Abstract
Background:
Bone mineral density (BMD) decreases postrenal transplantation. Evidence demonstrating the effects of bisphosphonates on BMD and fracture risk beyond 1-year posttransplant is sparse in existing literature, but remains essential to enhance clinical outcomes in this population.
Objective:
Our study aimed to systematically review and meta-analyze the current literature on the use of any bisphosphonate in the adult renal transplant population beyond the first year of renal transplant to determine its effect on BMD and fracture incidence.
Design:
We conducted a systematic review and meta-analysis of primary research literature that included full-text, English-language, original randomized clinical trials (RCTs) and observational studies.
Setting:
Patient data were primarily captured in an outpatient setting across various studies.
Patients:
Our population of interest was patients older than 18 years who received deceased/living donor kidney transplantation and any bisphosphonate with a follow-up greater than 12 months posttransplantation.
Measurements:
The primary outcome was change in BMD from baseline. Secondary outcomes were the incidence of fractures and effects of other confounders on bone health.
Methods:
We included RCTs and observational studies that satisfied our inclusion criteria. Each study was analyzed for risk of bias and data were extrapolated to analyze for overall statistical significance accounting for heterogeneity of studies.
Results:
Sixteen studies (N = 1762) were analyzed. The follow-up ranged from 12 to 98 months. There was a nonsignificant improvement in BMD with bisphosphonate treatment persisting into the second and third years posttransplant at the lumbar spine. The calculated standardized mean BMD difference was −0.29 (−0.75 to 0.17), P = .22. Only 5 studies reported a total of 43 new fractures. Prednisone (P < .01), low body weight (P < .001), low body mass index (P < .01), and male gender (P < .05) correlated with reduced lumbar and femoral BMD.
Limitations:
Limitations of this review include the use of BMD as a surrogate outcome, the bias of the included studies, and the incomplete reporting data in numerous analyzed studies.
Conclusions:
We demonstrate no statistically significant benefit of bisphosphonate treatment on BMD beyond the first year postrenal transplantation. Despite heterogeneity of treatment, a differential nonsignificant improvement in lumbar spine BMD was consistent and may be clinically relevant.
Trial Registration:
PROSPERO CRD42019125593
Keywords: renal transplantation, osteodystrophy, bisphosphonate
Abrégé
Contexte:
La densité minérale osseuse (DMO) décroit à la suite d’une greffe rénale. Les données probantes faisant état des effets des bisphosphonates sur la DMO et le risque de fracture au-delà d’un an post-greffe sont rares dans la littérature, mais demeurent essentielles pour améliorer les résultats cliniques pour cette population.
Objectif:
L’étude actuelle visait à réaliser une revue systématique et une méta-analyse de la littérature faisant état de l’usage des bisphosphonates dans une population de greffés rénaux adultes, au-delà de la première année post-greffe, afin de connaître les effets de cette médication sur la DMO et sur l’incidence de fractures.
Type d’étude:
Une revue systématique et une méta-analyse de la littérature ont été réalisées à partir d’articles rédigés en anglais, présentant les résultats d’essais cliniques et d’études observationnelles.
Cadre:
Dans les différentes études, les données provenaient principalement de patients suivis sur une base externe.
Sujets:
Notre population d’intérêt était constituée de patients adultes ayant subi une greffe rénale provenant d’un donneur décédé ou vivant, ayant reçu un traitement par un bisphosphonate et ayant été suivis pendant plus de douze mois post-transplantation.
Mesures:
L’issue principale était une variation de la DMO par rapport à la valeur initiale. L’incidence de fractures et les effets des autres facteurs de confusion sur la santé osseuse constituaient les issues secondaires.
Méthodologie:
Ont été inclus les essais cliniques et les études observationnelles qui répondaient à nos critères d’inclusion. Chaque étude a fait l’objet d’une analyse des risques de biais et les données ont été extrapolées pour analyser la signification statistique de l’ensemble en tenant compte de l’hétérogénéité des études.
Résultats:
Seize études (n=1762) ont été analysées. La période de suivi variait de 12 à 98 mois. Une amélioration non significative de la DMO du rachis lombaire ayant persisté dans la deuxième et la troisième année post-greffe a été observée à la suite d’un traitement par un bisphosphonate. La moyenne normalisée calculée des variations de la DMO s’établissait à -0,29 (-0,75 à 0,17; p=0,22). Seules cinq études ont rapporté de nouvelles fractures, pour un total de 43 fractures. La prise de prednisone (p<0,01), un faible poids (p<0, 001), un faible IMC (p<0,01) et le fait d’être un homme (p<0,05) ont corrélé avec une DMO lombaire ou fémorale réduite.
Limites:
Le recours à la DMO comme issue intermédiaire, les biais contenus dans les études incluses et le fait que plusieurs des études analysées comportaient des données incomplètes constituent les limites de l’étude.
Conclusion:
Nous n’avons pu démontrer un avantage statistiquement significatif sur la DMO à poursuivre un traitement par les bisphosphonates au-delà de la première année suivant une greffe rénale. Malgré l’hétérogénéité du traitement, une amélioration non significative de la DMO lombaire a été observée et pourrait s’avérer pertinente sur le plan clinique.
What was known before
Rapid bone loss occurs in the first year postrenal transplantation but is a chronic disease. Existing systematic reviews identify a benefit of bisphosphonates in reducing bone mineral density (BMD) loss in the first 12 months posttransplant.
What this adds
This current review adds there is no statistically significant benefit to bisphosphonate treatment on BMD beyond 1 year postrenal transplantation.
Introduction
End-stage renal disease (ESRD) is associated with renal osteodystrophy (osteitis fibrosa, adynamic bone disease, and osteomalacia).1-4 A well-functioning renal allograft ameliorates many metabolic abnormalities associated with the development of mineral and bone disorders (MBDs) of ESRD. However, renal transplant recipients are particularly susceptible to bone damage due to a multitude of factors including preexisting bone disorders, immunosuppression, and alteration in the renal-bone metabolism axis.5,6
Bone mineral density (BMD) measured by dual-energy X-ray absorptiometry (DEXA) has been shown to decrease below 2 standard deviations (SDs) posttransplantation,7 with estimated 3%-7% loss in the lumbar spine in the first year.8-11 Ongoing vertebral bone loss (approximately 2%/yr) has been demonstrated in longitudinal evaluation of BMD in 70 renal transplant recipients.12
There are few studies demonstrating that low BMD predicts fractures in renal recipients. Akaberi et al13 showed that low hip BMD predicted fractures in 238 renal recipients. The prevalence of fractures in the posttransplant population is up to 4-fold greater than pretransplant statistics, ranging widely between 5% and 44%, likely due to variations in observation time, definitions of fractures included, and the presence of diabetes.14-17
There is currently no established strategy for the prevention of posttransplant osteopenia and osteoporosis. Bisphosphonates, which inhibit osteoclast activity, are widely accepted as a treatment for osteopenia and osteoporosis in the general population.18 They have also been shown to protect against bone loss in the renal transplant recipients,1,7,19,20 although a specific risk is the potential to exacerbate a baseline low-turnover adynamic state.1,21,22
The currently available research on bisphosphonate use in the renal transplant population is limited to analysis of randomized clinical trial (RCTs) within the first year posttransplant18,23-25 The first year posttransplant is wrought with confounding factors including the physiologic adjustments in metabolism, the uremic effects of ESRD, the fluctuant nature of the posttransplant course, and aggressive immunosuppression.
Our study aimed to systematically review and meta-analyze the current literature on the use of any bisphosphonate in the adult renal transplant population beyond the first year of renal transplant to determine its effect on BMD and fracture incidence.
Methods
Eligibility Criteria
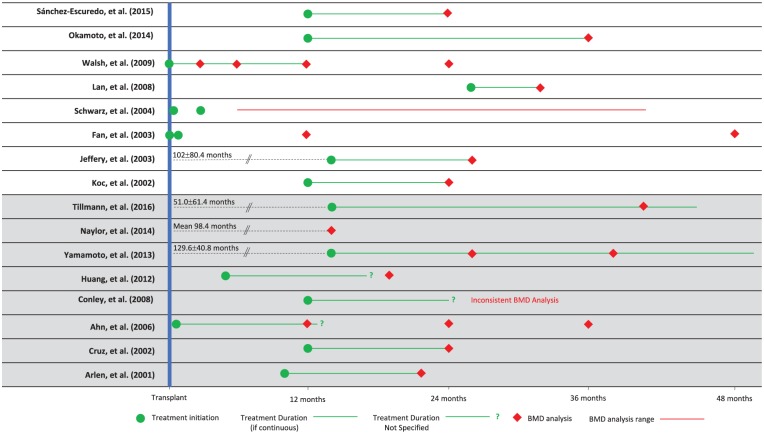
We conducted a systematic review and meta-analysis of primary research literature that included full-text, English-language, original RCTs and observational studies. Our population of interest was patients older than 18 years who received deceased/living donor kidney transplantation and any bisphosphonate with a follow-up greater than 12 months posttransplantation (Figure 1). Supplemental Table S1 summarizes the inclusion and exclusion criteria of our review.
Figure 1.
Summary of study timelines in postrenal transplant patients on bisphosphonate therapy.
Note. BMD = bone mineral density.
Search Strategy
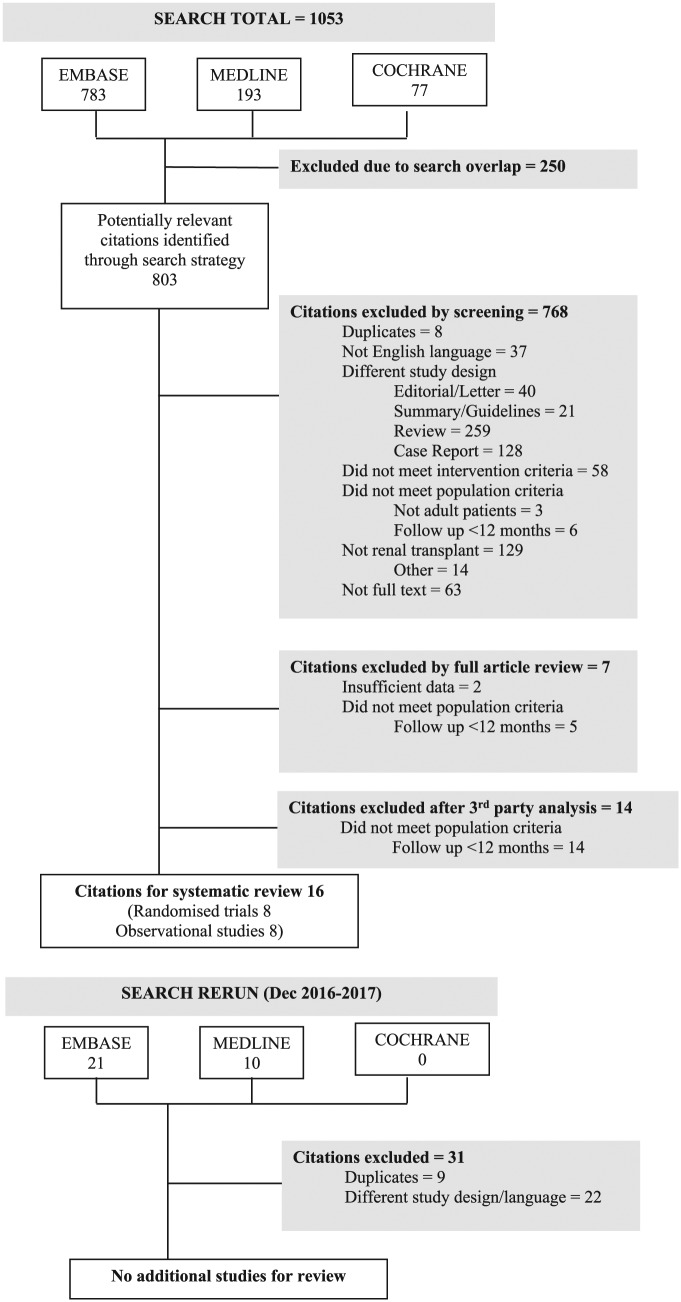
Electronic searches were performed in MEDLINE, EMBASE, and the Cochrane Register of Controlled Trials (CENTRAL) between 1946 and 2017 (Figure 2). A sample search strategy is outlined in Supplemental Figure 1.
Figure 2.
Schema of literature search.
Data Extraction and Outcome Measures
Each included study was assessed in conjunction by 2 authors (A.L. and A.W.) for data extraction (Tables 1-4). The primary outcome was the change in BMD from baseline. The secondary outcomes were the incidence of fractures and the effects of other confounders that may modify the risk of osteoporosis and fractures.
Table 1.
Duration of Bisphosphonate Therapy and Follow-up in Postrenal Transplant Patients.
| Study | Treatment | Concomitant treatment | Treatment start date | Treatment duration | Time of outcome analysis from treatment initiation |
|---|---|---|---|---|---|
| Sánchez-Escuredo et al26 | Oral ibandronate 150 mg monthly (n = 35) Control: Oral risedronate 35 mg weekly (n = 34) |
CaCO3 2500 mg Vitamin D 800 IU |
At least 12 months posttransplant | 12 months | 12 months |
| Okamoto et al27 | Oral alendronate 35 mg per week (n = 5) Control: no bisphosphonates (n = 7) |
Not specified | At least 12 months posttransplant | 24 months | 24 months |
| Walsh et al28 | IV pamidronate 1 mg/kg within 14 days of transplant and 1,
4, 8, and 12 months after transplant (n =
46) Control: no intervention (n = 47) |
Calcium CaCO3 500 mg Cholecalciferol 400 IU |
At transplantation | 12 months | 3, 6, 12, and 24 months |
| Lan et al29 | Alendronate 70 mg/wk for 6 months (n = 23) Control: no intervention (n = 23) |
CaCO3 800 mg Calcitriol 0.25 µg for 6 months |
Treatment: 25.9 ± 10.6 months Control: 27.1 ± 12.4 months |
6 months | 6 months |
| Schwarz et al30 | IV zoledronic acid 4 mg at 2 weeks and 3 months (n =
9) Control: placebo (n = 10) |
Calcium citrate 1000 mg daily for first 6 months | At time of transplantation | 2 doses—at 2 weeks and 3 months posttransplant | 6 to 36 months |
| Fan et al31 | IV pamidronate 0.5 mg/kg at the time of transplant and 1
month later (n = 9) Control: 500 mL NS (n = 8) |
Not specified | At the time of transplantation | 2 doses—at the time of transplant and 1 month later | 12 and 48 months |
| Jeffery et al32 | Alendronate 10 mg/d open label (n = 57; 46 completed 1
year) Control: Calcitriol 0.25 µg/d (n = 60; 51 completed 1 year) |
Calcium 500 mg/d | 102 ± 80.4 months (8.5 ± 6.7 years) posttransplantation | 12 months | 12 months |
| Koc et al33 | 1: Alendronate 10 mg/d (n = 8) 2: Calcitriol 0.5 µg/d (n = 8) Control: no intervention (n = 8) |
Calcium 1000 mg/d | Minimum duration of renal transplant was 12 months | Study period: 12 months | 12 months |
| Tillmann et al34 | IV ibandronate 3 mg every 3 months (average dose 12.0 ± 6.7
g) (n = 30) Control: No intervention (n = 30) |
Not specified | 51.0 ± 61.4 months after transplant First BMD measured at minimum 14 months posttransplant |
Average 19.3 ± 11 months | 26.8 ± 12.1 months after first measurement |
| Naylor et al35 | Unspecified bisphosphonate, grouped into “osteoporosis
treatment”—includes >90% bisphosphonate, nasal
calcitonin, raloxifene, systemic estrogen (n =
329) No comparator |
— | — | — | Mean 98.4 months (8.2 years) follow-up from study start |
| Yamamoto et al36 | Oral alendronate 35 mg/wk (n = 24) No control group |
Not specified | 129.6 ± 40.8 months (10.8 ± 3.4 years) posttransplant |
36 months treatment period | 12 and 24 months |
| Huang et al37 | Alendronate 70 mg/wk (n = 41) Control: no intervention (n = 35) |
Not specified | At least 5 months posttransplantation | Duration of treatment unclear | 14 ± 1.6 months |
| Conley et al38 | Bisphosphonate as prescribed by treating physician (n =
315) Control: no intervention (n = 239) |
Intervention: Calcium = 170 (71.1%) Vitamin D = 35 (14.6%) Active vitamin D = 11 (4.6%) Control: Calcium = 220 (69.8%) Vitamin D = 83 (26.3%) Active vitamin D = 39 (12.4%) |
12 months posttransplant | Treatment duration not specified | Not consistent |
| Ahn et al39 | Oral alendronate sodium or risedronate sodium (dose not
specified) Control: alfacalcidol (dose not specified) (n = 294, total) |
Calcium (dose not specified) | In study group, within 1 month of transplant | Treatment duration not specified | 12, 24, and 36 months posttransplant |
| Cruz et al40 | Oral alendronate 10 mg/d (n = 29, high risk) Control: no intervention (n = 28, low risk) |
None | 12 months posttransplant | Study period: 12 months | 12 months |
| Arlen et al41 | Oral etidronate disodium 400 mg for 2 weeks of every 12
weeks (n = 25) Control: no intervention (n = 24) |
Calcium replacement and Vitamin D at the discretion of the
treating physicians Patients held medication during the 2 weeks of treatment Intervention: CaCO3 (n = 3) Calcitriol (n = 4) Control: CaCO3 (n = 3) Calcitriol (n = 8) |
Treatment: 10.4 ± 5.3 months after
transplant Control: 10.7 ± 4.5 months |
Study period: 12 months | 12 months |
Note. IU = international unit; IV = intravenous; NS = normal saline; BMD = bone mineral density. White is RCT, Grey shaded region is Observational trials.
Table 2.
Baseline Characteristics of Included Studies.
| Study | Year | Population (male/female) | Mean age (mean ± SD) | Time after transplant | Intervention | Comparator | Funding support |
|---|---|---|---|---|---|---|---|
| Randomized control trials | |||||||
| Sánchez-Escuredo et al | 2015 | 77 (19/58) |
Ibandronate: 63 ± 12 Risedronate: 64 ± 10 |
Ibandronate: 20 ± 8 months Risedronate: 18 ± 7 months |
Ibandronate 150 mg/month Vitamin D 800 IU CaCO3 2500 mg (n = 38) |
Risedronate 35 mg/wk Vitamin D 800 IU CaCO3 2500 mg (n = 39) |
NA |
| Okamoto et al | 2014 | 12 (8/4) |
Alendronate: 52.8 ± 12.6 Control: 52.9 ± 7.3 |
Alendronate: 59.6 ± 59.5 months Control: 45.3 ± 42.3 months |
Alendronate 35 mg/wk for 24 months (n = 5) |
No intervention (n = 7) |
MSD K.K. |
| Walsh et al | 2009 | 93 (69/24) |
Treatment: 46.1 ± 12.77 Control: 46.1 ± 12.93 |
0 | Pamidronate IV 1 mg/kg peri-op, 1, 4, 8 and 12
months CaCO3 500 mg Cholecalciferol 400 units (n = 46) |
CaCO3 500 mg Cholecalciferol 400 units (n = 47) |
Novartis |
| Lan et al | 2008 | 46 (19/27) |
Treatment: 40.2 ± 18.5 Control: 39.4 ± 17.3 |
Treatment: 25.9 ± 10.6 months Control: 27.1 ± 12.4 months |
Alendronate 70 mg/wk CaCO3 800 mg/d Calcitriol 0.25 µg/d (n = 23) |
CaCO3 800 mg/d Calcitriol 0.25 µg/d (n = 23) |
Not reported |
| Schwartz et al | 2004 | 19 (not reported) |
Not reported | 0 | Two infusions of 4 mg zoledronic acid at 2 weeks and 3
months posttransplant (n = 9) |
Placebo (n = 10) |
Not reported |
| Fan et al | 2003 | I26 (26/0) |
Treatment: 46.2 (21.1-67.1) Control: 41.5 (21.3-65) Mean age calculated with only patients with BMD measurements at 4 years |
Not reported | Pamidronate IV 0.5 mg/kg in 500 mL NS peri-op and at 1
month (n = 14) |
500 mL NS (n = 12) |
Not reported |
| Jeffrey et al | 2003 | 117 (97 completed treatment) (71/26) |
Treatment: 44.8 ± 11.6 Control: 45.9 ± 10.8 Mean age documented is at the time of transplant and calculated with only patients who completed treatment |
Treatment: 85.2 ± 62.4 months Control: 115.2 ± 81.6 months Mean duration calculated with only patients who completed treatment |
Alendronate 10 mg/d open label Calcium (unspecified dose) (n = 60) 46 completed treatment |
Calcitriol 0.25 µg/d Calcium (unspecified dose) (n = 57) 51 completed treatment |
Not reported |
| Koc et al | 2002 | 24 (17/7) |
Alendronate: 34.3 ± 8.9 Calcitriol: 40.5 ± 8.1 Control: 35.5 ± 8.4 |
Alendronate: 48.7 ± 50.1 months Calcitriol: 47.4 ± 46.4 months Control: 41.5 ± 37.1 months |
I1: Alendronate 10 mg/d Calcium 1000 mg/d (n = 8) I2: Calcitriol 0.5 µg/d Calcium 1000 mg/d (n = 8) |
Calcium 1000 mg/d (n = 8) |
Not reported |
| Observational studies | |||||||
| Naylor et al | 2014 | 326 (199/27) |
46.1 ± 12 | 6.12 (2.4-24) months | >90% bisphosphonates, nasal calcitonin, raloxifene, systemic estrogen | No control group | NA |
| Yamamoto et al | 2013 | 24 (12/12) |
52 ± 7.8 | 129.6 ± 40.8 months | Alendronate 35 mg/d | No control group | NA |
| Huang et al | 2012 | 76 (36/40) |
Treatment: Male: −51.9 ± 9.0 Female: −53.3 ± 8.8 Control: Male: −48 ± 10.4 Female: −49.7 ± 7.6 |
Treatment: Male: −103.7 ± 59.4 Female: −53.3 ± 8.8 Control: Male: −92 ± 68.1 Female: −61.4 ± 39.6 |
Alendronate 70 mg/wk (n = 34) |
No intervention (n = 42) |
NA |
| Conley et al | 2008 | 554 (320/234) |
Treatment: 45.9 ± 0.7 Control: 46.9 ± 0.2 |
Not reported | Bisphosphonate, type not described Calcium = 170 (71.1), Vitamin D = 35 (14.6), Active vitamin D = 11 (4.6) (n = 315) |
Calcium = 220 (69.8) (NS) Vitamin D = 83 (26.3) (P = .001) Active vitamin D = 39 (12.4) (P = .003) (n = 239) |
Not reported |
| Ahn et al | 2006 | 294 | Not reported | Within 1 month | Calcium Alfacalcidol or bisphosphonate (alendronate or risendronate) |
No intervention | Not reported |
| Cruz et al | 2002 | 58 (39/19) |
Treatment: 48.6 ± 2.0 Control: 46.2 ± 2.0 |
Treatment: 97.2 ± 8.4 months Control: 84 ± 10.8 months |
Alendronate 10 mg/d (n = 29) |
No intervention (n = 29) |
NCRR grant, National Kidney Foundation of Connecticut, NIH |
| Arlen et al | 2001 | 49 (29/20) |
Treatment: 41 ± 13 Control: 42 ± 12 |
Treatment: 10.4 ± 5.2 months Control: 10.8 ± 4.5 months |
Etidronate disodium 400 mg for 2 weeks out of every
12 CaCO3 (n = 3) Calcitriol (n = 4) (n = 25) |
No intervention CaCO3 (n = 3) Calcitriol (n = 8, P < .03) (n = 24) |
Not reported |
| Tillmann et al | 2001 | 60 (24/36) |
Treatment: 47.9 ± 13.4 Control: 45.7 ± 11.4 Mean age documented is at the time of transplant |
Treatment: 51 ± 61.4 months Control 59.6 ± 59.7 months |
Ibandronate 3 mg IV Q3 months (n = 30) |
No intervention (n = 30) |
None |
Note. IU = international unit; NA = not applicable; IV = intravenous; BMD = bone mineral density; NS = normal saline; NCRR = National Center for Research Resources; NIH = National Institutes of Health.
Table 3.
Change in Bone Mineral Density in Postrenal Transplant Patients Between Bisphosphonate and Control Groups.
| Study | BMD | ||
|---|---|---|---|
| Lumbar | Femoral neck | Other | |
| Sánchez-Escuredo et al26
I1: n = 35 I2: n = 34 |
T-score at pretreatment/12 months I1: −1.7 ± 0.8/−1.4 ± 0.6 I2 −1.9 ± 0.8/−1.5 ± 0.8 |
T-score at pretreatment/12 months I1: −2.1 ± 0.7/−1.8 ± 0.9 I2: −2.2 ± 0.6/−1.8 ± 0.8 |
|
| Okamoto et al27
I: n = 5 C: n = 7 |
NA | NA | Total BMD: % change from baseline I: 1.86% ± 0.85%, P < .05 No other raw data provided |
| Walsh et al28
I: n = 46 C: n = 47 |
% change in BMD at 12 months from baseline AMTD: 7.78%, 95% CI: 5.15-10.41, P < .001 Significant difference between groups at 24 months No raw data |
% change in BMD at 12 months from baseline AMTD: 2.51%, 95% CI: −0.33 to 5.35, P = .08 No significant difference between groups at 24 months No raw data |
Ward’s area: Significant increase in I vs C at both 12 and 24 months AMTD: 5.83%, 95% CI: 2.19-9.45, P < .01 Total hip: Significant increase in I vs C at both 12 and 24 months AMTD: 2.79%, 95% CI: 0.92-4.67, P < .01 |
| Lan et al29
I: n = 23 C: n = 23 |
Mean g/cm2 at pretreatment/6 months I: (L1) 0.781 ± 0.117/0.820 ± 0.114, NS C: (L1) 0.760 ± 0.062/0.771 ± 0.069, NS |
Mean g/cm2 at pretreatment/6 months I: 0.650 ± 0.107/0.731 ± 0.109, P < .05 C: 0.657 ± 0.061/0.676 ± 0.060, NS Significant difference between groups at 6 months posttreatment,P < .05 |
Trochanter: Mean g/cm2 at pretreatment/6 months I: 0.524 ± 0.093/ 0.572 ± 0.103, NS C: 0.54 ± 0.082/ 0.561 ± 0.079, NS |
| Schwarz et al30
I: n = 9 C: n = 10 |
Z-score at 6 months to 32 months posttreatment I: No difference C: No difference NS between groups No raw data |
Z-score at 6 months to 32 months posttreatment I: −1.6 (2.9) to −1.2 (1.9), P < .05 between groups, P < .05 C: −1.3 (2.6) to −0.2 (3.6), P < .05 between groups, P < .05 median and range |
|
| Fan et al31
I: n = 9 C: n = 8 |
Mean g/cm2 at baseline/1 year/4 years I: 1.15 ± 0.07/(1 year) 1.11 ± 0.05, NS/(4 years) 1.10 ± 0.04, NS C: 1.27 ± 0.07/(1 year) 1.2 ± 0.5, P < .05/(4 years) 1.21 ± 0.08, NS |
Mean g/cm2 at baseline/1 year/4 years I: 0.93 ± 0.05/(1 year) 0.94 ± 0.04, NS/(4 years) 0.88 ± 0.04, NS C: 1.08 ± 0.07/ (1 year) 0.98 ± 0.06, P < .05/(4 years) 0.94 ± 0.06, P < .01 |
|
| Jeffery et al32
I: n = 57 C: n = 60 |
Mean g/cm2 at pretreatment/12 months I: 0.984 ± 0.149/1.025 ± 0.143, P < .001 C: 1.014 ± 0.15/1.034 ± 0.146, P < .01 NS between groups at 12 months (P = .082) |
Mean g/cm2 at pretreatment/12 months I: 0.809 ± 0.092/0.836 ± 0.107, P < .001 C: 0.830 ± 0.144/0.857 ± 0.125, P < .05 NS between groups at 12 months (P = .96) |
|
| Koc et al33
I1: n = 8 I2: n = 8 C: n = 8 |
Mean g/cm2 at 12 months/pretreatment I1: 1.050 ± 0.086/1.122 ± 0.094, P < .01 I2: 0.963 ± 0.142/ 1.034 ± 0.119, P < .05 C: 1.082 ± 0.187/1.095 ± 0.142, NS % change of BMD I1: 8.15% ± 9.2% I2: 6.89% ± 4.03% C: 0.06% ± 1.41% P < .05 compared with control |
Mean g/cm2 at 12 months/pretreatment I1: 0.826 ± 0.121/ 0.902 ± 0.092, P < .05 I2: 0.816 ± 0.121/0.902 ± 0.092, NS C: 0.933 ± 0.082/0.947 ± 0.082, NS % change of BMD I1: 9.34 ± 10.47% I2: 8.51 ± 13.8% C: 1.92 ± 2.52% NS compared with control |
|
| Tillmann et al34
I: n = 30 C: n = 30 |
Z-score at pre-treatment/26.8 ± 12.1 months I: −2.25 ± 1.11/−1.78 ± 1.30, P < .05 C: −0.52 ± 1.52/−0.28 ± 1.55, P < .05 Change in BMD I: 0.055 ± 0.066 C: 0.033 ± 0.079 NS between groups, P = .217 |
Z-score at pretreatment/26.8 ± 12.1 months I: −1.97 ± 0.85/−1.73 ± 0.71, P < .05 C: −0.69 ± 1.31/−0.55 ± 1.12, P < .05 Change in BMD I: 0.025 ± 0.077 C: 0.013 ± 0.106 NS between groups, P = .647 |
|
| Naylor et al35
n = 329 |
Z-score −0.4 ± 1.6 At median 6 months (baseline) −0.2 ± 1.6, P < .001 vs baseline At mean 2.7 years +0.5 ± 1.5, P < .001 vs baseline At mean 8.2 years |
Z-score −0.7 ± 1.1 At median 6 months (baseline) −0.6 ± 1, P < .01 vs baseline At mean 2.7 years +0.1 ± 1.5, P < .001 vs baseline At mean 8.2 years |
Hip Z-score −0.7 ± 1.1 At median 6 months (baseline) −0.6 ± 1.1, P < .01 vs baseline At mean 2.7 years −0.5 ± 1.1, P < .001 vs baseline At mean 8.2 years |
| Yamamoto et al36
n = 24 |
Mean g/cm2 pretreatment/12 months/24
months (Baseline) 0.80 ± 0.11/(12-month) 0.78 ± 0.12, NS/(24-month) 0.79 ± 0.15, NS |
||
| Huang et al37
I: n = 34 C: n = 42 |
For 76 included patients T-score at pretreatment/14 ± 1.6 months −1.53 ± 1.24/−1.32 ± 1.26, P < .001 BMD: Mean g/cm2 at pretreatment/14 ± 1.6 months 0.90 ± 0.14/0.92 ± 0.14, P = .001 |
T-score at pretreatment/14 ± 1.6 months −2.45 ± 0.96/−2.42 ± 1.02, NS BMD: Mean g/cm2 at pretreatment/14 ± 1.6 months 0.68 ± 0.12/ 0.69 ± 0.13, NS |
Hip: T-score at pretreatment/14 ± 1.6 months −1.76 ± 0.97/−1.68 ± 1.07, NS BMD: Mean g/cm2 at pretreatment/14 ± 1.6 months 0.81 ± 0.14/0.81 ± 0.14, NS |
| Conley et al38
I: n = 315 C: n = 239 |
T-score at mean 1.2 ± 0.05 years posttransplant/2.5 ± 0.05
years posttransplant I: −1.4 ± 1.3/−1.0 ± 1.3 C: 1.3 ± 0.5/0 ± 1.4 P < .001 between groups |
T-score at mean 1.2 ± 0.05 years posttransplant/2.5 ± 0.05
years posttransplant I: −1.9 ± 1/−1.7 ± 1 C: 1.0 ± 0/−1.0 ± 1.0 P < .001 between groups |
|
| Ahn et al39
n = 294 |
Mean different in T-score at 12, 24, and 36 months compared
with baseline 1 year C/I: −0.51 ± 0.66/−0.13 ± 0.73, P < .05 (n = 275/19) 2 year C/I: −0.76 ± 0.74/−0.4 ± 0.79, P < .05 (n = 151/45) 3 year C/I: −0.83 ± 0.83/−0.5 ± 0.8, P = .10 (n = 74/32) |
Mean different in T-score at 12, 24 and 36 months compared
with baseline 1 year C/I: −0.22 ± 0.69/0.13 ± 0.55, P < .05 (n = 273/19) 2 year C/I: −0.38 ± 0.82/−0.1 ± 0.77, P = .20 (n = 150/44) 3 year C/I: −0.43 ± 0.85/0.20 ± 0.94, P = .21 (n = 75/31) |
|
| Cruz et al40
I: n = 29 C: n = 28 |
T-score at pretreatment/12 months I: −1.71 ± 0.19/no raw data result (change in T-score: + 3.4% ± 0.6%, P < .001) C: −0.70 ± 0.24/no raw data result |
T-score at pretreatment/12 months I: −1.43 ± 0.13/−1.34 ± 0.14(P < .01) (change in T-score +1.6 ± 0.6%, P < .001) C: −1.10 ± 0.15/no raw data result |
Total femur: T-score at pretreatment/12 months I: −1.43 ± 0.13/−1.34 ± 0.14 (P < .01) (change in T-score: +1.6 ± 0.6%, P < .001) C: −0.67 ± 0.16/no raw data result |
| Arlen et al41
I: n = 25 C: n = 24 |
Mean g/cm2 at pretreatment/23.3 ± 6.6
months I: 0.981 ± 0.138/1.021 ± 0.140 C: 1.134 ± 0.168/1.143 ± 0.175 Change in % BMD (I vs C) 4.3% ± 6.1% vs 0.55 ± 5.3%, P < .05 |
Mean g/cm2 at pretreatment/23.3 ± 6.6 months
(change in % BMD) I: 0.784 ± 0.102/ 0.810 ± 0.110 C: 0.867 ± 0.137/ 0.893 ± 0.147 Change in % BMD (I vs C) 3.4 ± 6.5% vs 3.2 ± 6.4% NS |
Trochanter: Mean g/cm2 at pretreatment/23.3 ± 6.6 months (change in % BMD) I: 0.619 ± 0.094/0.683 ± 0.126 C: 0.725 ± 0.116/ 0.738 ± 0.109 Change in % BMD (I vs C) 10.3 ± 11.9% vs 2.2 ± 5.7%, P < .05 |
Note. BMD = bone mineral density; I = intervention; C = control; n = number; AMTD = adjusted mean treatment difference; CI = confidence interval. White is RCT, Grey shaded region is Observational trials.
Table 4.
Fracture Incidence in Postrenal Transplant Patients Between Bisphosphonate and Control Groups.
| Study | Protocol | Baseline fractures | New fracture incidence | Findings | ||
|---|---|---|---|---|---|---|
| Walsh et al28 | Spine radiographs at baseline, 12, 24
months Blinded interpretation using Genant et al42 scale |
N = 23/93 total 12/46 in intervention group (1 axial) 11/47 in control group |
At 12 months | At 24 months | 4.2% (−7.3 to 16.6) difference between groups at
12 months (P = .7) 8.4% (−3.7 to 22.2) between groups at 24 months (P = .3) |
|
| I (n = 46): | 2 | 2 (3.3%/yr) |
||||
| C (n = 47): | 4 | 6 (6.4%/yr) |
||||
| Schwarz et al30 | Not formally assessed as endpoint | Not assessed | Between 6 months and 3 years: I (n = 9): 2 vertebral fractures C (n = 10): 2 vertebral fractures |
No analysis done | ||
| Yamamoto et al36 | Nontraumatic (low energy) fractures Assessed via personal interviews and medical records |
N = 7/24 (4 wrist, 2 rib, 1 leg, 1 cuboidal) |
4 patients with 5 fractures during 3-year
period (2 leg, 1 lumbar spine, 1 hip, 1 humeral) |
New fractures correlated with higher intact PTH levels
(pg/mL) at baseline: Fracture (−) = 116.0 ± 52.6 Fracture (−) = 255.0 ± 3.0 (P < .0001) |
||
| Conley et al38 | Self-reported Counted if occurring between BMD1 and BMD2 (both occurring >1 year posttransplant) |
I: 56/315 C: 16/239 Significantly more patients with fracture in intervention group (P = .0002) |
I: 16 C: 7 (P < .05) Increase in bone density between BMD1 and BMD2 did not prevent late fractures |
Treatment associated with decreased probability of
fracture-free survival (HR = 0.40; 95% CI = 0.29-0.73,
P = .001) No association found between rate of bone loss and fractures, regardless of the bisphosphonate therapy |
||
| Arlen et al41 | Not formally assessed as endpoint | Not assessed | I (n = 25): n = 2 C (n = 24): n = 1 |
All patients who sustained fractures were from high-risk treatment group (BMD lower than mean baseline of control group) | ||
Note. I = intervention; C = control; BMD = bone mineral density; PTH = parathyroid hormone; HR = hazard ratio; CI = confidence interval. White is RCT, Grey shaded region is Observational trials.
Bias Assessment
Articles were independently assessed by each reviewer (A.L. and A.W.), and dichotomized to low/high risk of bias based on standardized scoring systems. An RCT was considered low risk if it satisfied a score of 8 or more based on the Cochrane Risk of Bias Tool Criteria (Supplemental Table S2).43 An observational trial was considered low risk if it satisfied a score of 3 or more based on the Newcastle-Ottawa Criteria (Supplemental Table S3).44
Statistical Analysis
A standardized mean difference (SMD) and its 95% confidence interval (CI) were calculated to account for heterogeneity of different units of pre- and posttransplant measurements.45 Using the 95% CIs, the SDs were then derived.46 Subsequently, forest/funnel plots were created using the Cochrane Collaboration RevMan v5.3 software.46 A random effects model was used to account for clinical heterogeneity of the meta-analyzed studies. Values of I2 >50% and P < .10 were considered to indicate significant heterogeneity.
Results
Description of the Search
The search strategy yielded 1084 articles between 1946 and 2017. All titles and abstracts were reviewed independently by 2 authors (A.L. and A.W.) in accordance with inclusion criteria (Figure 2). Thirty-five articles were fully reviewed. Fourteen articles were differentially categorized between reviewers. These were independently reviewed and resolved by a third author (D.T.W.) to ascertain eligibility.
Description of Studies
Sixteen studies met full inclusion criteria (Table 1), including 8 randomized trials26-33 and 8 observational studies.34-41 Two RCTs28,30 and 7 observational studies,34-36,38-41 were considered to have low risk of bias (Supplemental Figures S2 and S3).
The total sample size was 1762 patients; 683 patients were treated with bisphosphonates while the remaining were allocated to various comparison groups. Bisphosphonates used included alendronate, alendronate/risedronate, pamidronate, zolendronate, ibandronate, and etidronate. Comparators included no therapy, calcium and/or vitamin D, calcitriol, placebo, an alternative bisphosphonate, and no control group. Baseline characteristic data of each study are summarized in Table 2.
From the RCTs, 226 patients were prescribed a bisphosphonate with a concomitant treatment with Ca and/or vitamin D in 212 patients. Bisphosphonate-treatment duration ranged from 1 to 24 months, with a follow-up duration of 12 to 24 months (Table 1). From the observational studies, 457 patients were prescribed a bisphosphonate. Of these patients, 223 had a concomitant treatment with Ca and/or vitamin D. Precise treatment was not clearly specified in an additional 624 patients. The range for bisphosphonate treatment was 12 to 36 months with a follow-up duration of 12 to 98.4 months (Table 1).
BMD measurement was performed using DEXA in all studies, with results most often expressed as T-scores representing the number of SDs that the measurement falls from the mean of a young population. A T-score of −1 to −2.5 describes osteopenia and less than −2.5 is diagnostic of osteoporosis.47 Results were also reported as bone mineral content or Z-scores that describe the number of SDs from the mean value of gender and age-matched adults (Z-score less than or equal to −2 suggests abnormal bone loss).
Change in BMD
One year posttransplant
Thirteen studies demonstrated at least one site of improvement in BMD, while the other 3 studies30,32,36 showed nonsignificant changes. However, only 2 studies28,39 were able to capture patient data from the immediate peri-transplant period. At 12 months posttransplant, Walsh et al28 identified a significant change in BMD in the intervention group vs control group, at the lumbar spine, +2.3% vs −5.7%, adjusted mean treatment difference (AMTD) 7.78%, P < .001. T-scores were also significantly different in the intervention vs control group at both the lumbar spine (−0.13 ± 0.73 vs −0.51 ± 0.66, P < .05) and the femoral neck (0.13 ± 0.55 vs −0.22 ± 0.69, P < .05) at 12 months.39 Both intervention groups showed significant improvement with bisphosphonate persisting into the second28,39 and the third year39 posttransplantation at the lumbar spine, with no significant difference at the femoral neck (Table 3).
One year postinitiation of bisphosphonate
Thirteen studies captured BMD data at least 12 months postinitiation of bisphosphonate treatment. Only 2 of these studies did not exhibit a significant increase in BMD32 or Z-score30 in the intervention vs control group in the lumbar spine at 12 and 32 months posttreatment.32
Unlike the lumbar spine, all studies showed minimal change in BMD measurements at the femoral neck except for 3 studies31,33,39 that showed a significant change (Table 3). Result interpretation of the significantly different Z-scores between groups posttreatment was inconclusive in the study by Tillmann et al34 as pretreatment measurements were also different. Cruz et al40 also found different T-scores at the femoral neck posttreatment (change in T-score +1.6% ± 0.6%, P < .001), but did not provide raw data of the control group to allow for comparison.
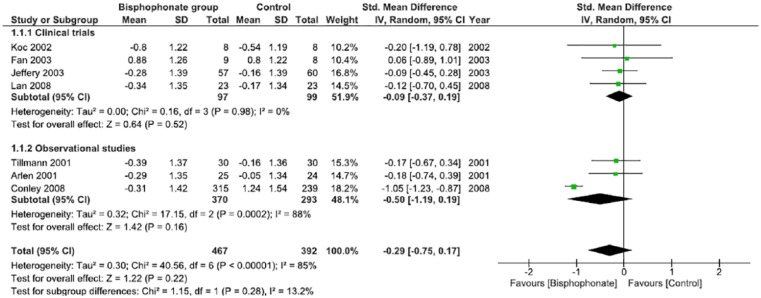
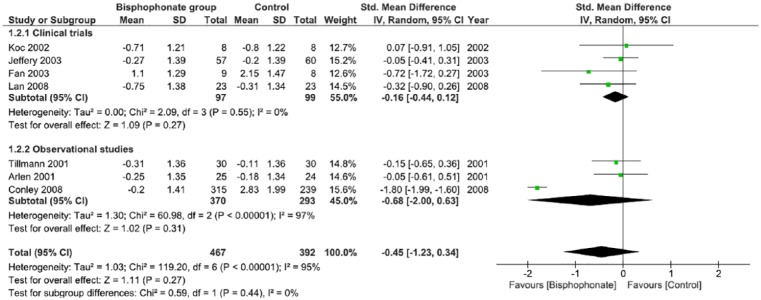
Using the calculated SMD between the intervention (bisphosphonate) and control groups, Figures 3 and 4 summarize the study findings that include pre and postbisphosphonate treatment information on BMD, at the lumbar spine and femoral neck, respectively. Data from 4 RCTs29,31-33 and 3 observational studies34,38,41 were included in analysis. There is a nonsignificant improvement in BMD favoring the use of bisphosphonates, as evidenced at both the lumbar spine and femoral neck. There was no statistical heterogeneity noted when a random effects model was used. The funnel plot demonstrates reasonable dispersion (Supplemental Figure S4).
Figure 3.
Change in bone mineral density at the lumbar spine in postrenal transplant patients between bisphosphonate and control groups.
Note. CI = confidence interval.
Figure 4.
Change in bone mineral density at the femoral neck in postrenal transplant patients between bisphosphonate and control groups.
Note. CI = confidence interval.
Fracture Incidence
Fracture incidence was low but only reported in 5 studies28,30,36,38,41 with a total of 43 new fractures. Conley et al38 reported benefit from bisphosphate treatment and decreased fracture rates (hazard ratio [HR] = 6.7, 95% CI = 6-6284, P < .01), despite only a small subset of patients (n = 3) with baseline osteoporosis at the femoral neck. Conley et al38 noted that bisphosphonate treatment was associated with decreased probability of fracture-free survival (HR = 0.40, 95% CI = 0.29-0.73, P = .001) in the initial analysis, even though treatment was associated with significant reduction in bone loss at the femoral neck (HR = 1.56, 95% CI = 1.21-2.06, P < .001) and lumbar spine (HR = 1.48, 95% CI = 1.13-1.98, P < .01). However, after adjusted analysis, no association was identified between bone loss and fractures regardless of the bisphosphonate treatment.38
Confounding Factors Affecting BMD
Immunosuppression
Four studies32,35,37,39 examined the effects of steroids on bone health. At baseline, patients with osteoporosis received a greater cumulative steroid dose than patients with osteopenia (1326.5 mg vs 724.5 mg; P < .01).37 In a univariate analysis, prednisolone use was associated with osteoporosis (odds ratio [OR] = 5.18; 95% CI = 1.6-16.4, P < .01).37 Jeffery et al32 described prednisone as an independent predictor of low BMD (multivariate, P < .01). Alternatively, Naylor et al35 found greater glucocorticoid exposure was not associated with a significant change in BMD at the lumbar spine, total hip, and femoral neck (P > .05), regardless of whether the patient had received osteoporosis treatment before. Similarly, no BMD differences were observed, 1 year posttransplant, in recipients receiving steroids.39 The effects of cyclosporine on BMD were examined in 2 studies37,39 and demonstrated no effects up to 1 year posttransplant.
Body mass index
Three studies32,35,39 found that low body weight (P < .001) and body mass index (BMI) (P < .01) were correlated with reduced lumbar and femoral BMD in a univariate analysis.32 Greater BMI was associated with a better BMD.35,39
Gender
Five studies32,34,35,37,39 examined the role of gender in BMD posttransplantation. Only one study32 identified a baseline association between female gender and reduced lumbar and overall BMD (P < .05). The other studies found no significant difference in gender with respect to change in BMD, although bone density may change differentially depending on site in males and females.35,37 Alendronate increased the BMD at the lumbar spine and the hipbone in males (P < .05), but only at the lumbar spine in females (P < .05).37 Male gender was also associated with a greater improvement in lumbar spine BMD in patients receiving osteoporosis treatment (P < .01).35
Diabetes
Three studies32,37,39 examined the role of diabetes in bone loss, but none investigated the duration or control of diabetes pretransplantation. One study32 identified pretransplantation diabetes as an independent risk factor for low BMD (P < .001), while the other 2 found a greater reduction in T-score at the lumbar spine in nondiabetic recipients (−0.52 ± 0.67 vs −0.15 ± 0.50, P < .01).39 Consequently, diabetes was not a significant predictive factor in BMD (OR = 0.6).37
Hemodialysis (HD) pretransplant
Only one study examined the impact of pretransplant HD duration on BMD.39 The mean change reduction in T-score at the lumbar spine in the first year posttransplant was significantly greater in recipients who had been on HD for ≥12 months compared with those who had experienced dialysis <12 months (−0.67 ± 0.79 vs −0.39 ± 0.57, P = .001).39
Smoking
In a multivariate analysis,37 smoking was not a risk factor of BMD change posttransplantation (see Supplemental Table S2).
Discussion
This systematic review and meta-analysis is the first to investigate the bisphosphonate effects on increasing BMD and fracture prevention beyond the first year postkidney transplantation. A recently published meta-analysis by Wang et al25 demonstrated that bisphosphonate treatment in general had a beneficial effect on BMD changes at both the lumbar spine and femoral neck, which is congruent with previous studies and established practice guidelines.48 Although prior studies have shown that the most rapid decrease in lumbar spine BMD occurs within the first year posttransplantation (estimated at 3%-7%), we recognize declining BMD to be a problem of longer chronicity, often confounded by several factors unique to the immediate posttransplant period.8-11,25
In our study, we demonstrate no statistically significant benefit of bisphosphonate treatment on BMD beyond the first year posttransplant. There was heterogeneity in studies’ treatment choice and duration, but a nonsignificant improvement in lumbar spine BMD was consistently seen, while the effect appeared inconsistent in the femoral neck. Two studies28,39 captured peri-transplant patients’ data providing a baseline comparison, and both groups showed significant improvement with bisphosphonate treatment at the lumbar spine and femoral neck beyond 1 year posttransplant, when treatment was initiated at the time of transplantation and lasted for at least 1 year. This significant improvement persisted into the second28,39 and third year39 at the lumbar spine but not at the femoral neck.
As bone loss progresses beyond the first year posttransplantation,9,12 we also analyzed the effects of bisphosphonates on BMD at least 12 months posttreatment initiation, regardless of the time from initial transplantation. Although bisphosphonate protocols varied widely across studies, 11 studies26-28,31,33,34,36,37,39-41 identified a significant increase in BMD at 12 months posttreatment as compared with baseline.
This review demonstrated no statistically significant change in BMD beyond 1 year with bisphosphonates. Thus, the benefit of bisphosphonates may be only evident within 1 year of transplant. This is an important observation, as the protective effect may be limited to the time with highest corticosteroid dosing, and other pre- and posttransplant factors such as inflammation or bone disease. This likely indicated that there is no benefit to bisphosphonate use in renal transplant recipients beyond 1 year posttransplantation.
Despite the significant changes found with the bisphosphonate treatment at the lumbar vertebral levels, only 3 studies31,33,39 displayed improvement at the femoral neck level. Although this does not translate directly to a lower fracture risk, this may extrapolate into ongoing hip fracture risks with greater protective effect at the lumbar spines in this population. This is a clinical consideration when risk-stratifying patients based on their BMD and fracture risks posttransplant. A significant limitation is that none of the studies captured fracture incidence as the sole primary outcome, likely given the paucity of fracture events.
Only one study38 was able to interpret fracture results beyond reporting incidence and found no difference (HR = 0.40; 95% CI = 0.29-0.73, P = .001) in fracture rate after bisphosphonate treatment in their adjusted analysis.38 Thus, the clinical significance of bisphosphonate therapy on patient morbidity with fracture prevention remains to be established.
Steroid use in both the early and long-term posttransplant periods has been shown to cause increased bone loss.37 Specifically, prednisone doses of >7.5 mg/d results in trabecular bone loss in most patients.41 Calcineurin inhibitors have also been implicated in bone loss in animal models.37 While all studies that examined steroid use reaffirmed their deleterious impact on bone health, the effect of bisphosphonates in patient populations that had received higher cumulative steroid doses was not congruent. Only Huang et al37 was able to demonstrate that bisphosphonates resulted in a greater improvement in BMD at the lumbar spine in those with osteoporosis at baseline versus osteopenic patients.
Previous systematic reviews on a similar population highlighted limitations of few studies and small sample sizes. Thus, the wide scope of literature analyzed in this study sought to address this by including both observational and randomized control trials with appropriate bias analysis. A second strength of this study is our focus on long-term effectiveness of bisphosphonate treatment. It is important to recognize that the predominant population to which these data apply is well beyond the initial 12-month posttransplant and that these are the patients who carry the burden of bone disease. Our findings on bisphosphonate treatment in BMD preservation beyond 12 months posttransplant highlight limited evidence supporting the use of bisphosphonates on renal osteodystrophy.
Limitations of this review include the use of BMD as a surrogate outcome, the bias of the included studies, and the incomplete reporting data in numerous analyzed studies. The most clinically relevant outcome is the incidence of fractures. Our review assessed BMD as the primary outcome, acknowledging that BMD is not an accurate indicator of clinically meaningful patient outcomes and quality of life. Other indicators, such as bone biopsy, should be considered as a surrogate outcome in the future, keeping in mind that biopsy is expensive, invasive, and biopsy-based treatment guidelines are not yet available. We had limited information on the bone turnover state of patients to identify patients who would potentially benefit from anti-resorptive therapy. Last, we recognize that high risk of bias was identified in 44% of our included studies. We elected to include these studies given the limited sample size of the renal transplant population. The conclusions drawn from this review, however, did not change based on this bias assessment.
In conclusion, our review finds no statistical evidence for improvement in BMD in renal transplant patients beyond the first year posttransplantation with the use of bisphosphonates. We detected a differential improvement in BMD favoring the lumbar spine more so than the femoral neck, which may have clinical implications despite nonsignificance. However, the limitations of this review highlight the need for randomized control trials in patients with quantified bone turnover status evaluating fracture risk. Also quantifying other surrogate outcomes such as bone biopsy is necessary to provide more definitive evidence for the use of bisphosphates for current practice guidelines. In our future work, we plan to explore the evidence on the safety profile of bisphosphonates in this unique population with a focus on graft function. Finally, anti-resorptive and anabolic therapies are alternatives to bisphosphonates in bone mineral diseases in the general population and investigation into the use of these therapies in the renal transplant population is an avenue to further treatment options.
Supplemental Material
Supplemental material, Supplemental_Tables_-_clean for Effect of Bisphosphonates on Bone Health in Adult Renal Transplant Patients: Beyond the First Year Posttransplant—A Systematic Review and Meta-Analysis by Alyssa Lip, Ashley Warias, M. Khaled Shamseddin, Benjamin Thomson and D. Thiwanka Wijeratne in Canadian Journal of Kidney Health and Disease
Footnotes
List of Abbreviations: AMTD, adjusted mean treatment difference; BMD, bone mineral density; BMI, body mass index; CI, confidence interval; CKD, chronic kidney disease; DEXA, dual energy X-ray absorptiometry; ESRD, end-stage renal disease; MBD, mineral and bone disorders; RCT, randomized control trial; SD, standard deviation.
Ethics Approval and Consent to Participate: No ethics was required as our study reviewed existing literature.
Consent for Publication: AL and AW independent screened and reviewed all articles. AL and AW are the primary authors of this manuscript. MKS, BT, and DTW provided supervision and clinical expertise. All authors have consented for publication.
Availability of Data and Materials: All data is provided in the article and supplementary tables. Primary data can be obtained directly from the original articles.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Alyssa Lip  https://orcid.org/0000-0002-1255-8840
https://orcid.org/0000-0002-1255-8840
Benjamin Thomson  https://orcid.org/0000-0001-6323-3467
https://orcid.org/0000-0001-6323-3467
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Coco M, Glicklich D, Faugere MC, et al. Prevention of bone loss in renal transplant recipients: a prospective, randomized trial of intravenous pamidronate. J Am Soc Nephrol. 2003;14(10):2669-2676. [DOI] [PubMed] [Google Scholar]
- 2. Ziegler R, Kasperk C. Glucocorticoid-induced osteoporosis: prevention and treatment. Steroids. 1998;63(5-6):344-348. [DOI] [PubMed] [Google Scholar]
- 3. Sherrard DJ, Hercz G, Pei Y, et al. The spectrum of bone disease in end-stage renal failure: an evolving disorder. Kidney Int. 1993;43(2):436-442. [DOI] [PubMed] [Google Scholar]
- 4. Malluche H, Faugere M. Renal bone disease 1990: an unmet challenge for the nephrologist. Kidney Int. 1990;38:193-211. [DOI] [PubMed] [Google Scholar]
- 5. Kalantar-Zadeh K, Molnar MZ, Kovesdy CP, Mucsi I, Bunnapradist S. Management of mineral and bone disorder after kidney transplantation. Curr Opin Nephrol Hypertens. 2012;21(4):389-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nishimura J, Ikuyama S. Glucocorticoid-induced osteoporosis: pathogenesis and management. J Bone Miner Metab. 2000;18(6):350-352. [DOI] [PubMed] [Google Scholar]
- 7. Grotz W, Nagel C, Poeschel D, et al. Effect of ibandronate on bone loss and renal function after kidney transplantation. J Am Soc Nephrol. 2001;12(7):1530-1537. [DOI] [PubMed] [Google Scholar]
- 8. Rodino MA, Shane E. Osteoporosis after organ transplantation. Am J Med. 1998;104(5):459-469. [DOI] [PubMed] [Google Scholar]
- 9. Julian BA, Laskow DA, Dubovsky J, Dubovsky EV, Curtis JJ, Quarles LD. Rapid loss of vertebral mineral density after renal transplantation. N Engl J Med. 1991;325(8):544-550. [DOI] [PubMed] [Google Scholar]
- 10. Horber FF, Casez JP, Steiger U, Czerniak A, Montandon A, Jaeger P. Changes in bone mass early after kidney transplantation. J Bone Miner Res. 1994;9(1):1-9. [DOI] [PubMed] [Google Scholar]
- 11. Kwan JT, Almond MK, Evans K, Cunningham J. Changes in total body bone mineral content and regional bone mineral density in renal patients following renal transplantation. Miner Electrolyte Metab. 1992;18(2-5):166-168. [PubMed] [Google Scholar]
- 12. Pichette V, Bonnardeaux A, Prudhomme L, Gagné M, Cardinal J, Ouimet D. Long-term bone loss in kidney transplant recipients: a cross-sectional and longitudinal study. Am J Kidney Dis. 1996;28(1):105-114. [DOI] [PubMed] [Google Scholar]
- 13. Akaberi S, Simonsen O, Lindergård B, Nyberg G. Can DXA predict fractures in renal transplant patients. Am J Transplant. 2008;8(12):2647-2651. [DOI] [PubMed] [Google Scholar]
- 14. Omidvar B, Ghorbani A, Shahbazian H, Beladi Mousavi SS, Shariat Nabavi SJ, Alasti M. Comparison of alendronate and pamidronate on bone loss in kidney transplant patients for the first 6 months of transplantation. Iran J Kidney Dis. 2011;5(6):420-424. [PubMed] [Google Scholar]
- 15. Cohen A, Shane E. Osteoporosis after solid organ and bone marrow transplantation. Osteoporos Int. 2003;14(8):617-630. [DOI] [PubMed] [Google Scholar]
- 16. Casez JP, Lippuner K, Horber FF, Montandon A, Jaeger P. Changes in bone mineral density over 18 months following kidney transplantation: the respective roles of prednisone and parathyroid hormone. Nephrol Dial Transplant. 2002;17(7):1318-1326. [DOI] [PubMed] [Google Scholar]
- 17. Nouri-Majalan N, Sanadgol H, Rahimian M, Soleimani H. Bone mineral density in kidney transplant recipients and patients on hemodialysis: a comparison with healthy individuals. Iran J Kidney Dis. 2008;2(3):154-159. [PubMed] [Google Scholar]
- 18. Palmer SC, Strippoli GF, McGregor DO. Interventions for preventing bone disease in kidney transplant recipients: a systematic review of randomized controlled trials. Am J Kidney Dis. 2005;45(4):638-649. [DOI] [PubMed] [Google Scholar]
- 19. Fan SL, Almond MK, Ball E, Evans K, Cunningham J. Pamidronate therapy as prevention of bone loss following renal transplantation. Kidney Int. 2000;57(2):684-690. [DOI] [PubMed] [Google Scholar]
- 20. El-Agroudy AE, El-Husseini AA, El-Sayed M, Mohsen T, Ghoneim MA. A prospective randomized study for prevention of postrenal transplantation bone loss. Kidney Int. 2005;67(5):2039-2045. [DOI] [PubMed] [Google Scholar]
- 21. Weber TJ, Quarles LD. Preventing bone loss after renal transplantation with bisphosphonates: we can . . . but should we? Kidney Int. 2000;57(2):735-737. [DOI] [PubMed] [Google Scholar]
- 22. ten Dam MA, Hilbrands LB, Wetzels JF. Nephrotic syndrome induced by pamidronate. Med Oncol. 2011;28(4):1196-1200. [DOI] [PubMed] [Google Scholar]
- 23. Wilson LM, Rebholz CM, Jirru E, et al. Benefits and harms of osteoporosis medications in patients with chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med. 2017;166(9):649-658. [DOI] [PubMed] [Google Scholar]
- 24. Kan SL, Ning GZ, Chen LX, Zhou Y, Sun JC, Feng SQ. Efficacy and safety of bisphosphonates for low bone mineral density after kidney transplantation: a meta-analysis. Medicine (Baltimore). 2016;95(5):e2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang J, Yao M, Xu JH, Shu B, Wang YJ, Cui XJ. Bisphosphonates for prevention of osteopenia in kidney-transplant recipients: a systematic review of randomized controlled trials. Osteoporos Int. 2016;27(5):1683-1690. [DOI] [PubMed] [Google Scholar]
- 26. Sánchez-Escuredo A, Fuster D, Rubello D, et al. Monthly ibandronate versus weekly risedronate treatment for low bone mineral density in stable renal transplant patients. Nucl Med Commun. 2015;36(8):815-818. [DOI] [PubMed] [Google Scholar]
- 27. Okamoto M, Yamanaka S, Yoshimoto W, Shigematsu T. Alendronate as an effective treatment for bone loss and vascular calcification in kidney transplant recipients. J Transplant. 2014; 2014: 269613. doi: 10.1155/2014/269613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Walsh SB, Altmann P, Pattison J, et al. Effect of pamidronate on bone loss after kidney transplantation: a randomized trial. Am J Kidney Dis. 2009;53(5):856-865. [DOI] [PubMed] [Google Scholar]
- 29. Lan G, Peng L, Xie X, Peng F, Wang Y, Yu S. Alendronate is effective to treat bone loss in renal transplantation recipients. Transplant Proc. 2008;40:3496-3498. [DOI] [PubMed] [Google Scholar]
- 30. Schwarz C, Mitterbauer C, Heinze G, Woloszczuk W, Haas M, Oberbauer R. Nonsustained effect of short-term bisphosphonate therapy on bone turnover three years after renal transplantation. Kidney Int. 2004;65(1):304-309. [DOI] [PubMed] [Google Scholar]
- 31. Fan SL, Kumar S, Cunningham J. Long-term effects on bone mineral density of pamidronate given at the time of renal transplantation. Kidney Int. 2003;63(6):2275-2279. [DOI] [PubMed] [Google Scholar]
- 32. Jeffery JR, Leslie WD, Karpinski ME, Nickerson PW, Rush DN. Prevalence and treatment of decreased bone density in renal transplant recipients: a randomized prospective trial of calcitriol versus alendronate. Transplantation. 2003;76(10):1498-1502. [DOI] [PubMed] [Google Scholar]
- 33. Koc M, Tuglular S, Arikan H, Ozener C, Akoglu E. Alendronate increases bone mineral density in long-term renal transplant recipients. Transplant Proc. 2002;34:2111-2113. [DOI] [PubMed] [Google Scholar]
- 34. Tillmann F, Schmitz M, Jager M, Krauspe R, Rump LC. Ibandronate in stable renal transplant recipients with low bone mineral density on long-term follow-up. Int Urol Nephrol. 2016;48(2):279-286. [DOI] [PubMed] [Google Scholar]
- 35. Naylor KL, Garg AX, Hodsman AB, Rush DN, Leslie WD. Long-term changes in bone mineral density in kidney transplant recipients. Transplantation. 2014;98(12):1279-1285. [DOI] [PubMed] [Google Scholar]
- 36. Yamamoto S, Suzuki A, Sasaki H, et al. Oral alendronate can suppress bone turnover but not fracture in kidney transplantation recipients with hyperparathyroidism and chronic kidney disease. J Bone Miner Metab. 2013;31(1):116-122. [DOI] [PubMed] [Google Scholar]
- 37. Huang WH, Lee SY, Weng CH, Lai PC. Use of alendronate sodium (Fosamax) to ameliorate osteoporosis in renal transplant patients: a case-control study. PLoS ONE. 2012;7(11):e48481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Conley E, Muth B, Samaniego M, et al. Bisphosphonates and bone fractures in long-term kidney transplant recipients. Transplantation. 2008;86(2):231-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ahn H, Kim HJ, Kim YS, et al. Risk factors for changes in bone mineral density and the effect of antiosteoporosis management after renal transplantation. Transplant Proc. 2006;38:2074-2076. [DOI] [PubMed] [Google Scholar]
- 40. Cruz DN, Brickel HM, Wysolmerski JJ, et al. Treatment of osteoporosis and osteopenia in long-term renal transplant patients with alendronate. Am J Transplant. 2002;2(1):62-67. [DOI] [PubMed] [Google Scholar]
- 41. Arlen DJ, Lambert K, Ioannidis G, Adachi JD. Treatment of established bone loss after renal transplantation with etidronate1. Transplantation. 2001;71(5):669-673. [DOI] [PubMed] [Google Scholar]
- 42. Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8(9):1137-1148. [DOI] [PubMed] [Google Scholar]
- 43. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wells G, et al. Newcastle-Ottawa Quality Assessment Scale Cohort Studies. Ontario, Canada: University of Ottawa; 2014. [Google Scholar]
- 45. Lipsey MW, Wilson DB. Practical Meta-Analysis. Thousand Oaks, CA: Sage; 2001. [Google Scholar]
- 46. Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Vol. 4 West Sussex, England: John Wiley; 2011. [Google Scholar]
- 47. Wei GS, Jackson JL, Hatzigeorgiou C, Tofferi JK. Osteoporosis management in the new millennium. Prim Care. 2003;30(4):711-741. [DOI] [PubMed] [Google Scholar]
- 48. EBPG Expert Group on Renal Transplantation. European best practice guidelines for renal transplantation. Section IV: long-term management of the transplant recipient. IV. 8. Bone disease. Nephrol Dial Transplant. 2002;17:43. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_Tables_-_clean for Effect of Bisphosphonates on Bone Health in Adult Renal Transplant Patients: Beyond the First Year Posttransplant—A Systematic Review and Meta-Analysis by Alyssa Lip, Ashley Warias, M. Khaled Shamseddin, Benjamin Thomson and D. Thiwanka Wijeratne in Canadian Journal of Kidney Health and Disease