Abstract
Background/Methods:
This study determines the co-expression of mammaglobin-A, vascular endothelial growth factor receptor-3 (VEGFR3) and Ki67 by immunohistochemistry (IHC) in tissue samples from 80 patients undergoing breast surgery (cancer or benign disease). The tissue expression was compared with the tumour histopathology and Kaplan Meier 5-year survival analysis was performed.
Results:
Positive breast tissue expression was observed in 53% samples for mammaglobin, 41% Ki67 and 65% VEGFR3 with a significant positive correlation between Ki67 and VEGFR3 co-expression. Ki67 and VEGFR3 expression correlated with the breast tumour grade and Ki67 expression also correlated with oestrogen receptor (ER) status. At 5 years post-operatively, 6/80 patients had died and 3 patients were alive but had cancer recurrence. High Ki67 expression significantly correlated with poor survival (disease-free and overall).
Conclusions:
In this study, VEGFR3 and Ki67 expression but not mammaglobin-A correlated with breast tumour pathology. Positive Ki67 expression was also associated with a poor 5-year survival outcome.
Keywords: Mammaglobin, breast cancer, Ki67, vascular endothelial growth factor (VEGF)
Introduction
Breast cancer is the most common cancer in the United Kingdom accounting for 15% of all cancers and worldwide, over 1.38 million women are diagnosed per year.1 Despite an increase in breast cancer incidence, the survival rates for breast cancer are actually improving.2
Breast tumour invasion and metastasis involves a complex cascade of events including angiogenesis, local invasion, intravasation, survival of circulating tumour cells in blood and lymphatic vessels, extravasation and growth at the secondary site, usually lymph and bone. Each stage involves numerous biological factors and research continues to determine the expression of these and new biological factors and whether they have diagnostic or prognostic potential.
Human mammaglobin was first identified in 1996 of which there are two isotypes, mammaglobin-A and mammaglobin-B, these share homology but demonstrate different expression/function.3 Mammaglobin-A is predominantly expressed in breast tissue,3 demonstrates positive immunohistochemical staining in up to 80% breast tumours, and this expression in breast tumours is up to 10-fold greater than that in normal breast tissue.3,4 Due to this, it has been proposed that mammaglobin-A could be a potential molecular diagnostic marker for breast cancer. In comparison, mammaglobin-B has been found to be expressed in endometrial cancer, salivary glands, and gastrointestinal cancers.5,6
While the majority of previous research has determined the levels of mammaglobin-A mRNA in peripheral blood from patients with breast cancer using reverse transcriptase polymerase chain reaction,7–9 little has been elucidated regarding the tumour protein expression. Three previous immunohistochemical studies have shown a correlation between high levels of mammaglobin-A protein expression with oestrogen receptor (ER) and progesterone receptor (PR) status, low tumour grade and a lack of axillary node invasion.10–12 These results suggest that less aggressive tumours are associated with overexpression of mammaglobin-A in breast cancer.
The only previous studies looking at the relationship between mammaglobin-A expression and survival have been in circulating tumour cells in peripheral blood and the results have been conflicting.7–9 Tumour cells require new blood vessels (angiogenesis) and lymphatic vessels (lymphangiogenesis) to facilitate growth and encourage metastasis or else they become necrotic and apoptotic,13 and this is true of breast cancer.14,15 The vascular endothelial growth factor (VEGF) family is involved in both tumour angiogenesis and lymphangiogenesis16–19 and consists of several VEGF factors (VEGF-A, -B, -C, and -D and placental growth factor). These VEGF factors bind with varying specificities to three endothelial transmembrane tyrosine kinase receptors known as VEGFR1, VEGFR2 and VEGFR3.16
The roles of the VEGF factors have been extensively studied in breast and other cancers; however, few previous studies have determined the expression of the receptors. VEGF-C and VEGF-D bind to VEGFR3 and have been shown to control lymphangiogenesis.13,17,20–23 Previous studies have shown that VEGFR3 expression was higher in breast tumour tissue when compared to normal breast tissue.24 VEGFR3 has been shown to promote lymphangiogenesis, angiogenesis, tumour cell proliferation, motility, and survival in breast and other cancers.17,24,25 In addition, it has been suggested that VEGFR3 functions as a survival signal in early breast cancer26 and may have independent angiogenic activities.27,28
The Ki67 antigen was identified in 1983 by Gerdes and colleagues and is now known to be expressed in all cycling cells in varying amounts depending on the cell-cycle phase.29 Ki67 is the most common immunohistochemical marker of cell proliferation30 and levels have been repeatedly shown to have a strong correlation with tumour grade in breast and other cancers.29 In addition, high Ki67 levels have been shown to be a marker of poor prognosis.31 The Ki67 Labelling Index (LI) is the percentage of Ki67-positive tumour cells and this has been shown to predict treatment response.32,33 However, Ki67 is still not recommended for use in routine diagnostic laboratories for breast cancer due to the lack of standardisation and guidelines in the methodology.34
In summary, the expression of mammaglobin-A, VEGFR3 and Ki67 are potential markers that are associated with different breast cancer stages and therefore the likely prognosis. Mammaglobin-A is associated with low-grade breast tumours and benign breast tissue and therefore associated with a good prognosis. Ki67 is a marker of poor prognosis and is associated with high grade tumours that are locally aggressive. VEGFR3 is thought to be associated with lymphoangiogenesis and therefore would be expected to signify the metastatic behaviour of the tumour.
While all three factors have been studied individually in breast cancer, no previous study has looked at the co-expression of these factors. Therefore, this study aims to determine whether there is an association between mammaglobin-A expression and VEGFR3 or Ki67 expression and whether the expression of any of these factors is associated with pathology, disease-free and/or overall 5-year survival in breast cancer patients.
Methods
Eighty patients who had undergone breast surgery for either breast cancer or benign disease at University Hospital of North Tees between October 2007 and June 2010 were selected to allow pathological representation across the sample. The study had ethics approval and all patients had given informed consent.
Immunohistochemical analysis
Formalin fixed paraffin embedded tissue samples were retrieved from the histology tissue archives. Five 4-µm sections were cut using a microtome and were mounted on charged slides, allowed to air dry and then placed into a 70oC oven overnight.
IHC was performed on a Benchmark XT automated staining machine (Ventana, Arizona, USA) using a biotin-free technique with the following primary antibodies; a rabbit monoclonal antibody raised against mammaglobin-A (Clone 31AF; Cell Marque), a mouse monoclonal antibody against VEGFR3 (clone KLT9; (Leica Biosystems, Newcastle-upon-Tyne, UK) diluted to 1:75 or a rabbit monoclonal primary antibody raised against Ki67 (clone – 30-9) (Ventana, Arizona, USA).
The tissue sections were deparaffinised and rehydrated using EZ Prep and Reaction Buffer (Ventana, Arizona, USA). These were then immersed in a pH 8.4 ethylenediaminetetraacetic acid (EDTA) buffer at 97°C for heat-mediated antigen retrieval for either 90 minutes, 8 minutes, or 60 minutes for mammaglobin, VEGFR3, or Ki67, respectively. All slides were then incubated for 4 minutes in 3% hydrogen peroxide (H2O2) before being incubated with the primary antibody; for 56 minutes for the mammaglobin, 40 minutes for VEGFR3, or 8 minutes for Ki67, all at 37°C.
Sections were then treated for 8 minutes with ultraViewMultimerIg (Ventana, Arizona, USA), which is the secondary antibody labelled with horseradish peroxidase (HRP), following by 3,3’ diaminobenzidine (DAB) chromogen for a further 8 minutes and then H2O2 for another 8 minutes. UltraView Copper reagent (Ventana, Arizona, USA) was then added for 4 minutes to enhance the DAB. The slides were then counterstained in haematoxylin and bluing reagent (Ventana, Arizona, USA) for 4 minutes each.
Positive and negative controls
For mammaglobin, a composite control block was used containing negative, weak positive and strong positive breast tumour tissue. For VEGFR3, human placental tissue was used as a positive control and human appendix tissue was used as a positive control for Ki67 both as per the manufacturer’s guidelines. Negative controls were carried out by omitting the primary antibody on the known positive tissue for all factors.
Evaluation of IHC
The evaluation of the immunohistochemical staining was performed by two independent clinical scientists on a double header microscope (Nikon OPTIPHOT-2) and a consensus was reached on each slide. Ten high power (x400) fields were selected and at least 500 tumour cells were counted per slide. Both of the scorers were blind to the clinical information of each sample.
In order for comparisons to be made with previous immunohistochemical results, the same scoring systems for positive staining were used as outlined below. For mammaglobin-A, positivity was denoted by >10% of the tumour/lesional cells showing mammaglobin-A positivity as observed by cytoplasmic staining. VEGFR3 tissue expression was described as negative where there was an absence or weak positive expression (<10% of cytoplasm of tumour cells) and positive for moderate (>10% but <50%) or strong positive (>50%) as previously described.19 Ki67 expression was scored as a percentage of positive nuclear stained tumour cells (0%-100%), regardless of staining intensity whereby ⩽10% positive cells was evaluated as low Ki67 expression and >10% as high Ki67 expression as previously described.35,36
The receptor status for PR, ER, and HER2 was determined for invasive tumours by IHC. ER and PR were reported using the Allred approach, which takes into account the percentage of actual tumour cells staining positively by IHC; from no cells scoring 0 to all cells scoring 5. It also determines the intensity of staining in these cells, from very low intensity scoring 0 to high intensity scoring 3. The two scores are added together to give a total positive score of 8/8. Scores 0 to 2 are deemed negative, 3 to 4 weak positive, and 5 to 8 strong positive.
HER2 scoring takes into account any membrane staining on the tumour cells. The four scores for IHC are 0 (no membrane staining), 1+ faint/ partial staining, 2+ weak to moderate complete membrane staining in greater than 10% tumour cells, and 3+ tumours exhibit strong, complete membrane staining in greater than 10% tumour cells. Tumours scoring 0 and 1+ are deemed negative, 3+ are positive, and 2+ require further testing by FISH to establish HER2 status.
Clinical and pathological parameters
The tissue expression of these factors was correlated with the following clinical and pathological parameters; histological grade, tumour size, receptor status (ER, PR and HER2 where available), metastatic status and tumour subtype. Five-year survival status for each patient was determined by hospital systems and or reviewing patient notes.
Statistical analysis
The relationship between the expression of mammaglobin-A, Ki67, VEGFR3 and the clinical/pathological parameters was evaluated using Pearson’s chi-squared (χ2) test and Fisher’s exact probability test when n < 5. Five-year survival analysis was determined by Kaplan Meier for both disease-free and overall survival and the log-rank test was used to determine difference in survival for each factor. All statistical analysis was performed using SPSS v.21.0 (IBM-Corporation, Illinois, Chicago). A P-value of <.05 was considered statistically significant.
Results
Demographics
Eighty women undergoing surgery for breast cancer or benign disease were recruited, and they had a median age at surgery of 60.5 years old with a range from 23 to 82 years old. The surgical procedures included wide local excisions and mastectomies with or without lymph node sampling. There were 46 left-sided and 34 right-sided breast procedures.
The pathological and clinical characteristics of the 80 breast tumours are summarised in Table 1. The most frequent tumour subtype was invasive ductal carcinoma and the mean tumour size was 21.8 mm (±15 mm), with a range of 5 to 100 mm. There were 7 benign cases, 8 patients with DCIS, 20 grade 1, 23 grade 2, and 22 grade 3 breast tumours. Lymph nodes were sampled in 65 patients and in 23 (36%) patients, lymphatic metastasis was present at surgical resection.
Table 1.
Pathological and clinical characteristics of the breast tissue samples (where available).
| Characteristics | Number (%) | |
|---|---|---|
| Tumour subtype (n = 80) | Benign | 7 (9%) |
| DCIS | 8 (10%) | |
| Invasive ductal | 37 (46%) | |
| Invasive lobular | 7 (9%) | |
| Invasive tubular | 12 (15%) | |
| Invasive other | 9 (11%) | |
| Tumour size (n = 72) | Tis | 7 (10%) |
| T1 | 39 (54%) | |
| T2 | 24 (33%) | |
| T3 | 2 (3%) | |
| Tumour grade (n = 65) | Grade 1 | 20 (31%) |
| Grade 2 | 23 (35%) | |
| Grade 3 | 22 (34%) | |
| Positive receptor status | ER status (n = 64) | 49 (77%) |
| PR status (n = 59) | 43 (73%) | |
| HER2 (n = 64) | 16 (25%) | |
| Lymphatic metastasis (n = 65) | N0 | 42 (65%) |
| N1 | 17 (26%) | |
| N2 | 4 (6%) | |
| N3 | 2 (3%) | |
DCIS: ductal carcinoma in situ.
Immunohistochemical staining
The oestrogen (ER), progesterone (PR), and HER2 receptor status is summarised in Table 1 when this was reported by pathology. Positive mammaglobin-A and VEGFR3 expression was observed in 52.5% (42/80) and 65% (52/80), respectively. In all, 43% (34/80) of samples had a high Ki67 Labelling Index (LI) as determined by the cut-off point of 10%. Examples of positive staining for each factor are shown in Figures 1 and 2.
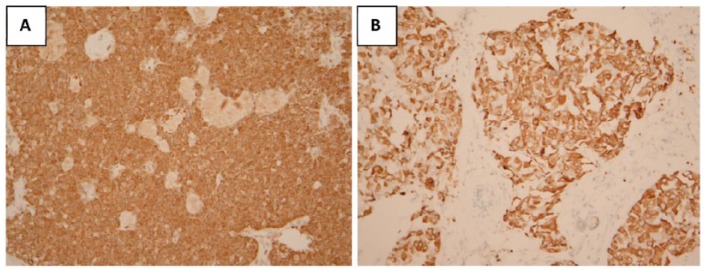
Figure 1.
Examples of positive mammaglobin immunohistochemical staining (x100) in (A) DCIS and (B) invasive ductal carcinoma. DCIS indicates ductal carcinoma in situ.
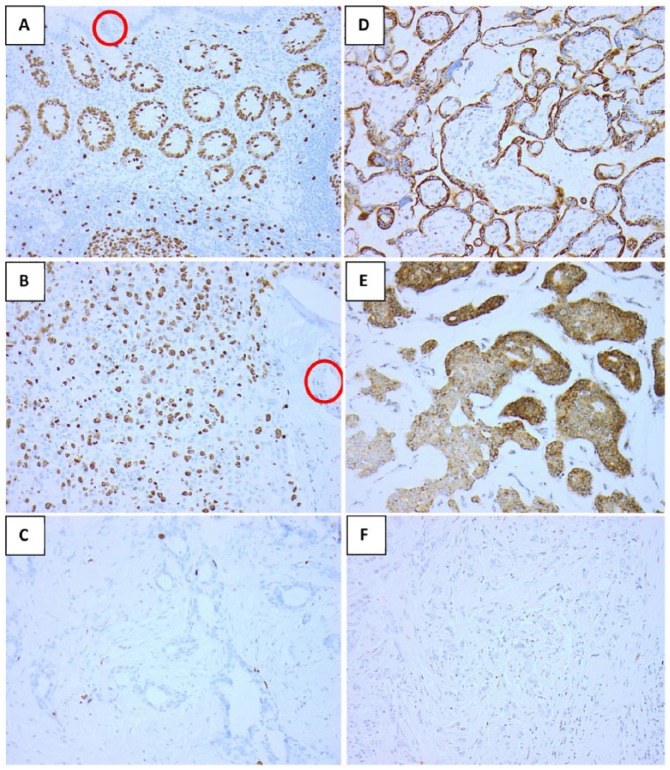
Figure 2.
Examples of immunohistochemical staining for Ki67 and VEGFR3: (A) positive Ki67 control of normal human appendix, with internal negative control of the lumen (circled), (B) high Ki67 in breast cancer with internal negative control of the blood vessel circled and (C) low Ki67 in breast cancer, (D) positive VEGFR3 control of normal human placenta (cytoplasmic staining), (E) positive VEGFR3 and (F) negative VEGFR3 in two breast cancer patient samples (all x200). VEGFR3 indicates vascular endothelial growth factor receptor-3.
The association between immunohistochemical staining between the factors is summarised in Table 2; no association was found between mammaglobin-A tissue expression with either VEGFR3 or Ki67 positivity. However, there was a significant association between VEGFR3 expression and Ki67 expression, with high Ki67 LI correlating to positive VEGFR3 expression.
Table 2.
Relationship between immunohistochemical staining and pathological status.
| Ki67 (P-value) | VEGFR3 (P-value) | Mammaglobin A (P-value) | |
|---|---|---|---|
| Tumour grade | <.001* | .002* | .865 |
| ER | .187 | .195 | .365 |
| PR | .424 | .353 | .220 |
| HER2 | .665 | .353 | 1.000 |
| Lymph node involvement | .045* | .358 | 1.000 |
| Ki67 | – | .037* | .768 |
| VEGFR3 | .037* | – | .925 |
| Mammaglobin A | .768 | .925 | – |
ER: oestrogen receptor; PR: progesterone receptor; VEGFR3: vascular endothelial growth factor receptor-3.
P < .05.
Breast Tissue Expression and Tumour Pathology
The correlations between the immunohistochemical staining of each factor with tumour pathology are summarised in Table 2. The expression of both Ki67 and VEGFR3 was significantly associated with tumour grade in this study (P < .001 and P = .002 respectively), with high Ki67 LI and positive VEGFR3 expression found in the higher tumour grades (Figure 3). High Ki67 LI also significantly correlated with the presence of lymphatic invasion at surgical resection. There were no significant associations found between Ki67, mammaglobin-A or VEGFR3 expression with tumour receptor status (Table 2).
Figure 3.
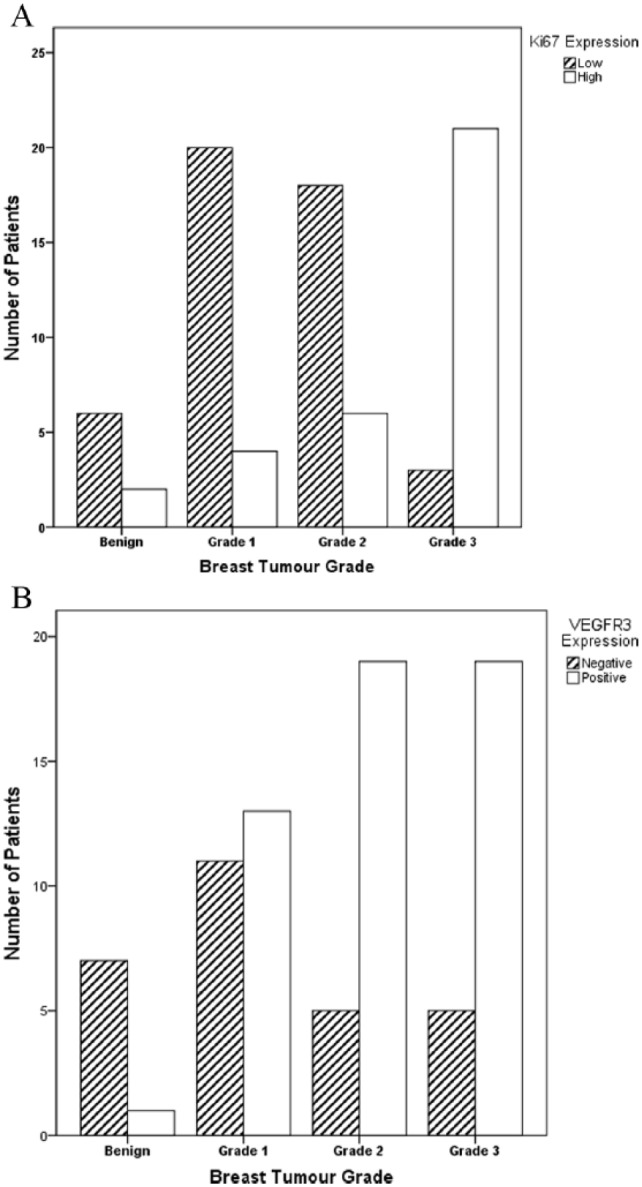
Bar charts illustrating the correlation between (A) Ki67 and (B) VEGFR3 expression with breast tumour grade. VEGFR3 indicates vascular endothelial growth factor receptor-3.
Post-operative treatment
Eight patients went on to have further surgery on the same breast due to incomplete tumour resection margins. The 73 patients with either DCIS or invasive cancer had various post-operative therapies which are summarised in Figure 4.
Figure 4.
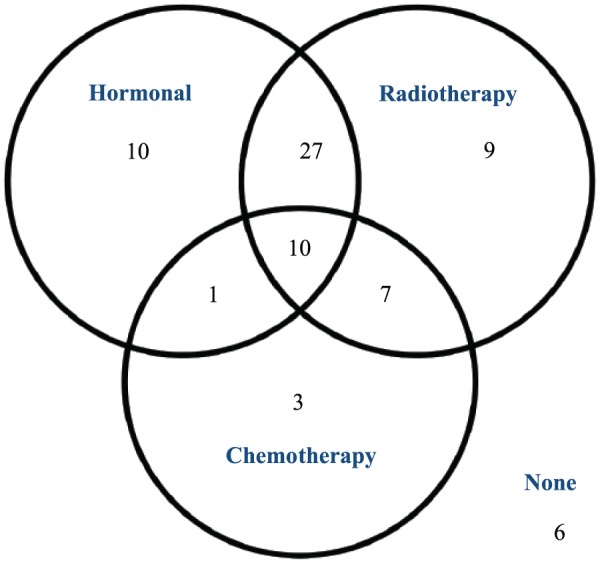
Post-operative therapies for DCIS and cancer patients. DCIS indicates ductal carcinoma in situ.
Survival analysis
At 5 years post-operatively, the disease status of the study patients was 6 patients had died, 3 others were alive but with cancer recurrence and the remaining 71 patients were alive and well. Kaplan Meier 5-year survival analysis was performed for both disease-free and overall survival for the expression of the studied factors.
There was no association between mammaglobin-A or VEGFR3 tissue expression with 5-year survival; however, high Ki67 expression significantly correlated with poor survival for both disease-free and overall 5-year survival (Figure 5).
Figure 5.
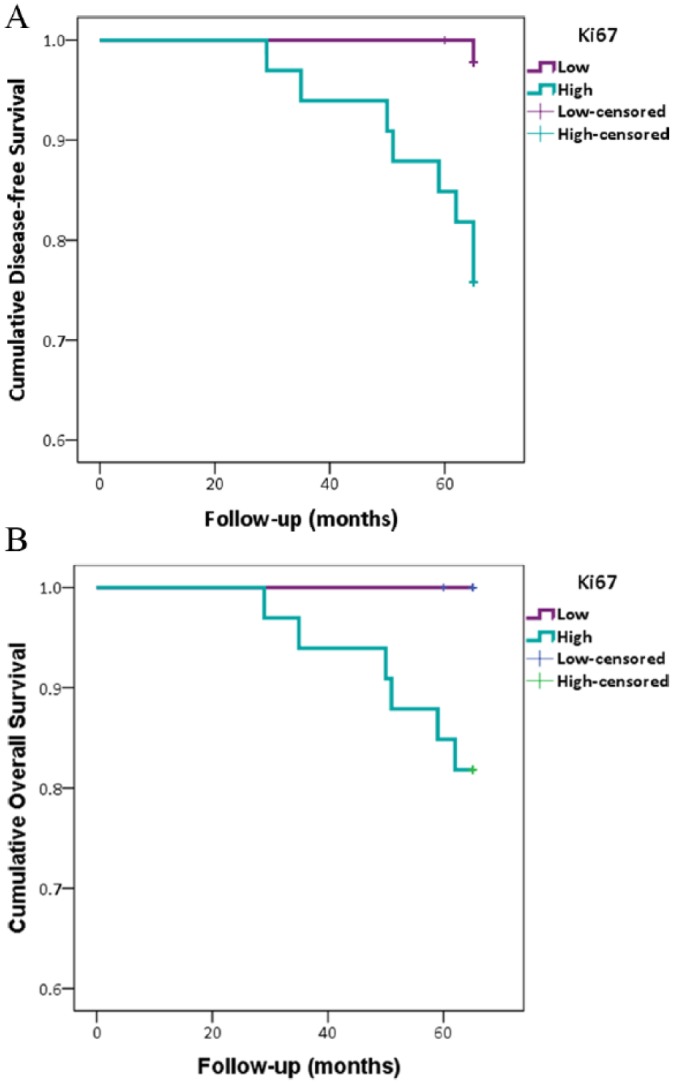
High Ki67 labelling index was associated with poor survival. Kaplan Meier curves illustrating Ki67 expression with (A) disease free survival (P = .002) and (B) overall 5-year survival.
Discussion
Previous studies have looked at the expression of mammaglobin-A, VEGFR3, and Ki67 individually in breast cancer; however, to our knowledge, this is the first study to look at the association between these three factors in the same samples.3,4,17,34
Positive mammaglobin-A expression was only found in 53% of breast samples studied, which is lower than some previous studies that have reported expression in as high as 80% of breast tumour samples; however, the higher levels quoted were looking at mammaglobin-A mRNA expression rather than the protein.3,4
As a result of mammaglobin-A being specifically expressed in breast tissue,4 it has been proposed as a potential molecular diagnostic marker for breast cancer. Previous immunohistochemical studies have shown a correlation between high levels of mammaglobin and the expression of ER and PR, low levels of Ki67, low tumour grade, and a lack of axillary node invasion.10,11 Mammaglobin-A expression has also been shown to negatively correlate with axillary node metastasis.11 These results suggest that less aggressive tumours are associated with overexpression of mammaglobin in breast cancer. In this study, no association was found between mammaglobin expression and tumour grade, receptor status, or tumour pathology, which is in contrast to these previous studies.10,11
Positive VEGFR3 expression was observed in 65% breast samples studied which is in agreement with one previous study23 but higher than another (48%).25 VEGFR3 expression was significantly associated with the breast tumour grade, and this is consistent with previous results whereby VEGFR3 was shown to affect tumour angiogenesis, which is associated with higher grade tumours.16,24,25 However, VEGFR3 was not significantly associated with metastasis, but this is still in agreement with a previous study.25 Despite VEGFR3 promoting lymphangiogenesis, positive expression was not associated with positive lymph node status in this study and this confirms previous results.25
In this study, 43% tumour samples had high Ki67 LI. Previous studies have shown Ki67 to be expressed in low levels in healthy normal breast tissue with Ki67 expression progressively increasing from benign to DCIS to invasive breast cancer.37 Ki67 was significantly associated with tumour grade in this study, with high Ki67 LI being found in the higher tumour grades. This corroborates with other studies on Ki67 in breast,23 with high proliferation being found in high-grade tumours. There was also a significant association between Ki67 and the presence of metastasis which again has been shown in previous studies.35,37,38
In this study, no significant associations were found between any of the studied factors with receptor status (ER, PR or HER2). For mammaglobin-A expression, this conflicts with previous studies that have shown a significant association between mammaglobin-A expression with both ER and PR status.10,11 Some previous studies have also found an inverse relationship between high Ki67 expression with ER and PR positive tumours, with ER and PR positivity being shown in the least proliferating tumours.37 No previous studies were found comparing VEGFR3 expression and receptor status in breast cancer.
There is limited information on the association between the tissue expressions of the three studied factors in breast cancer. This study found no association between positive mammaglobin-A expression with either VEGFR3 or Ki67 expression. The only previous study comparing mammaglobin and VEGFR3 expression did find a significant correlation but did not state whether this was positive or negative.12 However, based on previous results on the individual expression of these factors with breast tumour grade, mammaglobin-A and VEGFR3 expression may demonstrate a negative correlation due to their opposing association with breast tumour grade.
One previous study compared mammaglobin and Ki67 expression and found a significant correlation of high mammaglobin to low Ki67 LI which was attributed to mammaglobin being found in less aggressive phenotypes.11 There was a significant association found between VEGFR3 expression and Ki67 expression in this study, with high Ki67 LI correlating to positive VEGFR3 expression. This could be because tumours with high cell proliferation also show increased levels of angiogenesis and lymphangiogenesis. Previous studies have found VEGF expression to correlate with Ki67, but none have looked at VEGFR3 and Ki67.
There are a number of reasons for discrepancies in results between studies some of which will be discussed below. There is the potential for numerous technical differences between immunohistochemical procedures, for example, use of different primary antibodies with varying specificities, the laboratory procedure/protocol and how positive staining for a particular factor was defined.11 The main reason Ki67 is not recognised as a prognostic marker of breast cancer despite the significant association with both pathology and survival is due to a lack of standardisation in the methodology. This is down to inter-laboratory and inter-observer differences from the handling of the tissue, the antibody, and immunohistochemical protocol used to the selection of the tumour area to be counted and differences in the cut off determined for positive staining.37 This lack of standardisation is also likely with the other factors.
Another reason may be down to differences in sample sizes, as the present study was relatively small10,11 and the actual tissue samples studied, for example, whether normal breast tissue samples and benign cases were included.11
There have been limited previous studies determining the association between mammaglobin-A, VEGFR3, or Ki67 with survival following breast cancer diagnosis. In this study, Ki67 was the only factor found to correlate with both disease-free and overall 5-year survival. Previous results looking at the association between Ki67 and survival have been conflicting. A meta-analysis demonstrated a significant correlation between Ki67 and overall survival whereas the association with disease-free survival was marginal.39 However, another study found no correlation with either overall or disease-free survival.40
For mammaglobin, the majority of previous studies have been RNA expression in circulating breast tumour cells in peripheral blood rather than protein expression in breast tissue. Two of these studies found a significant association between peripheral blood mammaglobin positivity and shorter disease-free survival,7,8 and another have found no correlation.9 One previous immunohistochemical study found a negative mammaglobin expression correlated with prolonged survival.12 To our knowledge, no previous studies have determined the association between VEGFR3 and survival.
Conclusion
This is the first study to compare the co-expression of mammaglobin-A with VEGFR3 and Ki67 expression in breast cancer. Further understanding of how mammaglobin-A interacts with other markers could help identify whether mammaglobin-A itself can be used as a diagnostic tool. This study found positive VEGFR3 and Ki67 expression correlated with breast tumour pathology; however, no associations were found with mammaglobin-A. In addition Ki67 expression was found to correlate with both 5-year disease-free and overall survival analysis. Further research is needed to determine the interactions between these factors and it may be that with a larger sample size, stronger associations are found both between the co-expression of these factors and with pathology/survival.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded from a grant from the Kay Smith Fund.
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author’s Note: This manuscript is an original research article to determine the patho-biological expression of three factors that may be involved in breast tumour invasion and metastasis. The tissue expression of these factors was correlated with the tumour histopathology to see if they may be useful markers of breast cancer progression. Five-year survival analysis was also performed to see if the tissue expression could help predict prognosis and or breast tumour recurrence or metastasis.
Author Contributions: E.B. and P.B. conceived and designed the experiments. E.B. and N.W. analysed the data. E.B. and N.W. wrote the first draft of the manuscript. E.B. and N.W. contributed to the writing of the manuscript. E.B., N.W., L.H., J.F., D.W., and P.B agreed with manuscript results and conclusions. E.B. and N.W. jointly developed the structure and arguments for the paper. E.B. Made critical revisions and approved final version. All authors reviewed and approved of the final manuscript.
Ethical Approval: The study had ethics approval and all patients gave consent for their tissue and data to be used in research.
References
- 1. Cancer Research UK. Breast cancer statistics. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer#heading-Zero. 2013. Accessed June 17, 2019.
- 2. Cancer Research UK. Breast cancer survival statistics. http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer/survival#heading-Two. 2014. Accessed September 14, 2016.
- 3. Watson MA, Fleming TP. Mammaglobin, a mammary-specific member of the uteroglobin gene family, is overexpressed in human breast cancer. Cancer Res. 1996;56:860–865. [PubMed] [Google Scholar]
- 4. Tanaka Y, Amos KD, Fleming TP, et al. Mammaglobin A is a tumour-associated antigen in human breast carcinoma. Surgery. 2003;133:74–80. [DOI] [PubMed] [Google Scholar]
- 5. Tassi RA, Bignotti E, Falchetti M, et al. Mammaglobin B expression in human endometrial cancer. Int J Gynaecol Cancer. 2008;18:1090–1096. [DOI] [PubMed] [Google Scholar]
- 6. Aihara T, Fujiwara Y, Ooka M, Sakita I, Tamaki Y, Monden M. Mammaglobin B as a novel marker for detection of breast cancer micrometastases in axillary lymph nodes by reverse transcription-polymerase chain reaction. Breast Cancer Res Treat. 1999;58:137–140. [DOI] [PubMed] [Google Scholar]
- 7. Ntoulia M, Stathopoulou A, Ignatiadis M, et al. Detection of Mammaglobin A-mRNA-positive circulating tumor cells in peripheral blood of patients with operable breast cancer with nested RT-PCR. Clin Biochem. 2006;39:879–887. [DOI] [PubMed] [Google Scholar]
- 8. Zach O, Kasparu H, Krieger O, Hehenwarter W, Girschikofsky M, Lutz D. Detection of circulating mammary carcinoma cells in the peripheral blood of breast cancer patients via a nested reverse transcriptase polymerase chain reaction assay for mammaglobin mRNA. J Clin Oncol. 1999;17:2015–2019. [DOI] [PubMed] [Google Scholar]
- 9. Marques AR, Teixeira E, Diamond J, et al. Detection of human mammaglobin mRNA in serial peripheral blood samples from patients with non-metastatic breast cancer is not predictive of disease recurrence. Breast Cancer Res Treat. 2009;114:223–232. [DOI] [PubMed] [Google Scholar]
- 10. Baker L, Hall L, France J, Noor L, Wilson D, Bhaskar P. Mammaglobin – a expression in primary and recurrent breast cancer. Eur J Surg Oncol. 2013;39:476. [Google Scholar]
- 11. Nunez-Villar MJ, Martinez-Arribas F, Pollan M, et al. Elevated mammaglobin (h-MAM) expression in breast cancer is associated with clinical and biological features defining a less aggressive tumour phenotype. Breast Cancer Res. 2003;5:R65–R70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu X-L, Huang Y, Chen P. Expression of hMAM, VEGF-C and VEGFR-3 in breast cancer tissues and its relation with prognosis. Acad J Second Milit Med Univ. 2013;34:1078–1082; Abstract Only (in Chinese). [Google Scholar]
- 13. McMahon G. VEGF receptor signaling in tumor angiogenesis. Oncologist. 2000;5:3–10. [DOI] [PubMed] [Google Scholar]
- 14. Kubota Y. Tumor angiogenesis and anti-angiogenic therapy. Keio J Med. 2012;61:47–56. [DOI] [PubMed] [Google Scholar]
- 15. Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis – correlation in invasive breast carcinoma. New Eng J Med. 1991;324:1–8. [DOI] [PubMed] [Google Scholar]
- 16. Lohela M, Bry M, Tammela T, Alitalo K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol. 2009;21:154–165. [DOI] [PubMed] [Google Scholar]
- 17. Mylona Alexandrou P, Mpakali A, Giannopoulou I, et al. Clinicopathological and prognostic significance of vascular endothelial growth factors (VEGF)-C and -D and VEGF receptor 3 in invasive breast carcinoma. Eur J Surg Oncol. 2007;33:294–300. [DOI] [PubMed] [Google Scholar]
- 18. Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. [DOI] [PubMed] [Google Scholar]
- 19. Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- 20. Laakkonen P, Waltari M, Holopainen T, et al. Vascular endothelial growth factor receptor 3 is involved in tumor angiogenesis and growth. Cancer Res. 2007;67:593–599. [DOI] [PubMed] [Google Scholar]
- 21. Skobe M, Hawighorst T, Jackson DG, et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192–198. [DOI] [PubMed] [Google Scholar]
- 22. Veikkola T, Jussila L, Makinen T, et al. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. EMBO J. 2001;20:1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Raica M, Cimpean AM, Ceasus R, Ribatti D. Lymphatic microsvessel density, VEGF-C, and VEGFR-3 expression in different molecular types of breast cancer. Anticancer Res. 2011;31:1757–1764. [PubMed] [Google Scholar]
- 24. Kurenova EV, Hunt DL, He D, et al. Vascular endothelial growth factor receptor-3 promotes breast cancer cell proliferation, motility and survival in vitro and tumor formation in vivo. Cell Cycle. 2009;8:2266–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Filho AL, Martins A, Araújo Costa SM, Schmitt FC. VEGFR-3 expression in breast cancer tissue is not restricted to lymphatic vessels. Pathol Res Pract. 2005;201:93–99. [DOI] [PubMed] [Google Scholar]
- 26. Su JL, Yang PC, Shih JY, et al. The VEGF/Flt-4 axis pormotes invasion and metastasis of cancer cells. Cancer Cell. 2006;9:209–223. [DOI] [PubMed] [Google Scholar]
- 27. Kurenova EV, Hunt DL, He D, Magis AT, Ostrov DA, Cance WG. Small molecule chloropyramine hydrochloride (C4) targets the binding site of focal adhesion kinase and vascular endothelial growth factor receptor 3 and suppresses breast cancer growth in vivo. J Med Chem. 2009;52:4716–4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haiko P, Makinen T, Keskitalo S, et al. Deletion of vascular endothelial growth factor C (VEGF-C) and VEGF-D is not equivalent to VEGF receptor 3 deletion in mouse embryos. Mol Cell Biol. 2008;28:4843–4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 2010;11:174–183. [DOI] [PubMed] [Google Scholar]
- 30. Kos Z, Dabbs DJ. Biomarker assessment and molecular testing for prognostication in breast cancer. Histopathology. 2016;68:70–85. [DOI] [PubMed] [Google Scholar]
- 31. Urruticoechea A, Smith IE, Dowsett M. Proliferation marker Ki-67 in early breast cancer. J Clin Oncol. 2005;23:7212–7220. [DOI] [PubMed] [Google Scholar]
- 32. Faneyte IF, Schrama JG, Peterse JL, Remijnse PL, Rodenhuis S, van de Vijver MJ. Breast cancer response to neoadjuvant chemotherapy: predictive markers and relation with outcome. Br J Cancer. 2003;88:406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Petit T, Wilt M, Velten M, et al. Comparative value of tumour grade, hormonal receptors, Ki-67, HER-2 and topoisomerase II alpha status as predictive markers in breast cancer patients treated with neoadjuvant anthracycline-based chemotherapy. Eur J Cancer. 2004;40:205–211. [DOI] [PubMed] [Google Scholar]
- 34. Dowsett M, Nielsen TO, A’Hern R, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011;103:1656–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tawfik K, Kimler BF, Davis MK, Fan F, Tawfik O. Ki-67 expression in axillary lymph node metastases in breast cancer is prognostically significant. Hum Pathol. 2013;44:39–46. [DOI] [PubMed] [Google Scholar]
- 36. Koda M, Sulkowska M, Kanczuga-Koda L, et al. The effect of chemotherapy on Ki-67, Bcl-2 and Bak expression in primary tumors and lymph node metastases of breast cancer. Oncol Rep. 2007;18:113–119. [PubMed] [Google Scholar]
- 37. Kontzoglou K, Palla V, Karaolanis G, et al. Correlation between Ki67 and breast cancer prognosis. Oncology. 2013;84:219–225. [DOI] [PubMed] [Google Scholar]
- 38. Pathmanathan N, Balleine RL. Ki67 and proliferation in breast cancer. J Clin Pathol. 2013;66:512–516. [DOI] [PubMed] [Google Scholar]
- 39. Stuart-Harris R, Caldas C, Pinder SE, Pharoah P. Proliferation markers and survival in early breast cancer: a systematic review and meta-analysis of 85 studies in 32,825 patients. Breast. 2008;17:323–334. [DOI] [PubMed] [Google Scholar]
- 40. Pinto AE, Andre S, Pereria T, Nobega S, Soares J. Prognostic comparative study of S phase fraction and Ki67 index in breast carcinoma. J Clin Path. 2001;54:543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]