Abstract
Introduction. Cerebral palsy (CP) is caused by an injury to the developing brain, and abnormal gross motor function is a hallmark of CP. Properly structured exercises on land have been reported to be effective in improving functional performance in children with CP while only few have been documented on aquatic therapy. Objective. To investigate the effect of a 10-week aquatic exercise training program on gross motor function in children with spastic CP. Methods. Thirty participants aged 1 to 12 years were randomized into the experimental and control groups. Both groups received manual passive stretching and functional training exercises, depending on their level of motor impairment, either in water (temperature 28°C to 32°C) or on land. Each exercise training session lasted for about 1 hour 40 minutes, twice per week for 10 weeks in both groups. Measurement of gross motor function was done using Gross Motor Function Measure (GMFM-88) at baseline and after 4 weeks, 8 weeks, and 10 weeks of intervention. Both groups were compared for differences in change in gross motor function using Mann-Whitney U test. The level of significance was set at P < .05. Results. Only the experimental group showed significant improvement (P < .05) in all dimensions of gross motor function except for walking, running, and jumping (P = .112). Statistically significant difference (P < .05) was found between both groups for all dimensions of gross motor function after 10 weeks of intervention. Conclusion. Aquatic exercise training program is effective in the functional rehabilitation of children with spastic CP.
Keywords: aquatic exercise, gross motor function, spastic, cerebral palsy
Introduction
Cerebral palsy (CP) is the most common motor disability in childhood and it is associated with lifelong motor impairments.1 It is a disabling condition that affects a child’s life and that of his/her family irreversibly, and usually, it is a nonprogressive condition, but improvement over time is challenge.2 CP is a common neurologic problem in children and is reported as occurring in approximately 2 to 2.5 of 1000 live births globally.3 Reports by El-Tallawy et al4 also showed a prevalence of 2 per 1000 births in Africa. In Nigeria, CP is the second most common disorder seen at the pediatric neurology clinics after epilepsy.5,6 Majority (75%) of children with CP suffer from the spastic form.6,7
Abnormal gross and fine motor function and organization, reflecting abnormal motor control, are the core features of CP.8 Loss of selective motor control, abnormal muscle tone, imbalance of power between muscle agonists and antagonists, and impaired body balance mechanisms influence the growth of the child’s muscles and bones, which might result in reduced muscle elasticity, reduced joint range of motion, and disturbed bone and joint development.9 These motor problems can lead to difficulties with walking, feeding, and swallowing; coordinated eye movements; articulation of speech; and secondary problems with musculoskeletal function, behavior, and participation in society.8
A factor that matters most to a child and their family is the ability to perform daily activities.10 Mesterman et al11 reported that 30% to 50% of the Israeli, 8 to 30 years old with CP, require assistance in activities of daily living and for the tasks of dressing, shower, and mobility outside of home, and 50% to 60% of the participants aged below 18 years reported the need for assistance. After achieving a maximum independence level, social function and mobility skills have been reported to deteriorate in youth with CP from the age of 14 years onward.12
Cerebral palsy falls into the category of dynamic disabilities, which means that the physical conditions of individuals with CP can be altered under the influence of physical activities and exercises.13,14 Regular exercise is necessary for the health of children and adults, but in those with CP, the ability to exercise is adversely affected by their motor impairment.1 Various therapeutic exercises, such as strengthening, stretching, balance training, and functional task–oriented training, are commonly used in the rehabilitation of children with CP.15 These exercises, which are mostly carried out on land, have been shown to improve gross motor function in children with a diagnosis of CP.16,17 Despite the wide use of these exercises, some studies have reported challenges accompanying their usage. For instance, a survey carried out in Canada among parents of children with CP demonstrated that stretching was a form of exercise most frequently identified as painful by parents (93% of those reporting pain) and the one with the highest mean pain intensity.18
Aquatic or water intervention is one of the most popular supplementary treatments for children with neuromotor impairments, particularly CP.19 The intervention may provide safe and beneficial alternative low-impact exercise for children with disabilities,20 but there is still lack of evidence-based studies documenting the effects.21 A 2011 systematic review by Blohm22 looked at 8 studies, including 3 randomized controlled trials. All 8 studies reported that aquatic interventions, either as a major component or as a stand-alone intervention, were beneficial for children and adolescents with CP. Benefits reported in the studies included improvement in gross motor skills and maintaining improvements for 3 to 6 months after the intervention, improvement in function including walking efficiency, lower limb muscle strength, balance, and reduced spasticity. Aquatic therapy is advantageous because water can provide antigravity positioning, as well as buoyancy for weight reduction and decreased compressive forces on joints, resulting in a more fluid active movement for children who would not be able to do certain activities on land.23,24
With the dearth of available evidences showing the effect of aquatic therapy on gross motor function in children with CP, it has become necessary to investigate the effect of a structured aquatic exercise training program on gross motor function in children with CP.
Methods
A total of 30 children not older than 12 years of age who were diagnosed with spastic CP participated in this study. They were recruited from a developmental center in Lagos, Nigeria, where they were undergoing their rehabilitation. They were screened for eligibility based on the inclusion and exclusion criteria of the study. Children with associated neurodevelopmental conditions were excluded from this study.
Ethical Approval and Informed Consent
Ethical approval was sought and obtained from the Health Research and Ethics Committee of the Lagos University Teaching Hospital, Idi-Araba, Lagos (Ref No.: ADM/DCST/HREC/APP/1525).
Written informed consents were sought and obtained from the parents of the participants prior to the commencement of the study.
Procedure
Following the permission from the service director of the center, the participants’ case files were assessed prior to the commencement of the study to ascertain participants’ diagnoses and the type of CP. The participants were assessed by obtaining a detailed history and carrying out physical examination. Sociodemographic data such as age and gender were also obtained from their case files. The participants who met the criteria for the study were then randomly assigned into experimental and control groups. Their mobility level was determined using Gross Motor Function Classification System (GMFCS)–Expanded and Revised, while Gross Motor Function Measure–88 (GMFM-88) was used to measure their gross motor function.
Assessment Protocol
Mobility Level Assessment
This was carried out using the GMFCS–Expanded and Revised according to standard.25 Patients were carefully observed in order to ascertain their mobility level.
Gross Motor Function Assessment
This was carried out using the GMFM-88. Participants were observed for functional performance at various domains of the instrument.
All measurements were taken at baseline, end of fourth week, end of eighth week, and after 10 weeks of intervention. However, the mobility level was only assessed at baseline to describe the participants in terms of their motor impairments. All participants were ensured not to participate in any other form of treatment throughout the period of the study, and parents were advised to report to the researcher any complaints they have about their children at any point during the research period.
Intervention
All participants in both groups participated in a total of 20 treatment sessions for 10 consecutive weeks of 2 sessions per week. Concomitant use of antispastic drugs in any form was discouraged throughout the study.
Experimental Group
The participants in the experimental group received a treatment protocol adopted from the studies of Salem and Godwin.26 Participants received exercise training in water, 2 times a week for 10 weeks with the exercised parts fully immersed in water. The water temperature was between 28°C and 32°C throughout the entire duration of intervention. Two physiotherapists were involved in the treatment of each of the participants in a treatment session. The exercise protocol consisted of 2 categories of exercises as follows.
Exercise 1 (manual passive stretching)
This consisted of moving the joint involving the spastic group of muscles passively away from the direction of primary function and holding this position for 60 seconds in fully lengthened position of the muscle groups. This procedure was repeated 5 times for each part giving a total duration of 5 minutes.
Exercise 2 (functional training)
All participants were functionally trained according to their level of functional impairment in 4 levels with training in each level lasting for 15 minutes. These 4 levels were the following:
Level 1: 2-point kneeling exercise training
Level 2: Sitting education/training
Level 3: Standing education/training
Level 4: Walking education/training
Control Group
All participants in the land-based exercise group received same treatment protocol as water-based exercise group as described above except that all exercises were carried out on land. These exercises also included manual passive stretching and functional training: 2-point kneeling, sitting, standing, and walking, with same frequency and duration as water-based exercise group.
Post-Intervention Assessments
Participants were reassessed at the end of the fourth week, eighth week, and 10th week of intervention for changes in gross motor function using the GMFM-88. All assessments were carried out by blinded assessors who did not participate in the treatment of the participants but were trained on the assessment procedures.
Data Analysis
Statistical Package for Social Sciences (SPSS Inc, Chicago, IL) 21.0 version for Windows package program was used to perform data analysis. Demographic and quantitative data were expressed in terms of frequency, mean, and standard deviation. Independent t test was used to compare the age of participants in both groups. Friedman test was used to compare the baseline, end of fourth week, eighth week, and 10th week post-intervention changes in degree of spasticity and gross motor function of participants within each group, while Mann-Whitney U test was used to compare the change in spasticity and gross motor function between both groups. All statistical tests were performed at the .05 level of significance (P < .05).
Results
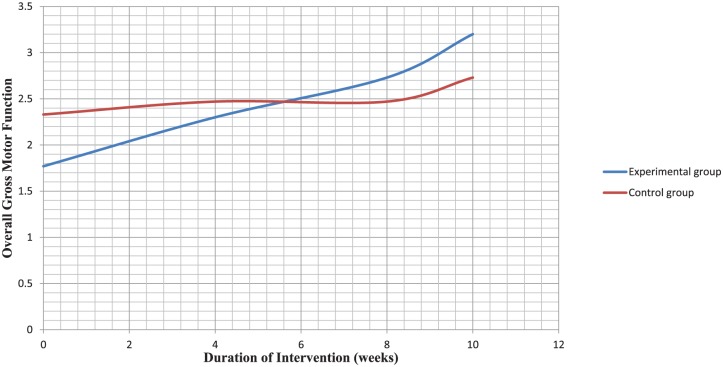
Both groups did not differ significantly in age and mobility level (Table 1). Comparison of mean rank values of the clinical outcome variables at baseline also revealed no significant difference in gross motor function between both groups (Table 2). There was significant difference in all dimensions of gross motor function (Table 3) among participants in the experimental group. The pattern of change in overall gross motor function across the duration of intervention is compared between both groups in Figure 1.
Table 1.
Comparison of Age and Mobility Level Between Both Groups at Baseline.
| Variables | All Participants (n) | Water-Based (n) | Land-Based (n) | Statistics | P |
|---|---|---|---|---|---|
| Mobility level: | |||||
| GMFCS II | 1 | 1 | 0 | U = 106.500 | .781 |
| GMFCS III | 11 | 5 | 6 | ||
| GMFCS IV | 16 | 7 | 9 | ||
| GMFCS V | 2 | 2 | 0 | ||
| Total | 30 | 15 | 15 | ||
| Age (years): | |||||
| 5.20 ± 2.43 | 4.93 ± 1.98 | 5.41 ± 2.85 | t = −0.559 | .105 | |
Abbreviations: GMFCS, Gross Motor Function Classification System; , mean ± standard deviation.
Table 2.
Mean Rank Comparison of Gross Motor Function Between Both the Groups at Baseline.
| Variables | Mean Rank |
U | P | |
|---|---|---|---|---|
| Water-Based | Land-Based | |||
| Lying and rolling | 16.53 | 15.60 | 97.000 | .518 |
| Sitting | 15.40 | 15.60 | 111.000 | .950 |
| Crawling and kneeling | 15.73 | 15.27 | 109.000 | .883 |
| Standing | 15.53 | 15.47 | 112.000 | .983 |
| Walking, running, and jumping | 16.13 | 14.87 | 103.000 | .662 |
| Overall gross motor function | 15.83 | 15.17 | 107.500 | .836 |
Table 3.
Comparison of Mean Rank Changes in Gross Motor Function in Experimental Group of Participants Across the Duration of Intervention.
| Variables | Mean Rank |
F | P | |||
|---|---|---|---|---|---|---|
| Baseline | 4th Week | 8th Week | 10th Week | |||
| Lying and rolling | 1.97 | 2.30 | 2.97 | 3.00 | 16.019 | .001* |
| Sitting | 1.90 | 2.30 | 2.87 | 2.93 | 17.727 | .001* |
| Crawling and kneeling | 2.17 | 2.30 | 2.57 | 2.97 | 12.600 | .006* |
| Standing | 2.23 | 2.50 | 2.50 | 2.77 | 8.000 | .046* |
| Walking, running, and jumping | 2.30 | 2.57 | 2.57 | 2.57 | 6.000 | .112 |
| Overall gross motor function | 1.77 | 2.30 | 2.73 | 3.20 | 20.753 | .000* |
Significant at P < .05.
Figure 1.
Comparison of mean rank change pattern in overall gross motor function between both groups across the duration of intervention.
Discussion
The aim of the present study was to investigate the effect of aquatic exercise training program on gross motor function in children with CP. Participants in both groups were similar in age and mobility level as well as in baseline parameters for gross motor function. This suggests that both groups were homogenous and therefore comparable and that results obtained could not be attributed to confounding variables of the participants or to chance.
Of the 30 participants involved in this present study, there was a preponderance of females compared with males (ratio 2.3:1). This finding is not in line with those found in previous similar studies6,27,28 where males were found to predominate. No obvious explanation could be given for the predominance of females compared with males in this study. It is, however, worthy of note that most of these previous studies were conducted in hospitals while participants for this study were recruited from a developmental center for children.
Majority (86.7%) of the participants recruited for this study were found to be older than 2 years. This counters the report of a study carried in Nigeria by Adekoje et al6 that included participants of the same age range as the present study. They reported that majority (63.4%) of their participants were aged 2 years and below. Other similar previous studies14,16,27,29,30 have also presented contrasting reports of age distribution, which are attributable to difference in inclusion criteria of participants. The findings that most participants in this study were older than 2 years may partly be due to the fact that developmental centers are not always the first choice for relatives seeking rehabilitation for their children or wards with CP. Therefore, the choice of a developmental center may be due to frustration of relatives as a result of delayed response of their handicapped children to treatment in the hospitals in the first few years of life and had to resort to these centers to relieve them of the burden when most of the children would have been older.
The most predominant type of spastic CP in the present study was the quadriplegic type (83.3%). This is similar to reports from earlier studies in Nigeria.6,31,32 It is, therefore, not surprising that the majority of the participants in the present study had severe motor impairments as reflected by their mobility level that was mostly GMFCS IV (53.3%). On the other hand, reports from studies in other parts of the world showed that diplegic type constituted the majority.14,16,28,29,30 There was no specific reason to account for this variation, but geographical differences may be considered.
The result of this study demonstrated that 10 weeks of aquatic exercise training program brought about significant improvement in gross motor function in the dimensions of lying and rolling, sitting, crawling, and kneeling, and standing as well as in the overall score. This may be attributed to the buoyancy effect of water, which provides antigravity positioning, weight reduction in water, and decreased compressive forces on joints, resulting in more fluid motor function for children who would not be able to do certain activities on land.22-24 This may consequently make functional training easier in water and may be responsible for the carry over effect in improvement of functional performance on land. These findings have been supported by previous studies.28,30 The study by Chrysagis et al28 found out that a 10-week aquatic training program improved the gross motor function in standing, which is consistent with the findings of this study. Declerck30 also reported significant improvement in motor function following 10 weeks of aquatic training in 14 youths with CP, which was found to translate into functional independence and improved self-care. These previous studies also documented significant improvement in walking, running, and jumping, which is contrary to the findings of this study. This variation may be attributed to methodological differences and inclusion criteria. While their studies both included ambulatory children with CP who could walk with or without support, the present study included both ambulatory and nonambulatory participants with majority not being ambulatory.
Comparison between both the experimental and control groups revealed that there was statistically significant difference on all dimensions of gross motor function in favor of aquatic exercise training program, and this significant difference was not observed until after the 10th week of intervention. This gives credence to aquatic exercise training as a beneficial intervention in the rehabilitation of children with spastic CP.
The result from this study, therefore, showed that aquatic exercise training program is effective in the rehabilitation of children with spastic CP. The exercise regimen should, however, be properly structured and extended to at least 10 weeks as done in this study and adhered to, so as to produce desired results.
Conclusion
From the findings of this study, it can be concluded that aquatic exercise training program is a veritable tool for the improvement of gross motor function in children with spastic cerebral palsy.
Footnotes
Author Contributions: BIA: Conception of research idea, data collection, documentation of results, data analysis and preparation of manuscript; CAG: Supervision of research, data analysis and proof-reading of manuscript; DOO: Supervision of research and proof-reading of manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Bolarinwa Isaac Akinola  https://orcid.org/0000-0002-0960-0147
https://orcid.org/0000-0002-0960-0147
References
- 1. Aisen ML, Kerkovich D, Mast J, et al. Cerebral palsy: clinical care and neurological rehabilitation. Lancet Neurol. 2011;10:844-852. [DOI] [PubMed] [Google Scholar]
- 2. Chahine NHA, Wehbe TW, Hilal RA, Zoghbi VV, Melki AE, Habib EB. Treatment of cerebral palsy with stem cells: a report of 17 cases. Int J Stem Cells. 2016;9:90-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shevell M, Dagenais L, Oskoui M. The epidemiology of cerebral palsy: new perspectives from a Canadian registry. Semin Pediatr Neurol. 2013;20:60-64. [DOI] [PubMed] [Google Scholar]
- 4. El-Tallawy HN, Farghaly WM, Shehata GA, Metwally NA, Rageh TA, Abo-Elfetoh N. Epidemiology of cerebral palsy in El-Kharga District-New Valley (Egypt). Brain Dev. 2011;33:406-411. [DOI] [PubMed] [Google Scholar]
- 5. Lagunju IA, Okafor OO. An analysis of disorders seen at the Paediatric Neurology Clinic, University College Hospital, Ibadan, Nigeria. West Afr J Med. 2009;28:38-42. [DOI] [PubMed] [Google Scholar]
- 6. Adekoje TO, Ibeabuchi MN, Lesi FE. Anthropometry of children with cerebral palsy at the Lagos University Teaching Hospital. J Clin Sci. 2016;13:96-104. http://www.jcsjournal.org/text.asp?2016/13/3/96/185245 [Google Scholar]
- 7. Dimitrijević L, Aleksandrović M, Madić D, Okiĉić T, Radovanović D, Daly D. The effect of aquatic intervention on the gross motor function and aquatic skills in children with cerebral palsy. J Hum Kinet. 2012;32:167-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol. 2007;109:8-14. [PubMed] [Google Scholar]
- 9. Koop SE. Musculoskeletal growth and development. In: Gage JR, Schwarts MH, Koop SE, Novacheck TF. eds. The Identification and Treatment of Gait Problems in Cerebral Palsy. 2nd ed. London, England: MacKeith Press; 2009. [Google Scholar]
- 10. Levitt S. The clinical picture for therapy and management. In: Treatment of Cerebral Palsy and Motor Delay. 4th ed. Hoboken, NJ: Blackwell; 2004:1-13. [Google Scholar]
- 11. Mesterman R, Leitner Y, Yifat R, et al. Cerebral palsy—long-term medical, functional, educational, and psychosocial outcomes. J Child Neurol. 2010;25:36-42. [DOI] [PubMed] [Google Scholar]
- 12. Kerr C, McDowell BC, Parkes J, Stevenson M, Cosgrove AP. Age-related changes in energy efficiency of gait, activity, and participation in children with cerebral palsy. Dev Med Child Neurol. 2011;53:61-67. [DOI] [PubMed] [Google Scholar]
- 13. Lockette KF, Keyes AM. Conditioning With Physical Disabilities. Champaign, IL: Human Kinetics; 1994. [Google Scholar]
- 14. Jorgić B, Dimitrijević L, Lambeck J, Aleksandrović M, Okicić T, Madić D. Effects of aquatic programs in children and adolescents with cerebral palsy: systematic review. Sport Sci. 2012;5:49-56. [Google Scholar]
- 15. Franki I, Desloovere K, De Cat J, et al. The evidence-base for basic physical therapy techniques targeting lower limb function in children with cerebral palsy: a systematic review using the International Classification of Functioning, Disability and Health as a conceptual framework. J Rehabil Med. 2012;44:385-395. [DOI] [PubMed] [Google Scholar]
- 16. Fragala MA, Goodgold S, Dumas HM. Effects of lower extremity passive stretching: pilot study of children and youth with severe limitations in self-mobility. Pediatr Phys Ther. 2003;15:167-175. [DOI] [PubMed] [Google Scholar]
- 17. Pin T, Dyke P, Chan M. The effectiveness of passive stretching in children with spastic cerebral palsy. Dev Med Child Neurol. 2006;48:855-862. [DOI] [PubMed] [Google Scholar]
- 18. Hadden KL, von Baeyer CL. Pain in children with cerebral palsy: common triggers and expressive behaviours. Pain. 2002;99:281-288. [DOI] [PubMed] [Google Scholar]
- 19. Getz M, Hutzler Y, Vermeer A. Effects of aquatic interventions in children with neuromotor impairments: a systematic review of the literature. Clin Rehabil. 2006;20:927-936. [DOI] [PubMed] [Google Scholar]
- 20. Fragala-Pinkham M, Haley SM, O’Neil ME. Group aquatic aerobic exercise for children with disabilities. Dev Med Child Neurol. 2008;50:822-827. [DOI] [PubMed] [Google Scholar]
- 21. Declerck M. Effect of Aquatic Intervention on the Gross Motor Function and Quality of Life of Children with Cerebral Palsy [thesis]. Edinburgh, Scotland: University of Edinburgh; 2010. [Google Scholar]
- 22. Blohm D. Effectiveness of aquatic interventions for children with cerebral palsy: systematic review of current literature. J Aquatic Phys Ther. 2011;19:19-29. [Google Scholar]
- 23. Cole A, Becker B. Comprehensive Aquatic Therapy. 2nd ed. Philadelphia, PA: Elsevier; 2004. [Google Scholar]
- 24. Kelly M, Darah J. Aquatic exercise for children with cerebral palsy. Dev Med Child Neurol. 2005;47:838-842. [DOI] [PubMed] [Google Scholar]
- 25. Russell DJ, Rosenbaum PL, Avery LM, Lane M. Gross Motor Function Measure (GMFM-66 and GMFM-88) User’s Manual. London, England: MacKeith Press; 2002. [Google Scholar]
- 26. Salem Y, Godwin EM. Effects of task-oriented training on mobility function in children with cerebral palsy. Neurorehabilitation. 2009;24:307-313. [DOI] [PubMed] [Google Scholar]
- 27. El-Maksoud GMA, Sharaf MA, Rezk-Allah SS. Efficacy of cold therapy on spasticity and hand function in children with cerebral palsy. J Adv Res. 2011;2:319-325. [Google Scholar]
- 28. Chrysagis DN, Douka A, Nikopoulos M, Apostolopoulou F, Koutsouki D. Effects of an aquatic program on gross motor function of children with spastic cerebral palsy. Biol Exerc. 2009;5:13-25. [Google Scholar]
- 29. Park ES, Park CI, Lee HJ, Cho YS. The effect of electrical stimulation on the trunk control in young children with spastic diplegic cerebral palsy. J Korean Med Sci. 2001;16:347-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Declerck M. Swimming and the Physical, Social and Emotional Well-Being of Youth With Cerebral Palsy [thesis]. Edinburgh, Scotland: University of Edinburgh; 2014. [Google Scholar]
- 31. Nottidge VA, Okogbo ME. Cerebral palsy in Ibadan, Nigeria. Dev Med Child Neurol. 1991;33:241-245. [DOI] [PubMed] [Google Scholar]
- 32. Iloeje SO, Ejike-Orji I. Compliance by cerebral palsy (CP) patients attending a child neurology service in a developing country: a preliminary study. West Afr J Med. 1993;12:1-5. [PubMed] [Google Scholar]