Abstract
Background:
BK virus-associated nephropathy (BKVN) is an important cause of chronic allograft dysfunction. The objective of our study was to evaluate the prognosis of BKVN.
Methods:
We retrospectively reviewed the data of 133 renal transplant recipients with BKVN treated at the First Affiliated Hospital of Sun Yat-Sen University between July 2007 and July 2017. BK viral loads, graft function, and pathologic indexes were compared between initial diagnosis and last follow-up.
Results:
After a mean follow-up period of 14.4 (range, 0.3–109.6) months after diagnosis of BKVN, BK viruria, and BK viremia become negative in 19.5% and 90.2% of patients, respectively. The mean estimated glomerular filtration rate (eGFR) at last follow-up was lower than at diagnosis of BKVN (18.3 ± 9.2 vs. 32.8 ± 20.6 mL·min−1·1.73 m−2, t = 7.426, P < 0.001). Eight (6.0%) patients developed acute rejection after reducing immunosuppression. At last follow-up, the eGFR was significantly lower in patients with subsequent rejection than those without (21.6 ± 9.8 vs. 33.5 ± 20.9 mL·min−1·1.73 m−2, t = 3.034, P = 0.011). In 65 repeat biopsies, SV40-T antigen staining remained positive in 40 patients and became negative in the other 20 patients. The eGFR (42.6 ± 14.3 vs. 26.5 ± 12.3 mL·min−1·1.73 m−2), urine viral loads (median, 1.3 × 105vs. 1.4 × 107 copies/mL), and plasma viral load (median, 0 vs. 0 copies/mL) were all significantly lower in patients with negative SV40-T antigen staining than those with persistent BK involvement (all, P < 0.05). Five (3.8%) recipients lost their graft at diagnosis of BKVN, and 13 (9.8%) lost their graft during the follow-up period. The 1-, 3-, and 5-year graft survival rates after diagnosis of BKVN were 99.2%, 90.7%, and 85.7%, respectively. Higher pathologic stage correlated with lower allograft survival rate (χ2 = 6.341, P = 0.042).
Conclusion:
Secondary rejection and persistent histologic infection in BKVN lead to poor prognosis.
Keywords: Kidney transplantation, BK virus, Pathology, Rejection, Prognosis
Introduction
In the era of powerful immunosuppression for kidney transplantation, human BK virus (BKV) infection occurs in 10% to 60% of renal transplant (RT) recipients,[1–3] and the incidence of BK virus-associated nephropathy (BKVN) is as high as 10%.[4,5] About 10% to 50% of patients with BKVN develop progressive renal dysfunction leading to eventual allograft loss.[6,7] Subsequent rejection after reducing immunosuppression for management of BKVN usually complicates the clinical course and can accelerate deterioration of graft function.[8,9]
Current methods for identifying BKV infection include quantitative polymerase chain reaction (Q-PCR) assays for measuring BKV DNA load in urine and plasma, and quantitative assays of urine cytology by light microscopy or electron microscopy.[10] A definitive diagnosis of BKVN is established by renal biopsy and immunohistochemistry analysis. Since 2006, we have monitored RT recipients at our center for BKV infection.[2] With increasing experience of managing BKV infection in RT recipients, we have been consulted regarding cases of BKV infection from across the country between 2007 and 2017. Unfortunately, many of the patients referred from other centers had advanced BKVN and some patients developed graft loss.
In this study, we report the clinical features, treatments, and outcomes of 133 RT recipients with BKVN treated at our institution over the past 10 years. We believe this cohort has distinct clinical characteristics and is useful for understanding the progression and prognosis of BKVN.
Methods
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee of the First Affiliated Hospital of Sun Yat-Sen University (Approval No. [2009] 28). All patients provided written informed consent for participation in the study and to have their medical data used for research purposes.
Patient population
We performed a retrospective analysis of RT recipients who were treated for BKVN at the First Affiliated Hospital of Sun Yat-Sen University between July 2007 and July 2017. All patients were routinely followed up in our outpatient clinic every 1 to 3 months. Clinical data were retrospectively collected and analyzed. Of 146 patients with biopsy-proved BKVN, 13/133 (9.8%) were excluded due to incomplete clinical data. Thus, 133 patients were included in the study. Estimated glomerular filtration rate (eGFR) was calculated with the MDRD study equation.[11] The study endpoints were graft loss, defined as either total loss of graft function (return to dialysis or re-transplantation) or patient death with a functional graft.
Urine cytology
Urinary cytologic smears stained by the Papanicolaou method were evaluated for the presence of cells with intranuclear viral inclusions (decoy cells). The presence of decoy cells was semi-quantitatively recorded as number per 10 high power field.[12]
Quantitative determination of BKV DNA load
Urine and blood samples were collected before a biopsy was performed, and during scheduled follow-up appointments. Determination of BKV DNA load was performed by a Q-PCR assay (MJ Research, Waltham, MA, USA). Specimen collection and processing, sequences of the Q-PCR primers and TaqMan probe (targeting the BKV VP1 gene), the plasmid standard containing the targeted BKV VP1 gene, amplification protocols, PCR precautions, and quality assurance have been described elsewhere.[12] The BKV DNA load was expressed in BKV genome copies/mL. The lower limit of quantitation was 1000 copies/mL.
Allograft biopsy and pathologic diagnosis
The BKVN was defined by the presence of interstitial inflammation and tubulitis, a viral cytopathic effect in tubular epithelial cells, and was confirmed by immunohistochemical nuclear staining with anti-SV40 large T-antigen monoclonal antibody (mouse anti-SV40 large T-antigen monoclonal antibody; Oncogene Research Products, Cambridge, MA, catalogue number DP02, clone PAb 416) as previously described.[1] The histologic features of BKVN were classified using the American Society of Transplantation (AST) schema, and BKVN was classified as stage A, B, and C based on scoring of viral cytopathic changes, interstitial inflammation, tubular atrophy, and interstitial fibrosis.[1] Histologic viral load was assessed semi-quantitatively as the percentage of tubules that stained positive for BKV using a 4-tier system (<10%, 10–25%, 26–50%, and >50%).[13] Histologic lesions were scored using the Banff schema of renal allograft pathology. T-cell-mediated rejection and antibody-mediated rejection were defined by the Banff criteria.[14–17] A repeat renal biopsy was performed to evaluate the evolution of pathologic damage.
Management of BKVN and rejection
In patients with biopsy-proved BKVN, calcineurin inhibitor (CNI) dosage was reduced by 25% to 50%, or tacrolimus was switched to cyclosporine. In severely infected patients, the mycophenolate dosage was reduced or the patient was switched to mizoribine. Acute cellular rejection was treated with methylprednisolone with/without rabbit anti-human thymocyte globulin (rATG). Antibody-mediated rejection was treated with pulse steroids, intravenous immunoglobulin, rATG, and plasma exchange.
Statistical analysis
Normally distributed continuous variables were presented as mean ± standard deviation (SD), and non-normally distributed continuous variables as median (range, minimum to maximum). Groups were compared using Student's t-test for normally distributed data, and Mann-Whitney U-test for non-normally distributed data. Categorical data were presented as number and percentage (%), and compared by Pearson's Chi-squared test or Fisher's exact test (if an expected value was ≤5). Kaplan-Meier analysis was used to estimate overall allograft survival. All analyses were 2-tailed, and a value of P < 0.05 was considered to indicate statistical significance. All analyses were performed using IBM SPSS version 19 (IBM Corporation, Somers, NY, USA).
Results
Patient characteristics
The baseline demographic and transplant characteristics of the patients are shown in Table 1. From the time of RT, an indication for a biopsy occurred a mean of 5.1 (range, 0–28) months earlier than the biopsy was performed. Most recipients (93.2%) received an allograft biopsy because their serum creatinine increased more than 30% compared to the baseline value. The mean follow-up time for the 133 patients was 14.4 (range, 0.3–109.6) months after diagnosis of BKVN.
Table 1.
Demographic and clinical characteristics of 133 renal transplant recipients.

Diagnosis of BKVN
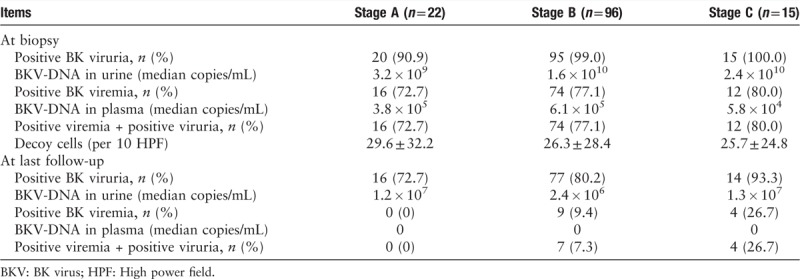
According to AST criterion of BKVN, 22 patients (16.5%) were stage A, 96 (72.2%) were stage B, and 15 (11.3%) were stage C. The median onset of biopsy-proven BKVN was 8.5, 14.4, and 27.0 months post-transplantation for stage A, B, and C patients, respectively. The BKV loads in urine and plasma are summarized in Table 2. At the time of biopsy, the incidence of BK viruria was higher than that of BK viremia (97.7% vs. 76.7%, χ2 = 26.438, P < 0.001). Of the 130 patients with BK viruria, 102 had BK viremia.
Table 2.
BKV DNA load in urine and plasma at biopsy and at last follow-up.
Renal allograft function and graft outcomes
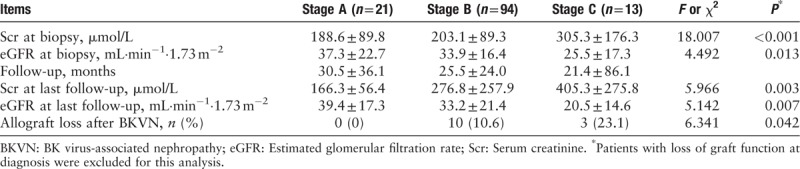
Allograft function at the time of biopsy and at last follow-up is shown in Table 3. The mean eGFR (32.8 ± 20.6 vs. 18.3 ± 9.2 mL·min−1·1.73 m−2, t = 7.426, P < 0.001) at last follow-up was lower than at diagnosis of BKVN. Five (3.8%) recipients lost their graft function at the time BKVN was diagnosed by biopsy, including 1 (4.5%) stage A patient (primary non-function caused by acute tubular injury and pre-existing hypertensive renal injury), 2 (2.1%) stage B patients, and 2 stage C patients.
Table 3.
Renal allograft function at biopsy and at last follow-up by BKVN stage.
During the follow-up period, 12 (9.4%) recipients lost their allograft function because of BKVN, and 1 (0.8%) recipient with a functioning graft died because of presumed viral encephalitis; however, an autopsy was not performed. Kaplan-Meier analysis revealed that allograft survival after RT was 99.2% at 1 year, 90.7% at 3 years, and 85.7% at 5 years. A higher BKVN pathologic stage was associated with lower allograft survival rate [Figure 1].
Figure 1.
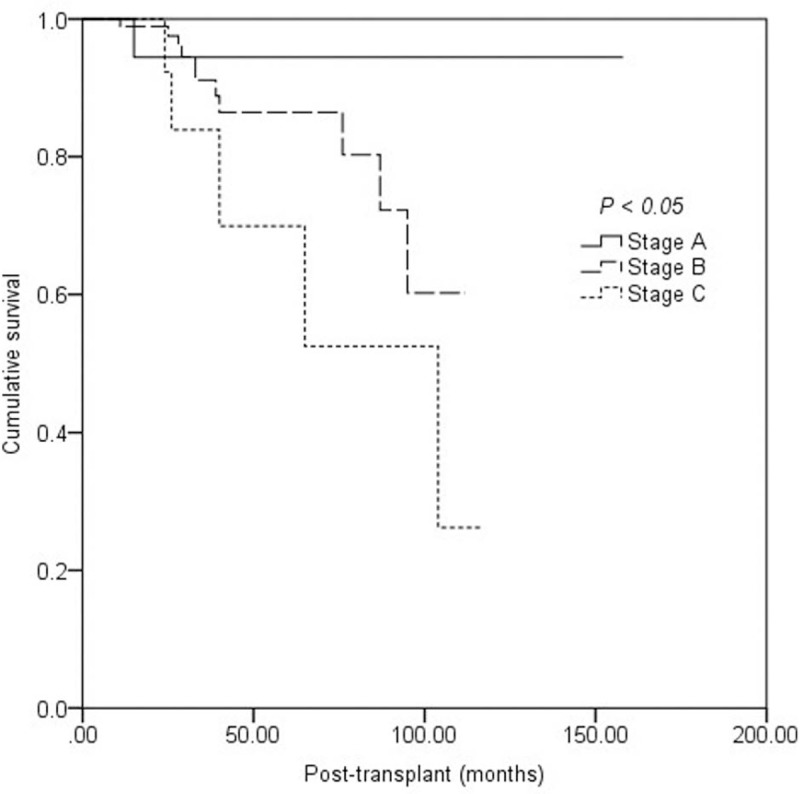
Kaplan-Meier estimate of death-censored graft survival in patients with stage A, B, and C BK virus-associated nephropathy.
Evolution of BK viruria and BK viremia and graft outcomes
The BKV DNA loads in urine and plasma at the time of biopsy and at the last follow-up are shown in Table 2. During follow-up period, positive BK viruria become negative in 26 (19.5%) patients within 16.7 ± 12.6 months. Positive BK viremia converted to negative in 120 (90.2%) patients within 24.8 ± 25.6 months. The median time for the viral load to decrease by more than 90% was 2.5 months in urine and 2.8 months in plasma. The median time for the plasma viral load to become negative was 4.0 months. At the last follow-up, serum creatinine (278.9 ± 245.5 vs. 248.8 ± 249.5 μmol/L, t = 0.557, P = 0.578), eGFR (31.5 ± 19.3 vs. 38.2 ± 25.0 mL·min−1·1.73 m−2, t = 1.506, P = 0.134), graft loss (4/26 vs. 9/107, χ2 = 0.498, P = 0.480), and subsequent rejection (1/26 vs. 7/107, χ2 = 0.003, P = 0.953.) were similar in patients with persistent BK viruria and in those without BK viruria. Similarly, serum creatinine (293.6 ± 251.6 vs. 271.0 ± 246.0 μmol/L, t = 0.303, P = 0.762), eGFR (33.0 ± 21.2 vs. 32.8 ± 20.6 mL·min−1·1.73 m−2, t = 0.033, P = 0.972), graft loss (3/13 vs. 10/120, χ2 = 1.779, P = 0.182), and subsequent rejection (0/13 vs. 8/120, χ2 = 1.120, P = 0.792) were all similar in patients with persistent BK viremia and those without BK viremia.
Rejection before BKVN and graft outcomes
Of the 133 recipients, 31 (23.3%) had a history of rejection and anti-rejection treatment before developing biopsy-proven BKVN. The mean period from rejection to BKVN diagnosis was 17.9 ± 21.9 months. At last follow-up, serum creatinine (323.5 ± 294.8 vs. 240.9 ± 201.2 μmol/L, t = 1.144, P = 0.255), eGFR (30.3 ± 23.2 vs. 33.6 ± 19.8 mL·min−1·1.73 m−2, t = 0.768, P = 0.444), graft loss (7/31 vs. 11/102, χ2 = 1.909, P = 0.167) were not different between patients with a history of rejection and those without.
Rejection after diagnosis of BKVN and graft outcomes
Of the patients who had repeat biopsies, 2 (1.5%) developed T-cell rejection (Banff 2013, arteritis ≥ 1), 5 (3.8%) developed acute antibody-mediated rejection (Banff 2013 criteria), and 1 (0.8%) developed mixed rejection. At last follow-up, serum creatinine (273.5 ± 252.2 vs. 264.8 ± 103.7 μmol/L, t = 0.098, P = 0.922) and graft loss (1/8 vs. 12/125, χ2 = 0.034, P = 0.854) were similar in patients with subsequent rejection and those without. However, the eGFR was significantly lower in patients with subsequent rejection than those without (21.6 ± 9.8 vs. 33.5 ± 20.9 mL·min−1·1.73 m−2, t = 3.034, P = 0.011).
Pathologic progression and graft outcomes
During a mean follow-up period of 25.0 (range, 1–109.6) months, 65 (48.9%) recipients received a second biopsy at a median of 16.5 months (range, 10 days to 69 months) after the initial biopsy. Seventeen (12.8%) recipients received a third biopsy at a median of 16.5 months (range 10 days to 69 months) after the initial biopsy. Two (1.5%) recipients received a fourth biopsy at 9.0 and 16.7 months, respectively, after the initial biopsy. In the 65 biopsies, SV40-T antigen staining remained positive in 40 patients and converted to negative in the other 20 patients. The time interval between initial biopsy and last biopsy was not different between patients with and without persistent BKV involvement in the graft. At the time of the last biopsy, eGFR and viral load in urine and in plasma were significantly lower in patients with negative SV40-T antigen staining than those with positive staining [Table 4]. The graft survival rate was similar in patients with positive and negative staining (18/20 vs. 40/45, χ2 = 0.018, P = 0.894).
Table 4.
Outcome of patients with and without persistent BKV involvement in repeat biopsies.

Compared with the initial biopsy, the degree of SV40-T antigen staining decreased (1.0 ± 0.9 vs. 1.7 ± 0.9, t = 4.904, P = 0.000), tubulitis decreased (1.0 ± 0.9 vs. 1.3 ± 0.9, t = 1.792, P = 0.054), interstitial inflammation decreased (1.3 ± 1.1 vs. 1.1 ± 0.9, t = 2.242, P = 0.230), tubular atrophy increased (1.9 ± 1.0 vs. 1.4 ± 0.9, t = 3.097, P = 0.001), and interstitial fibrosis increased (1.7 ± 0.9 vs. 1.4 ± 0.9, t = 2.055, P = 0.015) on subsequent biopsies.
Discussion
The BKV infections is a common infection after kidney transplantation, typically proceeding sequentially through viruria and viremia before resulting in a tubulointerstitial nephritis known as BKVN in up to 10% of patients.[1] The development of BKVN leads to renal allograft dysfunction and can result in graft loss in 10% to 70% of patients.[18] In this cohort, the overall allograft loss rate was 13.5%. The 1-, 3-, and 5-year graft survival rate after diagnosis of BKVN was 99.2%, 90.7%, and 85.7% respectively. Furthermore, higher pathologic stage at diagnosis correlated with lower graft survival rate, consistent with existing evidence.[6,19] Allograft function improved in stage A recipients, but gradually deteriorated in stage B and stage C recipients after a median follow-up of 25.0 months after diagnosis of BKVN.
In recent years, more attention has been given to BKVN[9]; however, in some centers BKV screening is not routinely performed after kidney transplantation. In this cohort, most recipients (93.2%) had deteriorated graft function when BKVN was diagnosed. Graft dysfunction, rather than a positive BKV screening test, was the main indication for biopsy. Because of this, biopsies were delayed by a mean of 5.1 months after there was an indication for a biopsy. It should be noted that many cases of BKVN in this study could have been diagnosed at an early stage if BKV screening was performed routinely and biopsy were performed in a timely manner. The median onset of biopsy-proved BKVN was 8.5, 14.4, and 27.0 months post-transplantation for stage A, B, and C patients, respectively. A delayed diagnosis of BKVN leads to more cases with advanced stage disease and worse graft function.
Currently, there are no effective antiviral agents for BKV infection, and decreasing immunosuppression intensity has been recommended for BKV infection and BKVN.[6] In this cohort, reduction of immunosuppression was effective in bringing about viremia clearance, consistent with many reports.[8,18,20] Specifically, changing from tacrolimus to a low dosage of cyclosporin A seemed to be effective in clearing BKV in the plasma and alleviating BKVN (data not shown). However, it seemed to be unsatisfactory for clearing BK viruria; 80.5% of patients remained positive for BK viruria at the last follow-up. On the contrary, graft outcomes were not different between patients with or without persistent BK viruria. The BKV may conceal and replicate in tubular or transitional epithelium under a low immunosuppression intensity, but cause no obvious injury when BK viremia is negative. These patients, however, need to be closely monitored because it has been reported that patients with persistent BK viruria have a worse prognosis.[21]
The diagnosis and treatment of secondary rejection after BKVN is a difficult problem. All treatment strategies based on a reduction in immunosuppression increase the risk of rejection because of insufficient immunosuppression.[20] The BKV itself may also promote rejection by mediating allosensitization and heterologous immunity.[22] Sawinski et al demonstrated that persistent BK viremia was a risk factor for, and precedes the development of, donor-specific antibody (DSA),[23] which has been proven to be associated with antibody-mediated rejection. We routinely monitor renal allograft function and DSA after initiating treatment for BKVN. During the follow-up period of 25 months after initiation of treatment for BKVN, 48.9% (65/133) of recipients received a repeat biopsy, and 12.3% (8/65) of recipients developed biopsy-proven acute rejection. This proportion is lower than the 8% to 50% described in the literature.[6] There are several reasons for a lower incidence of rejection after BKVN in this cohort. First, reduction of immunosuppression for treating BKVN is performed cautiously by clinicians at our center. A modest reduction of immunosuppression leads to a lower incidence of T-cell-mediated rejection (≥Banff IIA). Second, the baseline incidence of rejection at our center is not very high. Third, repeat biopsies were carried out in some patients, and rejection was confirmed only when endarteritis was present, because it is currently difficult to discriminate between BKVN and T-cell-mediated rejection with only tubulointerstitial inflammation.[24]
In this study, eGFR was significantly lower in patients with subsequent rejection than those without. Some investigations have reported that rejection secondary to BKVN carries a risk of graft loss.[6] Registry data have indicated that treatment for BKVN increases graft loss by 1.7-fold to 1.9-fold.[25] The number of patients in this study who developed rejection was not large enough to allow an effective statistical analysis of this issue.
In this study, repeat biopsies showed that SV40-T antigen staining intensity decreased, but remained positive in 40 patients and became negative in the other 20 patients. Actually, BKV cytopathic changes and corresponding SV40-T antigen staining are affected by time, particularly after immunosuppressant reduction.[6] Decreasing BK viremia, presumably reflecting diminished parenchymal viral replication, led to increasing difficulty in the identification of cytopathic changes on hematoxylin and eosin stains in repeat biopsies, even in cases with persistence of SV40 positivity. We observed that persistent BKV involvement in tissue was accompanied with higher BKV loads in urine and plasma, as well as worse outcomes during the follow-up period. Besides, renal scarring including interstitial fibrosis and tubular atrophy, still aggravated both in the cortex and the medulla. It has been reported that an increase in “ci” score from the first to the second biopsy significantly predicts graft loss.[6,19] Therefore, inhibiting renal sclerosis is key for delaying the deterioration of renal graft function.
This study had some limitations. Data were collected and analyzed retrospectively. Renal biopsies for diagnosing BKVN were performed based on indications, not based on a protocol, and repeat biopsies were performed in only some patients. Heterogeneity of treatment for BKVN could not be avoided as many patients came from other centers.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81770749); Natural Science Foundation of Guangdong Province (No. 2017A030313710); and Basic Scientific Research Fund of Sun Yat-Sen University (No. 17ykpy29).
Conflicts of interest
None.
Footnotes
How to cite this article: Chen XT, Yang SC, Li J, Deng RH, Chen WF, Qiu J, Chen LZ, Wang CX, Huang G. Prognosis of BK polyomavirus nephropathy: 10-year analysis of 133 renal transplant recipients at a single center. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000085
References
- 1.Hirsch HH, Randhawa P. BK polyomavirus in solid organ transplantation. Am J Transplant 2013; 13 Suppl 4:179–188. doi: 10.1111/ajt.12110. [DOI] [PubMed] [Google Scholar]
- 2.Huang G, Chen L, Qiu J, Wang C, Fei J, Deng S, et al. Prospective study of polyomavirus BK replication and nephropathy in renal transplant recipients in China: a single-center analysis of incidence, reduction in immunosuppression and clinical course. Clin Transplant 2010; 24:599–609. doi: 10.1111/j.1399-0012.2009.01141.x. [DOI] [PubMed] [Google Scholar]
- 3.Masutani K, Shapiro R, Basu A, Tan H, Wijkstrom M, Randhawa P. The Banff 2009 Working Proposal for polyomavirus nephropathy: a critical evaluation of its utility as a determinant of clinical outcome. Am J Transplant 2012; 12:907–918. doi: 10.1111/j.1600-6143.2012.03993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirsch HH, Knowles W, Dickenmann M, Passweg J, Klimkait T, Mihatsch MJ, et al. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med 2002; 347:488–496. doi: 10.1056/NEJMoa020439. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch HH, Steiger J. Polyomavirus BK. Lancet Infect Dis 2003; 3:611–623. doi: 10.1681/ASN.2014010119. [DOI] [PubMed] [Google Scholar]
- 6.Drachenberg CB, Papadimitriou JC, Chaudhry MR, Ugarte R, Mavanur M, Thomas B, et al. Histological evolution of BK virus-associated nephropathy: importance of integrating clinical and pathological findings. Am J Transplant 2017; 17:2078–2091. doi: 10.1111/ajt.14314. [DOI] [PubMed] [Google Scholar]
- 7.Wojciechowski D, Chandran S. BK virus infection after kidney transplantation: the data are mounting for a personalized approach. Transplantation 2016; 100:703–704. doi: 10.1097/TP.0000000000001067. [DOI] [PubMed] [Google Scholar]
- 8.Santeusanio AD, Lukens BE, Eun J. Antiviral treatment of BK virus viremia after kidney transplantation. Am J Health Syst Pharm 2017; 74:2037–2045. doi: 10.2146/ajhp160585. [DOI] [PubMed] [Google Scholar]
- 9.Blazquez-Navarro A, Dang-Heine C, Wittenbrink N, Bauer C, Wolk K, Sabat R, et al. BKV, CMV, and EBV interactions and their effect on graft function one year post-renal transplantation: results from a large multi-centre study. EBioMedicine 2018; 34:113–121. doi: 10.1016/j.ebiom.2018.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hariharan S. BK virus nephritis after renal transplantation. Kidney Int 2006; 69:655–662. doi: 10.1038/sj.ki.5000040. [DOI] [PubMed] [Google Scholar]
- 11.Huang G, Chen W, Wang C, Fei J, Deng S, Qiu J, et al. Noninvasive tool for the diagnosis of polyomavirus BK-associated nephropathy in renal transplant recipients. Diagn Microbiol Infect Dis 2013; 75:292–297. doi: 10.1016/j.diagmicrobio.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Huang G, Wu L, Yang S, Fei J, Deng S, Li J, et al. Factors influencing graft outcomes following diagnosis of polyomavirus-associated nephropathy after renal transplantation. PLoS One 2015; 10:e142460.doi: 10.1371/journal.pone.0142460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirsch HH, Brennan DC, Drachenberg CB, Ginevri F, Gordon J, Limaye AP, et al. Polyomavirus-associated nephropathy in renal transplantation: interdisciplinary analyses and recommendations. Transplantation 2005; 79:1277–1286. doi: 10.1097/01.TP.0000156165.83160.09. [DOI] [PubMed] [Google Scholar]
- 14.Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC. The Banff 97 working classification of renal allograft pathology. Kidney Int 1999; 55:713–723. [DOI] [PubMed] [Google Scholar]
- 15.Sis B, Mengel M, Haas M, Colvin RB, Halloran PF, Racusen LC, et al. Banff ’09 meeting report: antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant 2010; 10:464–471. doi: 10.1111/j.1600-6143.2009.02987.x. [DOI] [PubMed] [Google Scholar]
- 16.Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant 2014; 14:272–283. doi: 10.1111/ajt.12590. [DOI] [PubMed] [Google Scholar]
- 17.Loupy A, Haas M, Solez K, Racusen L, Glotz D, Seron D, et al. The Banff 2015 Kidney Meeting Report: current challenges in rejection classification and prospects for adopting molecular pathology. Am J Transplant 2017; 17:28–41. doi: 10.1111/ajt.14107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elfadawy N, Yamada M, Sarabu N. Management of BK polyomavirus infection in kidney and kidney-pancreas transplant recipients. Infect Disease Clin North Am 2018; 32:599–613. doi: 10.1016/j.idc.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Nickeleit V, Singh HK, Randhawa P, Drachenberg CB, Bhatnagar R, Bracamonte E, et al. The Banff Working Group Classification of Definitive Polyomavirus Nephropathy: morphologic definitions and clinical correlations. J Am Soc Nephrol 2018; 29:680–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ambalathingal GR, Francis RS, Smyth MJ, Smith C, Khanna R. BK polyomavirus: clinical aspects, immune regulation, and emerging therapies. Clin Microbiol Rev 2017; 30:503–528. doi: 10.1128/CMR.00074-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masutani K, Shapiro R, Basu A, Tan H, Ninomiya T, Randhawa P. Putative episodes of T-cell-mediated rejection in patients with sustained BK viruria but no viremia. Transplantation 2012; 94:43–49. doi: 10.1097/TP.0b013e318253e7a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seifert ME, Gunasekaran M, Horwedel TA, Daloul R, Storch GA, Mohanakumar T, et al. Polyomavirus reactivation and immune responses to kidney-specific self-antigens in transplantation. J Am Soc Nephrol 2017; 28:1314–1325. doi: 10.1681/ASN.2016030285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sawinski D, Forde KA, Trofe-Clark J, Patel P, Olivera B, Goral S, et al. Persistent BK viremia does not increase intermediate-term graft loss but is associated with de novo donor-specific antibodies. J Am Soc Nephrol 2015; 26:966–975. doi: 10.1681/ASN.2014010119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nankivell BJ, Renthawa J, Sharma RN, Kable K, O’Connell PJ, Chapman JR. BK virus nephropathy: histological evolution by sequential pathology. Am J Transpl 2017; 17:2065–2077. doi: 10.1111/ajt.14292. [DOI] [PubMed] [Google Scholar]
- 25.Bechert CJ, Schnadig VJ, Payne DA, Dong J. Monitoring of BK viral load in renal allograft recipients by real-time PCR assays. Am J Clin Pathol 2010; 133:242–250. doi: 10.1309/AJCP63VDFCKCRUUL. [DOI] [PubMed] [Google Scholar]


