Abstract
Background:
Endoscopic resection has been increasingly adopted for neoplasms in the major duodenal papilla. Previous studies have reached varying conclusions on whether prophylactic pancreatic stent (PS) placement is an effective measure against post-procedure complications. We aimed to investigate whether PS could reduce the incidence of post-procedure complications.
Methods:
The PubMed, Cochrane Library, and EMBASE databases were systematically searched from the inception dates to 25 December 2018 to identify all randomized controlled trials (RCTs) and retrospective cohort studies (RCSs) comparing prophylactic PS and no PS against post-procedure complications. The main outcomes measurements were post-procedure pancreatitis, bleeding, perforation and late papillary stenosis.
Results:
23 RCSs (1001 subjects) and 2 RCTs met the inclusion criteria. Meta-analysis of the RCSs showed that prophylactic PS decreased the odds of post-procedure pancreatitis (OR, 0.71; 95% CI, 0.36–1.40; p = 0.325) as well as late papillary stenosis (OR, 0.35; 95% CI, 0.07–1.75; p = 0.200; I2 =0%) and increased the odds of bleeding (OR, 1.32; 95% CI, 0.50–3.46; p = 0.572; I2 = 0%) and perforation (OR, 2.25; 95% CI, 0.33–15.50; p = 0.412; I2 = 0%) but not significantly. Sensitivity analysis illustrated prophylactic PS significantly decreased the risk of post-procedure pancreatitis (OR, 0.44; 95% CI, 0.24–0.80; p = 0.007).
Conclusions:
PS placement was prophylactic against post-procedure complications although not significantly. Sensitivity analysis suggests the significant effect of prophylactic PS against post-procedure pancreatitis. More RCTs are required to validate the statistical significance of our results and potentially relevant characteristics improving the prophylactic efficacy of stents.
Keywords: prophylactic pancreatic stent, post-procedure complications
Introduction
Tumors of the main duodenal papilla have a prevalence of only 0.04–0.12% in autopsy studies.1 Ampullary tumors may also occur sporadically or in patients with familial adenomatous polyposis (FAP).2 With the development of endoscopy, especially endoscopic retrograde cholangiopancreatography (ERCP), tumors in the papilla or ampulla are being gradually recognized. Adenomas arising in the major duodenal papilla or ampulla of Vater can potentially undergo the adenoma–carcinoma sequence, making complete removal mandatory for curative therapy.3 Currently, endoscopic papillectomy (EP) or endoscopic ampullectomy (EA) with curative intent is increasingly adopted for benign papillary tumors.
However, post-procedure complications may lead to an increase in morbidity and mortality, depending on the severity. Likewise, the social economic burdens resulting from the risk of procedure-related complications are not negligible. Pancreatitis is a common and potentially preventable short-term complication of EP. Other short-term complications include bleeding and perforation, while distal common pancreatic duct stricture is a common long-term complication. The routine placement of a pancreatic stent (PS) may be a supportive measure for the prevention of severe pancreatitis after EP. Nevertheless, mixed results have been obtained according to the current studies addressing prophylactic PS placement after EP to avoid these complications. A series of studies demonstrated that routine placement of a prophylactic PS may decrease the risk of these post-procedure complications.4–8 In contrast, some investigators reported that the existence of a PS did not correlate with subsequent pancreatitis after EP.9–11 Although prophylactic PSs are moderately recommended during papillectomy by the American Society for Gastrointestinal Endoscopy (ASGE), studies published to date have not reached consistent conclusions regarding whether prophylactic PSs should be routinely required for EP.12
Our systematic review and meta-analysis were performed to compare the efficacy of PS and no PS placement against post-procedure complications and validate whether PS placement is necessary or not.
Method
This meta-analysis was performed according to the Cochrane Handbook for Systematic Reviews of Interventions and presented based on Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines.13
Literature search
Studies were searched and identified by database searching of PubMed, EMBASE, and the Cochrane Library (including CENTRAL) from the January 1990, to 25 December 2018. In addition, we searched for available studies from review articles and abstracts of relevant conferences. The following keywords were involved in our searching: ‘endoscopic papillectomy’, ‘endoscopic ampullectomy’, ‘duodenal major papilla’, ‘ampulla’, ‘stent’, ‘pancreatic stent’. There were no language restrictions. We analyzed selected research for summary level data. PRISMA guidelines were applied for assessing search results. Two authors (Y.N.W. and M.Q.) screened titles and abstracts independently and screened selected research independently. Discrepancies were resolved by consensus.
Study selection
Selection criteria were articles and conference abstracts comparing the efficacy of prophylactic PSs and no PSs against post-procedure complications. Exclusion criteria were those studies not involving post-EP and ampullectomy complications (including pancreatitis, bleeding, perforation, late papillary stenosis) as a study endpoint or research comparing stents plus drugs or other novel stent-related procedures. In addition, case series were excluded from the analysis. There were no language restrictions. Both full-length article and conference abstract publications were selected. Research was included based on the following inclusion criteria: (1) trials reporting patients suffering neoplasms in major duodenal papilla or ampulla of Vater and receiving EP or EA; (2) every trial needed to contain both patients with prophylactic PS placement and without PS placement; (3) trials providing post-procedure complications data (at least including pancreatitis).
Data extraction and quality assessment
All data were independently extracted in duplicate by two authors (Y.N.W. and M.Q.) and reviewed by a third (J.B.H.) for agreement. The two independent investigators extracted data using a common data extraction form. The following data were extracted from each study: first author’s name, year of publication, country, percentage of female, mean age (upper and lower range), trial design, stent characteristic, number of patients with/without PSs, outcomes of post-procedure complications (pancreatitis, severity of pancreatitis, bleeding, perforation, late papillary stenosis), neoplasm position, percentage of patients with adenoma, percentage of patients undergoing pancreatic endoscopic sphincterotomy (EST), percentage of patients undergoing en-bloc, percentage of patients with FAP. We tried to contact the authors if we cannot extract required supplementary information about the data or trials.
To evaluate the quality of retrospective cohort studies (RCSs), we used the nine-point Newcastle–Ottawa scale (NOS) scale to assess the following three fundamental aspects of methodology: study participant selection (0–4), confounder adjustment (0–2), and outcome indicator determination (0–3). An RCS with 7–9 points NOS score was defined as a highly qualified study.14
Statistical analysis
RCS
The effects of PS placement were analyzed by calculating pooled estimates of post-procedure pancreatitis, the severity of pancreatitis, bleeding, perforation, late papillary stenosis. Separate analyses were performed for correlated outcomes by using odds ratio (OR). A statistically significant result was observed with a 95% confidence interval (CI) and a p value of <0.05. Subgroup analysis was performed to assess the effect of neoplasm position, a percentage of patients with adenoma, a percentage of adenoma, a percentage of patients undergoing EST, a percentage of patients undergoing en-bloc, a percentage of patients with FAP on post-procedure pancreatitis. A funnel plot was generated to evaluate publication bias among RCSs.15
All tests were two-tailed, and p < 0.05 was considered statistically significant. The statistical heterogeneity was evaluated by the I2 statistic and the Chi-square-based Q statistic in a random-effects model.16 We followed the general heterogeneity principle based on the suggestion in the Cochrane Handbook:13 I2 < 40% as ‘heterogeneity might not be important’ and >75% as ‘considerable heterogeneity’. Sensitivity analysis was also performed after restricting the studies to high-quality, characteristics of publication (full-length publications or abstracts), trials recruiting individuals without other accompanying diseases or particular conditions and per-protocol analysis. All the analyses described above were performed with STATA version 15.0 statistical software (Stata Corp., College Station, TX, USA).
Randomized controlled trials
Data from randomized controlled trials (RCTs) were also extracted as described previously. Outcomes of stents against post-procedure pancreatitis were stated. No further meta-analysis was implemented because of the limited number and quality of RCTs.
Results
Literature search and basic characteristics of studies
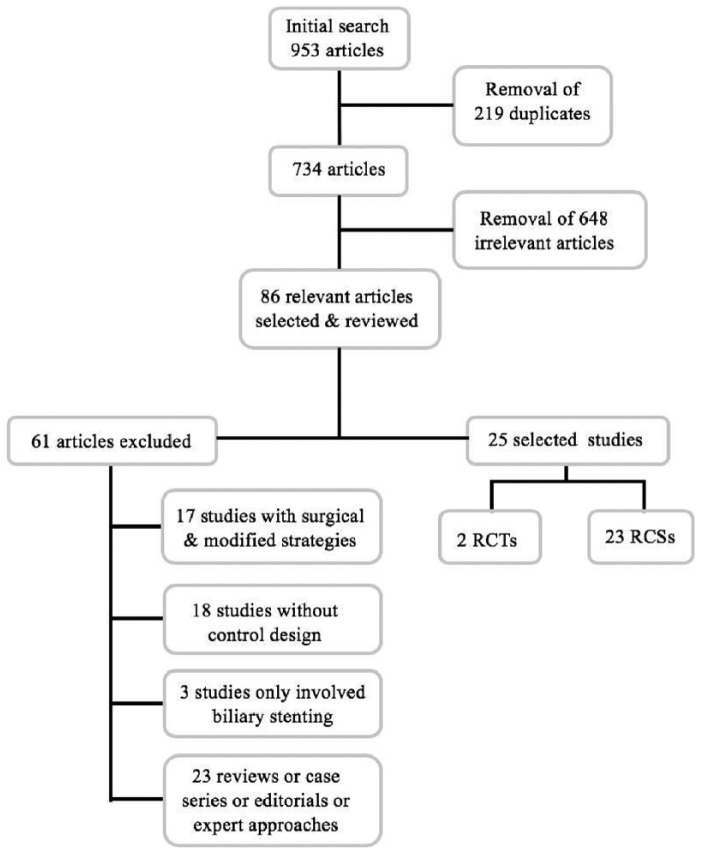
A total of 953 potentially eligible records were identified. After the removal of duplicates and screening of titles and abstracts, 734 studies were retained. The titles and abstracts of these records were screened for inclusion. Of these, 86 relevant articles were selected and reviewed by three independent authors (W.Y.N., Q.M. and H.J.B.). Ultimately, two RCTs (69 patients) and 23 nonrandomized studies (1001 patients) met the inclusion criteria and were selected for final review and analysis (Figure 1). Both RCTs and RCSs were separately analyzed. All RCSs were of adequate quality (NOS ⩾ 7). Overall, five conference abstracts were selected and included in the final analysis.
Figure 1.
Flow diagram for selecting eligible studies to include in the meta-analysis.
All RCSs were conducted in following countries: six in the United States, four in Korea, three in France, three in Japan, two in Brazil, two in Turkey, one in the Czech Republic, one in Greece, one in Finland, one in Italy and one in China. A detailed summary of these studies is presented in Table 1. The percentage of female patients varied from 7% to 66% among the studies. A total of 14 trials used stent sizes of 3–7F. Overall, five trials demonstrated the length of used stents: 3–7 cm.8,17–20 Flanged stents were used in four trials,8,9,18,21 whereas other trials did not provide information about the use of flanged or unflanged PSs. In five trials,8,9,11,21,22 the stents were made of polyethylene while other trials did not report their stent material.
Table 1.
Basic characteristics of included retrospective cohort studies.
| Study | Country | NOS score | %, females | Mean age, y (range) | Stent characteristics | PS group | No PS group | Neoplasm position | %, Adenoma | %, EST | %, en-bloc | %, FAP | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of no pancreatitis/pancreatitis (mild, moderate, severe) | No. of bleeding, perforation, hyperamylasemia, late papillary stenosis | No. of no pancreatitis/pancreatitis (mild, moderate, severe) | No. of bleeding, perforation, hyperamylasemia, late papillary stenosis | |||||||||||
| Zadorova et al.7 | Czech Republic | 8 | 44 | 68 (50–84) | 7Fr | 6/0 | NR | 8/2 | NR | MDP | 100 | 6 | NR | 0 |
| Norton et al.9 | USA | 9 | 54 | 42 (21–84) | straight, 5F, polyethylene, flanged | 6/2 | NR | 16/2 | NR | MDP | 97 | 8 | NR | 62 |
| Maguchi et al.24 | Japan | 8 | 42 | 66 (NR) | NR | 6/2 | 2, 1, NR, NR | 3/1 | 1,0, NR, NR | MDP | 83 | 0 | 92 | 0 |
| Catalano et al.5 | USA | 9 | 51 | NR (24–93) | 5–7Fr | 88/3 | NR, NR, NR, 2 | 10/2 | NR, NR, NR, 1 | MDP | 70 | 4 | NR | 30 |
| Cheng et al., 25 | USA | 9 | 60 | 59 (17–87) | 3F–5F | 37/4 | NR | 3/1 | NR | MDP | 91 | NR | 55 | 26 |
| Han et al.24 | Korea | 6 | 45 | 56 (32–79) | 5–7Fr, polyethylene | 11/0 | NR | 5/0 | NR | MDP | 50 | NR | 63 | 5 |
| Katsinelos et al.21 | Greece | 8 | 43 | 63 (42–76) | 5Fr, polyethylene, flanged | 4/0 | 0, NR, NR, NR | 9/1 | 1, NR, NR, NR | MDP | 100 | 100 | NR | 29 |
| Aiura et al.18 | Japan | 6 | 7 | 62 (54–73) | Straight, 5Fr, 5 cm, flanged | 4/1 | NR, 1, NR, 2 | 9/0 | NR, 0, NR, 0 | MDP | 71 | NR | 86 | 0 |
| Koya et al.26 | USA | CA | NR | NR | NR | 13/0 (0, 0, 0) | NR | 7/4 (2,2,0) | NR | AV | 100 | NR | NR | NR |
| Harano et al.27 | Japan | 6 | 39 | 67 (51–79) | 5Fr | 23/0 (0, 0, 0) | NR | 3/2 (2, 0, 2) | NR | MDP | 79 | NR | 61 | 0 |
| Patel et al.28 | USA | 8 | 58 | 54 (22–85) | 3Fr or 5Fr | 19/1 (1, 0, 0) | NR | 16/2 (2, 0, 0) | NR | AV | 100 | 100 | 95 | 27 |
| Jeanniard et al., 29 | France | 6 | 45 | 63 (35–79) | 5Fr or 7Fr | 22/4 | NR | 14/2 | NR | MDP | NR | NR | 81 | 12 |
| Salmi et al.17 | France | 8 | 55 | 64 (33–83) | 3 cm | 28/1 | NR | 20/5 | NR | AV | 53 | NR | 70 | 10 |
| Ardengh et al., 30 | Brazil | CA | NR | NR | NR | 7/5 | NR | 14/0 | NR | MDP | 65 | NR | NR | NR |
| Napoleon et al.31 | France | 9 | 53 | 57 (13–83) | NR | 41/7 (4, 2, 1) | NR, NR, NR, 1 | 30/15 (8, 4, 3) | NR, NR, NR, 1 | AV | 71 | NR | 73 | 23 |
| Ismail et al.19 | Finland | 8 | 38 | NR (28–91) | 5–7Fr, 3–7 cm | 31/1 | NR | 24/5 | NR | MDP | 57 | 2 | NR | 26 |
| Chang et al.11 | Korea | 8 | 34 | 55 (27–80) | 5–Fr, polyethylene | 48/6 (1, 5, 0) | NR, NR, NR, 0 | 26/2 (0, 2, 0) | NR, NR, NR, 1 | AV | 98 | 0 | 78 | NR |
| Bagci et al., 32 | Turkey | CA | 32 | 65 (28–82) | NR | 4/1 | NR | 11/3 | NR | AV | NR | NR | 58 | 5 |
| Paulino et al.,33 | Brazil | CA | 51 | 62 (46–87) | NR | 9/9 | NR | 36/5 | NR | AV | NR | NR | NR | NR |
| Gambitta et al.34 | Italy | CA | NR | NR | NR | 10/0 (0, 0, 0) | 2, NR, NR, NR | 2/3 (2, 0, 1) | 0, NR, NR, NR | AV | NR | NR | NR | NR |
| Shen et al.20 | China | 6 | 36 | NR | 5Fr, 3–7 cm | 45/5 | 21, 1, NR, 7 | 16/1 | 6, 0, NR, 4 | MDP | 100 | NR | 96 | 0 |
| Kang et al.10 | Korea | 6 | 33 | 61 (37–86) | NR | 48/12 | NR | 40/4 | NR | AV | 58 | 23 | 90 | 0 |
| Attila et al., 35 | Turkey | 6 | 66 | 64 (33–84) | NR | 26/1 | NR | 15/1 | NR | AV | 57 | NR | 64 | 5 |
FAP, familial adenomatous polyposis; NOS, Newcastle–Ottawa scale; PS, pancreatic stent; CA, conference abstract; NR, no report; MDP, major duodenal papilla; AV, ampulla of Vater.
No significant publication bias was identified by the evaluation of the funnel plot (Figure 2).
Figure 2.
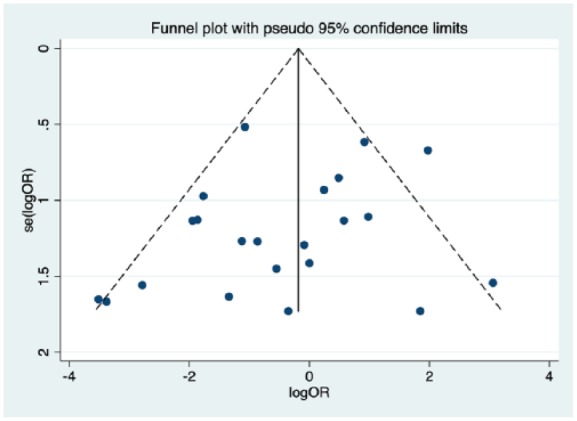
Funnel plot assessing for publication bias. No publication bias was noted.
Meta-analysis of RCSs
Post-procedure pancreatitis
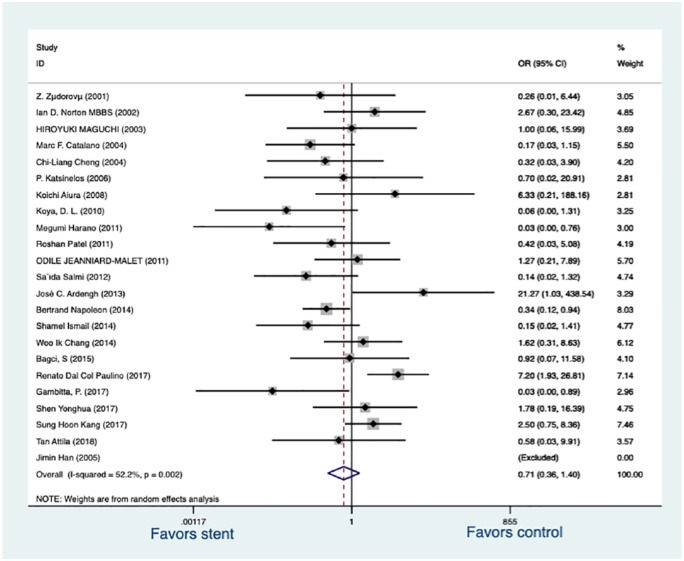
All trials followed the consensus definition (2012) for defining post-ERCP pancreatitis.23 Post-procedure pancreatitis was documented in 65 of 601 patients (10.82%) with a PS placed, compared with 63 of 400 patients (15.75%) without a PS placed. There was an OR reduction with prophylactic PS placement (OR, 0.71; 95% CI, 0.36–1.40; p = 0.325) but no statistically significant difference was demonstrated (Figure 3). Subgroup analysis was performed to evaluate the effect of neoplasm position, a percentage of patients with adenoma, a percentage of adenoma, a percentage of patients undergoing EST, a percentage of patients undergoing en-bloc, a percentage of patients with FAP on post-ER pancreatitis. In the studies analyzed, PS did not significantly reduce the risk of post-ER pancreatitis in the group with a neoplasm in the major duodenal papilla (OR, 0.73; 95% CI, 0.29–1.85; p = 0.512) nor in the group with a neoplasm in the ampulla of Vater (OR, 0.66, 95% CI, 0.24–1.83; p = 0.427). A significant difference for the effects of PS against post-ER pancreatitis was also not observed in the group with all patients suffering adenoma (OR, 0.72, 95% CI, 0.19–2.76; p = 0.626), neither in the group with a mixed proportion (50–98%) of patients suffering adenoma (OR, 0.60, 95% CI, 0.26–1.38; p = 0.232). The group with all patients undergoing EST did not exhibit a significant reduction of post-ER pancreatitis (OR, 0.50, 95% CI, 0.07–3.75; p = 0.504) between PS and no PS placement and the same is true in the group with mixed proportion (0–23%) of patients (OR, 0.84, 95% CI, 0.31–2.23; p = 0.719). Overall, three subgroups were established for the percentage of patients undergoing en-bloc resection: >90%, 70–90%, <70%. There was no significant difference for the efficacy of PS against post-ER pancreatitis among >90% subgroup (OR, 1.67, 95% CI, 0.67–4.20; p = 0.274), 70–90% subgroup (OR, 0.75, 95% CI, 0.39–1.45; p = 0.484), <70% subgroup (OR, 0.33, 95% CI, 0.08–1.28; p = 0.109), respectively. However, PS placement significantly reduced the incidence of post-ER pancreatitis in the group recruiting a mixed proportion (5–62%) patients with FAP (OR, 0.42, 95% CI, 0.23–0.76; p = 0.004) but the group without patients suffering FAP did not exhibit a difference between the effects of PS and no PS placement (OR, 1.03, 95% CI, 0.28–3.76; p = 0.961).
Figure 3.
Forrest plot of included RCSs demonstrating the effect of prophylactic pancreatic stents against post-procedure pancreatitis.
RCS, retrospective cohort study.
The severity of pancreatitis
Overall, six trials11,26–28,31,34 provided data on the severity of pancreatitis. Prophylactic PS significantly decreased the odds of mild (OR, 0.32; 95% CI, 0.13–0.81; p = 0.016; I2 = 0%) as well as severe pancreatitis (OR, 0.17; 95% CI, 0.03–0.90; p = 0.037; I2 = 0%). However, although a trend was noted, PS placement did not significantly decrease the odds of moderate pancreatitis (OR, 0.62; 95% CI, 0.20–1.95; p = 0.415; I2 = 0%).
Other complications
Overall, four studies20,21,24,34 reported data on post-endoscopic operation bleeding and three trials18,20,24 provided data on post-endoscopic operation perforation. Stents could increase the odds of post-procedure bleeding but not significantly (OR, 1.32; 95% CI, 0.50–3.46; p = 0.572; I2 = 0%) as well as post-procedure perforation (OR, 2.25; 95% CI, 0.33–15.50; p = 0.412; I2 = 0%). Overall, three trials demonstrated outcomes on late papillary stenosis. Stents decreased the odds of delayed papillary stenosis but not significantly (OR, 0.35; 95% CI, 0.07–1.75; p = 0.200; I2 = 0%).
Sensitivity analysis
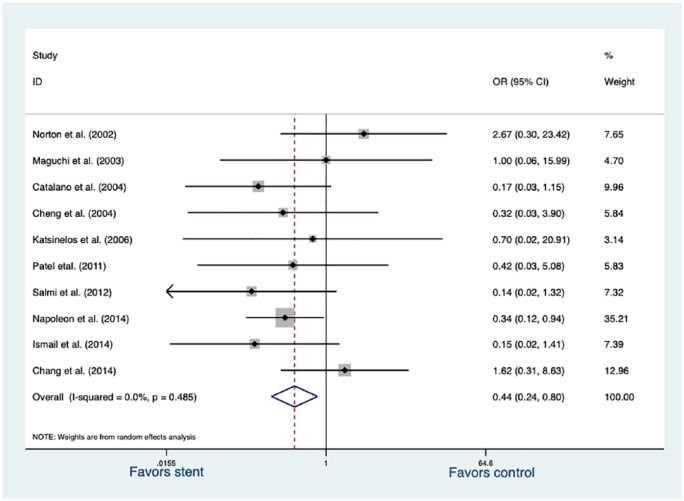
We conducted a sensitivity analysis after restricting the studies to full-length publication, high-quality research (NOS score 7–9 point). The selected 10 studies altered the results. Data were also analyzed by random effects, and different results were obtained. Post-procedure pancreatitis was documented in 27 of 335 patients (8.06%) with a PS placement, compared with 36 of 193 patients (18.65%) without a PS placement. A statistically significant OR reduction was observed (OR, 0.44; 95% CI, 0.24–0.80; p = 0.007; Figure 4).
Figure 4.
Forrest plot of 10 included full-length publications and high-quality RCSs demonstrating the effect of pancreatic stents against post-procedure pancreatitis.
RCS, retrospective cohort study.
Heterogeneity
To assess the relation between PS placement and post-procedure pancreatitis, significant heterogeneity was observed among all RCSs (I2 = 52.2%, p = 0.002) while the outcomes were stable at the severity of pancreatitis and other complications from related trials. In subgroup analysis for post-procedure pancreatitis, heterogeneity obviously existed in a group with patient suffering ampulla neoplasm (I2 = 65.9%, p = 0.002) and group with a mixed proportion of patients suffering adenoma (I2 = 52.7%, p = 0.011) between PS and no PS intervention. No or minor heterogeneity without statistical significance was evaluated in other forest plots and our sensitivity analysis.
Systematic review of RCTs
Overall, two studies met the inclusion criteria (69 patients). One full-length publication8 noted a statistically significant reduction in the incidence of post-procedure pancreatitis where one conference abstract36 concluded doubtful effectiveness of prophylactic PSs after EP based on their no significant difference of post-EP pancreatitis between stent group and control group. Studies were respectively reported from the United States and Korea. The incidence of pancreatitis in the stent group was 0% and 20%, respectively. The incidence of pancreatitis in the control group was 33% and 12%, respectively.
Discussion
Papillary or ampullary tumors will develop via an adenoma-to-carcinoma sequence, as observed in the colon.37 The efficacy of endoscopic resection for these neoplasms is increasingly validated. However, complications related to EP occur in up to 25% of patients.38 These complications include pancreatitis, bleeding, cholangitis, papillary stenosis and duodenal perforation. We only collected RCSs including conference abstracts in the statistical analysis. Due to the limited number, a systematic review for RCTs was conducted to better validate the evidence regarding the efficacy of PS in the prophylaxis of post-ER pancreatitis.
Our meta-analysis is the first summative study to demonstrate that PS placement decreases the odds of post-procedure pancreatitis but not significantly. PS significantly lowered the risk of mild and severe post-ER pancreatitis while the incidence of moderate post-ER pancreatitis was also lower for patients with PS placement. On sensitivity analysis without obvious heterogeneity, a prophylactic significance of PS placement was more convincing to reduce the risk of post-ER pancreatitis. In addition, PS potentially reduced the risk of post-procedure late papillary stenosis even if it was not statistically significant. So, this clinical issue requires accurate validation of whether PS placement is efficient or not.
On subgroup analysis, we found a significant reduction in post-ER pancreatitis after PS placement for patients with FAP. We are the first to demonstrate that prophylactic PS placement can significantly reduce the risk of post-ER pancreatitis for patients with FAP. But further precise trials are required to compare the difference of PS efficacy against post-procedure pancreatitis between patients with and without FAP. No any statistically significant difference in post-ER pancreatitis was observed concerning other subgroups (position of neoplasm, adenoma proportion in all pathological results, EST, en-bloc) but further trials are required to identify whether these variates are potential factors to influence the efficacy of PS against post-procedure complications. We found the lower en-bloc proportion in all patients contributed to the higher efficacy of PS placement against post-procedure pancreatitis. In other words, an en-bloc measure may be a potential risk factor of causing post-procedure pancreatitis for patients with prophylactic PS placement though it could reduce the risk of recurrence of neoplasm in major duodenal papilla and ampulla.
The characteristics (size, length and flange) of stents may also have an impact on the post-procedure outcomes. Compared with a 5–6F stent, unflanged 3F stents were reported to trigger a relatively lower incidence of post-ERCP pancreatitis.39 Whereas, it was concluded by other research that there was no difference in the incidence of post-ERCP pancreatitis between the long 3F stent group and short 5F stents.40 And a meta-analysis also indicated that shorter stents (<3 cm) can decrease the incidence of post-ERCP pancreatitis, although without statistical significance. However, the characteristics of stents involved in each study were not unified, making it difficult for us to perform subgroup analysis. Other elements like softer materials, and an unflanged design may contribute to improving the efficacy of PS against post-procedure complications.41,42 Additionally, it is unclear when the stents should be placed (before or after the procedure) and how long the stents should remain in place. All studies adopted the stent placement after the endoscopic procedure except in two studies that tried pre-ER placement. It is controversial whether a long duration may have an impact on pancreatic duct and a short duration may be enough to protect against the incidence of complications.43 The role of these stent characteristics on the efficacy of prophylactic PS against post-ER pancreatitis is worthy of further investigation.
All studies included in our meta-analysis did not implement any pharmacological therapy. In addition to prophylactic PS, rectal indomethacin is also moderately recommended during EP.12,44,45 This is a controversial issue, despite its effectiveness. Rectal indomethacin alone may replace PS for prophylaxis of post-ERCP pancreatitis than PS placement alone or the combination of indomethacin and PS placement.46 But another analysis reported that prophylactic PS placement was still the better choice against post-ERCP pancreatitis.47 The role of pharmacological agents in combination with PSs is required to be adequately investigated by a large-scale RCT.
The limitation of this meta-analysis is the lack of sufficient randomized trials which could make results more convincing. In addition to insufficient number of RCTs, these two RCTs also had a small sample size and contradictory results. The sample size of sole published RCTs was small partly because it was terminated early due to many incidences of post-papillectomy pancreatitis in the nonstented group. More RCTs with a wider spectrum of clinical background with larger sample sizes are required to be conducted in the future. Combining with the clinical experience of our institution, we personally suggest the potentially prophylactic effect of PS placement after EP and ampullectomy against post-procedure complications in actual clinical practice. Subgroup analysis of stent features on post-procedure pancreatitis was not achieved because the included trails showed a lack of stent data. Few studies stressed the importance of difference of stent characteristics on the efficacy against post-procedure complications until now. The issues mentioned above remain to be further investigated in the future. In addition, the short- and long-term consequences of a PS also need to be studied in detail. The studies included in our analysis did not evaluate the complications of stent placement in a systematic manner.
Conclusion
In summary, our systematic review and meta-analysis elucidated that PS placement is prophylactic against post-procedure complications, although not significantly. Sensitivity analysis suggests the significant effect of prophylactic PS against post-ER complications. More RCTs with a larger sample size are required to validate the statistical significance of our results and potentially relevant characteristics improving the prophylactic efficacy of stents.
Footnotes
Funding: This work was supported by Natural Science Foundation of China (No. 81760106) and Natural Science Foundation of Jiangxi Province (No. 20171BAB205012).
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Yining Wang  https://orcid.org/0000-0002-3454-9881
https://orcid.org/0000-0002-3454-9881
Contributor Information
Yining Wang, Department of Gastroenterology, The First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, China; Department of Liver Surgery and Transplantation, Liver Cancer Institute, Zhongshan Hospital, and Key Laboratory of Carcinogenesis and Cancer Invasion (Ministry of Education), Fudan University, Shanghai, China; Joint Programme of Nanchang University and Queen Mary University of London, Nanchang, China.
Miao Qi, Department of Gastroenterology, The First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, China; Joint Programme of Nanchang University and Queen Mary University of London, Nanchang, China.
Yuanzhen Hao, Department of Gastroenterology, The First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, China; Joint Programme of Nanchang University and Queen Mary University of London, Nanchang, China.
Junbo Hong, Department of Gastroenterology, The First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, 330006, China.
Reference
- 1. Rosenberg J, Welch JP, Pyrtek LJ, et al. Benign villous adenomas of the ampulla of Vater. Cancer 1986; 58: 1563–1568. [DOI] [PubMed] [Google Scholar]
- 2. Arvanitis ML, Jagelman DG, Fazio VW, et al. Mortality in patients with familial adenomatous polyposis. Dis Colon Rectum 1990; 33: 639–642. [DOI] [PubMed] [Google Scholar]
- 3. Bohnacker S, Soehendra N, Maguchi H, et al. Endoscopic resection of benign tumors of the papilla of Vater. Endoscopy 2006; 38: 521–525. [DOI] [PubMed] [Google Scholar]
- 4. Napoleon B, Alvarez-Sanchez MV, Leclercq P, et al. Systematic pancreatic stenting after endoscopic snare papillectomy may reduce the risk of postinterventional pancreatitis. Surg Endosc 2013; 27: 3377–3387. [DOI] [PubMed] [Google Scholar]
- 5. Catalano MF, Linder JD, Chak A, et al. Endoscopic management of adenoma of the major duodenal papilla. Gastrointest Endosc 2004; 59: 225–232. [DOI] [PubMed] [Google Scholar]
- 6. Desilets DJ, Dy RM, Ku PM, et al. Endoscopic management of tumors of the major duodenal papilla: refined techniques to improve outcome and avoid complications. Gastrointest Endosc 2001; 54: 202–208. [DOI] [PubMed] [Google Scholar]
- 7. Zadorova Z, Dvofak M, Hajer J. Endoscopic therapy of benign tumors of the papilla of Vater. Endoscopy 2001; 33: 345–347. [DOI] [PubMed] [Google Scholar]
- 8. Harewood GC, Pochron NL, Gostout CJ. Prospective, randomized, controlled trial of prophylactic pancreatic stent placement for endoscopic snare excision of the duodenal ampulla. Gastrointest Endosc 2005; 62: 367–370. [DOI] [PubMed] [Google Scholar]
- 9. Norton ID, Gostout CJ, Baron TH, et al. Safety and outcome of endoscopic snare excision of the major duodenal papilla. Gastrointest Endosc 2002; 56: 239–243. [DOI] [PubMed] [Google Scholar]
- 10. Kang SH, Kim KH, Kim TN, et al. Therapeutic outcomes of endoscopic papillectomy for ampullary neoplasms: retrospective analysis of a multicenter study. BMC Gastroenterology 2017; 17: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chang WI, Min YW, Yun HS, et al. Prophylactic pancreatic stent placement for endoscopic duodenal ampullectomy: a single-center retrospective study. Gut Liver 2014; 8: 306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. ASGE Standards of Practice Committee; Chathadi KV, Khashab MA, et al. The role of endoscopy in ampullary and duodenal adenomas. Gastrointest Endosc 2015; 82: 773–781. [DOI] [PubMed] [Google Scholar]
- 13. Higgins JPT, Green S. (eds). Cochrane handbook for systematic reviews of interventions Version 5.1.0. The Cochrane Collaboration; http://handbook.cochrane.org (2011, accessed 22 November 2017). [Google Scholar]
- 14. Wells GA, Shea B, O’Connell D, et al. The Newcastle–Ottawa scale (NOS) for assessing the quality of non-randomized studies in meta-analysis. Appl Eng Agric 2012; 18: 727–734. [Google Scholar]
- 15. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000; 56: 455–463. [DOI] [PubMed] [Google Scholar]
- 16. Deeks JJ, Atlman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. Hoboken: Wiley-Blackwell, 2008, pp. 285–312. [Google Scholar]
- 17. Salmi S, Ezzedine S, Vitton V, et al. Can papillary carcinomas be treated by endoscopic ampullectomy? Surg Endosc 2012; 26: 920–925. [DOI] [PubMed] [Google Scholar]
- 18. Aiura K, Hibi T, Handa K, et al. Endoscopic snare papillectomy for tumors of the major duodenal papilla. Digestive Endoscopy 2008; 20: 154–158. [Google Scholar]
- 19. Ismail S, Marianne U, Heikki J, et al. Endoscopic papillectomy, single-centre experience. Surg Endosc 2014; 28: 3234–3239. [DOI] [PubMed] [Google Scholar]
- 20. Shen YH CJ, Yao YL, Wu H, et al. Value of pancreatic stent placement for endoscopic resection of duodenal papilla adenoma. Chin J Dig Endosc 2017; 34: 427–430. [Google Scholar]
- 21. Katsinelos P, Paroutoglou G, Kountouras J, et al. Safety and long-term follow-up of endoscopic snare excision of ampullary adenomas. Surg Endosc 2006; 20: 608–613. [DOI] [PubMed] [Google Scholar]
- 22. Han J, Lee SK, Park DH, et al. Treatment outcome after endoscopic papillectomy of tumors of the major duodenal papilla. Korean J Gastroenterol 2005; 46: 110–119. [PubMed] [Google Scholar]
- 23. ASGE Standards of Practice Committee; Anderson MA, Fisher L, et al. Complications of ERCP. Gastrointest Endosc 2012; 75: 467–473. [DOI] [PubMed] [Google Scholar]
- 24. Maguchi H, Takahashi K, Katanuma A, et al. Indication of endoscopic papillectomy for tumors of the papilla of Vater and its problems. Digestive Endoscopy 2003; 15(Suppl.): S33–S35. [Google Scholar]
- 25. Cheng CL, Sherman S, Fogel EL, et al. Endoscopic snare papillectomy for tumors of the duodenal papillae. Gastrointest Endosc 2004; 60: 757–764. [DOI] [PubMed] [Google Scholar]
- 26. Koya DL, Pinkas H, Prieto R, et al. Prophylactic pancreatic duct stents prevent post-ampullectomy pancreatitis: a single center experience. Am J Gastroenterol 2010; 105: S61. [Google Scholar]
- 27. Harano M, Ryozawa S, Iwano H, et al. Clinical impact of endoscopic papillectomy for benign-malignant borderline lesions of the major duodenal papilla. J Hepatobiliary Pancreat Sci 2011; 18: 190–194. [DOI] [PubMed] [Google Scholar]
- 28. Patel R, Davitte J, Varadarajulu S, et al. Endoscopic resection of ampullary adenomas: complications and outcomes. Dig Dis Sci 2011; 56: 3235–3240. [DOI] [PubMed] [Google Scholar]
- 29. Jeanniard-Malet O, Caillol F, Pesenti C, et al. Short-term results of 42 endoscopic ampullectomies: a single-center experience. Scand J Gastroenterol 2011; 46: 1014–1019. [DOI] [PubMed] [Google Scholar]
- 30. Ardengh JC, Silva-Ney MV, Baron T, et al. Impact of technical modification of EUS-guided endoscopic papillectomy for ampullary neoplasm on the rate of post-resection acute pancreatitis. United Eur Gastroent J 2013; 1: A499. [Google Scholar]
- 31. Napoleon B, Gincul R, Ponchon T, et al. Endoscopic papillectomy for early ampullary tumors: long-term results from a large multicenter prospective study. Endoscopy 2014; 46: 127–134. [DOI] [PubMed] [Google Scholar]
- 32. Bagci S, Polat Z, Demirci H, et al. Endoscopic papillectomy of ampullary neoplasms: a single-center experience. United Eur Gastroent J 2015; 3: A528—A529. [Google Scholar]
- 33. Col Paulino RD, Rampazzo-Neto A, Varca M, et al. The influence of prophylactic plastic stent and main pancreatic duct size in post endoscopic papillectomy acute pancreatitis. Pancreatology 2017; 17: S38. [Google Scholar]
- 34. Gambitta P, Iannuzzi F, Pallotta S, et al. Pancreatic stent placement after endoscopic resection of ampullary tumors is mandatory. United European Gastroenterol 2017; J 5: A649. [Google Scholar]
- 35. Attila T, Parlak E, Alper E, et al. Endoscopic papillectomy of benign ampullary lesions: outcomes from a multicenter study. Turk J Gastroenterol 2018; 29: 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cho YD, Lee YS, Cha SW, et al. Prophylactic pancreatic stent placement after duodenal endoscopic snare papillectomy; prospective, randomized single center study. Gastrointestinal Endoscopy 2015; 81: AB162. [Google Scholar]
- 37. Fischer HP, Zhou H. Pathogenesis of carcinoma of the papilla of Vater. J Hepatobiliary Pancreat Surg 2004; 11: 301–309. [DOI] [PubMed] [Google Scholar]
- 38. Shemesh E, Nass S, Czerniak A. Endoscopic sphincterotomy and endoscopic fulguration in the management of adenoma of the papilla of Vater. Surg Gynecol Obstet 1989; 169: 445–448. [PubMed] [Google Scholar]
- 39. Andrade-Davila VF, Chavez-Tostado M, Davalos-Cobian C, et al. Rectal indomethacin versus placebo to reduce the incidence of pancreatitis after endoscopic retrograde cholangiopancreatography: results of a controlled clinical trial. BMC gastroenterology 2015; 15: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chahal P, Tarnasky PR, Petersen BT, et al. Short 5Fr vs long 3Fr pancreatic stents in patients at risk for post-endoscopic retrograde cholangiopancreatography pancreatitis. Clin Gastroenterol Hepatol 2009; 7: 834–839. [DOI] [PubMed] [Google Scholar]
- 41. Freeman ML. Pancreatic stents for prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis. Clin Gastroenterol Hepatol 2007; 5: 1354–1365. [DOI] [PubMed] [Google Scholar]
- 42. Choudhary A, Bechtold ML, Arif M, et al. Pancreatic stents for prophylaxis against post-ERCP pancreatitis: a meta-analysis and systematic review. Gastrointest Endosc 2011; 73: 275–282. [DOI] [PubMed] [Google Scholar]
- 43. Dultz G, Gerber L, Zeuzem S, et al. Prolonged retention of prophylactic pancreatic stents is not associated with increased complications. Pancreatology 2019; 19: 39–43. [DOI] [PubMed] [Google Scholar]
- 44. Elmunzer BJ, Scheiman JM, Lehman GA, et al. A randomized trial of rectal indomethacin to prevent post-ERCP pancreatitis. N Engl J Med 2012; 366: 1414–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rashdan A, Fogel EL, McHenry L, Jr, et al. Improved stent characteristics for prophylaxis of post-ERCP pancreatitis. Clin Gastroenterol Hepatol 2004; 2: 322–329. [DOI] [PubMed] [Google Scholar]
- 46. Elmunzer BJ, Higgins PD, Saini SD, et al. Does rectal indomethacin eliminate the need for prophylactic pancreatic stent placement in patients undergoing high-risk ERCP? post hoc efficacy and cost-benefit analyses using prospective clinical trial data. Am J Gastroenterol 2013; 108: 410–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li GD, Jia XY, Dong HY, et al. Pancreatic stent or rectal indomethacin-which better prevents post-ercp pancreatitis? A propensity score matching analysis. Medicine 2016; 95: e2994. [DOI] [PMC free article] [PubMed] [Google Scholar]