Abstract
The association of old age and chronic conditions, such as hypertension and obesity, can lead to larger decreases in the physical capacities of elderly, compared with their healthy counterparts. Physical exercise has been demonstrated to be efficient in postponing this phenomenon, mainly strength training. However, little is known about the effect of aerobic training on this condition. The aim of this work was to investigate the impact of 12 weeks of moderate-intensity aerobic training on the physical capacities of hypertensive obese older women. Aerobic power, lower limb muscle power, upper limb muscle strength, endurance, and flexibility of 19 hypertensive obese elders were evaluated. Afterward, patients were blindly randomized into control group (CG) and exercise group (EG). EG underwent three sessions/week of 60 min of moderate-intensity aerobic training, during 12 weeks. EG showed increases in VO2max compared with CG (p = .03) and increases in flexibility compared with basal moment (+21.6%; p = .01) after 12 weeks, whereas CG did not show any significant alterations. Moderate aerobic training is capable of inducing increases in maximal aerobic power and flexibility in hypertensive obese elderly. However, other essential physical capacities associated with independence in elderly people (i.e., muscle power and strength) were not responsive to this kind of protocol.
Keywords: old age, obesity, hypertension, physical capacities, aerobic training
Introduction
Aging process free of chronic degenerative diseases, also termed senescence, is associated with morphological (e.g., muscle atrophy; Coelho Júnior et al., 2015; Janssen, Baumgartner, Ross, Rosenberg, & Roubenoff, 2004; Janssen, Heymsfield, Wang, & Ross, 2000) and neuromuscular (e.g., decrease in muscle strength [MS] and muscle power [MP]; Bean et al., 2003; Lauretani et al., 2003; Reid et al., 2012; Reid & Fielding, 2012; Sallinen et al., 2010) alterations, which lead to impairment in the general functionality of older people. Besides its association with the development of geriatric syndromes, such as sarcopenia and frailty (Coelho Júnior et al., 2015; Fried et al., 2001; Iolascon et al., 2014; Sewo Sampaio et al., 2016), decrease in physical function leads to physical disability, impairment in general functionality, loss of autonomy, and institutionalization (Algilani et al., 2014; Millan-Calenti et al., 2010; Sharma, Parashar, & Mazta, 2014; Vaitkevicius et al., 2002).
Even if aging process is an independent risk factor for the development of functional impairment, some chronic degenerative diseases (e.g., hypertension and obesity)—generally associated with aging—can act as a trophic factor and increase the magnitude of impairment of the organic system caused by aging. Indeed, evidence in the literature demonstrates that obesity and hypertension during aging can cause a decrease in the capacity to perform activities of daily living, and consequently, physical disability, cognitive impairment, balance and gait impairment, increase in fall risk, and early death (Chien & Guo, 2014; Corona et al., 2014; Deschamps et al., 2002; Hausdorff, Herman, Baltadjieva, Gurevich, & Giladi, 2003; Rosano et al., 2011).
However, physical exercise, mainly strength training (Assumpção et al., 2008), seems to be a powerful tool to increase physical capacities in older people (Prestes et al., 2015). However, just few studies have been conducted on the capacity of aerobic exercise to elicit this phenomenon, mainly in pathological populations (i.e., hypertensive obese elderly). Considering safety, moderate-intensity aerobic exercise (MIE) has been recommended to be used in the initial phases of exercise training programs designed to improve health (Eckel et al., 2014; Pescatello et al., 2004), which increase and highlight the need for studies that verify this phenomenon.
Therefore, the aim of the present study was to record the impact of 12 weeks of MIE on maximal aerobic power (i.e., VO2max), muscular flexibility, MP of lower limbs, and resistance strength of upper limbs of hypertensive obese elderly.
Materials and Methods
This is an experimental study with random distribution of participants. This study was developed in accordance with the Declaration of Helsinki, in Resolution 196/96 of the National Health Council. All subjects signed a free consent form that explained the research objectives and risks to their integrity during the study. This study was approved by Ethics Committee of the Universidade Anhanguera de Leme.
Subjects
Nineteen hypertensive obese adults comprised the sample of the present study.
The exclusion criteria were use of hormone replacement and/or psychotropic drugs, cardiovascular disease (e.g., acute myocardial infarction, stroke, peripheral arterial disease, and transient ischemic disease), pulmonary disease, neurological or psychiatric disease (e.g., Parkinson’s or Alzheimer’s disease), musculoskeletal disorders, comorbidities associated with greater risk of falls, or any condition that could impair performing the sessions of exercise and evaluations. Eligibility for the present study was based on the analysis of medical records and the presence of a clinical diagnosis of hypertension and obesity—body mass index ≥28 kg/m² (World Health Organization, 2001), being present in all sessions of exercise, and age ≥60 years. Before the beginning of the experiments, all patients underwent medical evaluation and, posteriorly, were authorized to participate in the physical exercise program. Table 1 shows the general characteristics of the patients.
Table 1.
General Characteristics of Both Groups.
| CG (n = 8; 2♂) | EG (n = 10; 2♂) | p | |
|---|---|---|---|
| Age (years) | 61.7 ± 0.8 | 60.5 ± 0.2 | .26 |
| Body mass (kg) | 91.7 ± 10.1 | 81.8 ± 4.9 | .33 |
| Height (cm) | 168 ± 0.3 | 163 ± 0.7 | .20 |
| BMI (kg/m²) | 32.5 ± 3.7 | 30.5 ± 1.5 | .56 |
| VO2max (L min–1) | 21.1 ± 10.2 | 27.3 ± 11.0 | .24 |
| Muscle power | 10.7 ± 4.5 | 13.6 ± 3.9 | .20 |
| Muscle flexibility | 11.3 ± 7.3 | 16.3 ± 9.7 | .23 |
| Muscular endurance | 13.6 ± 4.7 | 14.3 ± 7.6 | .81 |
Note. M ± SD. CG = control group; EG = exercise group; BMI = body mass index.
Procedures
After the achievement of anamnesis, body composition measurement, and physical function evaluation, patients were randomized and allocated into two groups: exercise group (EG) and control group (CG). Physical function was evaluated before and after 12 weeks. All volunteers were instructed to refrain from physical exercise for 96 hr and from drinking coffee, alcohol, and energy drinks during the 24 hr before the tests. Although alimentary ingestion was not controlled, subjects were instructed to maintain normal diet during the study period. All evaluations occurred in the morning (07:00 a.m.-10:00 a.m.; pre and post protocol) and were performed 120 hr before and after the beginning and end of the physical exercise program, respectively.
Evaluations
Determination of VO2max
The determination of VO2max was performed in two steps. A priori, after placing the heart rate monitor (Oregon®, Brazil) on the left wrist, it was required that the patients perform, as quickly as possible, four laps around the athletics track (i.e., 400 m) of the university, totalizing 1,600 m (i.e., 1 mile inland) (Almeida et al., 2010). Due to the impairment of physical function generally present in this population, patients were allowed to decrease the pace or even walk during the performance of the test. Immediately after the end of the test, the heart rate was recorded to be, posteriorly, added in the final calculation.
Afterward, the calculation proposed by Pollock e Wilmore (1993) was used for VO2max quantification, which is VO2max = 6.952 + (0.0091 × W) – (0.0257 × A) + (0.5955 × G) – (0.2240 × TT) – (0.0115 × HR), where W = weight in pounds (weight in kg × 2.205), A = age in years, G = gender (1 = male and 0 = female), TT = time expended to perform the test, and HR = heart rate immediately after the end of the test (Pollock & Wilmore, 1993). During the performance of the test, all volunteers received verbal encouragement. The final result is expressed in liters per minute.
MP of lower limbs
Countermovement jump was performed to evaluate leg power. In the initial position, the volunteers stood on a jump platform (CEFISE® model Jump System Pro), their feet remained approximately parallel to the shoulder width, and their hands rested on the hips. When instructed, the volunteers flexed their knees at approximately 90° and jumped the maximum height possible. The greatest of three attempts, which were held at 3-min intervals, was considered for final evaluation. The values are expressed in centimeters (Hakkinen, Newton, et al., 1998; Ramirez-Campillo et al., 2014).
Muscular flexibility
For evaluation of muscular flexibility, a sit and reach test was performed. In summary, patients remained seated, with extended knees and the underside of the foot in contact with the tool. After the signal from the evaluator, the patient should move the scalometer the maximum possible, but performing just a trunk flexion. To avoid excessive movement, a researcher applied slight pressure on the knees of the patients. The highest value obtained in three attempts was considered for analysis. The values are measured in centimeters (Bertolla, Baroni, Cesar, Leal, & Oltramari, 2007; Matsudo, 2005).
Muscular endurance
To evaluate muscle endurance, a push-up test was performed. A priori, patients should perform with their feet in parallel, abdominals contracted, shoulder flexed and abducted, elbows straight, and hands in full contact with the ground. After the signal, patients performed the largest number of repetitions possible in 1 min. To register the repetition, the patients should flex the elbow to ~90° and perform to full extent. Different from men—who performed the test with knees extended, without touching the floor—women remained with the knees flexed and touching the floor. During the performance of the test, patients received verbal encouragement (Pollock & Wilmore, 2009).
MIE
EG underwent 36 MIE sessions, which occurred 3 days per week, with a 48-hr minimal interval between sessions along the 12 weeks. Exercise sessions were composed of two moments: warm-up (10 min) and main part (50 min). Warm-up comprised 5-min race technical exercises (e.g., dribbling, skipping) and 5-min light jogging. The main part was characterized by 50 min of an MIE at 60% of maximal HR (HRmax). HRmax was determined by the following formula: 205 – (0.42 × age), which was proposed by Sheffield, Holt, & Reeves (1965). A cardiac monitor (Oregon®) was used to monitor and ensure the required HR. All exercise sessions occur at an athletics track of the sports laboratory.
It is important to cite that a recent article of our group showed that environment is one of the factors that can interfere with the adherence of hypertensive patients in physical exercise programs (Asano et al., 2016). Thus, all experimental sessions were held in the athletics track of the university sports lab.
CG
The CG maintained their regular habits of life during the entire study period, without engaging in physical exercise programs.
Statistical analysis
Data were analyzed by intention-to-treat analysis. Wilcoxon and Levine tests were used to calculate data normality and homogeneity, respectively. Intragroup comparisons (Pre × Post Δ%) were performed by paired Student’s t test. Comparisons between the groups (CG × EG) in the basal moment and after intervention protocol were performed by unpaired Student’s t test. Level of significance was 5% (p < .05), and all procedures were performed using Statistical Package for the Social Sciences version 20.0 (IBM Corp., Armonk, NY, USA). Effect size (ES) was defined to be medium for values of Cohen’s d >0.2 but less than <0.5, good for values between 0.5 and 0.8, and large for values ≥0.8. Level of significance was 5% (p < .05), and all procedures were performed using Statistical Package for the Social Sciences version 20.0 (IBM Corp.). The power of the sample size was determined using G*Power version 3.1.9.2 (27) for a power (β) of 0.80 and 6.0 (ES).
Results
Table 1 shows the general characteristics of both groups (i.e., CG and EG) in the basal moment. There were no differences between the groups.
Figure 1 shows the Δ(%) results of VO2max on CG and EG. The intragroup comparisons did not demonstrate significant differences in CG (–10.5 ± 15.4%; p = 15; ES = 0.19) and EG (+32.4 ± 59.0%; p = .08; ES = 0.31). However, Student’s t test showed significant differences between CG and EG after 12 weeks (p = .03).
Figure 1.
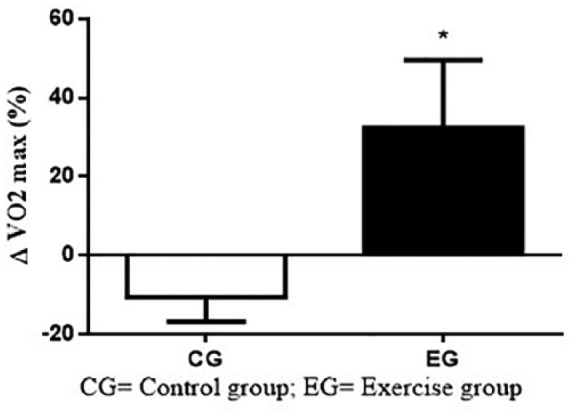
Δ(%) of VO2max on CG and EG.
Note. CG = control group; EG = exercise group.
Figure 2 shows the Δ(%) results of MP on CG and EG. The intragroup comparisons (CG = +19.3 ± 22.7%; p = 24; ES = 0.13; EG = +5.9 ± 18.6%; p = .15; ES = 0.22) and between-group comparisons (p = .19) did not show significant differences.
Figure 2.
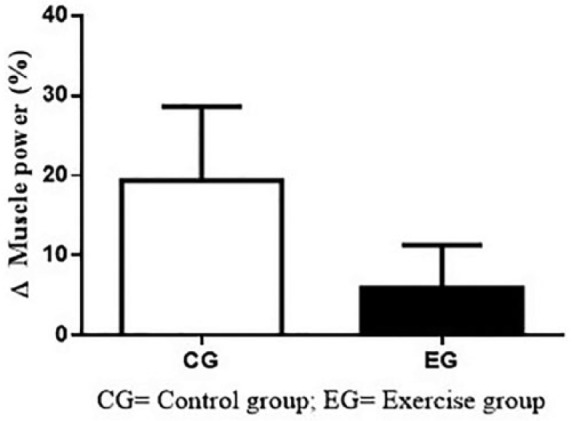
Δ(%) of muscle power on CG and EG.
Note. CG = control group; EG = exercise group.
Figure 3 shows the Δ(%) results of muscle flexibility on CG and EG. CG did not demonstrate significant differences during 12 weeks (+29.7 ± 74.3%; p = .37; ES = 0.00). However, EG showed a significant increase in flexibility after 12 weeks of MIE (+21.6 ± 24.5%; p = .01; ES = 0.39). There were no differences between groups (p = .60).
Figure 3.
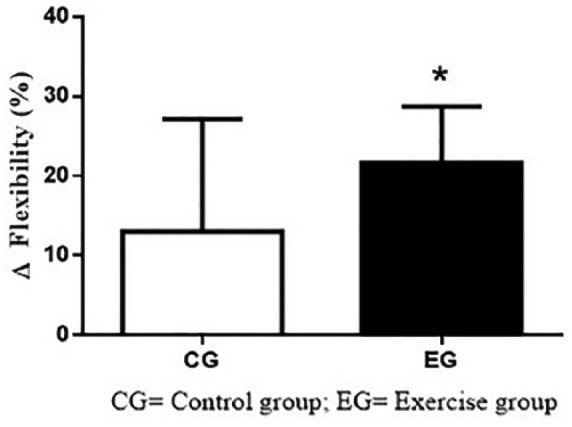
Δ(%) of muscle flexibility on CG and EG.
Note. CG = control group; EG = exercise group.
Figure 4 shows the Δ(%) results of muscular resistance on CG and EG. The intragroup comparisons (CG = +5.2 ± 16.8%; p = .46; ES = 0.23; EG = +8.6 ± 42.8%; p = .50; ES = 0.19) and between-group comparisons (p = .82) did not show significant differences.
Figure 4.
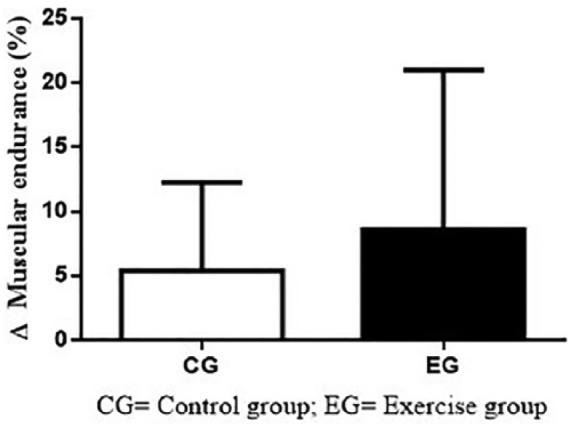
Δ(%) of muscular resistance on CG and EG.
Note. CG = control group; EG = exercise group.
Discussion
The aim of the present study was to record the impact of 12 weeks of MIE on VO2max, muscular flexibility, MP of lower limbs, and resistance strength of upper limbs in hypertensive obese elderly.
The main findings of the present study are that MIE causes a significant increase in VO2max and flexibility in hypertensive obese elderly. The cardiorespiratory function, generally evaluated by VO2max, has shown negative association with parameters associated with physiological health, as patients with maximum cardiorespiratory fitness show lower glucose levels and blood pressure values (i.e., systolic and diastolic pressure) than patients with low maximum cardiorespiratory fitness (Liu et al., 2014; Sui et al., 2012). Moreover, data from longitudinal experiments show that cardiorespiratory fitness presents a dose-dependent negative association with all-cause death, cardiovascular death, and cancer, even after adjustment for cofactors (e.g., smoking, blood glucose, and blood pressure; Blair et al., 1989).
Despite the lack of significant changes in intragroup comparisons, Cohen’s d index—which represents ES—shows medium classification to results of VO2max in EG. These results are probably due to the variation in sample results and sample size, which causes more interference in statistical test (i.e., Student’s t test) than in ES (Nakagawa & Cuthill, 2007). Indeed, the 95% confidence interval of mean to VO2max (i.e., 32.4%) changes in EG represent variation of −5.0% to 69.9%.
Evidence in the scientific literature—from experimental studies—corroborates with data of the present study and shows the effectiveness of MIE in eliciting changes in the maximal aerobic power of healthy elderly (Blumenthal et al., 1989; Hagberg et al., 1989; Kohrt et al., 1991; Park, Park, Kwon, Yoon, & Kim, 2003; Seals, Hagberg, Hurley, Ehsani, & Holloszy, 1984; Vaitkevicius et al., 2002).
Using a similar exercise approach to the present study, in 33 healthy elderly people, Blumenthal et al. (1989) conducted a 30-min MIE (70% of HR reserve) program, 3 days per week, for a period of 16 weeks. The results showed that physical exercise elicited an increase of 11.6% in maximal aerobic power in patients. These results are similar to those reported by Park et al. (2003), in which sedentary older women underwent 12 weeks of moderate aerobic training (50%-60% of HR reserve) and observed 6.2% increase in maximal aerobic power.
In one of the few studies that investigated hypertensive elderly patients—Vaitkevicius et al. (2002)—patients underwent 6 months of an MIE (~75% FCmáx) program. Elderly volunteers performed the aerobic training in a treadmill or cycle ergometer, twice a week, for 20 min. Results demonstrated that MIE elicited an increase of 6.5% in maximal aerobic power.
Even if the studies showed an increase in maximal aerobic power, there is a difference in magnitude between the aforementioned studies and the present study. Data from a meta-analytic regression, which evaluated 2,102 elderly people, showed that increase in maximal aerobic power is positively related to the frequency of sessions in the week and negatively to the initial condition of the volunteers (Huang, Gibson, Tran, & Osness, 2005). Furthermore, the volume of the session can be an independent factor associated with the magnitude of increase in maximal aerobic power (Algilani et al., 2014).
When evaluated together with the data of the present study, it is possible to infer that the high volume performed in the present study (i.e., 60 min) is the main reason for the differences in the magnitude of the increase in maximal aerobic power, in comparison with other studies. Vaitkevicius et al. (2002), for example, performed sessions with higher intensity (75% HRmax) and time of intervention (i.e., 6 months). However, the volume of the main part of the exercise session was ~12 min. Besides, despite the initial randomization of the patients, different initial levels of aerobic capacity can explain the dissimilarities and corroborate with the lack of significant changes in hypothesis test.
Another important result of the present study was the increase of 21.6% (ES, 0.39) in muscle flexibility in the EG, in conjunction with the lack of alterations in the CG. Evidence about the effect of aerobic training on muscle flexibility is still limited, mainly in elderly, collaborating to the absence of a consensus about the efficiency of this kind of intervention on physical capacity and impairing the discussion of the data (Fatouros et al., 2002; Marcinik, Hodgdon, Mittleman, & O’Brien, 1985).
In a well-designed study, Fatouros et al. (2002) studied the impact of 16 weeks of physical exercise on muscle flexibility of healthy elderly. The authors categorized volunteers to four different groups: strength training, aerobic exercise, concurrent (i.e., aerobic plus strength), and the CG. Results showed that aerobic training per se is not able to elicit significant changes in the sit and reach test. However—when the strength training was aggregated (i.e., concurrent training)—the magnitude of increase in muscle flexibility was identical (21% vs. 20%) with the strength training (Fatouros et al., 2002).
Several authors suggest that an increase in strength training—observed, predominantly, after strength training—can be one of the main factors that collaborate with an increase in muscle flexibility (Correia, Menêses, Aluísio, Cavalcante & Ritti-dias, 2014; Cyrino et al., 2004; Fatouros et al., 2002), which was shown in a systemic review (Correia et al., 2014). However, even if this physical capacity was just in part evaluated in the present study, data did not present significant alterations in the EG (+8.6%, p = .82). Besides, about the data from Fatouros et al. (2002), the researchers observed an increase in MS of the lower limbs in aerobic EG; however, this increase did not reflect in muscle flexibility (Fatouros et al., 2002).
Therefore, other mechanisms, such as increase in the synthesis of collagen, decrease in the activity of muscle spindle, and decrease in the activity of antagonist muscle (co-contraction) during muscular activity, can be associated with an increase in muscle flexibility observed in the EG (Correia et al., 2014; Fatouros et al., 2002; Hakkinen, Kallinen, et al., 1998). Anyway, it is probable that both factors are responsible for the increase in muscle flexibility.
Also, in the present study, there were no observed significant alterations in MP and muscular endurance. MP and MS have been extensively associated with the capacity of elderly population to perform the activities of daily living (i.e., get out of bed, cooking, sweeping the floor, walking to the market), corroborating strongly with them autonomy and independence, avoiding institutionalization (Lauretani et al., 2003; Reid et al., 2012; Reid & Fielding, 2012; Sallinen et al., 2010). Furthermore, maintaining MP—mainly of the lower limbs—during aging can aid in preventing falls, due its capacity to elicit fast muscle contraction (Bean et al., 2003; Izquierdo, Aguado, Gonzalez, Lopez, & Hakkinen, 1999).
In fact, evidence in the literature did not corroborate with the capacity of aerobic training to cause an increase in MS and MP in elderly (Chodzko-Zajko et al., 2009; Klitgaard et al., 1990). Klitgaard et al. (1990), for example, analyzed elderly who have been performing aerobic exercise in the last 20 years and observed that the MS of these volunteers was similar to that displayed by sedentary elderly and lower than that by sedentary young.
Since the 1970s, evidence in the literature has demonstrated the selectivity of MIE in causing adaptations of low-twitch muscle fibers, with distance runners showing a high percentage of this kind of muscle fibers (Costill et al., 1976; Hawley, 2002). This phenomenon may have collaborated with the lack of changes in physical capacities (i.e., MS and MP) observed in the present study, because increase in MS is associated with the intensity of physical training (Kalapotharakos et al., 2004; Raymond, Bramley-Tzerefos, Jeffs, Winter, & Holland, 2013; Steib, Schoene, & Pfeifer, 2010).
In the present study, the moderate intensity and long duration of physical exercise sessions lead to predominantly recruitment of type I fibers—which present low capacity to generate tension—being incapable to elicit the necessary stimulus to cause neuromuscular (e.g., increase in the recruitment of motor units, activation of agonists during concentric action) and morphological adaptations (e.g., increase in muscular cross-sectional area) associated with an increase in MS (Hawley, 2002).
Moreover, the stimulus from aerobic training was not enough to cause changes in muscular endurance. However, due the pathological condition of this group, it was thought that if aerobic training could collaborate with the improvement in physical mobility, volunteers could show an increase in the performance of the activities of daily life and, consequently, present an increase in MS. However, the experiment did not confirm this hypothesis.
Regarding MP, it is dependent on the specificity of muscle contraction, as programs aimed at increasing this physical capacity should compose concentric contractions performed as fast as possible, avoiding fatigue (Correa et al., 2012; Nogueira et al., 2009; Wallerstein et al., 2012), which did not occur in the present study. Besides, MP can also show an increase in response to improvement in MS (Fielding et al., 2002), which, newly, did not occur.
However, although other studies have been conducted in a similar way (Ramos et al., 2018) and despite the interesting results of the present study, some limitations such as the sample size—although small was believed that due to experimental design adopted the statistics used is enough to cause some inferences (Bujang & Adnan, 2016; Simel, Samsa, & Matchar, 1991; Sullivan & Feinn, 2012), the short time of intervention, the limited number of evaluations, and just one group of intervention should be considered. Such limitations also leave a path for further research that may improve future studies for this population.
Nevertheless, the present study showed that 12 weeks of moderate-intensity aerobic training performed in an open field and using a simple method to exercise prescription and control cause an increase in VO2max and muscle flexibility in hypertensive obese elderly. This result seems to be important, as both capacities are related to a positive prognosis in this population.
Conclusion
It is possible to conclude that hypertensive obese elderly who underwent 12 weeks of MIE did not show significant difference in MP and muscular resistance. But this training protocol shows a strategy to increase VO2max and muscle flexibility in this population.
Footnotes
Authors’ Note: All authors involved in this research declared the participation, as RMR; AIAM and COA for data collection, HJCJ; RYA and RCRP for data analysis, RSS; CBU; LCRL and SSA for write the rationale of the text and COA; JP and BR for revision and editing of the manuscript. In addition, all statements contained within the work are prohibited and based on scientific evidence. I am also aware that if necessary, it should be carried out to improve the work in question. Finally, I am aware of all the procedures proposed by Gerontology and Geriatric Medicine if the work in question is accepted for publication.
Ethical Approval: The Ethics Committee of the Universidade Anhanguera de Leme approved this experiment.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was fnanced in part by the Coordination of Improvement of Higher Education Personnel (CAPES-Brazil), Finance Code 001.
ORCID iDs: Hélio José Coelho-Júnior  https://orcid.org/0000-0001-7482-9514
https://orcid.org/0000-0001-7482-9514
Raul Cosme Ramos do Prado  https://orcid.org/0000-0001-7895-6019
https://orcid.org/0000-0001-7895-6019
Rodrigo Silveira da Silva  https://orcid.org/0000-0001-6330-1669
https://orcid.org/0000-0001-6330-1669
References
- Algilani S., Ostlund-Lagerstrom L., Kihlgren A., Blomberg K., Brummer R. J., Schoultz I. (2014). Exploring the concept of optimal functionality in old age. Journal of Multidisciplinary Healthcare, 7, 69-79. doi: 10.2147/JMDH.S55178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida J., Campbell C., Pardono E., da Costa Sotero R., Magalhães G., Simões H. (2010). Validade de equações de predição em estimar o VO2max de brasileiros jovens a partir do desempenho em corrida de 1.600m. Revista Brasileira de Medicina do Esporte, 16, 57-60. doi: 10.1590/S1517-86922010000100011 [DOI] [Google Scholar]
- Asano R. Y., Moraes N. F., Oliveira V. P., Martin K. K., de F. F., Leme T. M., . . . Junior H. (2016). Fatores associados a condição física ativa em pacientes com hipertensão arterial sistêmica: Um estudo transversal. Revista Brasileira De Ciência E Movimento, 24(1), 5-15. [Google Scholar]
- Assumpção C. D. O., Prestes J., Leite R. D., Urtado C. B., Neto J. B., Pellegrinotti Í. L. (2008). Efeito Do Treinamento De Força Periodizado Sobre a Composição Corporal E Aptidão Física Em Mulheres Idosas. Revista da Educação Física/uem, 19, 581-590. doi: 10.4025/reveducfis.v19i4.4014 [DOI] [Google Scholar]
- Bean J. F., Leveille S. G., Kiely D. K., Bandinelli S., Guralnik J. M., Ferrucci L. (2003). A comparison of leg power and leg strength within the InCHIANTI study: Which influences mobility more? The Journals of Gerontology, Series A: Biological Sciences & Medical Sciences, 58, 728-733. [DOI] [PubMed] [Google Scholar]
- Bertolla F., Baroni B. M., Cesar E., Leal P., Oltramari J. D. (2007). Effects of a training program using the Pilates method in flexibility of sub-20 indoor soccer athletes. Revista Brasileira de Medicina, 13(13), 198-202. doi: 10.1590/S1517-86922007000400002 [DOI] [Google Scholar]
- Blair S. N., Kohl H. W., 3rd, Paffenbarger R. S., Jr., Clark D. G., Cooper K. H., Gibbons L. W. (1989). Physical fitness and all-cause mortality. A prospective study of healthy men and women. Journal of the American Medical Association, 262, 2395-2401. [DOI] [PubMed] [Google Scholar]
- Blumenthal J. A., Emery C. F., Madden D. J., George L. K., Coleman R. E., Riddle M. W., . . . Williams R. S. (1989). Cardiovascular and behavioral effects of aerobic exercise training in healthy older men and women. Journal of Gerontology, 44(5), M147-M157. [DOI] [PubMed] [Google Scholar]
- Bujang M. A., Adnan T. H. (2016). Requirements for minimum sample size for sensitivity and specificity analysis. Journal of Clinical and Diagnostic Research, 10(10), YE01-YE06. doi: 10.7860/JCDR/2016/18129.8744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien M.-H., Guo H.-R. (2014). Nutritional status and falls in community-dwelling older people: A longitudinal study of a population-based random sample. PLoS ONE, 9(3), e91044. doi: 10.1371/journal.pone.0091044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodzko-Zajko W. J., Proctor D. N., Fiatarone Singh M. A., Minson C. T., Nigg C. R., Salem G. J., . . . Skinner J. S. (2009). American College of Sports Medicine position stand. Exercise and physical activity for older adults. Medicine and Science in Sports and Exercise, 41, 1510-1530. doi: 10.1249/MSS.0b013e3181a0c95c [DOI] [PubMed] [Google Scholar]
- Coelho Júnior H. J., Aguiar S., da S., Gonçalves I., de O., Sampaio R. A. C., . . . Asano R. Y. (2015). Sarcopenia is associated with high pulse pressure in older women. Journal of Aging Research, 2015, Article 109824. doi: 10.1155/2015/109824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona L. P., Pereira de, Brito T. R., Nunes D. P., da Silva Alexandre T., Ferreira Santos J. L., de Oliveira Duarte Y. A., Lebrao M. L. (2014). Nutritional status and risk for disability in instrumental activities of daily living in older Brazilians. Public Health Nutrition, 17, 390-395. doi: 10.1017/S1368980012005319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa C. S., LaRoche D. P., Cadore E. L., Reischak-Oliveira A., Bottaro M., Kruel L. F., . . . Pinto R. S. (2012). 3 Different types of strength training in older women. International Journal of Sports Medicine, 33, 962-969. doi: 10.1055/s-0032-1312648 [DOI] [PubMed] [Google Scholar]
- Correia M. A., Menêses A. L., Aluísio H. R. A. L., Cavalcante B., Ritti-dias R. M. (2014). Efeito do treinamento de força na flexibilidade: uma revisão sistemática. Revista Brasileira de Atividade Física & Saúde, 19, 3-11. doi: 10.12820/rbafs.v.19n1p3 [DOI] [Google Scholar]
- Costill D. L., Daniels J., Evans W., Fink W., Krahenbuhl G., Saltin B. (1976). Skeletal muscle enzymes and fiber composition in male and female track athletes. Journal of Applied Physiology, 40, 149-154. doi: 10.1152/jappl.1976.40.2.149 [DOI] [PubMed] [Google Scholar]
- Cyrino E. S., Oliveira A. R., Leite J. C., Porto D. B., Dias R. M. R., Segantin A. Q., . . . Santos V. A. (2004). Flexibility behavior after 10 weeks of resistance training. Revista Brasileira de Medicina, 10, 238-242. [Google Scholar]
- Deschamps V., Astier X., Ferry M., Rainfray M., Emeriau J. P., Barberger-Gateau P. (2002). Nutritional status of healthy elderly persons living in Dordogne, France, and relation with mortality and cognitive or functional decline. European Journal of Clinical Nutrition, 56, 305-312. doi: 10.1038/sj.ejcn.1601311 [DOI] [PubMed] [Google Scholar]
- Eckel R. H., Jakicic J. M., Ard J. D., de Jesus J. M., Houston Miller N., Hubbard V. S., . . . Tomaselli G. F. (2014). 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation, 129(25, Suppl. 2), S76-99. doi: 10.1161/01.cir.0000437740.48606.d1 [DOI] [PubMed] [Google Scholar]
- Fatouros I. G., Taxildaris K., Tokmakidis S. P., Kalapotharakos V., Aggelousis N., Athanasopoulos S., . . . Katrabasas I. (2002). The effects of strength training, cardiovascular training and their combination on flexibility of inactive older adults. International Journal of Sports Medicine, 23, 112-119. doi: 10.1055/s-2002-20130 [DOI] [PubMed] [Google Scholar]
- Fielding R. A., LeBrasseur N. K., Cuoco A., Bean J., Mizer K., Fiatarone Singh M. A. (2002). High-velocity resistance training increases skeletal muscle peak power in older women. Journal of the American Geriatrics Society, 50, 655-662. [DOI] [PubMed] [Google Scholar]
- Fried L. P., Tangen C. M., Walston J., Newman A. B., Hirsch C., Gottdiener J., . . . McBurnie M. A. (2001). Frailty in older adults: Evidence for a phenotype. The Journals of Gerontology, Series A: Biological Sciences & Medical Sciences, 56, M146-M156. [DOI] [PubMed] [Google Scholar]
- Hagberg J. M., Graves J. E., Limacher M., Woods D. R., Leggett S. H., Cononie C., . . . Pollock M. L. (1989). Cardiovascular responses of 70- to 79-yr-old men and women to exercise training. Journal of Applied Physiology, 66, 2589-2594. doi: 10.1152/jappl.1989.66.6.2589 [DOI] [PubMed] [Google Scholar]
- Hakkinen K., Kallinen M., Izquierdo M., Jokelainen K., Lassila H., Malkia E., . . . Alen M. (1998). Changes in agonist-antagonist EMG, muscle CSA, and force during strength training in middle-aged and older people. Journal of Applied Physiology, 84, 1341-1349. doi: 10.1152/jappl.1998.84.4.1341 [DOI] [PubMed] [Google Scholar]
- Hakkinen K., Newton R. U., Gordon S. E., McCormick M., Volek J. S., Nindl B. C., . . . Kraemer W. J. (1998). Changes in muscle morphology, electromyographic activity, and force production characteristics during progressive strength training in young and older men. The Journals of Gerontology, Series A: Biological Sciences & Medical Sciences, 53, B415-B423. [DOI] [PubMed] [Google Scholar]
- Hausdorff J. M., Herman T., Baltadjieva R., Gurevich T., Giladi N. (2003). Balance and gait in older adults with systemic hypertension. The American Journal of Cardiology, 91, 643-645. [DOI] [PubMed] [Google Scholar]
- Hawley J. A. (2002). Adaptations of skeletal muscle to prolonged, intense endurance training. Clinical and Experimental Pharmacology & Physiology, 29, 218-222. [DOI] [PubMed] [Google Scholar]
- Huang G., Gibson C. A., Tran Z. V., Osness W. H. (2005). Controlled endurance exercise training and VO2max changes in older adults: A meta-analysis. Preventive Cardiology, 8, 217-225. [DOI] [PubMed] [Google Scholar]
- Iolascon G., Di Pietro G., Gimigliano F., Mauro G. L., Moretti A., Giamattei M. T., . . . Brandi M. L. (2014). Physical exercise and sarcopenia in older people: Position paper of the Italian Society of Orthopaedics and Medicine (OrtoMed). Clinical Cases in Mineral and Bone Metabolism, 11, 215-221. [PMC free article] [PubMed] [Google Scholar]
- Izquierdo M., Aguado X., Gonzalez R., Lopez J. L., Hakkinen K. (1999). Maximal and explosive force production capacity and balance performance in men of different ages. European Journal of Applied Physiology and Occupational Physiology, 79, 260-267. doi: 10.1007/s004210050504 [DOI] [PubMed] [Google Scholar]
- Janssen I., Baumgartner R. N., Ross R., Rosenberg I. H., Roubenoff R. (2004). Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. American Journal of Epidemiology, 159, 413-421. [DOI] [PubMed] [Google Scholar]
- Janssen I., Heymsfield S. B., Wang Z. M., Ross R. (2000). Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. Journal of Applied Physiology, 89, 81-88. doi: 10.1152/jappl.2000.89.1.81 [DOI] [PubMed] [Google Scholar]
- Kalapotharakos V. I., Michalopoulou M., Godolias G., Tokmakidis S. P., Malliou P. V., Gourgoulis V. (2004). The effects of high- and moderate-resistance training on muscle function in the elderly. Journal of Aging and Physical Activity, 12, 131-143. [DOI] [PubMed] [Google Scholar]
- Klitgaard H., Mantoni M., Schiaffino S., Ausoni S., Gorza L., Laurent-Winter C., . . . Saltin B. (1990). Function, morphology and protein expression of ageing skeletal muscle: A cross-sectional study of elderly men with different training backgrounds. Acta Physiologica Scandinavica, 140, 41-54. doi: 10.1111/j.1748-1716.1990.tb08974.x [DOI] [PubMed] [Google Scholar]
- Kohrt W. M., Malley M. T., Coggan A. R., Spina R. J., Ogawa T., Ehsani A. A., . . . Holloszy J. O. (1991). Effects of gender, age, and fitness level on response of VO2max to training in 60-71 yr olds. Journal of Applied Physiology Bethesda, 71, 2004-2011. doi: 10.1152/jappl.1991.71.5.2004 [DOI] [PubMed] [Google Scholar]
- Lauretani F., Russo C. R., Bandinelli S., Bartali B., Cavazzini C., Di Iorio A., . . . Ferrucci L. (2003). Age-associated changes in skeletal muscles and their effect on mobility: An operational diagnosis of sarcopenia. Journal of Applied Physiology, 95, 1851-1860. doi: 10.1152/japplphysiol.00246.2003 [DOI] [PubMed] [Google Scholar]
- Liu J., Sui X., Lavie C. J., Zhou H., Park Y.-M. M., Cai B., . . . Blair S. N. (2014). Effects of cardiorespiratory fitness on blood pressure trajectory with aging in a cohort of healthy men. Journal of the American College of Cardiology, 64, 1245-1253. doi: 10.1016/j.jacc.2014.06.1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinik E. J., Hodgdon J. A., Mittleman K., O’Brien J. J. (1985). Aerobic/calisthenic and aerobic/circuit weight training programs for Navy men: A comparative study. Medicine and Science in Sports and Exercise, 17, 482-487. [DOI] [PubMed] [Google Scholar]
- Matsudo S. M. M. (2005). Avaliação do idoso: física e funcional. Londrina, Brazil: Midiograf. [Google Scholar]
- Millan-Calenti J. C., Tubio J., Pita-Fernandez S., Gonzalez-Abraldes I., Lorenzo T., Fernandez-Arruty T., Maseda A. (2010). Prevalence of functional disability in activities of daily living (ADL), instrumental activities of daily living (IADL) and associated factors, as predictors of morbidity and mortality. Archives of Gerontology and Geriatrics, 50, 306-310. doi: 10.1016/j.archger.2009.04.017 [DOI] [PubMed] [Google Scholar]
- Nakagawa S., Cuthill I. C. (2007). Effect size, confidence interval and statistical significance: A practical guide for biologists. Biological Reviews of the Cambridge Philosophical Society, 82, 591-605. doi: 10.1111/j.1469-185X.2007.00027.x [DOI] [PubMed] [Google Scholar]
- Nogueira W., Gentil P., Mello S. N. M., Oliveira R. J., Bezerra A. J. C., Bottaro M. (2009). Effects of power training on muscle thickness of older men. International Journal of Sports Medicine, 30, 200-204. doi: 10.1055/s-0028-1104584 [DOI] [PubMed] [Google Scholar]
- Park S.-K., Park J.-H., Kwon Y.-C., Yoon M.-S., Kim C.-S. (2003). The effect of long-term aerobic exercise on maximal oxygen consumption, left ventricular function and serum lipids in elderly women. Journal of Physiological Anthropology and Applied Human Science, 22, 11-17. [DOI] [PubMed] [Google Scholar]
- Pescatello L. S., Franklin B. A., Fagard R., Farquhar W. B., Kelley G. A., Ray C. A., & American College of Sports Medicine. (2004). American College of Sports Medicine position stand. Exercise and hypertension. Medicine & Science in Sports & Exercise, 36, 533-553. [DOI] [PubMed] [Google Scholar]
- Pollock M. L., Wilmore J. H. (1993). Exercícios na saúde e na doença: avaliação e prescrição para prevenção e reabilitação. Rio de Janeiro, Brazil: Guanabara Koogan. [Google Scholar]
- Prestes J., da Cunha Nascimento D., Tibana R. A., Teixeira T. G., Vieira D. C. L., Tajra V., . . . Navalta J. W. (2015). Understanding the individual responsiveness to resistance training periodization. Age, 37(3), 9793. doi: 10.1007/s11357-015-9793-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Campillo R., Castillo A., de la Fuente C. I., Campos-Jara C., Andrade D. C., Alvarez C., . . . Izquierdo M. (2014). High-speed resistance training is more effective than low-speed resistance training to increase functional capacity and muscle performance in older women. Experimental Gerontology, 58, 51-57. doi: 10.1016/j.exger.2014.07.001 [DOI] [PubMed] [Google Scholar]
- Ramos R. M., Coelho-Junior H. J., do Prado R. C. R., da Silva R. S., Asano R. Y., Prestes J., . . . Assumpção C. O. (2018). Moderate aerobic training decreases blood pressure but no other cardiovascular risk factors in hypertensive overweight/obese elderly patients. Gerontology & Geriatric Medicine, 4. doi: 10.1177/2333721418808645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond M. J., Bramley-Tzerefos R. E., Jeffs K. J., Winter A., Holland A. E. (2013). Systematic review of high-intensity progressive resistance strength training of the lower limb compared with other intensities of strength training in older adults. Archives of Physical Medicine and Rehabilitation, 94, 1458-1472. doi: 10.1016/j.apmr.2013.02.022 [DOI] [PubMed] [Google Scholar]
- Reid K. F., Doros G., Clark D. J., Patten C., Carabello R. J., Cloutier G. J., Fielding R. A. (2012). Muscle power failure in mobility-limited older adults: Preserved single fiber function despite lower whole muscle size, quality and rate of neuromuscular activation. European Journal of Applied Physiology, 112, 2289-2301. doi: 10.1007/s00421-011-2200-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K. F., Fielding R. A. (2012). Skeletal muscle power: A critical determinant of physical functioning in older adults. Exercise and Sport Sciences Reviews, 40, 4-12. doi: 10.1097/JES.0b013e31823b5f13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano C., Longstreth W. T. J., Boudreau R., Taylor C. A., Du Y., Kuller L. H., Newman A. B. (2011). High blood pressure accelerates gait slowing in well-functioning older adults over 18-years of follow-up. Journal of the American Geriatrics Society, 59, 390-397. doi: 10.1111/j.1532-5415.2010.03282.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallinen J., Stenholm S., Rantanen T., Heliovaara M., Sainio P., Koskinen S. (2010). Hand-grip strength cut points to screen older persons at risk for mobility limitation. Journal of the American Geriatrics Society, 58, 1721-1726. doi: 10.1111/j.1532-5415.2010.03035.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals D. R., Hagberg J. M., Hurley B. F., Ehsani A. A., Holloszy J. O. (1984). Endurance training in older men and women. I. Cardiovascular responses to exercise. Journal of Applied Physiology: Respiratory, Environmental and Exercise Physiology, 57, 1024-1029. doi: 10.1152/jappl.1984.57.4.1024 [DOI] [PubMed] [Google Scholar]
- Sewo Sampaio P. Y., Sampaio R. A. C., Coelho Junior H. J., Teixeira L. F. M., Tessutti V. D., Uchida M. C., Arai H. (2016). Differences in lifestyle, physical performance and quality of life between frail and robust Brazilian community-dwelling elderly women. Geriatrics & Gerontology International, 16, 829-835. doi: 10.1111/ggi.12562 [DOI] [PubMed] [Google Scholar]
- Sharma D., Parashar A., Mazta S. (2014). Functional status and its predictor among elderly population in a hilly state of North India. International Journal of Health & Allied Sciences, 3, 159-163. doi: 10.4103/2278-344X.138593 [DOI] [Google Scholar]
- Sheffield L. T., Holt J. H., Reeves T. J. (1965). Exercise graded by heart rate in electrocardiographic testing for angina pectoris. Circulation, 32, 622-629. [DOI] [PubMed] [Google Scholar]
- Simel D. L., Samsa G. P., Matchar D. B. (1991). Likelihood ratios with confidence: Sample size estimation for diagnostic test studies. Journal of Clinical Epidemiology, 44, 763-770. [DOI] [PubMed] [Google Scholar]
- Steib S., Schoene D., Pfeifer K. (2010). Dose-response relationship of resistance training in older adults: A meta-analysis. Medicine and Science in Sports and Exercise, 42, 902-914. doi: 10.1249/MSS.0b013e3181c34465 [DOI] [PubMed] [Google Scholar]
- Sui X., Jackson A. S., Church T. S., Lee D.-C., O’Connor D. P., Liu J., Blair S. N. (2012). Effects of cardiorespiratory fitness on aging: Glucose trajectory in a cohort of healthy men. Annals of Epidemiology, 22, 617-622. doi: 10.1016/j.annepidem.2012.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan G. M., Feinn R. (2012). Using effect size-or why the p value is not enough. Journal of Graduate Medical Education, 4, 279-282. doi: 10.4300/JGME-D-12-00156.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaitkevicius P. V., Ebersold C., Shah M. S., Gill N. S., Katz R. L., Narrett M. J., . . . Fleg J. L. (2002). Effects of aerobic exercise training in community-based subjects aged 80 and older: A pilot study. Journal of the American Geriatrics Society, 50, 2009-2013. [DOI] [PubMed] [Google Scholar]
- Wallerstein L. F., Tricoli V., Barroso R., Rodacki A. L. F., Russo L., Aihara A. Y., . . . Ugrinowitsch C. (2012). Effects of strength and power training on neuromuscular variables in older adults. Journal of Aging and Physical Activity, 20, 171-185. [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2001). Encuesta Multicêntrica: salud, bien estar y envejecimiento (SABE) en América Latina y el Caribe. Anales Da36a Reunión del Comité Asesor de Investigaciones em Salud. Retrieved from: http://iris.paho.org/xmlui/handle/123456789/45890


