Abstract
Inflammation has long been accepted as a key component of carcinogenesis. During inflammation, inflammasomes are potent contributors to the activation of inflammatory cytokines that lead to an inflammatory cascade. Considering the contributing role of inflammasomes in cancer progression, inflammasome inhibitors seem to have a promising future in cancer treatment and prevention. Here, we summarize the structures and signaling pathways of inflammasomes and detail some inflammasome inhibitors used to treat various forms of cancer, which we expect to be used in novel anticancer approaches. However, the practical application of inflammasome inhibitors is limited in regard to specific types of cancer, and the associated clinical trials have not yet been completed. Therefore, additional studies are required to explore more innovative and effective medicines for future clinical treatment of cancer.
Keywords: Inflammasome inhibitors, Therapeutics, Cancer
Background
Inflammasomes are multimeric proteins that promote immune responses and the programmed cell death process known as pyroptosis by the activation of caspase-1 in response to pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs). The inflammasome was first described by the team of Dr. Jürg Tschopp in 2002 [1], and this group discovered the features of the inflammasome in cold-associated periodic syndromes, gout and type 2 diabetes in follow-up studies [2]. However, emerging evidence indicates that inflammation triggered by viral or microbial infection plays a crucial role in tumorigenesis [3]. Inflammation associated with cancer progression is triggered by innate immune cells, including dendritic cells, natural killer (NK) cells, and macrophages [4]. Immune cells activated by tumors or tumor components might lead to antitumor immune responses through the recruitment of cytotoxic T cells or the promotion of cancer development by creating a proinflammatory context [5]. A key mechanism inducing inflammation in immune cells is orchestrated by the inflammasome. The activation of the inflammasome leads to the production of interleukin 1β (IL-1β) and interleukin 18 (IL-18) and initiates the programmed cell death process known as pyroptosis [6]. In view of the correlation between the inflammasome and cancer development, inflammasome inhibitors have drawn worldwide attention in the development of novel approaches for cancer treatment.
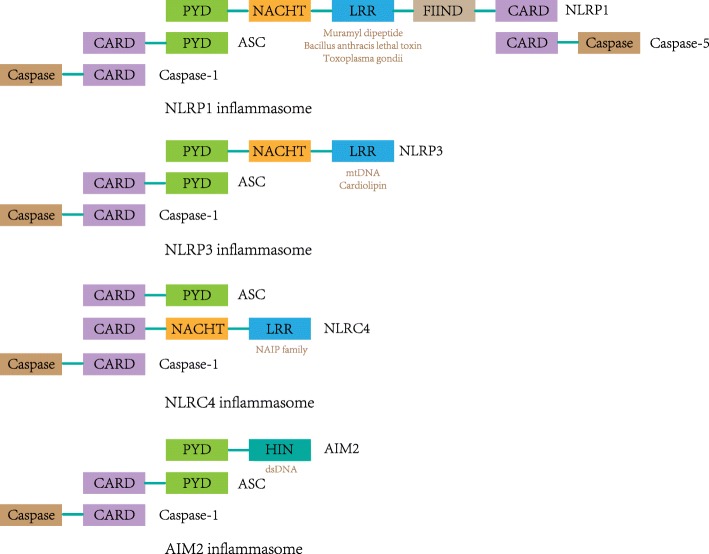
Inflammasomes consist of NOD (nucleotide oligomerization domain)-like receptors (NLRs), an apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) and caspase-1. The NLRs generally comprise a leucine-rich repeat (LRR) at the C-terminus, a caspase recruitment domain (CARD) or pyrin domain (PYD) at the N-terminus, and a nucleotide-binding oligomerization domain (NACHT) in the middle. The LRR domain is a sensor that receives signals from PAMPs and DAMPs, while the CARD or PYD interacts with the PYD domain in ASC [1]. Inflammasomes are categorized by their different NLRs such as NLRP1, NLRP3, NLRC4, and AIM2 for identification (Fig. 1). In comparison with NLRP3, NLRP1 has additional function-to-find domain (FIIND) and CARD domains at the N-terminus, which interact with caspase-5 [7]. Inflammasomes lacking a PYD, such as NLRC4, can directly bind with caspase-1 through the C-terminal CARD domain in an ASC-independent manner. However, it remains unclear how ASC interacts with the NLRC4 inflammasome complex [8, 9]. AIM2 consists of a C-terminal HIN domain and an N-terminal PYD, through which AIM2 can recruit ASC and caspase-1 to form the AIM2 inflammasome [10].
Fig. 1.
Structures of the NLRP1, NLRP3, NLRC4, and AIM2 inflammasomes. NLRP1 interacts with ASC and caspase-1 via an N-terminal PYD and binds caspase-5 to the complex via the C-terminal CARD. Muramyl dipeptide, Bacillus anthracis lethal toxin, and Toxoplasma gondii induce the activation of the NLRP1 inflammasome. NLRP3 interacts with ASC through an N-terminal PYD domain, which recruits caspase-1. NLRP3 is activated by the recognition of mtDNA and cardiolipin. The NLRC4 inflammasome is activated by the NAIP family, and it can recruit caspase-1 directly via its CARD in an ASC-independent manner. However, it remains unclear how ASC interacts with the NLRC4 inflammasome complex. The AIM2 inflammasome recruits ASC and caspase-1 through its N-terminal PYD domain and is activated by direct binding with dsDNA via its HIN domain
As a key regulator in inflammation, inflammasomes can activate inflammatory cytokines such as IL-1β and IL-18 in response to PAMPs or DAMPs [11]. The NLRP1 inflammasome is activated by muramyl dipeptide, Bacillus anthracis lethal toxin, and Toxoplasma gondii, and the NLRP3 inflammasome can be activated by the combination of mtDNA and cardiolipin. Recognition of NAIP family members induces the activation of the NLRC4 inflammasome, whereas the AIM2 inflammasome can be activated by direct binding with dsDNA via its HIN domain [12]. Inflammasome activation induces the production of IL-1β, which has been implicated in metabolic disorders. Studies have shown that IL-1β plays critical roles in type 2 diabetes and gout and that the blockade of IL-1β exhibits high efficacy in clinical trials [13, 14]. Moreover, the inflammasome is increasingly suspected of playing critical roles in autoinflammatory disorders, Alzheimer’s disease, and cancer [15].
In this review, we summarize the structures and functions of inflammasomes and the signaling pathway that activates inflammasomes, which induce inflammatory cascades. In this regard, multiple drugs that inhibit inflammasomes have been generalized as novel medications against various types of cancer, and some are worthy of further study. Finally, we list some inflammasome inhibitors whose anti-inflammatory activities are well proven. However, their antitumor activities remain to be discovered. Considering the correlation between inflammation and cancer development, these drugs are expected to be innovative therapeutics for cancer treatment.
Inflammasome signaling pathway
Canonical inflammasome activation requires two signals. The first signal, defined as priming, is the recognition of a DAMP or PAMP by pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs) and NLRs, which initiate innate and adaptive immunity. Here, we focus on the role of NLRs due to their necessity in forming the inflammasome complex. In response to the recognition of a PAMP or DAMP, NLRs oligomerize into homo- or heteroproteins and activate NF-κB. The activation of NF-κB induces the mRNA and protein expression of pro-IL-1β and pro-IL-18 [16]. The second signal is triggered by diverse stimuli activating NLRs, leading to the assembly of inflammasomes via the CARD domain in ASC, which can recruit caspase-1 and interact with NLRPs [17]. When caspase-1 is associated with NLRPs and ASC, the inflammasome complex promotes autocatalytic cleavage of caspase-1, forming the active form of the caspase-1 enzyme [18]. Active caspase-1 can activate pro-IL-1β and pro-IL-18, which are inflammatory cytokines that generate inflammatory responses [19]. Moreover, active caspase-1 also leads to the programmed cell death process termed pyroptosis in certain circumstances. In contrast to apoptosis, pyroptosis results in the rupture of the plasma membrane and release of DAMP molecules such as ATP and IL-1α into the extracellular space, which recruits more immune cells and further promotes the inflammatory cascade [20].
In contrast to the canonical pathway, the noncanonical pathway engages caspase-11 or caspase-8. In response to pathogens such as Escherichia coli, Citrobacter rodentium, or Vibrio cholera, caspase-11 is activated, leading to caspase-1-independent macrophage cell death and caspase-1-dependent IL-1β and IL-18 secretion [21]. In addition, the combination of fungi, Mycobacteria, and the dectin-1 receptor can trigger the formation of the noncanonical inflammasome complex consisting of MALT1, caspase-8, and ASC, which induces the activation of pro-IL-1β and the maturation of IL-1β [22].
Both the priming and the activation of the inflammasome are regulated by a deubiquitination mechanism. The application of G5, an inhibitor of deubiquitination, suggests the involvement of a deubiquitinating enzyme in the activation of the NLRP3 inflammasome. BRCC3, a deubiquitinating enzyme, was identified to regulate NLRP3 ubiquitination by targeting the LRR domain [23]. Moreover, IL-1R-associated kinase (IRAK) plays a critical role in NLRP3 priming by regulating NLRP3 deubiquitination, as demonstrated by using deficient mouse models. IRAK1 and IRAK4 interact with MyD88 in the transcriptional priming phase, whereas IRAK1 regulates posttranslational NLRP3 activation via the TRIF pathway [24]. Notably, the involvement of mitochondrial reactive oxygen species (mtROS) continues to be debated [25]. The deubiquitination pathway mediated by MyD88 is demonstrated to be mtROS dependent and can be inhibited by antioxidants; however, signaling by ATP can also induce the deubiquitination of NLRP in an mtROS-independent manner [26].
There also exist two molecules—heat shock protein 90 (HSP90) and the ubiquitin ligase-associated protein SGT1—that are important for NLRP3 activation. Downregulation of SGT1 expression by siRNA or chemical inhibition of HSP90 can significantly decrease inflammasome activity, leading to repression of NLRP3-mediated gout-like inflammation in mice. Moreover, the interaction of these molecules with NLRP3 is suggested to maintain NLRP3 in an inactive state. Once activating signals are detected, HSP90 and SGT1 dissociate from NLRP3, allowing inflammasome oligomerization [27].
Mitochondrial dysfunction is also involved in inflammasome activation. After the recognition of activating signals such as ATP or LPS, mitochondrial DNA is released into the cytosol and then bound directly by the NLRP3 inflammasome, leading to the activation of the inflammasome and maturation of caspase-1 [28]. During the binding of mtDNA and NLRP3, mitochondrial antiviral signaling protein (MAVS) and mitofusin2 (Mfn2) are thought to be implicated in NLRP3 activation; however, the actual interactions and functions of these proteins are not yet known [29, 30].
Thus, inflammasomes are essential in the immune system, and their roles in the activation of inflammation are incontrovertible. During inflammation, the stimulated inflammasome rapidly produces activated caspase-1, leading to cell pyroptosis and the release of inflammatory cytokines. Inflammatory cytokines are believed to participate in the processes of angiogenesis, metastasis, and epithelial-to-mesenchymal transition activation, which substantially contributes to cancer development [31]. Concerning the relationship between inflammation and cancer, the inflammasome appears to play a detrimental role in cancer due to its proinflammatory activity. However, the direct effect of inflammasome activation on cancer promotion remains controversial.
Contrasting roles of inflammasomes in cancer
Previous studies have shown that the activated inflammasome plays contrasting roles in cancer promotion and therapy [32]. A protective role for the inflammasome has mainly been observed in colitis-associated cancer. Dextran sulfate sodium (DSS) and azoxymethane (AOM) plus DSS mouse models show increases in the incidences of acute and recurrent colitis-associated cancer in mice lacking inflammasome genes, which are correlated with the levels of IL-1β and IL-18 at the tumor site [33–36]. Moreover, bone marrow reconstitution experiments have demonstrated increased inflammation and tumorigenesis in colitis-associated colon cancer in mice lacking NLRP1 [37]. Additionally, caspase-1-deficient mice have enhanced tumorigenesis as a result of increasing colonic epithelial cell proliferation in the early stage of cancer and reducing apoptosis in advanced colon cancer [38]. In other malignancies, NLRC4 suppresses the tumor growth of melanoma by stimulating tumor-associated macrophages and generating protective T cells [39]. In addition, elevating AIM2 expression by delivering an exogenous AIM2 promotor can significantly inhibit the proliferation and invasion of renal carcinoma [40]. Furthermore, the activation of NLRP1 by serine dipeptidases 8 (DPP8) and DPP9 mediates caspase-1-dependent pyroptosis in human acute myeloid leukemia [41]. This antitumor activity achieved by inhibiting NLRP1 is also exhibited in chronic myeloid leukemia [42].
However, the activation of the inflammasome can also facilitate tumor development. In a mouse model of intravenous injection of B16-F10 melanoma cells, researchers found that mice lacking NLRP3 had a significant decrease in lung metastases compared with wild-type mice and that the pathway was independent of caspase-1 and IL-1β [43]. An analysis of tissue-specific knockout mouse strains fully deficient in ASC used in a chemical-induced skin carcinogenesis model showed that ASC affected tumor proliferation in a dichotomous way: it favored tumor growth via a proinflammatory role in infiltrating cells, while it also limited keratinocyte proliferation and thus helped to suppress tumors [44]. However, ASC protein expression is repressed in metastatic melanoma compared with primary melanoma, and inflammasome-associated caspase-1 and IL-1β are inhibited when the ASC gene is inhibited in primary and metastatic melanoma cells [45]. Moreover, researchers have found that in animal and human breast cancer models, the inflammasome and IL-1β pathway promotes tumor proliferation and migration and that mice lacking inflammasome components exhibit notably suppressed tumor growth and lung metastasis [46]. Additionally, among risk factors for breast cancer, obesity has been associated with a poor clinical prognosis. Studies have found that the activation of the obesity-associated NLRC4 inflammasome drives breast cancer progression [47]. However, in pancreatic ductal adenocarcinoma, studies have demonstrated that the inhibition or deletion of NLRP3, ASC, or caspase-1 decreases tumor growth and metastasis by reprogramming innate and adaptive immunity in the tumor microenvironment [48]. A detrimental role for NLRP3 has also been observed in malignant mesothelioma [49]. AIM2, a subtype of inflammasome, was reported to be a cancer suppressor gene in early years. A recent study showed that AIM2 was highly expressed in non-small cell lung cancer (NSCLC) and promoted tumor development in an inflammasome-dependent manner [50]. As a molecule downstream of the inflammasome, IL-1β has been demonstrated to promote tumor progression by recruiting myeloid-derived suppressor cells, which might inhibit the antitumor immune response [51].
Considering the aforementioned findings, the inflammasome seems to play contrasting roles in cancer development. We hypothesize that different immune responses determine the role of the inflammasome in different types of cancer. In most malignancies, the activation of the inflammasome can lead to either immune surveillance against the tumor or an inflammatory response that promotes cancer development. In colon cancer, the activation of the inflammasome protects the epithelium from cancer invasion. A recent study found that mice deficient in IL-18 and IL-18 receptor but not wild-type mice are highly susceptible to AOM/DSS-induced colon cancer [52]. Considering that DSS induces mucosal damage in the intestinal epithelium, IL-18 secreted during inflammasome activation might be able to maintain the homeostasis of the epithelial barrier, which could account for its antitumor activity. On the other hand, this study showed that epithelial-derived IL-18 could directly interact with CD4 T cells, leading to the suppression of Th17 cell differentiation. However, IL-18 receptor is critical in Foxp3 Treg cells, which mediate the reduction in intestinal inflammation [53]. These findings suggest that the activation of the inflammasome induces the production of IL-18 and that IL-18 then reduces intestinal inflammation by repressing Th17 cells and elevating Treg function. The reduction in inflammation maintains the homeostasis of the intestinal epithelium, leading to the suppression of colon cancer. Further investigations are warranted to verify this hypothesis. The heterogeneity of the inflammasomes in various cancers suggests that inhibitor application should be tailored to the specific situation.
Antitumor effects of inflammasome inhibitors
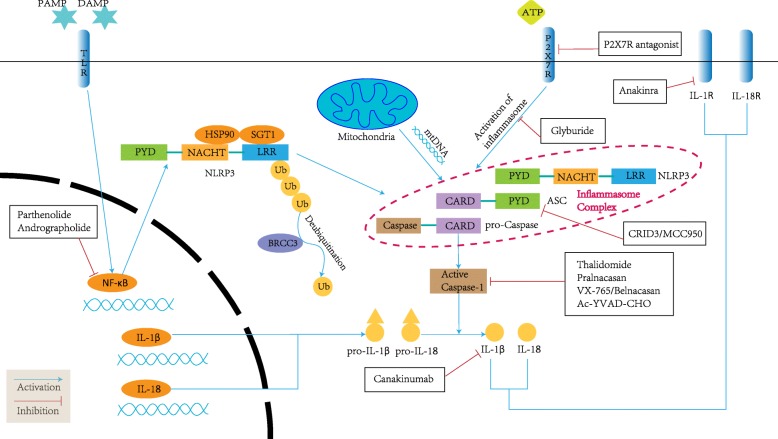
As excessive inflammation induced by the inflammasome can be a detrimental factor in multiple types of cancer, inflammasome inhibitors appear to be a promising approach for cancer prevention and treatment. Currently, many drugs and molecules have been shown to regulate inflammasome activity. However, some of them target the noncanonical signaling pathway of the inflammasome or indirectly affect the functions of the inflammasome by targeting other molecules. Here, we have listed the drugs targeting the canonical signaling pathway of the inflammasome and the antagonists most investigated in cancer treatment (Fig. 2; Table 1).
Fig. 2.
Signaling pathway and inhibitors of inflammasomes. The priming of the inflammasome is initiated by the recognition of a PAMP or DAMP, which mediates the activation of NF-κB. The activation of NF-κB induces the production of NLRP3 and the generation of pro-IL-1β and pro-IL-18. After deubiquitination and combination with mtDNA, NLRP3 interacts with ASC and caspase-1, forming the inflammasome complex. The inflammasome is activated by the recognition of P2X7R, leading to the cleavage of caspase-1. Active caspase-1 then promotes the secretion of IL-1β and IL-18, which is the key to inducing inflammasome-dependent inflammation. Inflammasome inhibitors target the upstream and downstream molecules in the inflammasome signaling pathway. Arrows denote an activating effect, and blunted lines denote targets inhibited by selective compounds
Table 1.
Studies and clinical trials of inflammasome inhibitors in cancer
| Drug | Target | Effective cancer type | Clinical trials | Reference |
|---|---|---|---|---|
| Thalidomide | Caspase-1 | Prostate cancer | Phase II | [54] |
| Multiple myeloma | Phase III | [55] | ||
| Anakinra | IL-1R | Melanoma | N/A | [56] |
| Breast cancer | [57] | |||
| Multiple myeloma | Phase II | [58] | ||
| P2X7R antagonist | P2X7R | Prostate cancer | N/A | [59] |
| Pancreatic ductal adenocarcinoma (PDAC) | [60, 61] | |||
| Osteosarcoma | [62] | |||
| Multiple myeloma | [63] | |||
| Head and neck squamous cell carcinoma | [64] | |||
| Colorectal cancer | [65] | |||
| Basal cell carcinoma | Phase I | [66] | ||
| Parthenolide | NF-κB | Gastric cancer | N/A | [67] |
| Colorectal cancer | [68] | |||
| Pancreatic adenocarcinoma | [69] | |||
| Nasopharyngeal carcinoma | [70] | |||
| Andrographolide | NF-κB | Insulinoma | N/A | [71] |
| Colorectal cancer | [72–74] | |||
| Breast cancer | [75, 76] | |||
| Multiple myeloma | [77] | |||
| Canakinumab | IL-1β | Lung cancer | Phase III (undergoing) | [78] |
Drugs already used in clinical applications
Thalidomide
In the past, thalidomide has mainly been used as a sedative or hypnotic drug to treat anxiety, insomnia, gastritis, and tension [79]. The antitumor activity of thalidomide was discovered when it was used for the treatment of erythema nodosum leprosum because of its antiangiogenic properties [80]. However, due to its potential to cause congenital defects, thalidomide analogs have mostly been applied to many types of cancer, including prostate cancer and multiple myeloma.
For the treatment of multiple myeloma, thalidomide has been approved for first-line therapy in combination with other chemotherapeutic drugs [81]. In patients with relapsed myeloma, few therapies are available. However, researchers have found that thalidomide has a practical antitumor effect on patients with advanced myeloma. According to statistics, 10% of patients experience complete or almost complete remission and 32% exhibit a decrease in serum or urine paraprotein levels. In most patients, the percentage of plasma cells in the bone marrow is reduced, and the hemoglobin level is elevated, indicating substantial antitumor activity against myeloma [55]. In a randomized phase II trial, the combination of thalidomide and docetaxel resulted in a significant reduction in the prostate-specific antigen level and an elevation of the median survival rate in patients with metastatic androgen-independent prostate cancer [54]. The mechanism of malignancy control with thalidomide might involve its antiangiogenic activity. Thalidomide was demonstrated to reduce the high levels of certain angiogenic factors, such as fibroblast growth factor (FGF) and vascular endothelial growth factor (VEGF) [82]. Moreover, thalidomide enhances cell-mediated immunity by directly interacting with cytotoxic T cells, which are lethal to tumor cells [83].
However, the application of thalidomide in other carcinomas has not shown significant efficacy. In unresectable hepatocellular carcinoma, thalidomide is tolerated in most patients with gradual dose escalation, but its monotherapeutic activity is modest compared with that of the combined therapy [84]. In a randomized, double-blinded, placebo-controlled trial, 722 patients with advanced non-small cell lung cancer were treated with thalidomide in combination with gemcitabine and carboplatin. The results showed that this treatment regimen did not improve the survival rate but did increase the risk of thrombotic events [85]. Moreover, this outcome was also demonstrated in a phase III clinical trial performed in France, and neuropathy was the most common adverse event observed [86]. Additionally, in patients with metastatic melanoma, the combination of thalidomide and dacarbazine or temozolomide shows limited efficacy. Constipation, peripheral neuropathy, fatigue, edema, and rash are attributed to thalidomide [87, 88].
Overall, thalidomide is widely used in the treatment of multiple myeloma and prostate cancer. Especially in multiple myeloma, the combination of melphalan-prednisone-thalidomide is deemed a standard therapy for patients ineligible for stem cell transplantation [89]. However, its antitumor activity has a moderate effect on other types of cancer.
Anakinra
Anakinra is a recombinant form of interleukin-1 receptor antagonist (IL-1Ra), which is commonly applied in the treatment of rheumatoid arthritis and autoinflammatory disease [90].
Previous studies with myeloma cells have shown that anakinra can significantly reduce IL-6 levels but does not increase myeloma cell death. However, a combination therapy of anakinra and dexamethasone induces cell death in myeloma cells [91]. In a study of breast cancer mouse models, anakinra decreased the growth of tumors in the bone and reduced the number of mice with bone metastasis from 90% (placebo) to 40% (treatment) or 10% (preventative). This study indicated that anakinra fails to increase tumor cell death but represses cell proliferation and angiogenesis [57]. Melanoma, the most dangerous type of skin cancer, has a poor prognosis. A study found that anakinra increases M1 macrophage polarization and decreases myeloid-derived suppressor cell numbers in mice with melanoma [56]. In phase II clinical trials, researchers investigated the roles of anakinra and low-dose dexamethasone in patients with smoldering or indolent multiple myeloma. The results showed that anakinra targets the progressed myeloma fraction in vivo and decreases the proliferation of myeloma cells [58]. Then, the antitumor activity of anakinra was mainly mediated by reducing angiogenesis. The administration of anakinra alleviated the levels of CD34-positive blood vessels and significantly reduced the expression of the endothelin 1 gene [57]. Moreover, previous studies have shown that IL-1β elevates the expression of VEGF and VEGF represses the activities of IL-1β [92, 93]. The inhibition of IL-1β by anakinra could obviously suppress the activity of VEGF, leading to an antiangiogenic effect.
Anakinra is usually used as a second-line treatment in rheumatoid arthritis, and subcutaneous injection of anakinra has been approved by the US FDA [94]. However, the antitumor applications of anakinra await further studies.
Drugs studied in clinical trials
P2X7R antagonist
Previous studies have demonstrated that P2X7 is highly expressed in prostate cancer, pancreatic ductal adenocarcinoma (PDAC), head and neck squamous cell carcinoma, colorectal cancer, and papilloma. When the expression of PX27 is downregulated by siRNA, the metastasis and invasion of prostate cancer cells are notably reduced through the PI3K/AKT and ERK1/2 pathways [59]. In PDAC, P2X7R allosteric inhibitor-treated cells exhibited attenuated tumor proliferation and invasion compared to untreated control cells [60]. In addition, extracellular ATP and BzATP, which have relatively high affinities for P2X7R, further impact cell survival and the complex function of P2X7R [61]. Moreover, P2X7R plays an important role in bone tumor growth and functions [95]. In osteosarcoma, P2X7R was proven to facilitate the growth and matrix invasion of tumor cells, which indicates the potential of P2X7R as a therapeutic target [62]. In another bone cancer, multiple myeloma, the activation of P2X7R was also deemed to affect cell necrosis in the RPMI-8226 cell line [63]. Furthermore, the inhibition of P2X7R can lead to decreased invasiveness in A253 cells, which are derived from an epidermoid carcinoma [64]. Since chronic inflammation is a key factor leading to colorectal cancer, P2X7R has been documented as a regulator in inflammatory responses. In colorectal cancer patients, high expression of P2X7R is significantly associated with tumor size and lymph node metastasis [65]. High expression of P2X7 enhances cancer proliferation, migration, invasion, and angiogenesis. Cancer cells can downregulate the expression of P2X7 to avoid apoptosis and use ATP as an invasion-promoting signal [96]. The activation of P2X7 promotes cancer invasion by releasing cathepsin and MMP-9 [97, 98]. Moreover, the P2X7-dependent release of VEGF promotes angiogenesis and contributes to cancer development [99]. These findings suggest that P2X7R antagonists alter the context of tumor cells, leading to the suppression of cancer progression.
In clinical trials, the safety and tolerability of a P2X7 antagonist were assessed in an open-label, phase I study, in which approximately 65% of patients with basal cell carcinoma showed a decrease in lesion area and the most common adverse event was an allergic reaction occurring at the treatment site [66]. These properties warrant additional studies to evaluate the potential of P2X7 antagonists in the treatment of not only skin cancer but also other malignancies.
Parthenolide
Parthenolide is a sesquiterpene lactone compound found in the herb named feverfew, which is used as an anti-inflammatory medicine [100]. While NF-κB has been reported to be a key factor regulating a number of genes that are crucial for tumor invasion and metastasis, parthenolide is considered a potential antitumor therapeutic drug that functions by inhibiting the NF-κB signaling pathway [101]. In gastric cancer, parthenolide significantly inhibits tumor cell growth and downregulates the phosphorylation of NF-κB. During a study of combined therapy with parthenolide and paclitaxel, the survival time of patients with gastric cancer was notably prolonged [67]. In addition, in pancreatic adenocarcinoma, tumor cell growth can be inhibited by parthenolide in a dose-dependent manner. After a higher concentration of parthenolide treatment is administered, internucleosomal DNA fragmentation indicative of apoptosis can be observed [69]. In a study of colorectal cancer models, intraperitoneal injection of parthenolide notably inhibited tumor proliferation and angiogenesis. By focusing on the Bcl-2 family in cancer cells, a parthenolide-mediated cell death signaling pathway was investigated and confirmed to be associated with colorectal cancer cell death [68]. In nasopharyngeal carcinoma, parthenolide induces tumor cell death through the NF-κB/COX-2 pathway. Using COX-2 inhibitors or knocking down COX-2 expression with siRNA or shRNA suppresses cancer stem-like cell phenotypes. Parthenolide exerts an inhibitory effect on NF-κB by suppressing both the phosphorylation of IκB kinase and the degradation of IκBα [70]. The mechanisms involved in the antitumor activity of parthenolide, including the inhibition of NF-κB, activation of JNK, activation of p53, and suppression of STAT3, have attracted great interest. Parthenolide sensitizes cancer cells to TNF-α-induced apoptosis by inhibiting NF-κB and activating JNK [102]. The administration of parthenolide can activate p53, leading to a reduction in cancer cell proliferation [103]. Moreover, parthenolide can inhibit the activation of STAT proteins by blocking their tyrosine phosphorylation, which is indispensable for STAT translocation into the nucleus and target gene activation [104].
In practical use, the low solubility and bioavailability of parthenolide limit its potential [105]. However, creating synthetic analogs of parthenolide may be a novel way to address this problem [106]. Currently, a clinical trial of parthenolide is being performed in allergic contact dermatitis [107]. Therefore, additional studies are required to exploit parthenolide as a novel antitumor drug.
Canakinumab
Canakinumab is a human monoclonal antibody targeting IL-1β but not IL-1α. In 2009, canakinumab was authorized by the US Food and Drug Administration and European Medicines Agency as a treatment for cryopyrin-associated periodic syndromes [108]. A randomized, double-blinded trial found that compared with controls, canakinumab at a dosage of 150 mg every 3 months led to a notable reduction in the rate of recurrent cardiovascular events [109]. When cancer is considered, canakinumab still deserves recognition. During a randomized, double-blinded, placebo-controlled trial of patients with lung cancers and atherosclerosis, researchers found that canakinumab could significantly decrease lung cancer mortality by targeting the IL-1β innate immunity pathway. It is worth mentioning that this antitumor effect is mostly detected in patients with lung adenocarcinoma or poorly differentiated large cell cancer, whereas meaningful assessments of the effects on patients with small cell lung cancers or squamous cell carcinomas have rarely been performed [78]. The chronic use of aspirin can reduce mortality in colorectal cancer and lung adenocarcinomas due to its anti-inflammatory activity [110, 111], and canakinumab is theorized to combat cancer in a similar way, according to its function of inhibiting the inflammasome [78].
Currently, the application of canakinumab as an antitumor drug is mainly focused on lung cancer. Canakinumab is being studied in phase III clinical trials in non-small cell lung cancer to evaluate its tolerability and efficacy compared with those of a placebo. Consequently, the completion of the clinical trials is warranted to determine whether canakinumab can be used safely and effectively in cancer treatment.
Andrographolide
Andrographolide is a labdane diterpenoid that has been isolated from the stem and leaves of Andrographis paniculata [112]. Numerous studies have validated the facts that andrographolide can inhibit cell invasion and induce cell death in various types of cancer cells. A recent study showed that andrographolide significantly reduces tumor cell proliferation at both the early stage and advanced stage of insulinoma by targeting the TLR4/NF-κB signaling pathway [71]. In addition, in colon cancer, andrographolide represses cell proliferation, elevates cell apoptosis, and activates caspase-3/9 in SW620 human colon cancer cells by inhibiting NF-κB, TLR4, MyD88, and MMP-9 signaling activation [72]. Among chemotherapeutic drugs, 5-fluorouracil (5-Fu) is the one most commonly used in colorectal cancer. Andrographolide can promote the 5-Fu-induced antitumor effect by repressing the level of phosphorylated cellular-mesenchymal to epithelial transition factor [73]. In colitis-associated cancer, andrographolide inhibits the NLRP3 inflammasome, protecting mice against dextran sulfate sodium-induced colon carcinogenesis [74]. In breast cancer, andrographolide suppresses breast cancer-induced osteolysis by inhibiting the NF-κB and ERK signaling pathway at a relatively low dose and by promoting apoptosis at a relatively high dose. Its antitumor activity correlates with the downregulation of the NF-κB signaling pathway [75, 76]. Moreover, andrographolide reduces proliferation and increases cell apoptosis by downregulating the protein expression of TLR4 and NF-κB in multiple myeloma [77]. The antitumor mechanisms of andrographolide include the inhibition of the NF-κB pathway [113], suppression of cyclins and cyclin-dependent kinases [114], and activation of the p53 protein [114], leading to reductions in cancer cell proliferation, invasion, and angiogenesis.
Clinical trials of andrographolide have mainly focused on inflammatory diseases such as acute upper respiratory tract infections [115, 116], and its antitumor activity has been demonstrated only in vitro. Therefore, more studies are required to investigate its application in cancer treatment.
Overall, clinical application was limited to only anakinra and thalidomide, and other drugs are still under assessment in clinical trials. All of these drugs inhibit the production and activation of inflammasome-associated molecules such as P2X7R, IL-1, NF-κB, and caspase-1, leading to the suppression of the inflammasome. As mentioned above, the antitumor mechanisms of these drugs involve the regulation of the expression of p53, NF-κB, STAT, and VEGF, leading to the suppression of tumor cell proliferation, metastasis, invasion, and angiogenesis. However, the direct interactions of inflammasome inhibitors involved in repressing cancer development are not yet known. Further studies are needed to explore the mechanisms in a more explicit way.
Potential antitumor drugs
Considering the correlation between inflammation and tumorigenesis, it is rational to expect that antagonists that inhibit the initiation of inflammation could be explored as potential antitumor drugs. In the inflammasome signaling pathway, there are many steps that could be targeted, such as the assembly and activation of inflammasomes, the synthesis of IL-1, and the generation of caspase-1. Several inhibitors targeting the above processes hold promise in developing novel drugs against cancer and are described below.
Glyburide
Glyburide is an antidiabetic drug in a class of medications known as sulfonylureas, which are commonly used in the therapy of type 2 diabetes [117]. Glyburide was demonstrated to block ATP-sensitive potassium channels in pancreatic B cells [118]. In placental inflammation-associated diseases, trophoblasts can secrete IL-1β through the NLRP3 pathway, which plays an important role in inflammation-associated pregnancy complications, and glyburide offers considerable therapeutic promise as an inhibitor of the NLRP3 inflammasome [119]. Moreover, glyburide was beneficial in human melioidosis in a study of 1160 patients with gram-negative sepsis due to its inhibitory effect on the inflammasome and subsequent suppression of the inflammatory response. Considering the role of the NLRP3 inflammasome in endotoxemia, the data suggest that glyburide can delay lipopolysaccharide (LPS)-induced lethality in mice [120].
However, since glyburide specifically inhibits the NLRP3 inflammasome in vitro, treatment requires the administration of a high dose in vivo, which causes hypoglycemia and is beyond its pharmacological action in type 2 diabetes. A recent finding suggests that the small molecule 16673-34-0, which is an intermediate substrate in the synthesis of glyburide, disrupts the synthesis of the NLRP3 inflammasome and limits infarct size in mouse models of myocardial infarction without affecting glucose metabolism [121]. Therefore, this substrate, which exhibits pharmacodynamics similar to those of glyburide, may be a novel inhibitor of the inflammasome with fewer side effects than glyburide.
CRID3/MCC950
The cytokine release inhibitory drugs (CRID3), also known as MCC950, are diarylsulfonylurea-containing compounds that inhibit the activation of the NLRP3 inflammasome both in mice in vivo and in human cells in vitro [122]. Researchers have found that CRID3 inhibits the secretion of IL-1β and caspase-1 in response to the NLRP3 and AIM2 inflammasomes but not in response to the NLRC4 inflammasome. In contrast to the NLRP3 inhibitors glyburide and parthenolide, CRID3 can prevent AIM2-dependent pyroptosis. Moreover, the potential target of CRID3 was identified as glutathione S-transferase omega 1 (GSTO1), a protein that has been demonstrated to interact with ASC [123, 124]. In a study of a spontaneous chronic colitis mouse model, MCC950 resulted in significant suppression of IL-1β secretion and activation of caspase-1, indicating a potential novel avenue for treatment of human colonic inflammation diseases [125].
Pralnacasan
Pralnacasan is an orally absorbed nonpeptide compound that inhibits interleukin 1β converting enzyme (ICE), which is also known as caspase-1 [126]. ICE exists in the plasma membrane of monocytic cells where it activates the precursors of IL-1β and IL-18 into their active forms. This process is considered to be downstream in the inflammasome signaling pathway [127, 128]. In a collagenase-induced osteoarthritis mouse model, pralnacasan has been demonstrated to reduce joint damage, indicating its potential as a disease-modifying drug for the therapy of osteoarthritis [129]. In dextran sulfate sodium (DSS)-induced murine colitis models, pralnacasan is able to ameliorate dextran sulfate sodium-induced colitis with almost no side effects. This process is probably mediated by the repression of the inflammatory cytokines IL-1β and IL-18 [130]. Researchers found that IL-18 mRNA and TNF-α mRNA levels were elevated in DSS-induced colitis, and the administration of pralnacasan significantly reduced the expression of IL-18 mRNA but did not affect the expression of TNF-α mRNA. Therefore, the therapeutic approach of combining TNF-α expression-reducing substances with pralnacasan appears to be a promising idea [131].
VX-765
VX-765, also known as belnacasan, is an inhibitor that decreases the activity of caspase-1. A study showed that the administration of VX-765 in rat models significantly reduced the number of seizures and delayed the time to seizure onset [132]. The same anticonvulsant effect of VX-765 has been exhibited in mice models in a dose-dependent manner [133]. Moreover, the application of VX-765 stops the accumulating deposition of amyloid β, indicating its potent therapeutic activity in Alzheimer’s disease [134]. In addition to its inhibitory effect on nervous system disease, VX-765 has also been proven to reduce infarct size in a rat model of myocardial infarction. In combination with an antiplatelet P2Y12 inhibitor, VX-765 exhibited a highly protective function when myocardial infarction occurred [135, 136].
Currently, clinical trials of VX-765 are mainly studying the treatment of epilepsy. A randomized, double-blinded, placebo-controlled phase II study of VX-765 in patients with treatment-resistant partial epilepsy was completed, and the results showed no statistically significant difference between the VX-765 group and the placebo group [137]. Consequently, a longer duration study is warranted to measure the clinical efficacy of VX-765.
Ac-YVAD-CHO
Ac-YVAD-CHO is a polypeptide with a sequence homologous to the known sequences of caspase substrates, which accounts for its ability to inhibit the activation of caspase-1 [138, 139]. Researchers have used Ac-YVAD-CHO as a therapeutic intervention in pancreatic carcinoma cells, and they found that the inhibition of caspase-1 leads to cell apoptosis. Moreover, according to their observations, caspase-1 was directly involved in antiapoptotic processes in pancreatic cancer [140]. Additionally, the administration of Ac-YVAD-CHO has been demonstrated to induce remission in rats with endotoxemia by decreasing the secretion of IL-1β and IL-18 [141].
Overall, caspase-1 and IL-1β, molecules downstream of the inflammasome, play major roles in the generation of inflammation, and the drugs mentioned above are commonly used in the treatment of inflammation-associated disease because they can reduce the functions of caspase-1 and IL-1β. However, their applications in cancer therapy remain unknown. Thus, further investigations are warranted to characterize the antitumor activities of these potent inflammasome inhibitors.
Conclusions
The role of the inflammasome in cancer development has received increasing attention in recent years. During the progression of cancer, excessive inflammation stimulated by the inflammasome is the generally accepted hypothesis explaining the detrimental effect of inflammasomes on multiple forms of cancer. In the downstream course of the inflammasome pathway, IL-1β and IL-18 are activated by caspase-1 to generate an inflammatory response. Therefore, drugs that can downregulate the functions of these cytokines seem to have therapeutic activities in inflammation-associated diseases.
In various in vitro experiments, inflammasome inhibitors have been shown to attenuate the proliferation and invasion of cancer cells. However, their antitumor activities are limited to specific types of cancer. In terms of practical applications, the clinical trials studying inflammasome inhibitors have mainly focused on multiple myeloma, in which thalidomide and anakinra are well studied. Otherwise, inflammasome inhibitors are mostly utilized in inflammatory diseases such as osteoarthritis, rheumatoid arthritis, and colon colitis. Considering the limited application of inflammasome inhibitors in cancer treatment, we are looking forward to more broad-spectrum and effective antitumor drugs. Several of the inflammasome inhibitors detailed above have been demonstrated to have the function of reducing inflammatory responses, indicating that inflammasome inhibitors could be novel candidates for the treatment of malignancies in which inflammation is involved as a major contributor.
The correlation between inflammasomes and cancer provides a promising approach for cancer therapy. The contrasting roles of inflammasomes in different cancers suggest the need for specific strategies when inhibitors are applied in cancer treatment. However, the inappropriate administration of inflammasome inhibitors might result in the repression of antitumor immunity and enhanced infection susceptibility and deterioration in autoinflammatory diseases. Consequently, the application of inflammasome inhibitors must be tailored to the specific type of cancer, and further studies are warranted to characterize the antitumor effects of these drugs.
Acknowledgements
Not applicable
Abbreviations
- AIM2
Absent in melanoma 2
- AOM
Azoxymethane
- ASC
Apoptosis-associated speck-like protein containing a caspase recruitment domain
- CARD
Caspase recruitment domain
- CRID3
Cytokine release inhibitory drugs
- DAMP
Danger-associated molecular pattern
- dsDNA
Double-stranded DNA
- DSS
Dextran sulfate sodium
- FIIND
Function-to-find domain
- GSTO1
Glutathione S-transferase omega 1
- HIN
Hematopoietic interferon-inducible nuclear
- ICE
Interleukin 1β converting enzyme
- IL-18
Interleukin 18
- IL-1β
Interleukin 1β
- JNK
c-Jun N-terminal kinase
- LPS
Lipopolysaccharide
- LRR
Leucine-rich repeat
- mtDNA
Mitochondrial DNA
- MyD88
Myeloid differentiation factor 88
- NACHT
N-terminus and a nucleotide-binding oligomerization domain
- NAIP
NLR family, apoptosis inhibitory protein
- NLRs
Nucleotide oligomerization domain-like receptors
- NOD
Nucleotide oligomerization domain
- PAMP
Pathogen-associated molecular pattern
- PDAC
Pancreatic ductal adenocarcinoma
- PYD
Pyrin domain
- STAT
Signal transducers and activators of transcription
Authors’ contributions
All authors have contributed to the preparation of this manuscript. All authors have read and approved the manuscript.
Funding
National Natural Science Youth Foundation of China (81602027); Youth Foundation of Xiangya Hospital Central South University (2015Q01); Natural Science Foundation General Program of Hunan Province (2018JJ3821); Project of scientific research plan of Hunan Provincial Health Commission (C2019185); Clinic and Rehabilitation research foundation of Sinobioway group (xywm2015I37)
Availability of data and materials
Not applicable
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ruimin Chang, Email: Changruimin@163.com.
Chunfang Zhang, Email: zhcf3801@csu.edu.cn.
References
- 1.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10(2):417–426. doi: 10.1016/S1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 2.Dagenais M, Skeldon A, Saleh M. The inflammasome: in memory of Dr. Jurg Tschopp. Cell Death Differ. 2012;19(1):5–12. doi: 10.1038/cdd.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balkwill F, Coussens LM. Cancer: an inflammatory link. Nature. 2004;431(7007):405–406. doi: 10.1038/431405a. [DOI] [PubMed] [Google Scholar]
- 4.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6(1):24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 5.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kantono M, Guo B. Inflammasomes and cancer: the dynamic role of the inflammasome in tumor development. Front Immunol. 2017;8:1132. doi: 10.3389/fimmu.2017.01132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chavarria-Smith J, Mitchell PS, Ho AM, Daugherty MD, Vance RE. Functional and evolutionary analyses identify proteolysis as a general mechanism for NLRP1 inflammasome activation. PLoS Pathog. 2016;12(12):e1006052. doi: 10.1371/journal.ppat.1006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan F, Kolb R, Pandey G, Li W, Sun L, Liu F, et al. Involvement of the NLRC4-inflammasome in diabetic nephropathy. PLoS One. 2016;11(10):e0164135. doi: 10.1371/journal.pone.0164135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broz P, von Moltke J, Jones JW, Vance RE, Monack DM. Differential requirement for Caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe. 2010;8(6):471–483. doi: 10.1016/j.chom.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin T, Perry A, Smith P, Jiang J, Xiao TS. Structure of the absent in melanoma 2 (AIM2) pyrin domain provides insights into the mechanisms of AIM2 autoinhibition and inflammasome assembly. The Journal of biological chemistry. 2013;288(19):13225–13235. doi: 10.1074/jbc.M113.468033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15(10):1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 12.Sutterwala FS, Haasken S, Cassel SL. Mechanism of NLRP3 inflammasome activation. Ann N Y Acad Sci. 2014;1319:82–95. doi: 10.1111/nyas.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malozowski S, Sahlroot JT. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;357(3):302–303. doi: 10.1056/NEJMc071324. [DOI] [PubMed] [Google Scholar]
- 14.So A, De Smedt T, Revaz S, Tschopp J. A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res Ther. 2007;9(2):R28. doi: 10.1186/ar2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menu P, Vince JE. The NLRP3 inflammasome in health and disease: the good, the bad and the ugly. Clin Exp Immunol. 2011;166(1):1–15. doi: 10.1111/j.1365-2249.2011.04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamkanfi M. Emerging inflammasome effector mechanisms. Nat Rev Immunol. 2011;11(3):213–220. doi: 10.1038/nri2936. [DOI] [PubMed] [Google Scholar]
- 17.Rathinam VA, Vanaja SK, Fitzgerald KA. Regulation of inflammasome signaling. Nat Immunol. 2012;13(4):333–342. doi: 10.1038/ni.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu D, Pan P, Su X, Zhang L, Qin Q, Tan H, et al. Interferon regulatory factor-1 mediates alveolar macrophage pyroptosis during LPS-induced acute lung injury in mice. Shock. 2016;46(3):329–338. doi: 10.1097/SHK.0000000000000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garaude J, Kent A, van Rooijen N, Blander JM. Simultaneous targeting of toll- and nod-like receptors induces effective tumor-specific immune responses. Sci Transl Med. 2012;4(120):120ra16. doi: 10.1126/scitranslmed.3002868. [DOI] [PubMed] [Google Scholar]
- 20.Fernandes-Alnemri T, Wu J, Yu JW, Datta P, Miller B, Jankowski W, et al. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007;14(9):1590–1604. doi: 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479(7371):117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 22.Gringhuis SI, Kaptein TM, Wevers BA, Theelen B, van der Vlist M, Boekhout T, et al. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1beta via a noncanonical caspase-8 inflammasome. Nat Immunol. 2012;13(3):246–254. doi: 10.1038/ni.2222. [DOI] [PubMed] [Google Scholar]
- 23.Py BF, Kim MS, Vakifahmetoglu-Norberg H, Yuan J. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol Cell. 2013;49(2):331–338. doi: 10.1016/j.molcel.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Fernandes-Alnemri T, Kang S, Anderson C, Sagara J, Fitzgerald KA, Alnemri ES. Cutting edge: TLR signaling licenses IRAK1 for rapid activation of the NLRP3 inflammasome. J Immunol. 2013;191(8):3995–3999. doi: 10.4049/jimmunol.1301681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bauernfeind F, Bartok E, Rieger A, Franchi L, Nunez G, Hornung V. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J Immunol. 2011;187(2):613–617. doi: 10.4049/jimmunol.1100613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juliana C, Fernandes-Alnemri T, Kang S, Farias A, Qin F, Alnemri ES. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J Biol Chem. 2012;287(43):36617–36622. doi: 10.1074/jbc.M112.407130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayor A, Martinon F, De Smedt T, Petrilli V, Tschopp J. A crucial function of SGT1 and HSP90 in inflammasome activity links mammalian and plant innate immune responses. Nat Immunol. 2007;8(5):497–503. doi: 10.1038/ni1459. [DOI] [PubMed] [Google Scholar]
- 28.Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36(3):401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ichinohe T, Yamazaki T, Koshiba T, Yanagi Y. Mitochondrial protein mitofusin 2 is required for NLRP3 inflammasome activation after RNA virus infection. Proc Natl Acad Sci U S A. 2013;110(44):17963–17968. doi: 10.1073/pnas.1312571110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subramanian N, Natarajan K, Clatworthy MR, Wang Z, Germain RN. The adaptor MAVS promotes NLRP3 mitochondrial localization and inflammasome activation. Cell. 2013;153(2):348–361. doi: 10.1016/j.cell.2013.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Germano G, Allavena P, Mantovani A. Cytokines as a key component of cancer-related inflammation. Cytokine. 2008;43(3):374–379. doi: 10.1016/j.cyto.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 32.Kolb R, Liu GH, Janowski AM, Sutterwala FS, Zhang W. Inflammasomes in cancer: a double-edged sword. Protein Cell. 2014;5(1):12–20. doi: 10.1007/s13238-013-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dupaul-Chicoine J, Yeretssian G, Doiron K, Bergstrom KS, McIntire CR, LeBlanc PM, et al. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity. 2010;32(3):367–378. doi: 10.1016/j.immuni.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 34.Allen IC, TeKippe EM, Woodford RM, Uronis JM, Holl EK, Rogers AB, et al. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med. 2010;207(5):1045–1056. doi: 10.1084/jem.20100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dupaul-Chicoine J, Arabzadeh A, Dagenais M, Douglas T, Champagne C, Morizot A, et al. The Nlrp3 inflammasome suppresses colorectal cancer metastatic growth in the liver by promoting natural killer cell tumoricidal activity. Immunity. 2015;43(4):751–763. doi: 10.1016/j.immuni.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 36.Zaki MH, Vogel P, Body-Malapel M, Lamkanfi M, Kanneganti TD. IL-18 production downstream of the Nlrp3 inflammasome confers protection against colorectal tumor formation. J Immunol. 2010;185(8):4912–4920. doi: 10.4049/jimmunol.1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams TM, Leeth RA, Rothschild DE, Coutermarsh-Ott SL, McDaniel DK, Simmons AE, et al. The NLRP1 inflammasome attenuates colitis and colitis-associated tumorigenesis. J Immunol. 2015;194(7):3369–3380. doi: 10.4049/jimmunol.1402098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu B, Elinav E, Huber S, Booth CJ, Strowig T, Jin C, et al. Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proc Natl Acad Sci U S A. 2010;107(50):21635–21640. doi: 10.1073/pnas.1016814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janowski AM, Colegio OR, Hornick EE, McNiff JM, Martin MD, Badovinac VP, et al. NLRC4 suppresses melanoma tumor progression independently of inflammasome activation. J Clin Invest. 2016;126(10):3917–3928. doi: 10.1172/JCI86953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chai D, Liu N, Li H, Wang G, Song J, Fang L, et al. H1/pAIM2 nanoparticles exert anti-tumour effects that is associated with the inflammasome activation in renal carcinoma. J Cell Mol Med. 2018;22(11):5670–5681. doi: 10.1111/jcmm.13842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson DC, Taabazuing CY, Okondo MC, Chui AJ, Rao SD, Brown FC, et al. DPP8/DPP9 inhibitor-induced pyroptosis for treatment of acute myeloid leukemia. Nat Med. 2018;24(8):1151–1156. doi: 10.1038/s41591-018-0082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu Z, Wang H, Wei S, Wang Z, Ji G. Inhibition of ER stress-related IRE1alpha/CREB/NLRP1 pathway promotes the apoptosis of human chronic myelogenous leukemia cell. Mol Immunol. 2018;101:377–385. doi: 10.1016/j.molimm.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 43.Chow MT, Sceneay J, Paget C, Wong CS, Duret H, Tschopp J, et al. NLRP3 suppresses NK cell-mediated responses to carcinogen-induced tumors and metastases. Cancer Res. 2012;72(22):5721–5732. doi: 10.1158/0008-5472.CAN-12-0509. [DOI] [PubMed] [Google Scholar]
- 44.Drexler SK, Bonsignore L, Masin M, Tardivel A, Jackstadt R, Hermeking H, et al. Tissue-specific opposing functions of the inflammasome adaptor ASC in the regulation of epithelial skin carcinogenesis. Proc Natl Acad Sci U S A. 2012;109(45):18384–18389. doi: 10.1073/pnas.1209171109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu W, Luo Y, Dunn JH, Norris DA, Dinarello CA, Fujita M. Dual role of apoptosis-associated speck-like protein containing a CARD (ASC) in tumorigenesis of human melanoma. J Invest Dermatol. 2013;133(2):518–527. doi: 10.1038/jid.2012.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo B, Fu S, Zhang J, Liu B, Li Z. Targeting inflammasome/IL-1 pathways for cancer immunotherapy. Sci Rep. 2016;6:36107. doi: 10.1038/srep36107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kolb R, Phan L, Borcherding N, Liu Y, Yuan F, Janowski AM, et al. Obesity-associated NLRC4 inflammasome activation drives breast cancer progression. Nat Commun. 2016;7:13007. doi: 10.1038/ncomms13007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Daley D, Mani VR, Mohan N, Akkad N, Pandian G, Savadkar S, et al. NLRP3 signaling drives macrophage-induced adaptive immune suppression in pancreatic carcinoma. J Exp Med. 2017;214(6):1711–1724. doi: 10.1084/jem.20161707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thompson JK, Shukla A, Leggett AL, Munson PB, Miller JM, MacPherson MB, et al. Extracellular signal regulated kinase 5 and inflammasome in progression of mesothelioma. Oncotarget. 2018;9(1):293–305. doi: 10.18632/oncotarget.22968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang M, Jin C, Yang Y, Wang K, Zhou Y, Zhou Y, et al. AIM2 promotes non-small-cell lung cancer cell growth through inflammasome-dependent pathway. J Cell Physiol. 2019. [DOI] [PubMed]
- 51.Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67(20):10019–10026. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salcedo R, Worschech A, Cardone M, Jones Y, Gyulai Z, Dai RM, et al. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18. J Exp Med. 2010;207(8):1625–1636. doi: 10.1084/jem.20100199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harrison OJ, Srinivasan N, Pott J, Schiering C, Krausgruber T, Ilott NE, et al. Epithelial-derived IL-18 regulates Th17 cell differentiation and Foxp3(+) Treg cell function in the intestine. Mucosal Immunol. 2015;8(6):1226–1236. doi: 10.1038/mi.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dahut WL, Gulley JL, Arlen PM, Liu Y, Fedenko KM, Steinberg SM, et al. Randomized phase II trial of docetaxel plus thalidomide in androgen-independent prostate cancer. J Clin Oncol. 2004;22(13):2532–2539. doi: 10.1200/JCO.2004.05.074. [DOI] [PubMed] [Google Scholar]
- 55.Singhal S, Mehta J, Desikan R, Ayers D, Roberson P, Eddlemon P, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341(21):1565–1571. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 56.Triozzi PL, Aldrich W. Effects of interleukin-1 receptor antagonist and chemotherapy on host-tumor interactions in established melanoma. Anticancer Res. 2010;30(2):345–354. [PubMed] [Google Scholar]
- 57.Holen I, Lefley DV, Francis SE, Rennicks S, Bradbury S, Coleman RE, et al. IL-1 drives breast cancer growth and bone metastasis in vivo. Oncotarget. 2016;7(46):75571–75584. doi: 10.18632/oncotarget.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lust JA, Lacy MQ, Zeldenrust SR, Dispenzieri A, Gertz MA, Witzig TE, et al. Induction of a chronic disease state in patients with smoldering or indolent multiple myeloma by targeting interleukin 1{beta}-induced interleukin 6 production and the myeloma proliferative component. Mayo Clin Proc. 2009;84(2):114–122. doi: 10.4065/84.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qiu Y, Li WH, Zhang HQ, Liu Y, Tian XX, Fang WG. P2X7 mediates ATP-driven invasiveness in prostate cancer cells. PLoS One. 2014;9(12):e114371. doi: 10.1371/journal.pone.0114371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Giannuzzo A, Pedersen SF, Novak I. The P2X7 receptor regulates cell survival, migration and invasion of pancreatic ductal adenocarcinoma cells. Mol Cancer. 2015;14:203. doi: 10.1186/s12943-015-0472-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giannuzzo A, Saccomano M, Napp J, Ellegaard M, Alves F, Novak I. Targeting of the P2X7 receptor in pancreatic cancer and stellate cells. Int J Cancer. 2016;139(11):2540–2552. doi: 10.1002/ijc.30380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adinolfi E, Cirillo M, Woltersdorf R, Falzoni S, Chiozzi P, Pellegatti P, et al. Trophic activity of a naturally occurring truncated isoform of the P2X7 receptor. FASEB J. 2010;24(9):3393–3404. doi: 10.1096/fj.09-153601. [DOI] [PubMed] [Google Scholar]
- 63.Farrell AW, Gadeock S, Pupovac A, Wang B, Jalilian I, Ranson M, et al. P2X7 receptor activation induces cell death and CD23 shedding in human RPMI 8226 multiple myeloma cells. Biochim Biophys Acta. 2010;1800(11):1173–1182. doi: 10.1016/j.bbagen.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 64.Bae JY, Lee SW, Shin YH, Lee JH, Jahng JW, Park K. P2X7 receptor and NLRP3 inflammasome activation in head and neck cancer. Oncotarget. 2017;8(30):48972–48982. doi: 10.18632/oncotarget.16903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fei Q, Xiao J, Hu B, Sun N, Wei Y, Zhu J. High expression of P2X7R is an independent postoperative indicator of poor prognosis in colorectal cancer. Human Pathol. 2017;64:61–68. doi: 10.1016/j.humpath.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 66.Gilbert SM, Gidley Baird A, Glazer S, Barden JA, Glazer A, Teh LC, et al. A phase I clinical trial demonstrates that nfP2X7-targeted antibodies provide a novel, safe and tolerable topical therapy for basal cell carcinoma. Br J Dermatol. 2017;177(1):117–124. doi: 10.1111/bjd.15364. [DOI] [PubMed] [Google Scholar]
- 67.Sohma I, Fujiwara Y, Sugita Y, Yoshioka A, Shirakawa M, Moon JH, et al. Parthenolide, an NF-kappaB inhibitor, suppresses tumor growth and enhances response to chemotherapy in gastric cancer. Cancer Genomics Proteomics. 2011;8(1):39–47. [PubMed] [Google Scholar]
- 68.Kim SL, Trang KT, Kim SH, Kim IH, Lee SO, Lee ST, et al. Parthenolide suppresses tumor growth in a xenograft model of colorectal cancer cells by inducing mitochondrial dysfunction and apoptosis. Int J Oncol. 2012;41(4):1547–1553. doi: 10.3892/ijo.2012.1587. [DOI] [PubMed] [Google Scholar]
- 69.Liu JW, Cai MX, Xin Y, Wu QS, Ma J, Yang P, et al. Parthenolide induces proliferation inhibition and apoptosis of pancreatic cancer cells in vitro. J Exp Clin Cancer Res. 2010;29:108. doi: 10.1186/1756-9966-29-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liao K, Xia B, Zhuang QY, Hou MJ, Zhang YJ, Luo B, et al. Parthenolide inhibits cancer stem-like side population of nasopharyngeal carcinoma cells via suppression of the NF-kappaB/COX-2 pathway. Theranostics. 2015;5(3):302–321. doi: 10.7150/thno.8387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang QQ, Ding Y, Lei Y, Qi CL, He XD, Lan T, et al. Andrographolide suppress tumor growth by inhibiting TLR4/NF-kappaB signaling activation in insulinoma. Int J Biol Sci. 2014;10(4):404–414. doi: 10.7150/ijbs.7723. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 72.Zhang R, Zhao J, Xu J, Jiao DX, Wang J, Gong ZQ, et al. Andrographolide suppresses proliferation of human colon cancer SW620 cells through the TLR4/NF-kappaB/MMP-9 signaling pathway. Oncol Lett. 2017;14(4):4305–4310. doi: 10.3892/ol.2017.6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Su M, Qin B, Liu F, Chen Y, Zhang R. Andrographolide enhanced 5-fluorouracil-induced antitumor effect in colorectal cancer via inhibition of c-MET pathway. Drug Des Devel Ther. 2017;11:3333–3341. doi: 10.2147/DDDT.S140354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guo W, Sun Y, Liu W, Wu X, Guo L, Cai P, et al. Small molecule-driven mitophagy-mediated NLRP3 inflammasome inhibition is responsible for the prevention of colitis-associated cancer. Autophagy. 2014;10(6):972–985. doi: 10.4161/auto.28374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhai Z, Qu X, Yan W, Li H, Liu G, Liu X, et al. Andrographolide prevents human breast cancer-induced osteoclastic bone loss via attenuated RANKL signaling. Breast Cancer Res Treat. 2014;144(1):33–45. doi: 10.1007/s10549-014-2844-7. [DOI] [PubMed] [Google Scholar]
- 76.Zhai Z, Qu X, Li H, Ouyang Z, Yan W, Liu G, et al. Inhibition of MDA-MB-231 breast cancer cell migration and invasion activity by andrographolide via suppression of nuclear factor-kappaB-dependent matrix metalloproteinase-9 expression. Mol Med Rep. 2015;11(2):1139–1145. doi: 10.3892/mmr.2014.2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gao H, Wang J. Andrographolide inhibits multiple myeloma cells by inhibiting the TLR4/NF-kappaB signaling pathway. Mol Med Rep. 2016;13(2):1827–1832. doi: 10.3892/mmr.2015.4703. [DOI] [PubMed] [Google Scholar]
- 78.Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ. Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet (London, England). 2017;390(10105):1833–1842. doi: 10.1016/S0140-6736(17)32247-X. [DOI] [PubMed] [Google Scholar]
- 79.Miller MT. Thalidomide embryopathy: a model for the study of congenital incomitant horizontal strabismus. Trans Am Ophthalmol Soc. 1991;89:623–674. [PMC free article] [PubMed] [Google Scholar]
- 80.Peng HL, Yi YF, Zhou SK, Xie SS, Zhang GS. Thalidomide effects in patients with hereditary hemorrhagic telangiectasia during therapeutic treatment and in Fli-EGFP transgenic zebrafish model. Chin Med J (Engl). 2015;128(22):3050–3054. doi: 10.4103/0366-6999.169068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Breitkreutz I, Anderson KC. Thalidomide in multiple myeloma--clinical trials and aspects of drug metabolism and toxicity. Expert Opin Drug Metab Toxicol. 2008;4(7):973–985. doi: 10.1517/17425255.4.7.973. [DOI] [PubMed] [Google Scholar]
- 82.Figg WD, Kruger EA, Price DK, Kim S, Dahut WD. Inhibition of angiogenesis: treatment options for patients with metastatic prostate cancer. Invest New Drugs. 2002;20(2):183–194. doi: 10.1023/A:1015626410273. [DOI] [PubMed] [Google Scholar]
- 83.Haslett PA, Corral LG, Albert M, Kaplan G. Thalidomide costimulates primary human T lymphocytes, preferentially inducing proliferation, cytokine production, and cytotoxic responses in the CD8+ subset. J Exp Med. 1998;187(11):1885–1892. doi: 10.1084/jem.187.11.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lin AY, Brophy N, Fisher GA, So S, Biggs C, Yock TI, et al. Phase II study of thalidomide in patients with unresectable hepatocellular carcinoma. Cancer. 2005;103(1):119–125. doi: 10.1002/cncr.20732. [DOI] [PubMed] [Google Scholar]
- 85.Lee SM, Rudd R, Woll PJ, Ottensmeier C, Gilligan D, Price A, et al. Randomized double-blind placebo-controlled trial of thalidomide in combination with gemcitabine and Carboplatin in advanced non-small-cell lung cancer. J Clin Oncol. 2009;27(31):5248–5254. doi: 10.1200/JCO.2009.21.9733. [DOI] [PubMed] [Google Scholar]
- 86.Pujol JL, Breton JL, Gervais R, Tanguy ML, Quoix E, David P, et al. Phase III double-blind, placebo-controlled study of thalidomide in extensive-disease small-cell lung cancer after response to chemotherapy: an intergroup study FNCLCC cleo04 IFCT 00-01. J Clin Oncol. 2007;25(25):3945–3951. doi: 10.1200/JCO.2007.11.8109. [DOI] [PubMed] [Google Scholar]
- 87.Ott PA, Chang JL, Oratz R, Jones A, Farrell K, Muggia F, et al. Phase II trial of dacarbazine and thalidomide for the treatment of metastatic melanoma. Chemotherapy. 2009;55(4):221–227. doi: 10.1159/000219435. [DOI] [PubMed] [Google Scholar]
- 88.Laber DA, Okeke RI, Arce-Lara C, Taft BS, Schonard CL, McMasters KM, et al. A phase II study of extended dose temozolomide and thalidomide in previously treated patients with metastatic melanoma. J Cancer Res Clin Oncol. 2006;132(9):611–616. doi: 10.1007/s00432-006-0114-8. [DOI] [PubMed] [Google Scholar]
- 89.Benboubker L, Dimopoulos MA, Dispenzieri A, Catalano J, Belch AR, Cavo M, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371(10):906–917. doi: 10.1056/NEJMoa1402551. [DOI] [PubMed] [Google Scholar]
- 90.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 91.Lust JA, Donovan KA. The role of interleukin-1 beta in the pathogenesis of multiple myeloma. Hematol Oncol Clin North Am. 1999;13(6):1117–1125. doi: 10.1016/S0889-8588(05)70115-5. [DOI] [PubMed] [Google Scholar]
- 92.Bellamy WT, Richter L, Sirjani D, Roxas C, Glinsmann-Gibson B, Frutiger Y, et al. Vascular endothelial cell growth factor is an autocrine promoter of abnormal localized immature myeloid precursors and leukemia progenitor formation in myelodysplastic syndromes. Blood. 2001;97(5):1427–1434. doi: 10.1182/blood.V97.5.1427. [DOI] [PubMed] [Google Scholar]
- 93.Nie L, Lyros O, Medda R, Jovanovic N, Schmidt JL, Otterson MF, et al. Endothelial-mesenchymal transition in normal human esophageal endothelial cells cocultured with esophageal adenocarcinoma cells: role of IL-1beta and TGF-beta2. Am J Physiol Cell Physiol. 2014;307(9):C859–C877. doi: 10.1152/ajpcell.00081.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Singh JA, Hossain A, Tanjong Ghogomu E, Kotb A, Christensen R, Mudano AS, et al. Biologics or tofacitinib for rheumatoid arthritis in incomplete responders to methotrexate or other traditional disease-modifying anti-rheumatic drugs: a systematic review and network meta-analysis. Cochrane Database Syst Rev. 2016;5:CD012183. doi: 10.1002/14651858.CD012183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Agrawal A, Gartland A. P2X7 receptors: role in bone cell formation and function. J Mol Endocrinol. 2015;54(2):R75–R88. doi: 10.1530/JME-14-0226. [DOI] [PubMed] [Google Scholar]
- 96.Roger S, Pelegrin P. P2X7 receptor antagonism in the treatment of cancers. Expert Opin Investig Drugs. 2011;20(7):875–880. doi: 10.1517/13543784.2011.583918. [DOI] [PubMed] [Google Scholar]
- 97.Lopez-Castejon G, Theaker J, Pelegrin P, Clifton AD, Braddock M, Surprenant A. P2X(7) receptor-mediated release of cathepsins from macrophages is a cytokine-independent mechanism potentially involved in joint diseases. J Immunol. 2010;185(4):2611–2619. doi: 10.4049/jimmunol.1000436. [DOI] [PubMed] [Google Scholar]
- 98.Gu BJ, Wiley JS. Rapid ATP-induced release of matrix metalloproteinase 9 is mediated by the P2X7 receptor. Blood. 2006;107(12):4946–4953. doi: 10.1182/blood-2005-07-2994. [DOI] [PubMed] [Google Scholar]
- 99.Hill LM, Gavala ML, Lenertz LY, Bertics PJ. Extracellular ATP may contribute to tissue repair by rapidly stimulating purinergic receptor X7-dependent vascular endothelial growth factor release from primary human monocytes. J Immunol. 2010;185(5):3028–3034. doi: 10.4049/jimmunol.1001298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kwok BH, Koh B, Ndubuisi MI, Elofsson M, Crews CM. The anti-inflammatory natural product parthenolide from the medicinal herb Feverfew directly binds to and inhibits IkappaB kinase. Chem Biol. 2001;8(8):759–766. doi: 10.1016/S1074-5521(01)00049-7. [DOI] [PubMed] [Google Scholar]
- 101.Nennig SE, Schank JR. The role of NFkB in drug addiction: beyond inflammation. Alcohol Alcohol. 2017;52(2):172–179. doi: 10.1093/alcalc/agw098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang S, Lin ZN, Yang CF, Shi X, Ong CN, Shen HM. Suppressed NF-kappaB and sustained JNK activation contribute to the sensitization effect of parthenolide to TNF-alpha-induced apoptosis in human cancer cells. Carcinogenesis. 2004;25(11):2191–2199. doi: 10.1093/carcin/bgh234. [DOI] [PubMed] [Google Scholar]
- 103.Gopal YN, Chanchorn E, Van Dyke MW. Parthenolide promotes the ubiquitination of MDM2 and activates p53 cellular functions. Mol Cancer Ther. 2009;8(3):552–562. doi: 10.1158/1535-7163.MCT-08-0661. [DOI] [PubMed] [Google Scholar]
- 104.Sobota R, Szwed M, Kasza A, Bugno M, Kordula T. Parthenolide inhibits activation of signal transducers and activators of transcription (STATs) induced by cytokines of the IL-6 family. Biochem Biophys Res Commun. 2000;267(1):329–333. doi: 10.1006/bbrc.1999.1948. [DOI] [PubMed] [Google Scholar]
- 105.Curry EA, 3rd, Murry DJ, Yoder C, Fife K, Armstrong V, Nakshatri H, et al. Phase I dose escalation trial of feverfew with standardized doses of parthenolide in patients with cancer. Invest New Drugs. 2004;22(3):299–305. doi: 10.1023/B:DRUG.0000026256.38560.be. [DOI] [PubMed] [Google Scholar]
- 106.Guzman ML, Rossi RM, Neelakantan S, Li X, Corbett CA, Hassane DC, et al. An orally bioavailable parthenolide analog selectively eradicates acute myelogenous leukemia stem and progenitor cells. Blood. 2007;110(13):4427–4435. doi: 10.1182/blood-2007-05-090621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sur R, Martin K, Liebel F, Lyte P, Shapiro S, Southall M. Anti-inflammatory activity of parthenolide-depleted Feverfew (Tanacetum parthenium) Inflammopharmacology. 2009;17(1):42–49. doi: 10.1007/s10787-008-8040-9. [DOI] [PubMed] [Google Scholar]
- 108.Lachmann HJ, Kone-Paut I, Kuemmerle-Deschner JB, Leslie KS, Hachulla E, Quartier P, et al. Use of canakinumab in the cryopyrin-associated periodic syndrome. N Engl J Med. 2009;360(23):2416–2425. doi: 10.1056/NEJMoa0810787. [DOI] [PubMed] [Google Scholar]
- 109.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 110.Cuzick J, Otto F, Baron JA, Brown PH, Burn J, Greenwald P, et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009;10(5):501–507. doi: 10.1016/S1470-2045(09)70035-X. [DOI] [PubMed] [Google Scholar]
- 111.Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet (London, England). 2011;377(9759):31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 112.Chakravarti RN, Chakravarti D. Andrographolide, the active constituent of Andrographis paniculata Nees; a preliminary communication. Ind Med Gaz. 1951;86(3):96–97. [PMC free article] [PubMed] [Google Scholar]
- 113.Bao Z, Guan S, Cheng C, Wu S, Wong SH, Kemeny DM, et al. A novel antiinflammatory role for andrographolide in asthma via inhibition of the nuclear factor-kappaB pathway. Am J Respir Crit Care Med. 2009;179(8):657–665. doi: 10.1164/rccm.200809-1516OC. [DOI] [PubMed] [Google Scholar]
- 114.Shi MD, Lin HH, Lee YC, Chao JK, Lin RA, Chen JH. Inhibition of cell-cycle progression in human colorectal carcinoma Lovo cells by andrographolide. Chem Biol Interact. 2008;174(3):201–210. doi: 10.1016/j.cbi.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 115.Chang J, Zhang RM, Zhang Y, Chen ZB, Zhang ZM, Xu Q, et al. Andrographolide drop-pill in treatment of acute upper respiratory tract infection with external wind-heat syndrome: a multicenter and randomized controlled trial. Zhong Xi Yi Jie He Xue Bao. 2008;6(12):1238–1245. doi: 10.3736/jcim20081206. [DOI] [PubMed] [Google Scholar]
- 116.Gabrielian ES, Shukarian AK, Goukasova GI, Chandanian GL, Panossian AG, Wikman G, et al. A double blind, placebo-controlled study of Andrographis paniculata fixed combination Kan Jang in the treatment of acute upper respiratory tract infections including sinusitis. Phytomedicine. 2002;9(7):589–597. doi: 10.1078/094471102321616391. [DOI] [PubMed] [Google Scholar]
- 117.Riddle MC. Editorial: sulfonylureas differ in effects on ischemic preconditioning--is it time to retire glyburide? J Clin Endocrinol Metab. 2003;88(2):528–530. doi: 10.1210/jc.2002-021971. [DOI] [PubMed] [Google Scholar]
- 118.Serrano-Martin X, Payares G, Mendoza-Leon A. Glibenclamide, a blocker of K+(ATP) channels, shows antileishmanial activity in experimental murine cutaneous leishmaniasis. Antimicrobial agents and chemotherapy. 2006;50(12):4214–4216. doi: 10.1128/AAC.00617-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tamura K, Ishikawa G, Yoshie M, Ohneda W, Nakai A, Takeshita T, et al. Glibenclamide inhibits NLRP3 inflammasome-mediated IL-1beta secretion in human trophoblasts. J Pharmacol Sci. 2017;135(2):89–95. doi: 10.1016/j.jphs.2017.09.032. [DOI] [PubMed] [Google Scholar]
- 120.Koh GC, Maude RR, Schreiber MF, Limmathurotsakul D, Wiersinga WJ, Wuthiekanun V, et al. Glyburide is anti-inflammatory and associated with reduced mortality in melioidosis. Clin Infect Dis. 2011;52(6):717–725. doi: 10.1093/cid/ciq192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Marchetti C, Chojnacki J, Toldo S, Mezzaroma E, Tranchida N, Rose SW, et al. A novel pharmacologic inhibitor of the NLRP3 inflammasome limits myocardial injury after ischemia-reperfusion in the mouse. J Cardiovasc Pharmacol. 2014;63(4):316–322. doi: 10.1097/FJC.0000000000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Coll RC, Robertson AAB, Chae JJ, Higgins SC, Muñoz-Planillo R, Inserra MC, et al. A small molecule inhibitior of the NLRP3 inflammasome is a potential therapeutic for inflammatory diseases. Nature Medicine. 2015;21(3):248. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Coll RC, Robertson A, Butler M, Cooper M, O'Neill LA. The cytokine release inhibitory drug CRID3 targets ASC oligomerisation in the NLRP3 and AIM2 inflammasomes. PLoS One. 2011;6(12):e29539. doi: 10.1371/journal.pone.0029539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Laliberte RE, Perregaux DG, Hoth LR, Rosner PJ, Jordan CK, Peese KM, et al. Glutathione s-transferase omega 1-1 is a target of cytokine release inhibitory drugs and may be responsible for their effect on interleukin-1beta posttranslational processing. J Biol Chema. 2003;278(19):16567–16578. doi: 10.1074/jbc.M211596200. [DOI] [PubMed] [Google Scholar]
- 125.Perera AP, Fernando R, Shinde T, Gundamaraju R, Southam B, Sohal SS, et al. MCC950, a specific small molecule inhibitor of NLRP3 inflammasome attenuates colonic inflammation in spontaneous colitis mice. Sci Rep. 2018;8(1):8618. doi: 10.1038/s41598-018-26775-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Randle JC, Harding MW, Ku G, Schönharting M, Kurrle R. ICE/Caspase-1 inhibitors as novel anti-inflammatory drugs. Expert Opin Investig Drugs. 2001;10(7):1207. doi: 10.1517/13543784.10.7.1207. [DOI] [PubMed] [Google Scholar]
- 127.Ayala JM, Yamin TT, Egger LA, Chin J, Kostura MJ, Miller DK. IL-1 beta-converting enzyme is present in monocytic cells as an inactive 45-kDa precursor. J Immunol. 1994;153(6):2592. [PubMed] [Google Scholar]
- 128.Kronheim SR, Mumma A, Greenstreet T, Glackin PJ, van Ness K, March CJ, et al. Purification of interleukin-1 beta \converting enzyme, the protease that cleaves the interleukin-1 beta precursor. Arch Biochem Biophys. 1992;296(2):698–703. doi: 10.1016/0003-9861(92)90629-B. [DOI] [PubMed] [Google Scholar]
- 129.Rudolphi K, Gerwin N, Verzijl N, Kraan PVD, Berg WVD. Pralnacasan, an inhibitor of interleukin-1β converting enzyme, reduces joint damage in two murine models of osteoarthritis. Osteoarthritis Cartilage. 2003;11(10):738–746. doi: 10.1016/S1063-4584(03)00153-5. [DOI] [PubMed] [Google Scholar]
- 130.Florian L, Christian B, Nikola L, Kathrin S, Britta S, Hans Anton L, et al. The interleukin-1 beta-converting enzyme inhibitor pralnacasan reduces dextran sulfate sodium-induced murine colitis and T helper 1 T-cell activation. J Pharmacol Exp Ther. 2004;308(2):583–590. doi: 10.1124/jpet.103.057059. [DOI] [PubMed] [Google Scholar]
- 131.Bauer C, Loher F, Dauer M, Mayer C, Lehr HA, Schönharting M, et al. The ICE inhibitor pralnacasan prevents DSS-induced colitis in C57BL/6 mice and suppresses IP-10 mRNA but not TNF-α mRNA expression. Dig Dis Sci. 2007;52(7):1642–1652. doi: 10.1007/s10620-007-9802-8. [DOI] [PubMed] [Google Scholar]
- 132.Maroso M, Balosso S, Ravizza T, Iori V, Wright CI, French J, et al. Interleukin-1beta biosynthesis inhibition reduces acute seizures and drug resistant chronic epileptic activity in mice. Neurotherapeutics. 2011;8(2):304–315. doi: 10.1007/s13311-011-0039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ravizza T, Lucas SM, Balosso S, Bernardino L, Ku G, Noe F, et al. Inactivation of caspase-1 in rodent brain: a novel anticonvulsive strategy. Epilepsia. 2006;47(7):1160–1168. doi: 10.1111/j.1528-1167.2006.00590.x. [DOI] [PubMed] [Google Scholar]
- 134.Flores J, Noel A, Foveau B, Lynham J, Lecrux C, LeBlanc AC. Caspase-1 inhibition alleviates cognitive impairment and neuropathology in an Alzheimer’s disease mouse model. Nat Commun. 2018;9(1):3916. doi: 10.1038/s41467-018-06449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yang XM, Downey JM, Cohen MV, Housley NA, Alvarez DF, Audia JP. The highly selective caspase-1 inhibitor VX-765 provides additive protection against myocardial infarction in rat hearts when combined with a platelet inhibitor. J Cardiovasc Pharmacol Ther. 2017;22(6):574–578. doi: 10.1177/1074248417702890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Audia JP, Yang XM, Crockett ES, Housley N, Haq EU, O'Donnell K, et al. Caspase-1 inhibition by VX-765 administered at reperfusion in P2Y12 receptor antagonist-treated rats provides long-term reduction in myocardial infarct size and preservation of ventricular function. Basic Res Cardiol. 2018;113(5):32. doi: 10.1007/s00395-018-0692-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Younus I, Reddy DS. A resurging boom in new drugs for epilepsy and brain disorders. Expert Rev Clin Pharmacol. 2018;11(1):27–45. doi: 10.1080/17512433.2018.1386553. [DOI] [PubMed] [Google Scholar]
- 138.Thornberry NA, Rano TA, Peterson EP, Rasper DM, Timkey T, Garcia-Calvo M, et al. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J Biol Chem. 1997;272(29):17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- 139.Talanian RV, Quinlan C, Trautz S, Hackett MC, Mankovich JA, Banach D, et al. Substrate specificities of caspase family proteases. The Journal of biological chemistry. 1997;272(15):9677–9682. doi: 10.1074/jbc.272.15.9677. [DOI] [PubMed] [Google Scholar]
- 140.Schlosser S, Gansauge F, Ramadani M, Beger HG, Gansauge S. Inhibition of caspase-1 induces cell death in pancreatic carcinoma cells and potentially modulates expression levels of bcl-2 family proteins. Febs Letters. 2001;491(1):104–108. doi: 10.1016/S0014-5793(01)02144-5. [DOI] [PubMed] [Google Scholar]
- 141.Boost K, Hoegl S, Hofstetter C, Flondor M, Stegewerth K, Platacis I, Pfeilschifter J, et al. Targeting caspase-1 by inhalation-therapy: effects of Ac-YVAD-CHO on IL-1 beta, IL-18 and downstream proinflammatory parameters as detected in rat endotoxaemia. Intensive Care Med. 2007;33(5):863. doi: 10.1007/s00134-007-0588-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable