Abstract
Corticotropin-releasing factor (CRF) receptor type 2 (CRF2) exists in both cardiomyocytes and neurocytes. The purpose of this research was to explore if chronic treatment with urocortin 2 (UCN2), a CRF2 receptor agonist, at different doses can improve prognosis and regulate the expression of CRF2 receptor and calcium handling proteins without any adverse effects on behavior in heart failure. Heart failure was established in Sprague-Dawley rats and was confirmed by echocardiography. Heart failure rats were injected intraperitoneally with UCN2 (5, 10, or 20 µg·kg−1·d−1) for 30 days. Survival rate, cardiac function, expressions of cardiac CRF2 receptor, RyR2, SERCA2, and hypothalamic and hippocampal c-FOS, CRF receptor type 1 (CRF1) and CRF2 receptor were determined. Behavior was evaluated by Morris Water-Maze and Open-Field tests. Results showed that chronic peripheral UCN2 treatment improved survival rate in a dose–response manner and increased cardiac function and expression of CRF2 receptor and SERCA2 in heart failure, especially at the high dosage. Moreover, cellular-fos (c-FOS), CRF1 receptor, and CRF2 receptor expressions of both hypothalamic and hippocampal tissues were significantly increased in high dosage group. Furthermore, the behavior tests suggested that chronic UCN2 treatment did not exacerbate stress/anxiety-like behavior in HF. In conclusion, chronic peripheral treatment with UCN2 increases survival in a dose–response manner in heart failure rats without inducing stress/anxiety-like behavior.
Keywords: urocortin 2, heart failure, cardiac function, survival rate, dose–response, stress
Introduction
Since corticotropin-releasing factor (CRF) was purified from animal hypothalamus in 1981, several CRF peptide ligand family members have been found such as urocortins 1, 2, 3 (UCN1, 2, 3) and sauvagine. The CRF receptor types 1, 2, and 3 (CRF1,2,3 receptors) are members of G protein-coupled receptors. The CRF1 receptor is abundant in the central and peripheral nervous systems. The CRF2 receptor is detected in cardiovascular, nervous, respiratory, digestive, reproductive, and endocrine systems. The CRF3 receptor is found just in the catfish.1-6 Corticotropin-releasing factor has a higher affinity for CRF1 receptor than CRF2 receptor. On the contrary, UCN2 and 3 display much higher affinity to CRF2 receptor. Sauvagine and UCN1 have similar affinity to both CRF1 receptor and CRF2 receptor.6,7
Heart failure (HF) is a severe disease influencing approximately 1% to 2% of the adults,8 and is also a common complication of myocardial infarction (MI). Moreover, HF independently increases the mortality of patients with MI. Even without MI, the 1-month and 1-year mortalities after an incident with decompensated HF are 10.4% and 30%, respectively.9,10 After diagnosis, the mortalities for those patients are as high as 50% in 5 years.11-13 The data from previous researches demonstrate that UCN2 (38 amino acids), a strong CRF2 receptor agonist, exhibited obvious inotropy and lusitropy by activation of CRF2 receptor on cardiomyocytes.6,14 In animal models, urocortins have beneficial effects on HF.6,15-17 The clinical studies also showed that UCN2 improves hemodynamic effect.18,19 Despite its therapeutic potential, however, it remains to be studied whether chronic treatment with UCN2 may reduce mortality of MI-induced HF, and, if so, by which mechanisms. For example, may chronic UCN2 treatment affect expression levels of CRF2 receptor, sarcoplasmic/endoplasmic reticulum calcium ATPase 2 (SERCA2) and type 2 ryanodine receptor (RyR2), and hence, improve calcium handling in heart tissue? On the other hand, cardiac reactions to stress and cognitive disability may lead to poor prognosis in both healthy people,20 and patients with coronary artery disease (CAD).21 The MI-related feelings of helplessness and fear22 as well as hyper-reactivity may also be related to poor prognosis in the patients with MI and HF. Corticotropin-releasing factor has a crucial effect on the stress reaction through activating the hypothalamus-pituitary-adrenal (HPA) axis. Thus, peripherally injected UCN2, a CRF-like peptide, may also cause central side effects. Therefore, this research was designed to assess the therapeutic impact of peripherally administered UCN2 in MI-induced HF and potential side effects on stress/anxiety-like behavior.
Materials and Methods
Animals
One-hundred eighty adult male Sprague-Dawley rats weighing approximately 200 to 250 g had free access to animal chow and water. All rats were placed in individual cages under room temperature (24°C-26°C) with 12 to 12 hour dark-light cycles. All experiments were approved by the Animal Experimental Ethical Committee of the university hospital (Certificate HKDL No. 2015-142) dealing with animal protection and conformed with the Guideline for the Care and Use of Laboratory Animals published by the US National Institutes of Health (No. 85-23, revised 1996).
Heart Failure Model
This study employed a MI model produced by ligation of the left anterior descending coronary artery (LAD) using a modified technique previously described in detail.23 Each rat was anaesthetized by inhalation of 2% isoflurane with careful monitoring of the level of anesthesia during the operation. Fifteen rats with sham operation without ligation of the LAD served as sham controls (Sham, n = 15). One-hundred sixty-five rats underwent permanent LAD ligation performed with a sterile suture. A successful operation was identified visually by the dramatic color change in the ischemic zone. After closing thorax, skin and muscles were stitched in layers. After recovery, each rat was warmed in an individual cage. The electrocardiography was monitored continuously during the operation. After the first 6 weeks, surviving MI rats were evaluated by closed-chest trans-thoracic echocardiography (Vevo 2100 Imaging System, Visual Sonics Inc). Echocardiography was performed by experienced experts, who were blinded to the study protocol. A 2-dimensional and M-mode echocardiography was conducted for determining left ventricular chamber volume and contractile parameters. Cardiac function was evaluated by left ventricular fractional shortening (LVFS) and ejection fraction (LVEF) based on the following formulas:
LVEF (%) = (LVEDV − LVESV)/LVEDV × 100 and
LVFS (%) = (LVEDD − LVESD)/LVEDD × 100.
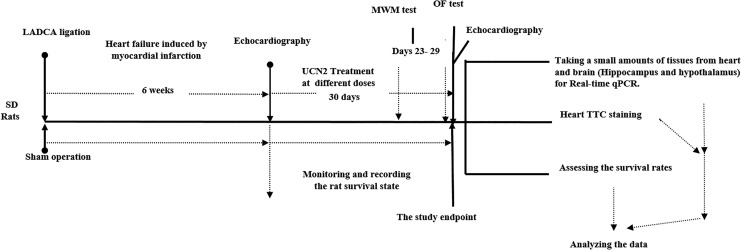
In this study, only MI rats with EF ≤42% were classified as severe HF. Apart from death, rats with EF >42% were considered as mild or no HF and were excluded from this study. Finally, 78 MI rats meeting the abovementioned criterion of severe HF were selected for further study. They were randomly assigned into the 0, 5, 10, and 20 UCN2-HF groups as follows: 0UCN2-HF (normal saline (NS), 200 µL·d−1 injected intraperitoneally, n = 20), 5UCN2-HF (UCN2 5 µg·kg−1·d−1, n = 20), 10UCN2-HF (UCN2 10 µg·kg−1·d−1, n = 20) and 20UCN2-HF (UCN2 20 µg·kg−1·d−1, n = 18). The rats in the 5, 10, and 20UCN2-HF groups were injected intraperitoneally with 5, 10, and 20 µg·kg−1·d−1 of UCN2 dissolved in 200 µL NS, respectively. Rats in the sham and 0UCN2-HF groups were injected intraperitoneally with 200 µL NS per day. Thirty days later, echocardiography was performed to assess cardiac function again for each surviving rat in the various groups. Hearts were taken from each surviving rat at day 30 post-treatment. One examiner was responsible for monitoring and recording the rat survival state each day for assessing the survival rates in the various groups (Figure 1).
Figure 1.
Schematic description of the experimental protocol and treatments, study events, and test timelines. LADCA indicates left anterior descending coronary artery; LVEF, left ventricular ejection fraction; MWM, Morris Water Maze test; OF test, Open-Field test; TTC staining, triphenyltetrazolium chloride staining.
Open Field and Morris Water Maze Tests
All surviving rats underwent the Morris Water Maze (MWM) test on posttreatment days 23 to 29 (6 consecutive days for training and 1 day for final test), and the Open Field (OF) test on posttreatment day 30. The 2 tests were performed as previously described with little modifications.24,25 Briefly, for the MWM test on posttreatment day 29, the escape platform was taken away, and then each rat was gently put into the water in a fixed quadrant of a round pool. The number of each rat passing through the area where the platform had been, the total distance traveled, the movement time, and the speed were recorded within 180 seconds. For the OF test, each rat was gently placed in a dark arena with 25 equal squares and recorded for 20 minutes on posttreatment day 30. Horizontal score was the numbers of squares passed by the rats, and vertical score was the times of vertical activities (front paws leaving the ground or climbing the walls).
Quantification of Myocardial Infarct Size
At the study end point, each surviving rat was euthanized by an overdose of pentobarbital sodium (0.1g·kg−1 body weight, ip) and thereafter MI size was quantified. The heart of each rat was rapidly excised, weighed, and frozen at −20°C, and then was sliced into 6 separate sections (slices), with the first slice at the level of the ligature. Each section was separately incubated in 1% triphenyltetrazolium chloride (TTC) in a pH 7.4 buffer solution (30°C). After triphenyltetrazolium chloride staining, the zone of infarction appeared pallid.26 The infarct area was isolated and weighed. The severity degree of MI was presented as a percentage of MI zone weight over whole heart weight. Small amounts of the remaining heart tissue were immediately weighed and stored in liquid nitrogen for further analyses. After the wash with normal saline, all rat brains were stored and frozen at −80°C. Before the RNA extraction, the pieces of hypothalamus and hippocampus were isolated,27,28 and frozen again at −80°C for further study.
Quantitative Real-Time Reverse Transcriptase-Polymerase Chain Reaction
The RNA extraction from the lysates of the heart, hippocampus, and hypothalamus was performed employing a Trizol kit (Sigma) in accordance with the relevant instructions. 3 µg of isolated RNA was appended in a 20 µL reaction mixture (Applied TaKaRa kit) containing 11 μL diethylpyrocarbonate-treated water, 4 µL 5× reverse transcription buffer, 1 µL oligo dT, 2 µL deoxyribonucleotide triphosphates, 1 µL RTase and 1 µL RNase inhibitor. Under the following conditions, 42°C for 70 minutes, 70°C for 10 minutes, reverse transcription was performed to produce complementary DNA using a thermal cycler machine. And then all complementary DNA products were frozen at −20°C for further study. The expression of CRF2 receptor, CRF1 receptor, RYR2, c-FOS, SERCA2, and GAPDH were studied by quantitative real-time reverse transcriptase-polymerase chain reaction (qRT-PCR), which was performed with the TaKaRa kit under the experimental conditions as follows: 15 minutes at 95°C, 40 cycles of 20 seconds at 95°C, 45 seconds at 60°C. The following primers and probe sequences were used: CRF1 receptor forward 5′GGAATGGAGACAGAGAAGGTTG3′ and reverse 5′AGCTGTCGCACACCCTAATC3′; CRF2 receptor forward 5′CCCGGGCCATGTCC-AT3′ and reverse 5′ACAGCGGCCGTCTGCTT3′; RYR2 forward 5′ACTGCTGGGCTACGG- CTAC3′ and reverse 5′CTGAAGATGCGGAACCTCTC3′; c-FOS forward 5′TCAGAGCATTGGCAGAAGGG3′ and reverse 5′GTGAGCTGCCAGGATGAACT3′; SERCA2 forward 5′GACATTGAAACATGCTTTCTAAGGGC3′ and reverse 5′TGAACTGACGATGAGCACTTTATTACG3′; GAPDH forward 5′CATCACTGCCACCCAGAAGACTG3′ and reverse 5′ATGCCAGTGAGCTTCCCGTTCAG3′. Each sample for qRT-PCR experiment was measured 3 times, and the 2−ΔΔ C t method was used to calculate the relative quantification.
Drugs and Dosages
The UCN2 was purchased from a Bio-Tech Company and applied via intraperitoneal injection at 5, 10, or 20 µg·kg−1·d−1 in the UCN2-treated groups.
Statistical Analysis
Values are expressed as mean ± SEM. Student t test as well as one-way analysis of variance followed by Tukey test was performed to assess differences among groups. The Kaplan-Meier method and log-rank test were used to estimate the cumulative survival rates and significance. P < .05 was considered to be statistically significant.
Results
Survival Rates
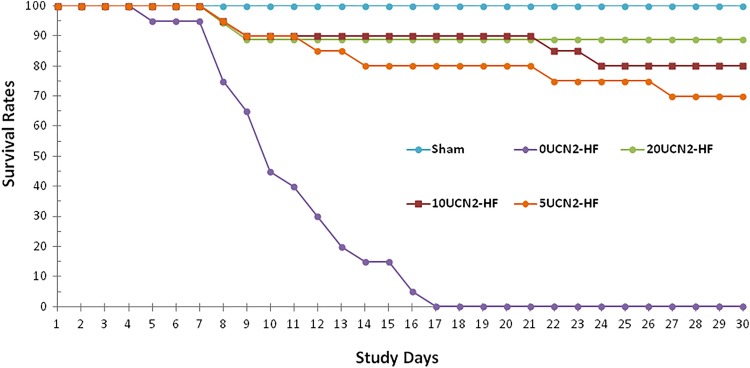
At the study end point, all animals from the 0UCN2-HF group had died, that is, the survival rate was 0%. Treatment with UCN2 for 30 days significantly improved the survival rates of the 5, 10, and 20UCN2-HF groups to 70%, 80%, and 89%, respectively (P < .001, vs 0UCN2-HF group, Figure 2), which acted in a dose–response manner. The survival rate of 20UCN2-HF group was higher than that of 5UCN-HF and 10UCN2-HF groups, but the difference between these groups had no statistical significance. The survival rates of the 10UCN2-HF and 20UCN-HF groups were no significant difference from the sham group (P = N.S.), which amounted to 100%. These results suggest that UCN2 markedly increased survival in HF induced by MI, especially at the higher dosages (10 and 20 µg·kg−1·d−1).
Figure 2.
Survival rates in the various animal groups. The survival rate in 0UCN2-HF was significantly lower compared to those in the other groups; UCN2 treatment significantly improved the survival rate in a dose–response manner, and the best results were observed in 20UCN2-HF group. UCN2 indicates urocortin 2.
Heart Infarct Size and Weight
Table 1 demonstrates the severity degrees of MI and heart weights in all surviving animals from each group. At the study end point, the rats had higher heart weight in the sham group than all HF groups treated with UCN2 (all P < .01 vs sham group; Table 1). Neither heart weight nor infarct size significantly differed between the 5, 10, and 20UCN2-HF groups (Table 1).
Table 1.
Heart Weight and Infarct Size of the Surviving Rats at the Study End Point.a
| Groups (# of Surviving/Total Rats) | Heart Weight (mg) | Infarct Size (mg) | Infarct Size/Whole Heart Weight (%) |
|---|---|---|---|
| Sham group (15/15) | 820 ± 17 | 0 | 0 |
| 5UCN2-HF group (14/20) | 697 ± 26b | 180 ± 6b | 25.9 ± 0.4%b |
| 10UCN2-HF group (16/20) | 677 ± 22b | 178 ± 7b | 26.2 ± 0.6%b |
| 20UCN2-HF group (16/18) | 681 ± 22b | 178 ± 10b | 26.1 ± 1.2%b |
a There were no surviving rats in the 0UCN2-HF group at the study end point.
b P < .01 vs sham group.
Cardiac Function
Although baseline parameters did not differ between 4 HF groups, all HF groups demonstrated LV dilation as evidenced by significantly increased diastolic and systolic (left ventricular internal diameter) LVIDs and LV volumes versus compared to that sham group at 6 weeks postoperation and after 30 days of treatment (Table 2). Moreover, all HF groups also exhibited significantly decreased LVEF and LVFS (Table 2 and Figure 3). Treatment with UCN2 at the high dosage (20 µg·kg− 1·d− 1) improved LV function as displayed by enhanced LVFS and LVEF compared to its baseline condition (Table 2 and Figure 3). It had no significant difference in LV volume, LVID, LVEF, and LVFS in both diastole and systole before and after treatment with UCN2 at medium and low dosages (10 and 5 µg·kg− 1·d− 1), although all these parameters appeared to show a trend toward improvement (Table 2). Taken together, echocardiography (Figure 3 and Table 2) and survival data (Figure 2) indicate that cardiac function and survival were significantly improved in the 20UCN2-HF group.
Table 2.
Echocardiographic Parameters in Different Groups.a
| Parameters/Groups | Sham | 0UCN2-HF | 5UCN2-HF | 10UCN2-HF | 20UCN2-HF | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline, n = 15 | Treated, n = 15 | Baseline, n = 20 | Treated, n = 0 | Baseline, n = 20 | Treated, n = 14 | Baseline, n = 20 | Treated, n = 16 | Baseline, n = 18 | Treated, n = 16 | |
| LVID, d (mm) | 5.95 ± 0.18 | 5.76 ± 0.15 | 8.68 ± 0.13b | – | 9.63 ± 0.21b | 9.16 ± 0.30 | 9.63 ± 0.29b | 8.96 ± 0.31 | 9.35 ± 0.27b | 8.75 ± 0.28 |
| LVID, s (mm) | 2.67 ± 0.21 | 2.24 ± 0.14 | 7.79 ± 0.15b | – | 8.20 ± 0.21b | 7.81 ± 0.33 | 8.28 ± 0.33b | 7.75 ± 0.32 | 8.10 ± 0.26b | 7.24 ± 0.33 |
| Ejection fraction (%) | 83.43 ± 2.36 | 88.74 ± 1.91 | 26.98 ± 1.39b | – | 27.99 ± 1.57b | 29.12 ± 1.91 | 28.20 ± 1.84b | 32.48 ± 1.81 | 26.24 ± 1.74b | 33.26 ± 2.90c |
| Fractional shortening (%) | 55.09 ± 3.05 | 61.24 ± 2.38 | 13.22 ± 0.74b | – | 13.87 ± 0.83b | 14.43 ± 1.01 | 14.0 ± 0.99b | 16.22 ± 0.97 | 12.91 ± 0.92b | 16.83 ± 1.64c |
| LV vol, d (uL) | 179.48 ± 12.19 | 166.08 ± 9.76 | 414.80 ± 13.36b | – | 525.31 ± 25.44b | 473.6 ± 33.01 | 530.52 ± 37.08b | 453.03 ± 34.92 | 494.91 ± 30.91b | 428.86 ± 29.89 |
| LV vol, s (uL) | 30.26 ± 4.99 | 18.53 ± 3.27 | 297.48 ± 13.0b | – | 370.17 ± 20.83b | 336.42 ± 30.47 | 386.27 ± 36.64b | 304.16 ± 29.18 | 362.24 ± 25.94b | 287.37 ± 28.22 |
a Values are expressed as mean ± SEM.
b Baseline or treated: P < .001, compared to sham group.
cSame group: P < .05, compared to sham group, student t test.
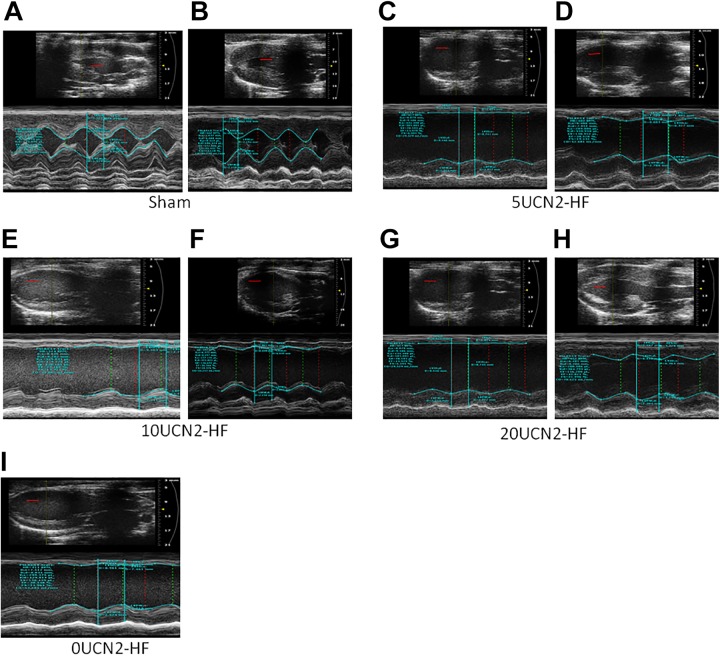
Figure 3.
Echocardiography measurements in the various groups. Note: A-I, original echocardiography images in different groups. A, sham group before treatment; (B) sham group post-treatment day 30; (C) 5UCN2-HF group before treatment; (D) 5UCN2-HF group post-treatment day 30; (E) 10UCN2-HF group before treatment; (F) 10UCN2-HF group post-treatment day 30; (G) 20UCN2-HF group before treatment; (H) 20UCN2-HF group post-treatment day 30; (I) 0UCN2-HF group before treatment. Scale bar 3 mm (in red). UCN2 indicates urocortin 2.
Cardiac Expression of CRF2 Receptor and Calcium-Handling Proteins
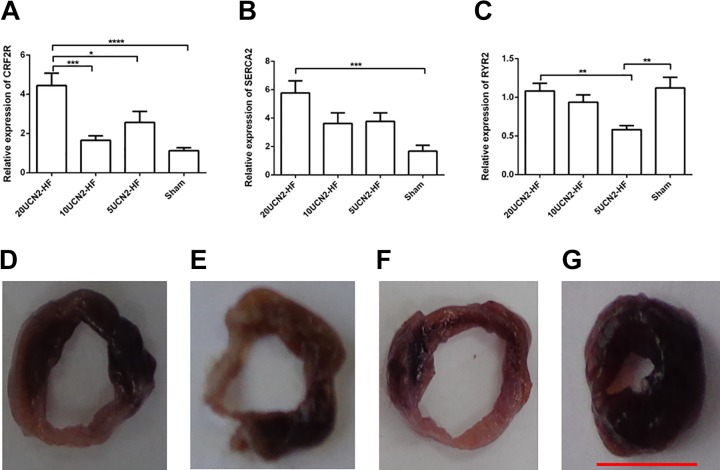
Figure 4 demonstrated that the cardiac CRF2 receptor expression was significant higher in the 20UCN2-HF group compared to the sham, 5UCN2-HF and 10UCN2-HF groups (Figure 4A; P < .05-.001). The 20UCN2-HF group also showed a higher level of cardiac SERCA2 expression than the sham group (Figure 4B; P < .01). The RYR2 expression was relatively constant, except for lower expression in the 5UCN2-HF group versus the sham and 20UCN2-HF groups (Figure 4C; P < .01).
Figure 4.
CRF2 receptor, SERCA2, and RYR expressions in heart tissue. CRF2 receptor (A), SERCA2 (B), and RYR (C) expressions of heart tissues in the various groups. Representative images of myocardial sections stained by triphenyltetrazolium chloride from 5UCN2-HF (D), 10UCN2-HF (E), 20UCN2-HF (F), and sham (G) groups. Data are shown as mean ± SEM. One-way ANOVA: CRF2 receptor (F [3,56] = 10.8, P < .001), SERCA2 (F [3,56] = 5.8, P < .01), RYR2 (F [3,56] = 5.7, P < .01); Post hoc Tukey test: **, P < .01; ***P < .001. Sham group, n = 14; 5UCN2-HF group, n = 14; 10UCN2-HF group, n = 16; 20UCN2-HF group, n = 16. Scale bar 10 mm (in red). ANOVA indicates analysis of variance; HF, heart failure; UCN2, urocortin 2.
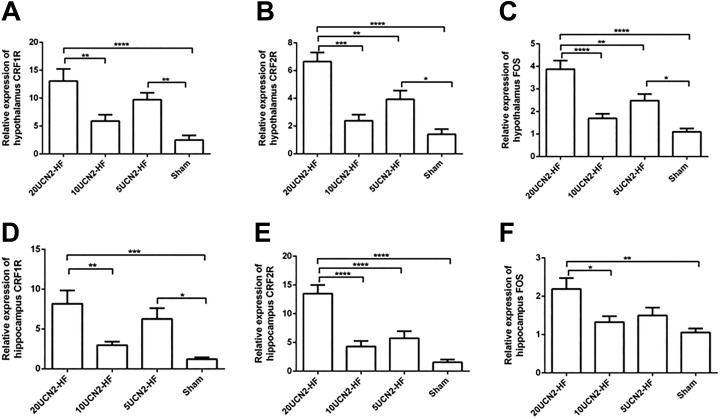
Expression of c-FOS and CRF Receptors in Hippocampus and Hypothalamus
The data also showed that CRF1 receptor, CRF2 receptor, and c-FOS expression levels of both hypothalamus (Figure 5A-C) and hippocampus (Figure 5D-F) tissues were obviously elevated in the 20UCN2-HF group versus the 10UCN2-HF and Sham groups (P < .01-.001). It revealed that CRF1 receptor, CRF2 receptor and c-FOS expression levels were up-regulated remarkably after treatment with UCN2 at 20 µg·kg− 1·d−1.
Figure 5.
CRF1 receptor, CRF2 receptor, and c-FOS expressions in hypothalamus and hippocampus. CRF1 receptor (A and D), CRF2 receptor (B and E), and c-FOS (C and F) expressions of hypothalamus and hippocampus in the various groups respectively. Data are shown as mean ± SEM. One-way ANOVA: hypothalamus CRF1 receptor (F [3,56] = 9.7, P < .001), hypothalamus CRF2 receptor (F [3,56] = 18.4, P < .001), hypothalamus c-FOS (F [3,56] = 19.3, P < .001), hippocampus CRF1 receptor (F [3,56] = 7.8, P < .001), hippocampus CRF2 receptor (F [3,56] = 20.7, P < .001) and hippocampus FOS (F [3,56] = 5.7, P < .01); Post hoc Tukey test: *, P < .05; **, P < .01; ***P < .001. Sham group, n = 14; 5UCN2-HF group, n = 14; 10UCN2-HF group, n = 16; 20UCN2-HF group, n = 16. ANOVA indicates analysis of variance; HF, heart failure; UCN2, urocortin 2.
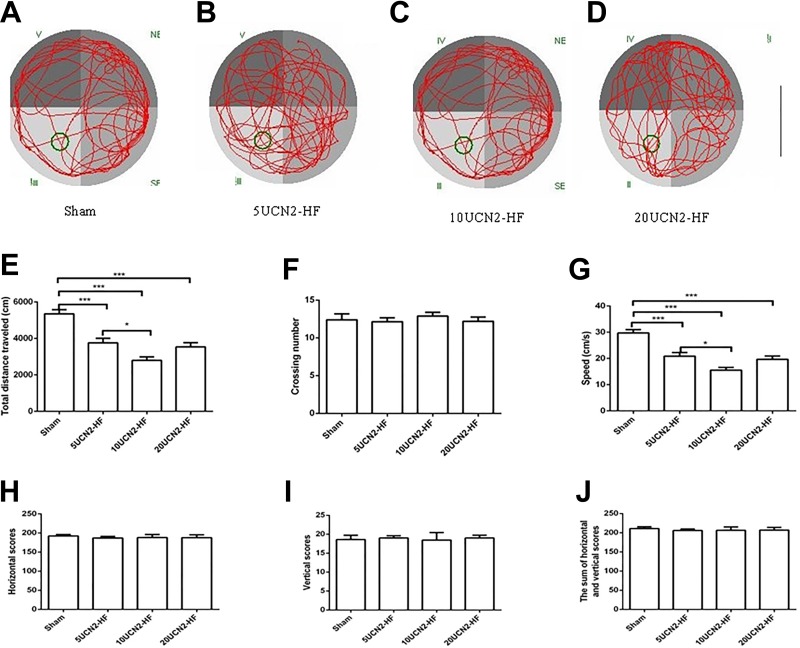
Results of MWM and OF Tests
The data of the MWM test are presented in Figure 6A-G. Although, no difference in crossing numbers was seen between 4 groups, it had differences in speed and total distance traveled between the groups in statistic (Figure 6E and G). Both the speed and total distance traveled were significantly lower in 3 UCN2-HF groups versus the Sham group (P < .001; Figure 6E and G). Furthermore, the data showed that the total distance traveled and speed were obviously increased in 5UCN2-HF group versus 10UCN2-HF group (P < .05). The 20UCN2-HF group tended to be more active than 10UCN2-HF group, displaying higher levels of total distance traveled and speed (Figures 6E and G); however, these differences had no statistical significance. The OF test did not show differences in horizontal score, vertical score and sum of horizontal and vertical scores between the UCN2-HF groups and the sham group (Figures 6H-J).
Figure 6.
Morris Water Maze and Open-Field tests. Morris Water Maze: original movement tracks of the rats of the sham (A, n = 15), the 5UCN2-HF (B, n = 14), the 10UCN2-HF (C, n = 16) and the 20UCN2-HF (D, n = 16) groups. Total distances traveled (E), crossing numbers (F) and speeds (G) of rats in the various groups; Open-Field test: Horizontal scores (H), vertical scores (I) and sum of horizontal and vertical scores (J) of rats from the various groups. Data are shown as mean ± SEM. One-way ANOVA: Total distances traveled (F [3,57] = 22.9, P < .001), crossing numbers (F [3,57] = 0.3, P = .82), speeds (F [3,57] = 23.1, P < .001), horizontal scores (F [3,57] = 0.1, P = .94), vertical scores (F [3,57] = 0.05, P = .99) and sum of horizontal and vertical scores (F [3,57] = 0.11, P = .95); Post hoc Tukey test *, P < .05; ***P < .001. Scale bar 0.8 m (in black). ANOVA indicates analysis of variance; HF, heart failure; UCN2, urocortin 2.
Discussion
This is the first report on the evidence that chronic peripheral treatment with UCN2 can significantly improve the survival in rats with HF induced by MI, and increase cardiac function and cardiac CRF2 receptor and SERCA2 expression, especially at the high dosage (20 µg·kg−1·day−1). Moreover, c-FOS, CRF1 receptor, and CRF2 receptor expression of both hypothalamus and hippocampus tissues was significantly increased by peripheral UCN2 treatment. Furthermore, the results of the open field test suggested that chronic treatment with UCN2 did not exacerbate stress/anxiety-like behavior in HF induced by MI.
Chronic Peripheral UCN2 Treatment Increases Heart Function and Survival Rate
Although several studies have shown that urocortins can improve cardiac contractility in both men and animals,6,16-19 researchers pointed out that use of some cardiac inotropes such as digoxin is associated with increased mortality in patients with HF.29 As far as I know, it is the first report of the experimental evidence for increasing survival rate by chronic peripheral treatment with UCN2 in a severe HF animal model. This effect was dose-dependent. It is clear that the CRF2 receptor is predominant in the heart.6 Previous studies have found that UCN2 exerts an inotropic6,14,16-19,30-33 effect in heart through CRF2 receptor-mediated activation of protein kinase A, which increases sarcoplasmic reticulum Ca2+ load and Ca2+ influx to elevate sarcoplasmic reticulum Ca2+ release and intracellular Ca2+ concentration.33 Urocortin 2 also increases phosphorylation of phospholamban,14 extracellular signal-regulated kinase 1/2, protein kinase B, endothelial nitric oxide synthase,34 Na+/Ca2+ exchange and action potential duration35 in a time- and dosage-dependent effect in cardiomyocytes. Furthermore, UCN2 inhibits rapidly activating delayed rectifier potassium current in hERG-HEK293 cells which help to prolong action potential duration,35 which, in turn, may enhance intracellular Ca2+ concentration and contribute to increase the cardiac contractility. But some researchers found that the expression level of cardiac CRF2 receptor significantly reduced in the patients with HF.36 In addition, in pharmacology, chronic use of certain agonists may also lead to downregulation of the relevant receptors. The present study found that chronic peripheral treatment of UCN2 in HF rats results in a significant upregulation of cardiac CRF2 receptor expression instead of downregulation. Furthermore, the elevated cardiac CRF2 receptor expression is compared well with the improved survival rate, the increased LV function (LVEF, LVFS) and the increased SERCA2 expression level of heart tissues. Hence, the present research—in combination with previous researches—suggests that chronic treatment with UCN2 can produce improved cardiac function in the setting of HF induced by MI via alterations in action potential duration, Ca2+ handling and expression of CRF2 receptor and SERCA2a. Similar to a previous report,37 this research also indicated that RyR2 expression was modestly reduced in HF animals.
Chronic Peripheral UCN2 Treatment and Behavior
Corticotropin-releasing factor has an important role in regulating the HPA axis, which is a common pathway in stress/anxiety-related responses. Stress behavior can be induced by intracerebroventricular administration of CRF.38 Moreover, c-FOS activation in the hypothalamus is related to stress.39 Furthermore, there is an association between hippocampus and cognitive function.40 As mentioned, the cardiovascular reactions to the stress may lead to a poor prognosis in both healthy people20 and patients with CAD, especially in patients with MI.21,22 But until now, no direct evidence can demonstrate whether chronic treatment with UCN2 leads to behavioral changes such as stress and cognitive decline in a HF animal model induced by MI. To explore this question, this study used UCN2, a highly selective CRF2 receptor agonist, to treat HF rats for about 1 month. Behavior of the rats was evaluated by the OF and MWM tests, which are commonly used to estimate stress and cognitive behavior, respectively. In addition, CRF1 receptor, CRF2 receptor, and c-FOS expression levels of hypothalamus and hippocampus were measured. They were increased in both hypothalamus and hippocampus tissues in the high dosage UCN2-HF group, indicating certain changes of the stress axis on the molecular level. The OF test, however, did not show differences in horizontal score, vertical score and sum of horizontal and vertical scores among groups, which demonstrated that UCN2, a CRF2 receptor agonist, did not result in stress or anxiety in a HF model induced by MI. In the MWM test, the total distance traveled and speed were significantly lower in the 3 UCN2-HF groups. However, it had, no significant difference in crossing number, which was used to estimate the cognitive function in the various groups. Hence, how could we explain that, on the one hand, behavioral tests suggest no aggravation of stress, whereas, on the other hand, there is increased expression of CRF1 receptor, CRF2 receptor, and c-FOS in hypothalamus and hippocampus in the high dose UCN2-HF rats? It is certainly true that increased CRF1 receptor and c-FOS expression levels could increase stress. The activation of CRF–CRF1 receptor system increases stress reactions, whereas the activation of UCNs–CRF2 receptor system can terminate the stress reactions.41-47 This study showed that CRF2 receptor expression levels in both hypothalamus and hippocampus were largely upregulated in the high dose UCN2-HF group. Thus, based on the previously discussed considerations, the present study suggests that the stress/anxiety reduction resulting from augmentation of CRF2 receptor expression levels may offset the effect of elevated stress/anxiety owing to increased CRF1 receptor and c-FOS expression levels.
Conclusions
The current research revealed that chronic treatment with UCN2 increased LVEF, LVFS, and cardiac CRF2 receptor and SERCA2 expressions, especially at the high dosage (20 µg·kg− 1·d− 1), in HF rats induced by MI, whereas the survival rate of these HF rats was obviously improved. Although treatment with high dose of Ucn2 elevated the expression of CRF1 receptor, CRF2 receptor, and c-FOS in both hypothalamus and hippocampus in the HF rats, behavioral tests did not show any evidence of aggravation of stress, which could be due to the augmentation of CRF2 receptor expression: the greatly increased CRF2 receptor expression may eliminate the stress induced by increased CRF1 receptor and c-FOS expressions in HF rats. In conclusion, chronic peripheral treatment with UCN2 at high dose increases heart function and survival in HF rats via elevated cardiac CRF2 receptor and SERCA2 expression without inducing stress/anxiety-like behavior.
Acknowledgments
The authors would like to thank Prof Dr Jens Kockskämper (Institute of Pharmacology and Clinical Pharmacy, University of Marburg, Germany) for his important comments and suggestions on this work. The authors also appreciate Le-Xin Yang (5-year-program undergraduate of clinical medicine, Shanghai Medical College, Fudan University) for her help during this study.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The present research was supported by the National Natural Science Foundation of China (No. 81570754 to Dr. LZ Yang), the Shanghai Natural Scientific Research Fund (No. 14ZR1424500 to Dr. LZ Yang) and the Shanghai Pu Jiang Talent Program (No. 10PJ1406900 to Dr. LZ Yang).
ORCID iD: Li-Zhen Yang  https://orcid.org/0000-0001-6473-8088
https://orcid.org/0000-0001-6473-8088
References
- 1. Hauger RL, Grigoriadis DE, Dallman MF, Plotsky PM, Vale WW, Dautzenberg FM. International union of pharmacology. XXXVI. Current status of the nomenclature for receptors for corticotropin-releasing factor and their ligands. Pharmacol Rev. 2003;55(1):21–26. [DOI] [PubMed] [Google Scholar]
- 2. Huising MO, Pilbrow AP, Matsumoto M, et al. Glucocorticoids differentially regulate the expression of CRFR1 and CRFR2α in MIN6 insulinoma cells and rodent islets. Endocrinology. 2011;152(1):138–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kimura Y, Takahashi K, Totsune K, et al. Expression of urocortin and corticotropinreleasing factor receptor subtypes in the human heart. J Clin Endocrinol Metab. 2002;87(1):340–346. [DOI] [PubMed] [Google Scholar]
- 4. Perrin MH, Vale WW. Corticotropin releasing factor receptors and their ligand family. Ann N Y Acad Sci. 1999;885:312–328. [DOI] [PubMed] [Google Scholar]
- 5. Takefuji M, Murohara T. Corticotropin-releasing hormone family and their receptors in the cardiovascular system. Circ J. 2019;83(2):261–266. doi:0.1253/circj.CJ-18-0428. [DOI] [PubMed] [Google Scholar]
- 6. Yang LZ, Tovote P, Rayner M, Kockskämper J, Pieske B, Spiess J. Corticotropin-releasing factor receptors and urocortins, links between the brain and the heart. Eur J Pharmacol. 2010;632(1-3):1–6. [DOI] [PubMed] [Google Scholar]
- 7. Perrin MH, Grace CR, Riek R, Vale WW. The three-dimensional structure of the N-terminal domain of corticotropin-releasing factor receptors: sushi domains and the B1 family of G protein-coupled receptors. Ann N Y Acad Sci. 2006;1070:105–119. [DOI] [PubMed] [Google Scholar]
- 8. Tanai E, Frantz S. Pathophysiology of heart failure. Compr Physiol. 2015;6(1):187–214. [DOI] [PubMed] [Google Scholar]
- 9. Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol. 2008;101(7):1016–1022. doi:10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 10. Chang PP, Wruck LM, Shahar E, et al. Trends in hospitalizations and survival of acute decompensated heart failure in four US communities (2005-2014): ARIC study community surveillance. Circulation. 2018;138(1):12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Writing committee members, Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology Foundation/American heart association task force on practice guidelines. Circulation. 2013;128(16):e240–e327. [DOI] [PubMed] [Google Scholar]
- 12. Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292(3):344–350. [DOI] [PubMed] [Google Scholar]
- 13. Levy D, Kenchaiah S, Larson MG, et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347(18):1397–1402. [DOI] [PubMed] [Google Scholar]
- 14. Yang LZ, Kockskämper J, Khan S, et al. cAMP- and Ca2 (+)/ calmodulin- dependent protein kinases mediate inotropic, lusitropic and arrhythmogenic effects of urocortin2 in mouse ventricular myocytes. Br J Pharmacol. 2011;162(2):544–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grossini E, Molinari C, Mary DA, Marino P, Vacca G. The effect of urocortin II administration on the coronary circulation and cardiac function in the anaesthetized pig is nitric-oxide-dependent. Eur J Pharmacol. 2008;578(2-3):242–248. [DOI] [PubMed] [Google Scholar]
- 16. Rademaker MT, Charles CJ, Espiner EA, Frampton CM, Lainchbury JG, Richards AM. Endogenous urocortins reduce vascular tone and enin-aldosterone/endothelin activity in experimental heart failure. Eur Heart J. 2005;26(19):2046–2054. [DOI] [PubMed] [Google Scholar]
- 17. Rademaker MT, Charles CJ, Espiner EA, Frampton CM, Lainchbury JG, Richards AM. Four-day urocortin-I administration has sustained beneficial haemodynamic, hormonal, and renal effects in experimental heart failure. Eur Heart J. 2005;26(19):2055–2062. [DOI] [PubMed] [Google Scholar]
- 18. Davis ME, Pemberton CJ, Yandle TG, et al. Urocortin 2 infusion in human heart failure. Eur Heart J. 2007;28(21):2589–2597. [DOI] [PubMed] [Google Scholar]
- 19. Rademaker MT, Richards AM. Urocortins: actions in health and heart failure. Clin Chim Acta.2017;474:76–87. [DOI] [PubMed] [Google Scholar]
- 20. Carroll D, Ginty AT, Der G, Hunt K, Benzeval M, Phillips AC. Increased blood pressure reactions to acute mental stress are associated with 16-year cardiovascular disease mortality. Psychophys. 2012;(49):1444–1448. [DOI] [PubMed] [Google Scholar]
- 21. Hagenaars SP, Harris SE, Clarke TK, et al. Polygenic risk for coronary artery disease is associated with cognitive ability in older adults. Int J Epidemiol. 2016;2016:dyv 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. von Känel R, Hari R, Schmid JP, Saner H, Begré S. Distress related to myocardial infarction and cardiovascular outcome: a retrospective observational study. BMC Psychiatry. 2011;11:98.doi:10. 1186/1471-244X-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pfeffer JM, Finn PV, Zornoff LA, Pfeffer MA. Endothelin-A receptor antagonism during acute myocardial infarction in rats. Cardiovasc Drugs Ther. 2000;14(6):579–587. [DOI] [PubMed] [Google Scholar]
- 24. Mikami Y, Toda M, Watanabe M, Nakamura M, Toyama Y, Kawakami Y. A simple and reliable behavioral analysis of locomotor function after spinal cord injury in mice. Technical note. J Neurosurg. 2002;97(suppl. 1):142–147. [DOI] [PubMed] [Google Scholar]
- 25. Morris RG, Steele RJ, Bell JE, Martin SJ. N-methyl-d-aspartate receptors, learning and memory: chronic intraventricular infusion of the NMDA receptor antagonist d-AP5 interacts directly with the neural mechanisms of spatial learning. Eur J Neurosci.2013;37(5):700–717. [DOI] [PubMed] [Google Scholar]
- 26. Vivaldi MT, Kloner RA, Schoen FJ. Triphenyltetrazolium staining of irreversible ischemic injury following coronary artery occlusion in rats. Am J Pathol. 1985;121(3):522–530. [PMC free article] [PubMed] [Google Scholar]
- 27. Clapp RH, Luckman SM. Proxyfan acts as a neutral antagonist of histamine H3 receptors in the feeding-related hypothalamic ventromedial nucleus. Br J Pharmacol. 2012;167(5):1099–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chiu K, Lau WM, Lau HT, So KF, Chang RC. Micro- dissection of rat brain for RNA or protein extraction from specific brain region. J Vis Exp. 2007;(7):269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vamos M, Erath JW, Benz AP, Lopes RD, Hohnloser SH. Meta-analysis of effects of digoxin on survival in patients with atrial fibrillation or heart failure: an update. Am J Cardiol. 2019;123(1):69–74. [DOI] [PubMed] [Google Scholar]
- 30. Domínguez-Rodríguez A, Mayoral-Gonzalez I, Avila-Medina J, et al. Urocortin-2 prevents dysregulation of Ca2+ homeostasis and improves early cardiac remodeling after ischemia and reperfusion. Front Physiol. 2018;9:813 doi:10.3389/fphys.2018.00813.eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang Y, Yao XQ, Lau CW, Chan YC, Tsang SY, Chan FL. Urocortin and cardiovascular protection. Acta Pharmacol Sin. 2004;25(3):257–265. [PubMed] [Google Scholar]
- 32. Giamouridis D, Gao MH, Lai NC, et al. Effects of urocortin 2 versus urocortin 3 gene transfer on left ventricular function and glucose disposal. JACC Basic Transl Sci. 2018;3(2):249–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang LZ, Kockskämper J, Heinzel FR, et al. Urocortin II enhances contractility in rabbit ventricular myocytes via CRF(2) receptor-mediated stimulation of protein kinase A. Cardiovasc Res. 2006;69(2):402–411. [DOI] [PubMed] [Google Scholar]
- 34. Walther S, Pluteanu F, Renz S, et al. Urocortin 2 stimulates nitric oxide production in ventricular myocytes via Akt- and PKA-mediated phosphorylation of eNOS at serine 1177. Am J Physiol Heart Circ Physiol. 2014;307(5):H689–H700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang LZ, Zhu YC. Urocortin2 prolongs action potential duration and modulates potassium currents in guinea pig myocytes and HEK293 cells. Eur J Pharmacol. 2015;758:97–106. [DOI] [PubMed] [Google Scholar]
- 36. Pilbrow AP, Lewis KA, Perrin MH, et al. Cardiac CRFR1 expression is elevated in human heart failure and modulated by genetic variation and alternative splicing. Endocrinology. 2016;157(12):4865–4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97(12):1314–1322. [DOI] [PubMed] [Google Scholar]
- 38. Tanaka M, Tomimatsu Y, Sakimura K, et al. Characterization of CRF1 R antagonists with differential peripheral vs central actions in CRF challenge in rats. Peptides. 2017;95:40–50. [DOI] [PubMed] [Google Scholar]
- 39. Lin X, Itoga CA, Taha S, et al. c-Fos mapping of brain regions activated by multi-modal and electric foot shock stress. Neurobiol Stress. 2018;8:92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fjalldal S, Follin C, Svärd D, et al. Microstructural white matter alterations and hippocampal volumes are associated with cognitive deficits in craniopharyngioma. Eur J Endocrinol. 2018;178(6):577–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aisenberg N, Serova L, Sabban EL, Akirav I. The effects of enhancing endocannabinoid signaling and blocking corticotrophin releasing factor receptor in the amygdala and hippocampus on the consolidation of a stressful event. Eur Neuropsychopharmacol. 2017;27(9):913–927. [DOI] [PubMed] [Google Scholar]
- 42. Bale TL, Contarino A, Smith GW, et al. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000;24(4):410–414. [DOI] [PubMed] [Google Scholar]
- 43. Coste SC, Kesterson RA, Heldwein KA, et al. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin- releasing hormone receptor-2. Nat Genet. 2000;24(4):403–409. [DOI] [PubMed] [Google Scholar]
- 44. Ferrer-Pérez C, Reguilón MD, Manzanedo C, Aguilar MA, Miñarro J, Rodríguez-Arias M. Antagonism of corticotropin-releasing factor CRF1Rs blocks the enhanced response to cocaine after social stress. Eur J Pharmacol. 2018;823:87–95. [DOI] [PubMed] [Google Scholar]
- 45. Kishimoto T, Radulovic J, Radulovic M, et al. Deletion of CRHR2 reveals an anxiolytic role for corticotropin-releasing hormone receptor-2. Nat Genet. 2000;24(4):415–419. [DOI] [PubMed] [Google Scholar]
- 46. Ryabinin AE, Tsoory MM, Kozicz T, et al. Urocortins: CRF’s siblings and their potential role in anxiety, depression and alcohol drinking behavior. Alcohol. 2012;46(4):349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Timpl P, Spanagel R, Sillaber I, et al. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat Genet. 1998;19(2):162–166. [DOI] [PubMed] [Google Scholar]