To the Editor: Ischemic stroke remains a major cause of death and disability worldwide and contributes to the rising costs of health care. Thrombolytic therapy with tissue plasminogen activator (tPA, alteplase) is beneficial for the treatment of acute ischemic stroke. However, only 40% to 50% of stroke patients show a significant improvement after treatment. In addition, the use of alteplase is restricted because symptomatic intracranial hemorrhage (sICH) occurs in 1.7% to 6.4% of treated patients.[1] 25-hydroxyvitamin D (25(OH)D), which is the major circulating metabolite of vitamin D, has been confirmed to be closely related to cerebrovascular disease (CVD). A series of studies indicated that vitamin D deficiency was associated with an increased risk of ischemic stroke, high stroke severity and poor functional outcomes.[2] The aim of this study was to examine the relationship between serum 25(OH)D levels and functional outcomes in patients treated with intravenous thrombolysis for acute ischemic stroke in a Chinese population.
We retrospectively analyzed patients with acute ischemic stroke who were treated with intravenous alteplase in our stroke unit from February 2014 to January 2017. All patients with a clinical diagnosis of ischemic stroke and a National Institutes of Health Stroke Scale (NIHSS) score of less than 24 received IV alteplase (0.9 mg/kg) within 4.5 hours of stroke onset after obtaining informed consent. The inclusion and exclusion criteria for receiving alteplase, as well as the thrombolytic protocol, strictly followed the European Cooperative Acute Stroke Study (ECASS) II and ECASS III criteria. None of the patients had a history of 25(OH)D deficiency or received oral replenishment. Patients with incomplete baseline data (43 cases, 41 lacking 25(OH)D level data) or those that were lost to follow-up (13 cases) were excluded. As such, a total 208 patients were enrolled in this study.
Data were collected by physicians with experience in acute stroke care using predefined criteria. The NIHSS and modified Rankin Scale (mRS) scores were evaluated by certified raters. This study was approved by the ethics committee of the Third Affiliated Hospital of Sun Yat-sen University. All subjects involved in the study provided written informed consent.
Blood samples were collected within 24 hours of hospital admission and after at least 8 hours of fasting. Serum 25(OH)D levels were measured with a commercially available enzyme-linked immunosorbent assay kit (Immunodiagnostic Systems Limited, Bolton, UK) according to the manufacturer's instructions. A 25(OH)D level <50 nmol/L was defined as deficient. The season at blood sampling, which was defined as spring (March to May), summer (June to August), fall (September to November), or winter (December to February), was also recorded for analysis.
Baseline characteristics, including age, sex, body mass index (BMI) and medical history (atrial fibrillation, coronary artery disease, current smoking, diabetes mellitus, hypertension, and previous stroke/transient ischemic attack [TIA]), were collected. Onset-to-treatment time and blood pressure (BP) on admission were recorded. Laboratory data examined included blood cell count, C-reactive protein (CRP) level and glucose level on admission; and total cholesterol (TC), high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), and homocysteine (HCY) levels within 24 hours after admission. Information on previous use of antiplatelet, anticoagulant, antidiabetic, and statin drugs (including type and dose) was collected.
Outcomes, which were defined as any intracerebral hemorrhage (ICH), symptomatic ICH (sICH), good functional outcome, all-cause death, and major disability, were assessed in the 3-month follow-up period after stroke onset. A sICH was defined as clinical neurological deterioration (an increment of the NIHSS scores of 4 or greater) in addition to any hemorrhage identified on CT/MRI. Outcomes were assessed by the mRS score at 3-month follow-up visit. Good functional outcome was defined as an mRS score of 0–1, and major disability was defined as an mRS score of 3–5.
All the statistical analyses were performed with SPSS for Windows, version 22.0 (SPSS Inc., Chicago, IL, USA). Continuous data were presented as the mean ± standard deviation (SD) or median (interquartile range, IQR), while categorical data were expressed as numbers and percentages. Continuous variables were analyzed using Student's t-test or Wilcoxon rank test. Categorical data were analyzed using the χ2 test or Fisher exact test if any expected value was below 5. Significance was established at a 2-tailed α level of 0.05. Cross-tables (2 × 2) were used to calculate odds ratios (ORs). Logistic regression analysis was used to estimate ORs and 95% confidence intervals (CIs) of the effect of 25(OH)D deficiency on ICH, good functional outcome, major disability, or all-cause death in all patients or in different lipid-level subgroups.
Of the 208 participants, there were 149 males (71.6%) and 59 females (28.4%) with a mean age of 63.5 ± 13.8 years and a mean serum 25(OH)D level of 56.0 ± 27.6 nmol/L. Baseline characteristics of the study population, subdivided according to quintiles of serum total 25(OH)D level, are summarized in Table 1.
Table 1.
Baseline characteristics of patients according to 25(OH)D levels.
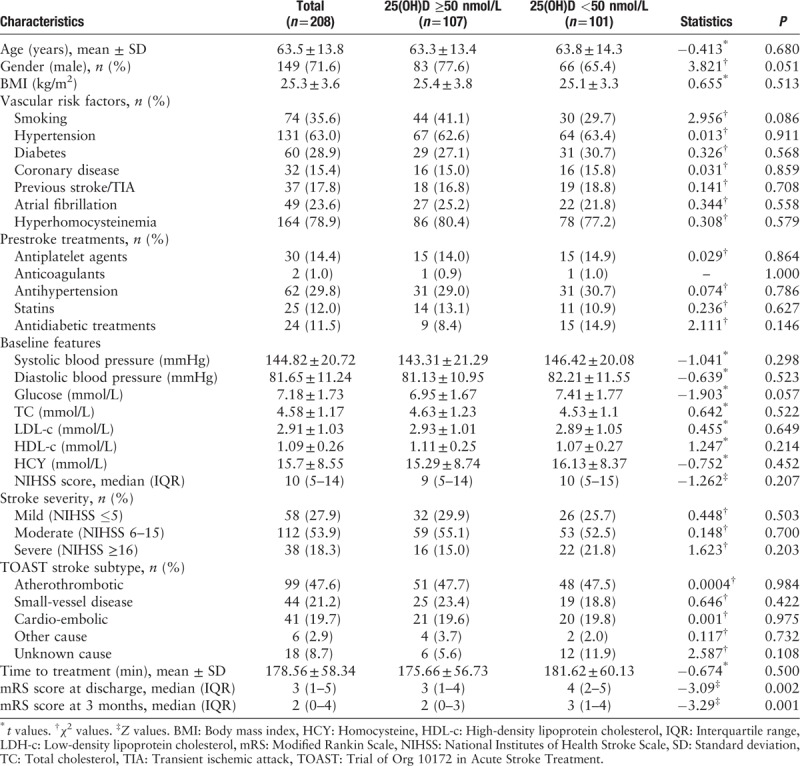
Patients with a low 25(OH)D level were more likely to be female (22.4% vs. 34.6%, P = 0.051), be a non-smoker (58.9% vs. 70.3%, P = 0.086), and have a higher baseline glucose level (6.95 ± 1.67 mmol/L vs. 7.41 ± 1.77 mmol/L, P = 0.057).
The median mRS score was high in patients with a low 25(OH)D level at discharge (3 [IQR]: 1–4 vs. 4 [IQR]: 2–5, P = 0.002) and at 3 months (2 [IQR]: 0–3 vs. 3 [IQR]: 1–4, P = 0.001). The distribution of mRS scores at 3 months according to 25(OH)D level is shown in Supplementary file 1. Patients in the high 25(OH)D level group were likely to have a low mRS score. Analysis according to mRS score at 3 months demonstrated that patients with an mRS score <4 had a higher 25(OH)D level than those with an mRS score ≥4 (P < 0.05) (Supplementary file 2).
There were 21 cases of hemorrhagic transformation: 9 (8.4%) in the high 25(OH)D level group and 12 (11.9%) in the low 25(OH)D level group (P = 0.409). There were 16 (7.7%) cases of sICH: 5 (4.7%) in the high 25(OH)D level group and 11 (10.9%) in the low 25(OH)D level group. There was a trend towards more patients in the low 25(OH)D level group having an sICH (OR = 0.401, 95% CI: 0.134–1.198, P = 0.102). Ninety-two (44.2%) patients had good functional outcomes at 3 months: 53 (49.5%) in the high 25(OH)D level group and 39 (38.6%) in low 25(OH)D level group. Seventy-nine (40.3%) patients developed a major disability: 36 (34.3%) in the high 25(OH)D level group and 43 (47.3%) in the low 25(OH)D level group. Fourteen (6.7%) patients died: 3 (2.8%) in the high 25(OH)D level group and 11 (10.9%) in the low 25(OH)D level group. There were trends towards more patients in the high 25(OH)D level group having good functional outcomes at 90 days (OR = 1.56, 95% CI: 0.899–2.708, P = 0.114) and more patients in the low 25(OH)D level group developing a major disability (OR = 0.582, 95% CI: 0.327–1.036, P = 0.066). More patients in the low 25(OH)D level group died (OR = 0.236, 95% CI: 0.064–0.872, P = 0.03). However, after adjusting for age, sex, BMI, BP, blood glucose, blood lipid level, smoking status, admission NIHSS, and season, the above differences were no longer statistically significant (Supplementary files 3 and 4).
Several studies have shown associations between stroke outcomes and inflammatory response factors such as CRP, leucocyte count, and neutrophil ratio. Since there is a close relation between 25(OH)D and inflammation, we analyzed leucocyte count, neutrophil count, neutrophil ratio, and CRP level on admission based on 25(OH)D level and found that patients in the low 25(OH)D level group displayed a trend towards higher leucocyte counts than patients in the high 25(OH)D level group ([8.63 ± 2.81] ×109/L vs. [7.93 ± 2.45] ×109/L, P = 0.059). However, neutrophil count and neutrophil ratio were not significantly different between the groups (data not shown). The low 25(OH)D level group had a significantly higher CRP level than the high 25(OH)D level group (7.3 [IQR]: 2.6–15.1 mg/L vs. 4.1 [IQR]: 1.7–8.7 mg/L, P = 0.004). These results indicated an increased inflammatory response in patients with a low 25(OH)D level (Supplementary file 5).
Several population-based, cross-sectional studies have found significant associations between 25(OH)D and blood lipid components. Furthermore, a recent study analyzed the relationship between 25(OH)D deficiency and clinical outcomes in ischemic stroke patients with different levels of various blood lipid components and found that serum 25(OH)D deficiency predicts poor outcome among acute ischaemic stroke patients with low HDL-c.[3] To determine whether blood lipid components affect the clinical outcomes in 25(OH)D deficient patients treated with intravenous thrombolysis for acute ischemic stroke, we subdivided the patients based on blood lipid component levels and made further analysis. According to Chinese guidelines on the Prevention and Treatment of Dyslipidemia in Adults in 2016, the cutoff points for normal lipid levels were TC, 5.2 mmol/L; LDL-c, 3.4 mmol/L; and HDL-c, 1.0 mmol/L. Detrimental effects of 25(OH)D deficiency on clinical outcomes at 3 months were observed in patients with a TC ≥ 5.2 mmol/L and LDL-c ≥ 3.4 mmol/L. In the case of 25(OH)D deficiency and intravenous thrombolysis, patients with a high TC or LDL-c level had a low chance of an good functional outcome and a higher risk of all-cause death (as shown in Supplementary file 6). After adjustment for age, sex, BMI, BP, blood glucose, TC, HLD-c, LDL-c, smoking status, admission NIHSS, and season, and the other two lipid levels, only patients with an LDL-c ≥3.4 mmol/L had a higher rate of all-cause death at 3 months (OR = 45.08, 95% CI: 3.13–1838.78, P = 0.014) compared to those with an LDL-c < 3.4 mmol/L.
In this study, we demonstrated that a low 25(OH)D level was associated with worse functional outcomes at 3 months in ischemic stroke patients who received alteplase, and a high TC or LDL-c level contributed to a low chance of having a good functional outcome and a high risk of all-cause death at 3 months.
It has been suggested that 25(OH)D has neuroprotective properties, and serum 25(OH)D level is a significant predictor of functional outcome in patients with acute ischemic stroke.[2] Previous studies have shown that 25(OH)D deficiency is associated with an increased risk of ischemic stroke, severe stroke and high mortality. Although there are no data regarding mortality and ischemic stroke with 25(OH)D deficiency after intravenous thrombolysis in the literature, our results are consistent with those of previous studies conducted on stroke patients, irrespective of the acute treatment administered. A recent study by Daumas et al[4] showed that a low serum 25(OH)D level was associated with worse functional outcomes in patients with acute ischemic stroke treated with intravenous thrombolysis. Our data from a Chinese population provided a similar result. On this basis, we also analyzed subgroups of patients with different blood lipid component levels and found that a high TC (≥5.2 mmol/L) or LDL-c level (≥ 3.4 mmol/L) was associated with a low frequency of good functional outcomes and a high frequency of all-cause death at 3 months, suggesting a synergism between 25(OH)D and blood lipids. Previous studies have found an association between 25(OH)D level and blood lipid levels. 25(OH)D deficiency might cause adipocyte calcium influx and promote the expression of lipase, resulting in increased lipid synthesis and increased serum levels of numerous blood lipid factors in patients.[5] Furthermore, many studies have shown that intravenous thrombolysis combined with statins (a class of cholesterol-lowering drugs) improves the outcomes of patients with acute ischemic stroke at 3 months. Therefore, 25(OH)D might act in conjunction with blood lipids to regulate the pathophysiological process of stroke, especially in acute ischemic stroke patients treated with intravenous thrombolysis.
Several mechanisms have been found to explain the protective effect of 25(OH)D in patients with ischemic stroke, including blood-brain barrier protection, antioxidation and immunomodulatory potential, and inflammatory response regulation.[2] Our data showed an increased CRP level and leucocyte count in patients with 25(OH)D deficiency, suggesting that 25(OH)D exerts protective effects by regulating the inflammatory response at an early stage of stroke.
In conclusion, a significant association was observed between a low 25(OH)D level and worse outcomes at 3 months in Chinese patients treated with intravenous thrombolysis for acute ischemic stroke. Patients with 25(OH)D deficiency might have high all-cause mortality in patients with co-occurring high LDL-c.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Wei L, Chen C, Dai YQ, Ding L, Li HY, Lin YJ, Wu HT, Wu Z, Lu ZQ. Serum 25-hydroxyvitamin D deficiency predicts poor outcomes among acute ischemic stroke patients receiving intravenous thrombolysis. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000084
References
- 1.Wahlgren N, Ahmed N, Davalos A, Ford GA, Grond M, Hacke W, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet 2007; 369:275–282. doi: 10.1016/S0140-6736(07)60149-4. [DOI] [PubMed] [Google Scholar]
- 2.Zhou R, Wang M, Huang H, Li W, Hu Y, Wu T. Lower vitamin D status is associated with an increased risk of ischemic stroke: a systematic review and meta-analysis. Nutrients 2018; 10:277.doi: 10.3390/nu10030277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu T, Zhong C, Peng Y, Chen CS, Wang J, Ju Z, et al. Serum 25-hydroxyvitamin D deficiency predicts poor outcome amongst acute ischaemic stroke patients with low high density lipoprotein cholesterol. Eur J Neurol 2016; 23:1763–1768. doi: 10.1111/ene.13121. [DOI] [PubMed] [Google Scholar]
- 4.Daumas A, Daubail B, Legris N, Jacquin-Piques A, Sensenbrenner B, Denimal D, et al. Association between admission serum 25-Hydroxyvitamin D levels and functional outcome of thrombolyzed stroke patients. J Stroke Cerebrovasc Dis 2016; 25:907–913. doi: 10.1016/j.jstrokecerebrovasdis.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Wang JM, Ye SD, Li SM, Hu W. Correlations of 25(OH)D level with blood lipid, inflammatory factors and vascular endothelial function in diabetic patients. Eur Rev Med Pharmacol Sci 2018; 22:731–735. doi: 10.26355/eurrev_201802_14303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


