving systemic therapy agents into the adjuvant setting for localized renal cell carcinoma.
- 7.Gross-Goupil M, Kwon TG, Eto M. et al. Axitinib versus placebo as an adjuvant treatment for renal cell carcinoma: results from the Phase III, randomized ATLAS trial. Ann. Oncol. 29(12), 2371–2378 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haas NB, Manola J, Uzzo RB. et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomized, Phase III trial. Lancet 387(10032), 2008–2016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The ASSURE trial evaluated patients randomized to adjuvant sunitinib, sorafenib or placebo for pT1b (grade 3–4), pT2-4, or TanyN+ M0 renal cell carcinoma and found no difference in DFS or OS at a median follow-up of 5.8 years.
- 9.Motzer RJ, Haas NB, Donskov F. et al. Randomized Phase III trial of adjuvant pazopanib versus placebo after nephrectomy in patients with localized or locally advanced renal cell carcinoma. J. Clin. Oncol. 35(35), 3916–3923 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The PROTECT trial evaluated patients randomized to adjvuant pazopanib or placebo for pT2 (grade 3–4), pT3-4, or TanyN1 renal cell carcinoma and found no difference in DFS for the 600 mg dosing but suggested improved DFS for the 800 mg dosing. No difference in OS was observed.
- 10.Ravaud A, Motzer RJ, Pandha HS. et al. Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. N. Engl. J. Med. 375(23), 2246–2254 (2016). [DOI] [PubMed] [Google Scholar]; •• S-TRAC evaluated patients randomized to adjuvant sunitinib or placebo for pT3-4 or TanyN+ renal cell carcinoma and found an improved DFS but no difference in OS at a median follow-up of 5.4 years.
- 11.Haas NB, Manola J, Dutcher JP. et al. Adjuvant treatment for high-risk clear cell renal cancer: updated results of a high-risk subset of the ASSURE randomized trial. JAMA Oncol. 3(9), 1249–1252 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bex A, Albiges L, Ljungberg B. et al. Updated European Association of Urology Guidelines regarding adjuvant therapy for renal cell carcinoma. Eur. Urol. 71(5), 719–722 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Jonasch E. Updates to the management of kidney cancer. J. Natl Compr. Cancer Netw. 16(5S), 639–641 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Remark R, Alifano M, Cremer I. et al. Characteristics and clinical impacts of the immune environments in colorectal and renal cell carcinoma lung metastases: influence of tumor origin. Clin. Cancer Res. 19(15), 4079–4091 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Perut F, Cenni E, Unger RE, Kirkpatrick CJ, Giunti A, Baldini N. Immunogenic properties of renal cell carcinoma and the pathogenesis of osteolytic bone metastases. Int. J. Oncol. 34(5), 1387–1393 (2009). [PubMed] [Google Scholar]
- 16.Weinstock M, McDermott D. Targeting PD-1/PD-L1 in the treatment of metastatic renal cell carcinoma. Ther. Adv. Urol. 7(6), 365–377 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Motzer RJ, Escudier B, McDermott DF. et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 373(19), 1803–1813 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Motzer RJ, Tannir NM, McDermott DF. et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N. Engl. J. Med. 378(14), 1277–1290 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Checkmate 214 was a trial which shifted the landscape for metastatic renal cell carcinoma demonstrating an improved response and OS for patients assigned to the combination of ipilimumab and nivolumab over sunitinib in the first-line setting for intermediate- and poor-risk patients.
- 19.Powles T, Albiges L, Staehler M. et al. Updated European Association of Urology Guidelines recommendations for the treatment of first-line metastatic clear cell renal cancer. Eur. Urol. 73(3), 311–315 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Rini BI, Tannir NM, Escudier B. et al. Characterization of response to nivolumab plus ipilimumab (N+I) or sunitinib (S) in patients (Pts) with previously untreated advanced renal cell carcinoma (arcc): Checkmate 214. Ann. Oncol. 29(Suppl. 8), viii309–viii310 (2018). [Google Scholar]
- 21.Birtle AJ, Chester JD, Jones RJ. et al. Results of POUT: a Phase III randomised trial of perioperative chemotherapy versus surveillance in upper tract urothelial cancer (UTUC). J. Clin. Oncol. 36(6 Suppl.), Abstract 407 (2018). [Google Scholar]
- 22.Brant A, Kates M, Chappidi MR. et al. Pathologic response in patients receiving neoadjuvant chemotherapy for muscle-invasive bladder cancer: is therapeutic effect owing to chemotherapy or TURBT? Urol. Oncol. 35(1), 34.e17–34.e25 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Grossman HB, Natale RB, Tangen CM. et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N. Engl. J. Med. 349(9), 859–866 (2003). [DOI] [PubMed] [Google Scholar]
- 24.Liao RS, Gupta M, Schwen ZR. et al. Comparison of pathological stage in patients treated with and without neoadjuvant chemotherapy for high risk upper tract urothelial carcinoma. J. Urol. 200(1), 68–73 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Nanda RLM, Yau C, Asare S. et al. Pembrolizumab plus standard neoadjuvant therapy for high-risk breast cancer (BC): results from I-SPY 2. J. Clin. Oncol. 35(15 Suppl.), Abstract 506 (2017). [Google Scholar]
- 26.Forde PM, Chaft JE, Smith KN. et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N. Engl. J. Med. 378(21), 1976–1986 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Necchi A, Anichini A, Raggi D. et al. Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive urothelial bladder carcinoma (PURE-01): an open-label, single-arm, Phase II study. J. Clin. Oncol. 36(34), 3353–3360 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Powles T, Rodriguez-Vida A, Duran I. et al. A Phase II study investigating the safety and efficacy of neoadjuvant atezolizumab in muscle invasive bladder cancer (ABACUS). J. Clin. Oncol. 36(15 Suppl.), Abstract 4506 (2018). [Google Scholar]
- 29.Liu J, Blake SJ, Yong MC. et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov. 6(12), 1382–1399 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Harshman LC, Drake CG, Haas NB. et al. Transforming the perioperative treatment paradigm in non-metastatic RCC – a possible path forward. Kidney Cancer 1(1), 31–40 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pierorazio PM, Patel HD, Johnson MH. et al. Distinguishing malignant and benign renal masses with composite models and nomograms: a systematic review and meta-analysis of clinically localized renal masses suspicious for malignancy. Cancer 122(21), 3267–3276 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Patel HD, Johnson MH, Pierorazio PM. et al. Diagnostic accuracy and risks of biopsy in the diagnosis of a renal mass suspicious for localized renal cell carcinoma: systematic review of the literature. J. Urol. 195(5), 1340–1347 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Srivastava A, Patel HD, Joice GA. et al. Incidence of T3a up-staging and survival after partial nephrectomy: size-stratified rates and implications for prognosis. Urol. Oncol. 36(1), 12.e7–12.e13 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Patel HD, Iyoha E, Pierorazio PM. et al. A systematic review of research gaps in the evaluation and management of localized renal masses. Urology 98, 14–20 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farber NJ, Wu Y, Zou L. et al. Challenges in RCC imaging: renal insufficiency, post-operative surveillance, and the role of radiomics. Kidney Cancer J. 13(4), 84–90 (2015). [PMC free article] [PubMed] [Google Scholar]