Abstract
Background:
Vasovagal syncope (VVS) is common in children and greatly affect both physical and mental health. But the mechanisms have not been completely explained. This study was designed to analyze the gut microbiota in children with VVS and explore its clinical significance.
Methods:
Fecal samples from 20 VVS children and 20 matched controls were collected, and the microbiota were analyzed by 16S rRNA gene sequencing. The diversity and microbiota compositions of the VVS cases and controls were compared with the independent sample t test or Mann-Whitney U test. The correlation between the predominant bacteria and clinical symptoms was analyzed using Pearson or Spearman correlation test.
Results:
No significant differences in diversity were evident between VVS and controls (P > 0.05). At the family level, the relative abundance of Ruminococcaceae was significantly higher in VVS children than in controls (median [Q1, Q3]: 22.10% [16.89%, 27.36%] vs. 13.92% [10.31%, 20.18%], Z = −2.40, P < 0.05), and LEfSe analysis revealed Ruminococcaceae as a discriminative feature (linear discriminant analysis [LDA] score > 4, P < 0.05). The relative abundance of Ruminococcaceae in VVS patients was positively correlated with the frequency of syncope (r = 0.616, P < 0.01). In terms of its correlation with hemodynamics, we showed that relative abundance of Ruminococcaceae was negatively correlated with the systolic and diastolic pressure reduction at the positive response in head-up tilt test (HUTT; r = −0.489 and −0.448, all P < 0.05), but was positively correlated with the mean pressure drop and decline rate (r = 0.489 and 0.467, all P < 0.05) as well as diastolic pressure drop and decline rate at the HUTT positive response (r = 0.579 and 0.589, all P < 0.01) in VVS patients.
Conclusion:
Ruminococcaceae was the predominant gut bacteria and was associated with the clinical symptoms and hemodynamics of VVS, suggesting that gut microbiota might be involved in the development of VVS.
Keywords: Vasovagal syncope, Gut microbiota, Ruminococcaceae, Children
Introduction
Vasovagal syncope (VVS) is the most common form of syncope, accounting for more than one-half of children presenting to the emergency department.[1] The peak onset age of VVS is between 13 and 15 years.[2] Patients with VVS are often triggered by long-term standing or by emotional stimuli with or without presyncope symptoms. VVS is a benign self-limiting process but recurrent seizures may affect both the quality of daily life and mental health.[3] Therefore, further clarification of the pathogenesis of this disease is very important and valuable for clinical practice.
The pathogenesis of VVS is perplexing and includes the Bezold–Jarisch reflex, autonomic nervous system dysfunction, excessive activation of the renin-angiotensin system (RAS), and vasoactive substances.[4–7] These mechanisms often interact or coexist but are not completely understood. With the continuous development of genetic technology, the role of gut microbiota in a variety of diseases has attracted great attention in the past few years.[8] Previous studies have shown that the diversity and composition of gut microbiota play important pathophysiological roles in cardiovascular diseases, such as hypertension and atherosclerosis.[9] The main mechanisms for these diseases involve vasomotor dysfunction caused by a variety of neurological and humoral factors,[10] which exhibit some similarities to the pathogenesis of VVS. Many precursor symptoms of VVS are often accompanied by gastrointestinal disorders manifested as nausea, vomiting, and abdominal pain. These abdominal symptoms were previously thought to be related to autonomic dysfunction. Therefore, we hypothesized that gut microbiota might be involved in the mechanisms for VVS. However, the role of gut microbiota in VVS has not yet been elucidated. Therefore, the present study was undertaken to explore the change and significance of gut microbiota in VVS of children.
Methods
Ethical approval
This study was approved by the Peking University First Hospital and informed consent was obtained from all of the patients before their enrolment in this study. All procedures performed in studies were in accordance with the ethical standards of the institutional and/or national research committee and with the Helsinki Declaration and its later amendments or comparable ethical standards.
Subjects
The VVS group consisted of 20 children diagnosed with VVS who were recruited from the Department of Pediatrics of Peking University First Hospital from July 2016 to January 2017. While, the control group consisted of 20 age- and sex-matched healthy children.
The VVS inclusion criteria were as follows: (1) aged between 5 and 18 years, (2) the diagnostic criteria of VVS are met,[11] and (3) a normal routine stool examination. The exclusion criteria were as follows: (1) structural abnormalities of the cardiovascular, neurological or metabolic systems as observed by electrocardiogram (ECG), echocardiography, blood biochemistry, cerebral magnetic resonance imaging (MRI) or computed tomography (CT) scan; (2) body mass index (BMI) > 24 kg/m2; (3) a history of antibiotic or probiotic use within 8 weeks; (4) an onset of constipation or diarrhoeal disease within 8 weeks.
The healthy children inclusion criteria were as follows: (1) aged between 5 and 18 years; (2) no syncope, dizziness, paleness and so on; and (3) a normal routine stool examination. The exclusion criteria were as follows: (1) a history of structural or functional abnormalities; (2) a history of antibiotic or probiotic use within the last 8 weeks; (4) an onset of constipation or diarrhoeal disease within the last 8 weeks.
All participants were analyzed for general characteristics and demographic data. The children with VVS were examined for the syncope frequency and clinical data during the head-up tilt test (HUTT).
Head-up tilt test
After fasting for 4 h before the test, participants were instructed to lie down on a HUTT bed (HUT-821, Beijing Che-Chi Pharmaceutical Technology Co., Ltd., China) for 10 min in a quiet, dark environment. A non-invasive continuous blood pressure monitor (Finapres Medical System-FMS, FinometerPRO, FMS Company, Netherlands) was used to record heart rate and blood pressure, and ECG was recorded. The head of the bed frame was then tilted upward to a 60° angle and the changes in heart rate, blood pressure, and ECG were recorded. The test was terminated when a positive reaction was detected or after 45 min of HUTT if no positive reaction was detected.
The positive criteria for HUTT were as follows:[11] decreased blood pressure; decreased heart rate; a junctional escape rhythm that occurred after sinus arrest; a second- or third-degree atrioventricular block; and a pause of asystole of more than 3 seconds. A fall in blood pressure and heart rate was defined as follows: systolic pressure ≤80 mmHg (1 mmHg = 0.133 kPa) or diastolic pressure ≤50 mmHg or a drop in mean blood pressure ≥25%. Bradycardia was defined as follows: a heart rate decline to <75 beats/min in 4 to 6-year-old, <65 beats/min in 7 to 8-year-old, <60 beats/min in children over 8 years old.
Fecal sample collection and microbiota analysis
Fecal samples were collected from all participants in the morning following an overnight fast using disposable sterile forceps. Each fecal sample was immediately packaged in a liquid nitrogen container and stored at −80°C until DNA extraction.
Genomic DNA from fecal samples was extracted using the CTAB method. A frozen aliquot (200 mg) of each fecal sample was briefly immersed in 1000 μl of 2% cetyltrimethylammonium bromide (CTAB) buffer with 20 μl of lysozyme. The sample was incubated at 65°C for 2 h and then centrifuged at 3000 × g for 5 min, and the supernatant was saved. An equal volume of hydroxybenzene (pH 8.0): chloroform: isoamyl alcohol at a ratio of 25:24:1 was added to the saved supernatant. The mixed sample was then centrifuged at 3000 × g for 10 min and the supernatant was saved. An equal volume of chloroform: isoamyl alcohol at a ratio of 24:1 was added to the supernatant. The mixed sample was centrifuged at 3000 × g for 10 min and the supernatant was saved. A three-quarter volume of isopropanol was added to the saved supernatant. The sample was mixed well, precipitated at −20°C, and centrifuged at 3000 × g for 10 min. The supernatant was discarded. The saved pellet was washed with 1 ml of 75% ethanol twice and dried. A total of 51 μl of ddH2O and 1 μl of RNase A were added to hydrolyze the RNA. The samples were placed at 37°C for 15 min and stored at −20°C before DNA amplification by polymerase chain reaction (PCR).
The hypervariable region (V4) of the bacterial 16 S rRNA gene was amplified from fecal DNA extracts using the modified universal bacterial primer pair 515F (GTGCCAGCMGCCGCGGTAA) and 806R (GGACTACHVGGGTWTCTAAT). All PCR analyses were carried out with the Phusion® High-Fidelity PCR Master Mix with GC Buffer (New England Biolabs, Ipswich, MA, USA). An initial PCR sample contained approximately 10 ng of template DNA, 0.2 μmol/L of forward and reverse primers, and 12.5 μL of 2 × Taq PCR Mix in a total volume of 25 μL. The reaction was carried on a Veriti 96-well Thermal Cycler (Applied Biosystems, USA) using the following program: initial denaturation at 98°C for 1 min, 30 cycles of denaturation at 98°C for 10 s, annealing at 50°C for 30 s, and elongation at 72°C for 30 s, with a final extension step at 72°C for 5 min.
An equal volume of 1 × loading buffer (containing SYBR green) was mixed with the PCR products and the mixtures were subjected to gel electrophoresis on 2% agarose gels. Samples with bright bands between 400 and 450 bp were selected for further experiments. PCR products of the same sample were mixed in equidensity ratios and the mixed products were purified with the GeneJET gel extraction kit (Thermo Scientific).
Sequencing libraries were generated using the TruSeq® DNA PCR-Free Sample Preparation Kit according to the manufacturer's recommendations, and index codes were added. The library quality was assessed on the Qubit@ 2.0 Fluorometer (Thermo Scientific) and Agilent Bioanalyzer 2100 system. Lastly, the library was sequenced on an Illumina HiSeq 2500 platform, and 250-bp paired-end reads were generated.
Paired-end reads were assigned to each sample according to unique barcodes and were truncated by cleaving the barcode and primer sequence. The paired-ends were merged by FLASH, a fast and accurate analysis tool designed to merge overlapping reads. The splicing sequences were termed raw tags, and the effective tags were ultimately obtained after filtering and removing chimera sequences from the raw tags.
Sequences were analyzed using UPARSE software (v7.0.1001, Robert C. Edgar, USA) and assigned to the same operational taxonomic units (OTUs) according to sequences with more than 97% similarity. Representative sequences for each OTU were selected, and taxonomic information was annotated using the mothur method and SILVA SSU rRNA as a template database. Rapid multiple sequence alignment was conducted with the MUSCLE software (v3.8.31) to determine the phylogenetic relationships of the representative sequences of all obtained OTUs.
Statistical analysis
IBM SPSS 22.0 (International Business Machines Corporation, USA) was used for data analysis. The Shapiro-Wilk test was used to check the normality of the data distribution. Measurement data with normal distributions were expressed as the mean ± standard deviation (SD), while enumeration data with non-normal distributions were expressed as the median (Q1, Q3). Parametric data with normal distributions were compared with the independent sample t test and the correlation was examined using Pearson correlation test. The Mann-Whitney U test was used for comparisons and the Spearman correlation test was used for correlations for the enumeration data with non-normal distributions.
Alpha and beta diversity-weighted UniFrac were calculated for each sample using the Qiimer software (version 1.9.1, Pathogen and Microbiome Institute, Northern Arizona University, USA) based on the rarefied OTU counts. Alpha diversity was applied to analyze the complexity of the species diversity in each sample based on six indexes: the observed-species, Chao1, ACE, Shannon, Simpson, and Good's coverage indexes. These indexes were used to evaluate the uniformity and richness of each sample.[12] A beta diversity analysis was used to evaluate differences in the species complexity between samples based on diversity-weighted UniFrac.[13] Rarefaction curves and principal coordinate analysis (PCoA) were displayed using R software (v2.15.3). Differences in beta diversity between the two groups were tested based on the UniFrac distance using Anosim, which was used to examine whether the differences between groups were significantly greater than the differences within groups; this algorithm was included in the R vegan package. The specific bacterial taxa differentiating the two groups were characterized using the LEfSe method, which emphasized statistical significance and biological relevance. An effect size threshold of 4 was used for the biomarkers in the two groups. P-values <0.05 were considered statistically significant for all tests.
Results
Demographic features
The VVS group comprised a total of 20 children (7 boys and 13 girls), with a mean age of 11.1 ± 2.0 years. The control group comprised 20 children (8 boys and 12 girls), with a mean age of 10.7 ± 2.5 years. No significant differences were evident in terms of age, gender distribution, height, weight, and BMI between the two groups [Table 1]. A total of 2,922,206 effective tags were generated from all samples with an average of 73,055 ± 11,305 effective tags per sample. A total of 7012 OTUs were obtained. The OTUs for each sample are shown in Figure 1A.
Table 1.
Comparison of clinical characteristics in the study groups.
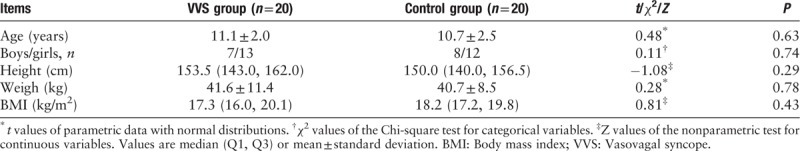
Figure 1.
Characteristics of the microbiota in the 2 groups. (A) Distribution diagram of the OTUs of each sample for all subjects. The abscissa represents the samples, and the ordinate represents the number of OTUs. (B) Rarefaction curves (1) based on observed-species indexes and (2) based on Shannon indexes. (1) The abscissa represents the number of randomly selected sequences, and the ordinate represents the annotated number of OTUs based on the selected sequences. (2) The abscissa represents the number of randomly selected sequences and the ordinate represents the calculated Shannon index based on the selected sequences. (C) Venn graph. Venn graph showing the unique OTUs of VVS group (green color), the unique OTUs of the healthy control group (blue color), and the common OTUs in the two groups (cyan color). (D) PCoA plot. PCoA plot showing the dispersal of microbiota from the VVS patients (green dot) and healthy controls (red square). PCoA1, principal coordinate analysis 1 for 47.04% of the total variation; PCoA2, principal coordinate analysis 2 for 13.07% of the total variation. HG: Healthy group; OTUs: Operational taxonomic units; PC1: The first principal coordinate; PC2: The second principal coordinate; PCoA: Principal coordinate analysis; VVS: Vasovagal syncope.
Rarefaction curve
The rarefaction curves based on the observed-species and Shannon indexes were nearly asymptotic with the increased sequencing, indicating that the sequencing data were enough to cover virtually all species in all samples and to reveal microbial information, as shown in Figure 1B.
OTUs between the two groups
Venn graph 5942 OTUs were obtained in the VVS group, while 4547 OTUs were obtained in the control group. The common number of OTUs was 3478; 2464 were unique to the VVS group and 1069 were unique to the control, as shown in Figure 1C.
Alpha diversity between the two groups
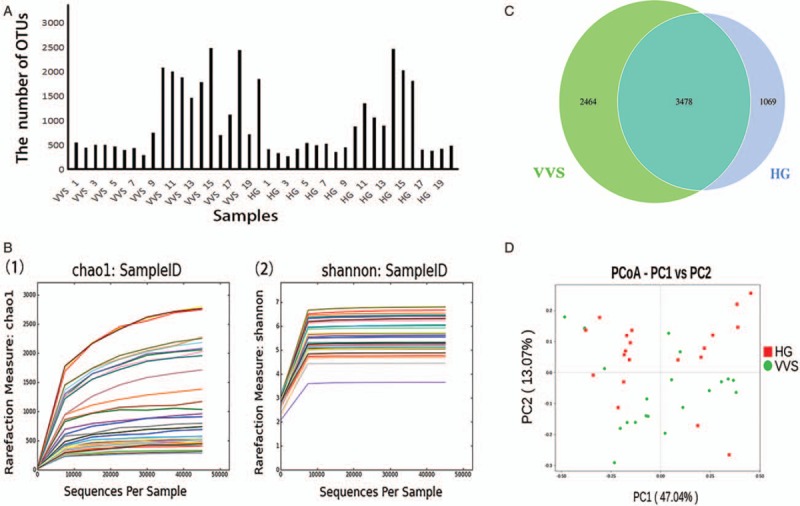
There was no significant difference in alpha diversity between the two groups [Table 2].
Table 2.
Comparison of alpha diversity in the study groups.
Principal coordinate analysis
The PCoA was performed to obtain the principal coordinates and to visualize complex multidimensional data. The closer and more aggregated in the graph the UniFrac was, the more similar the composition of the species in each sample would be, as shown in Figure 1D. No significant difference was evident between the two groups as tested by Anosim (R = 0.013, P > 0.05).
Gut microbiota structural diversity at different levels
At the phylum level, Firmicutes, Bacteroidetes, and Actinobacteria were the predominant bacteria in the two groups, and no difference was evident for the two groups at the phylum level (Z = −1.05, P > 0.05; t = 0.16, P > 0.05; Z = 0.70, P > 0.05, Figure 2A). At the family level, Lachnospiraceae, Bacteroidaceae, and Ruminococcaceae were the predominant bacteria in the two groups, and the relative abundance of Ruminococcaceae was higher in the VVS group than in the control group (median [Q1, Q3]: 22.10% [16.89%, 27.36%] vs. 13.92% [10.31%, 20.18%], Z = −2.40, P < 0.05, Figure 2B).
Figure 2.
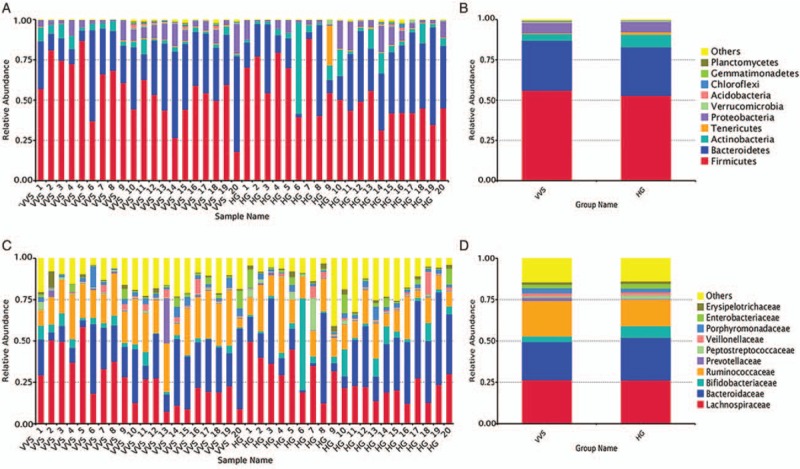
The distribution of gut microbiota in phylum level and family level of the two groups. (A) Top 10 bacteria at the phylum level of each sample. (B) Top 10 bacteria at the phylum level of each group. (C) Top 10 bacteria at the family level of each sample. (D) Top 10 bacteria at the family level of each group. HG: Healthy group; VVS: Vasovagal syncope.
Differential bacterial analysis
The LEfSe method was used to compare the gut microbiota compositions of the VVS group and control group to identify the specific bacterial taxa associated with VVS.[14] A cladogram representing the structures of the fecal microbiota and the predominant bacteria in the VVS group was generated. The LEfSe analysis revealed Ruminococcaceae as the discriminative feature (LDA > 4, P < 0.05, Figure 3A and B) at the family level.
Figure 3.
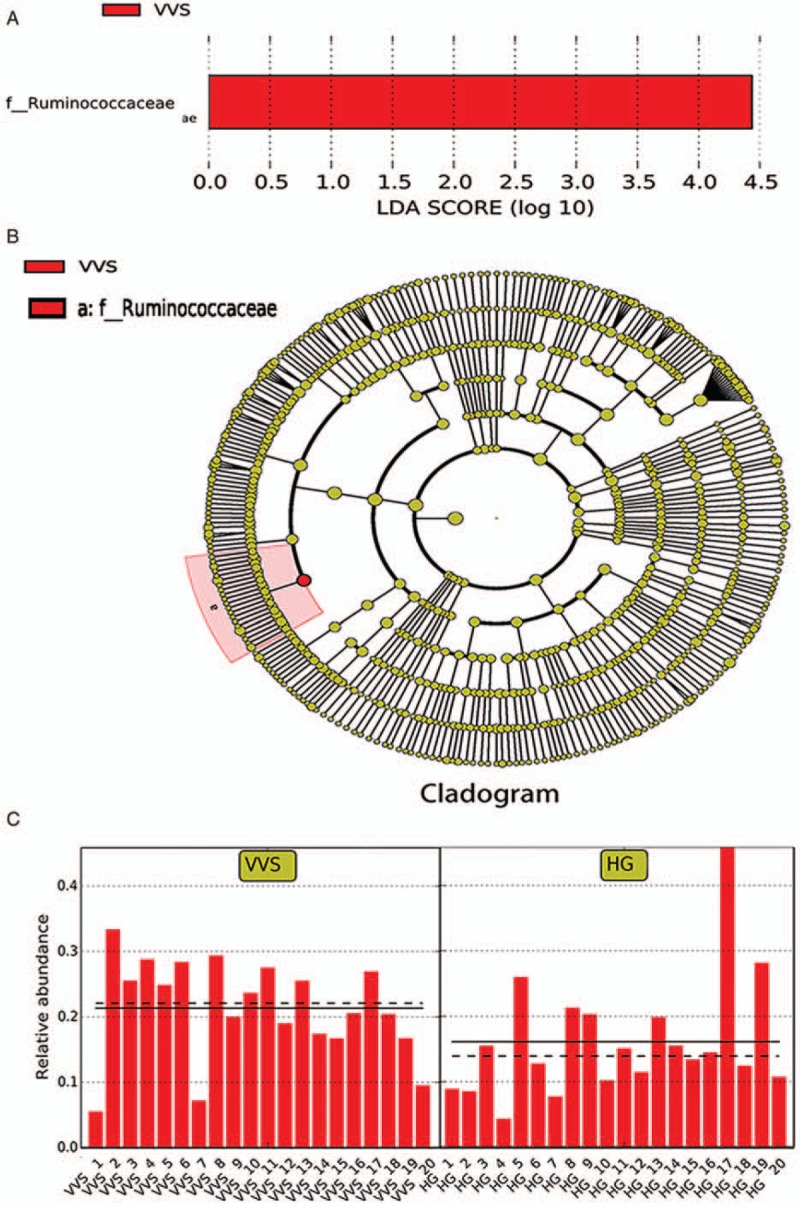
The predominant bacteria in vasovagal syncope group using LEfSe. (A) Histogram of the LDA scores. Histogram of the LDA scores computed for bacteria that were differentially abundant between the VVS and healthy children; VVS-enriched bacteria are indicated with a positive LDA score. The LDA score indicates the effect size and ranking of each differentially abundant taxon. (B) Cladogram. In the cladogram, circles radiating from the inside to the outside, with the diameter positively correlated with the relative abundance, represent the different classification levels of kingdom, phylum, class, order, family, genus, and species. Yellow indicates no significant difference between the two groups and red indicates an important role in the VVS group. (C) Relative abundance of Ruminococcaceae in each sample. The solid line shows the average, and the dotted line shows the median. HG: Healthy group; LDA: Linear discriminant analysis; VVS: Vasovagal syncope.
Associations between relative abundance of Ruminococcaceae and clinical data
We analyzed the associations between the relative abundance of Ruminococcaceae and clinical symptoms, including syncope frequency, the time to the positive response in HUTT, systolic, diastolic and mean pressure in baseline and at the positive response in HUTT. We also assessed the association between relative abundance of Ruminococcaceae and the reduction and decline rate of systolic, diastolic, and mean pressure at the positive response in HUTT. The relative abundance of Ruminococcaceae was positively correlated with the frequency of syncope (r = 0.616, P < 0.01, Figure 4A), negatively correlated with systolic and diastolic pressure (r = −0.489, P < 0.05, Figure 4B; r = −0.448, P < 0.05, Figure 4C), and positively correlated with the reduction and decline rate of diastolic pressure at the positive response of HUTT (r = 0.579, P < 0.01, Figure 4D; r = 0.589, P < 0.01, Figure 4E). The relative abundance of Ruminococcaceae was also positively correlated with the mean pressure drop and decline rate at the positive response of HUTT (r = 0.489, P < 0.05, Figure 4F; r = 0.467, P < 0.05, Figure 4G).
Figure 4.
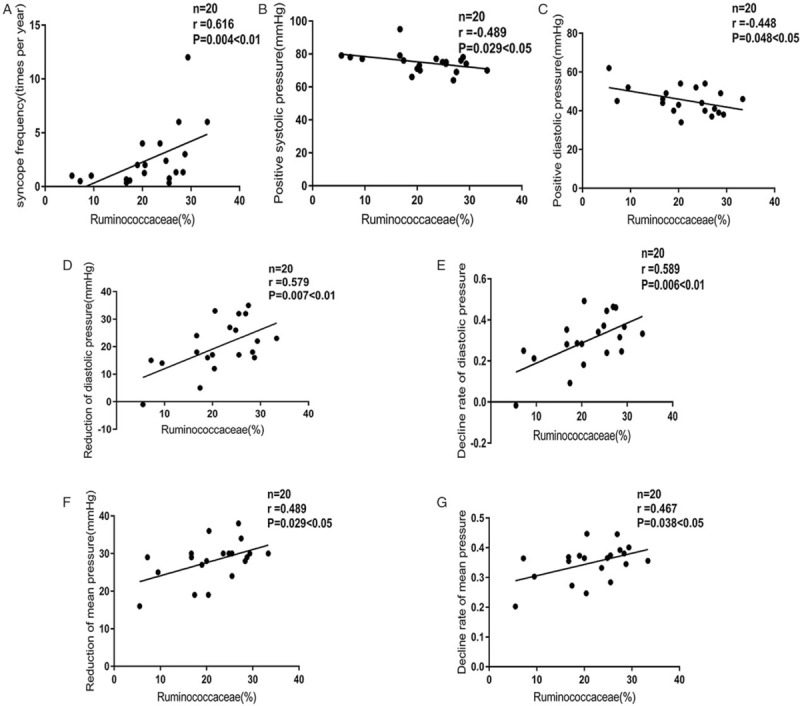
Correlation between the relative abundance of Ruminococcaceae in the vasovagal syncope group and clinical symptoms. (A) Correlation between the relative abundance of Ruminococcaceae in the VVS group and syncopal frequency. (B) Correlation between the relative abundance of Ruminococcaceae in the VVS group and the systolic pressure during the time to HUTT positivity. (C) Correlation between the relative abundance of Ruminococcaceae in the VVS group and the diastolic pressure during the time to HUTT positivity. (D) Correlation between the relative abundance of Ruminococcaceae in the VVS group and the reduction in diastolic pressure during the positive time of HUTT. (E) Correlation between the relative abundance of Ruminococcaceae in the VVS group and the decline rate of diastolic pressure during the time to HUTT positive response. Decline rate of diastolic pressure = (basic diastolic pressure – diastolic pressure during the HUTT positive time)/basic diastolic pressure. (F) Correlation between the relative abundance of Ruminococcaceae in the VVS group and a reduction in the mean pressure during the time to HUTT positive response. 1 mmHg = 0.133 kPa. Mean pressure = (systolic pressure + 2 × iastolic pressure)/3. (G) Correlation between relative abundance of Ruminococcaceae in the VVS group and the decline rate of the mean pressure during the time to HUTT positive response. Mean pressure = (systolic pressure + 2 × diastolic pressure)/3; the decline rate of the mean pressure = (basic mean pressure – the mean pressure during the HUTT positive time)/basic mean pressure. HUTT: Head-up tilt test; VVS: Vasovagal syncope.
Discussion
VVS is the most common form of syncope in children. The pathogenesis of VVS is complex and remains to be elucidated. With the development of genetic techniques, advances in the microbiome have suggested a relationship between gut microbiota dysbiosis and cardiovascular disease. Complex mechanisms are involved in the pathogenesis of cardiovascular disease and include vasomotor dysfunction caused by a variety of neurohumoral regulatory processes, which exhibit similarities to the pathogenesis of VVS. Clinically, we found VVS children often had concomitant abdominal pain, nausea, and other gastrointestinal discomforts as a prodromal symptom, which was previously thought to be related to autonomic regulation. However, the role of gut microbiota in VVS has not been investigated. Therefore, we determined the difference between the gut microbiota of VVS children and that of healthy children and then explored its significance in the development of the disease.
In our study, we found that no significant differences in alpha and beta diversity were evident between the VVS and control groups. Ruminococcaceae was the predominant bacteria in the gut microbiota composition of the VVS group according to the results of the LEfSe method, and its relative abundance was positively correlated with the syncopal frequency (P < 0.05), negatively correlated with systolic and diastolic pressure of the time to positive response in HUTT (P < 0.05), positively correlated with the reduction and decline rate of diastolic pressure at the positive response in HUTT (P < 0.01), and positively correlated with the mean pressure drop and decline rate (P < 0.05). Therefore, we speculate that the increased relative abundance of Ruminococcaceae may be involved in the development of VVS.
Ruminococcaceae, comprising Firmicutes, Clostridia, and Clostridiales, is a commensal bacteria that colonize the caecum and colon.[15] Maria et al[16] observed an anxiety mouse model before and after inducing anxiety and found an obvious increase in the relative abundance of Ruminococcaceae after anxiety was induced and a positive correlation with the level of anxiety determined by the dark box. These results suggested that Ruminococcaceae might be associated with anxiety. Emotional disorders including anxiety are common triggers of VVS, and recurrent syncope may also result in increased anxiety. In our study, we found a positive correlation between the relative abundance of Ruminococcaceae and syncopal frequency. However, whether the increased abundance of Ruminococcaceae is a cause or an effect of the increasing frequency of syncope remains unknown.
The main function of Ruminococcaceae is to produce short-chain fatty acids (SCFA), such as butyric acid and acetic acid.[17] In our study we found that the relative abundance of Ruminococcaceae in the VVS group was significantly increased, suggesting that its metabolite SCFA level was possibly increased compared with that of the control group. SCFA are final products of complex carbohydrates that are cleaved by intestinal microbiota.[18] SCFA can enter the bloodstream under the actions of anaerobic bacteria and participate in various metabolic processes such as affecting the energy supply, regulating immune responses and suppressing inflammatory cytokine production. These processes serve as protective measures against many chronic diseases.[19] During the past years, studies have shown that butyrate and acetate can improve hypertension[20] and that the specific mechanism might be related to angiotensin II (AngII). Kim et al[21] found that the blood pressure of mice injected with AngII and butyrate was significantly lower than that of mice injected with AngII alone, and the relative abundance of butyrate-producing bacteria in the intestinal tracts of the mice injected with AngII was significantly reduced. To further investigate the mechanism, Wang et al[22] injected AngII into unilaterally nephrectomized Sprague-Dawley rats and divided them into an experimental group with injected butyrate and an untreated control group. The authors found that the elevated blood pressure induced by AngII was significantly suppressed and that renal injury and inflammation were improved in the butyrate-injected Sprague-Dawley rats, the mechanism of which was related to renin receptor regulation. These studies suggested that SCFA could decrease blood pressure and that the mechanism might be related to the regulation of RAS, which was similar to the pathogenesis of VVS. Therefore, we speculated that the higher the relative abundance of Ruminococcaceae and the higher its metabolite concentrations, namely SCFA, the more obvious the effects of vasodilation and blood pressure drop would be. This was consistent with our findings.
In addition, as the understanding of gut microbiota has progressed, increasing studies have shown that the gut-brain axis is a bidirectional regulatory pathway.[23] As the central nervous system regulates intestinal function, the gut and autonomic nervous system regulate brain activity through microbes and their metabolites via the microbe-gut-brain axis, with the central pathway through the vagus nerve.[24] Studies have shown that fasting can damage the vagus nerve, suggesting that the vagus nerve is involved in regulating the axis.[25] Moreover, probiotic intake can reduce anxiety but the effect disappears when the vagus nerve is cut, further confirming this conclusion.[26] An imbalance in the autonomic nervous system is a component of VVS pathogenesis, and SCFA can be used as receptors for enteric nerves.[27] Therefore, it is likely that increasing the relative abundance of SCFA-producing bacteria Ruminococcaceae may lead to imbalanced autonomic nervous system function, which can cause VVS; however, the specific mechanism remains unclear and should be further investigated. Previous studies have shown that SCFA can reduce insulin resistance in obese patients[28] and that among healthy people the relative abundance of Ruminococcaceae is higher in low-BMI subjects than in high-BMI subjects,[29] consistent with previous findings showing that VVS is more likely to occur in low-BMI cohorts.[30]
In conclusion, no significant differences in alpha and beta diversity were evident between the VVS and healthy children. The predominant bacteria in the gut microbiota composition of the VVS group was Ruminococcaceae. A correlation exists between gut Ruminococcaceae and clinical syncopal frequency and hemodynamics. The results suggest that the disturbed gut microbiota may be involved in the development of VVS. Future studies should include further investigation into the pathogenesis of VVS and implementation of new methodologies for assessing VVS.
Funding
This work was supported by a grant from the National Youth Top-notch Talent Support Program, Clinical Scientists Program of Peking University and Beijing Science and Technology Program (No. Z171100001017253).
Conflicts of interest
None.
Footnotes
How to cite this article: Bai W, Chen S, Tang CS, Qi JG, Cui QH, Xu M, Du JB, Jin HF. Gut microbiota analysis and its significance in vasovagal syncope in children. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000086
References
- 1.Massin MM, Bourguignont A, Coremans C, Comté L, Lepage P, Gérard P. Syncope in pediatric patients presenting to an emergency department. J Pediatr 2004; 145:223–228. doi: 10.1016/j.jpeds.2004.01.048. [DOI] [PubMed] [Google Scholar]
- 2.Sheldon RS, Sheldon AG, Connolly SJ, Morillo CA, Klingenheben T, Krahn AD, et al. Age of first faint in patients with vasovagal syncope. J Cardiovasc Electrophysiol 2006; 17:49–54. doi: 10.1111/j.1540-8167.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- 3.Flevari P, Leftheriotis D, Repasos E, Katsaras D, Katsimardos A, Lekakis J, et al. Fluoxetine vs. placebo for the treatment of recurrent vasovagal syncope with anxiety sensitivity. Europace 2016; 19:127–131. doi: 10.1093/europace/euw153. [DOI] [PubMed] [Google Scholar]
- 4.Sheriff DD, Nådland IH, Toska K. Role of sympathetic responses on the hemodynamic consequences of rapid changes in posture in humans. J Appl Physiol 2010; 108:523.doi: 10.1152/japplphysiol.01185.2009. [DOI] [PubMed] [Google Scholar]
- 5.Stewart JM, Sutton R, Kothari ML, Goetz AM, Visintainer P, Medow MS. Nitric oxide synthase inhibition restores orthostatic tolerance in young vasovagal syncope patients. Heart 2017; 103:1711–1718. doi: 10.1136/heartjnl-2017-311161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saadjian AY, Lévy S, Franceschi F, Zouher I, Paganelli F, Guieu RP. Role of endogenous adenosine as a modulator of syncope induced during tilt testing. Circulation 2002; 106:569–574. doi: 10.1161/01.CIR.0000023924.66889.4C. [DOI] [PubMed] [Google Scholar]
- 7.Shinohara T, Ebata Y, Ayabe R, Fukui A, Okada N, Yufu K, et al. Cardiac autonomic dysfunction in patients with head-up tilt test-induced vasovagal syncope. Pacing Clin Electrophysiol 2015; 37:1694–1701. doi: 10 1111/pace 12484. [DOI] [PubMed] [Google Scholar]
- 8.Benson AK, Kelly SA, Legge R, Ma F, Low SJ, Kim J, et al. Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proc Natl Acad Sci U S A 2010; 107:18933–18988. doi: 10.1073/pnas.1007028107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2015; 472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Idelman G, Smith DLH, Zucker SD. Bilirubin inhibits the up-regulation of inducible nitric oxide synthase by scavenging reactive oxygen species generated by the toll-like receptor 4-dependent activation of NADPH oxidase. Redox Biol 2015; 5:398–408. doi: 10.1016/j.redox.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subspecialty Group of Cardiology, the Society of Pediatrics, Chinese Medical Association; Editorial Board, Chinese Journal of Pediatrics; Subspecialty Group of Cardiology, the Society of Pediatries, Beijing Medical Association; Professional Board of Syncope in Children, Pediatrician Society, Chinese Medical Doctor Association. Guideline for diagnosis of syncope in children (updated in 2016) (in Chinese). Natl Med J China 2016; 54:246–250. doi: 10.3760/cma.j.issn.0578-1310.2016.04.003. [Google Scholar]
- 12.Li B, Zhang X, Guo F, Wu W, Zhang T. Characterization of tetracycline resistant bacterial community in saline activated sludge using batch stress incubation with high-throughput sequencing analysis. Water Res 2013; 47:4207–4216. doi: 10.1016/j.watres.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 13.Lozupone C, Lladser ME, Dan K, Stombaugh J, Knight R. UniFrac: an effective distance metric for microbial community comparison. ISME J 2011; 5:169–172. doi: 10.1038/ismej.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, et al. Metagenomic biomarker discovery and explanation. Genome Biol 2011; 12:R60.doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donaldson GP, Lee SM, Mazmanian SK. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol 2015; 14:20–32. doi: 10.1038/nrmicro3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maria BBK, Lukasz K, Bratbo SD, Pang W, Nielsen DS, Josefsen K, et al. Gut microbiota composition is correlated to grid floor induced stress and behavior in the BALB/c mouse. PLoS One 2012; 7:e46231.doi: 10.1371/journal.pone.0046231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hooda S, Boler BMV, Serao MCR, Brulc JM, Staeger MA, Boileau TW, et al. 454 pyrosequencing reveals a shift in fecal microbiota of healthy adult men consuming polydextrose or soluble corn fiber. J Nutr 2012; 142:1259–1265. doi: 10.3945/jn.112.158766. [DOI] [PubMed] [Google Scholar]
- 18.Wong JM, De SR, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol 2006; 40:235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, et al. Innate immunity and intestinal microbiota in the development of type 1 diabetes. Nature 2008; 455:1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, et al. Gut microbiota dysbiosis is linked to hypertension. Hypertension 2015; 65:1331–1340. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim S, Wang G, Lobaton G, Li E, Yang T, Raizada M. OS 05-10 the microbial metabolite, butyrate attenuates angiotensin ii-induced hypertension and dysbiosis. J Hypertens 2016; 34 Suppl 1:e60.doi: 10.1097/01.hjh.0000500010.38755.52. [Google Scholar]
- 22.Wang L, Zhu Q, Lu A, Liu X, Zhang L, Xu C, et al. Sodium butyrate suppresses angiotensin II-induced hypertension by inhibition of renal (pro)renin receptor and intrarenal renin-angiotensin system. J Hypertens 2017; 35:1899–1908. doi: 10. 1097/HJH. 0000000000001378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sang HR, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain–gut–enteric microbiota axis. Nat Rev Gastroenterol Hepatol 2009; 6:306–314. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol 2012; 10:735–742. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- 25.Khasar SG, Reichling DB, Green PG, Isenberg WM, Levine JD. Fasting is a physiological stimulus of vagus-mediated enhancement of nociception in the female rat. Neuroscience 2003; 119:215–221. doi: 10.1016/S0306-4522(03)00136-2. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Wang BR, Zhang XJ, Xu Z, Ding YQ, Ju G. Evidences for vagus nerve in maintenance of immune balance and transmission of immune information from gut to brain in STM-infected rats. World J Gastroenterol 2002; 8:540–545. doi: 10.3748/wjg.v8.i3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raybould HE. Gut chemosensing: interactions between gut endocrine cells and visceral afferents. Auton Neurosci 2010; 153:41–46. doi: 10.1016/j.autneu.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, He G, Peng Y, Zhong W, Wang Y, Zhang B. Sodium butyrate alleviates adipocyte inflammation by inhibiting NLRP3 pathway. Sci Rep 2015; 5:12676.doi: 10.1038/srep12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clarke SF, Murphy EF, O'Sullivan O, Lucey AJ, Humphreys M, Hogan A, et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014; 63:1913–1920. doi: 10.1136/gutjnl-2013-306541. [DOI] [PubMed] [Google Scholar]
- 30.Yamada T, Yanagimoto S. Dose-response relationship between the risk of vasovagal syncope and body mass index or systolic blood pressure in young adults undergoing blood tests. Neuroepidemiology 2017; 49:31–33. doi: 10.1159/000479698. [DOI] [PubMed] [Google Scholar]


