Abstract
Background
In the era of precision medicine, chemotherapy is still considered the cornerstone of treatment for lung cancer patients without gene mutations. How to reduce the toxicity and increase the efficiency of chemotherapy is worth exploring. This study aimed to investigate the curative effects and safety of hyperthermia combined with chemotherapy (HCT) for advanced patients with non-small cell lung cancer (NSCLC), especially those with malignant pleural effusion.
Methods
We retrospectively evaluated medical records of 93 patients with advanced NSCLC (stage IIIB-IV) from March 2011 to January 2014. The patients were divided into HCT and chemotherapy (CT) groups. The HCT group was treated with gemcitabine and cisplatin (GP) regimen combined with regional radiofrequency deep hyperthermia, while the CT group was treated with GP regimen only. Those with malignant pleural effusion extra underwent thoracentesis and intrapleural injection chemotherapy combined with hyperthermic or not. Clinical treatment results and adverse reactions were compared and analyzed after treatment. SPSS 19.0 software (SPSS Inc., USA) was used for statistical data processing. P values less than 0.05 were accepted to be statistically significant.
Results
Among the 93 patients, HCT group included 48 patients (16 patients with malignant pleural effusion), CT group included 45 patients (10 patients with malignant pleural effusion). There was no significant difference between the two groups in patient characteristics. The overall response rate (ORR) of pleural effusions was much better in HCT group than that in CT group (81.2% vs. 40.0%, P = 0.046). The patients in HCT group had lower incidence rate of weakness (12.5% vs. 46.7%, χ2 = 13.16, P < 0.001) and gastrointestinal (25.0% vs. 77.8%, χ2 = 25.88, P < 0.001) adverse reactions than that in CT group. The objective tumor response and survival showed no significant differences.
Conclusions
Hyperthermia combined with chemotherapy might lead to the development of better therapeutic strategy for advanced NSCLC with malignant pleural effusion patients. Also, it could greatly reduce the chemotherapy toxic effects in the incidence of weakness and gastrointestinal adverse reactions in advanced NSCLC patients.
Keywords: Advanced non-small cell lung cancer, Chemotherapy, Combination therapy, Hyperthermia
Introduction
Lung cancer is the most common cause of cancer-related deaths worldwide.[1] Patients with lung cancer have a dismal chance of survival, with a 5-year survival rate of only 17.1%, and the rate is much worse for those with stage IV disease, only 3.6%.[2] Non-small cell lung cancer (NSCLC) accounted for more than 85% of all lung cancer cases. Most of the patients with NSCLC present with advanced, unresectable disease (at stage IIIB or IV). The efficacy of surgical treatment for advanced NSCLC is always contraindicated due to poor condition of the patients or presence of multiple metastatic lesions.
Recently, hyperthermia treatment has become an important method for NSCLC patients, as it could not only induce direct cytotoxicity for cancer cells, but also act as a chemosensitizer and radiation sensitizer.[3] Hyperthermia may increase tumor perfusion and could be utilized to improve drug delivery. Simultaneously, the increased tumor oxygenation is expected to increase radiosensitivity. Numerous studies have suggested that hyperthermia, chemotherapy, and radiotherapy exerted a synergistic effect in the treatment of colorectal cancer, advanced head and neck cancer, prostate cancer, ovarian cancer, esophagus cancer, and lung cancer.[4–11] Chemotherapy is an important therapy for advanced NSCLC and can improve the overall survival of patients. Gemcitabine plus cisplatin (GP) is the standard first-line chemotherapeutic regimen for advanced NSCLC. Gemcitabine is a nucleoside analog that possesses a unique mechanism of action, providing wide range of antitumor activity. Cisplatin induces formation of intra- and inter-strand crosslinks, inducing DNA strand breaks in the replication of cells. Gemcitabine combined with cisplatin is currently used as a first-line regimen for advanced NSCLC. This regimen is associated with a significant progression-free survival (PFS) of NSCLC patients than those treated with paclitaxel and cisplatin. But GP regimen more likely causes grade 3/4, hematologic, renal toxicity and gastrointestinal adverse events.[12]
Hence, this study aimed to evaluate the clinical efficacy and safety of combining gemcitabine, cisplatin, and hyperthermia for the treatment of patients with advanced NSCLC.
Methods
Ethical approval
This study was approved by the Committee for the Ethics Review of Research Involving Human Subjects of the Shanxi Medical University. Written informed consent was obtained from each patient.
Patient characteristics
We retrospectively evaluated the medical records of 93 inoperable patients with advanced NSCLC from March 2011 to January 2014 from the Department of Oncology, The First Hospital of Shanxi Medical University. All the patients were treated with chemotherapy (gemcitabine and cisplatin) combined with or without hyperthermia treatment. Other inclusion criteria were as follows: (1) histologically or cytologically documented cases with stage IIIB or IV NSCLC; (2) Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0 to 2; (3) No prior chemo- or radiotherapy; (4) adequate functioning of major internal organs; (5) NSCLC patients with malignant pleural effusion, but not brain metastases; and (6) patients with best supportive care, at least until disease progression. The following information of the patients was obtained from the patient charts, including age, gender, performance status, histological subtype, tumor stage, type of treatment, and clinical response to treatment. These patients were grouped as follows: (A) hyperthermia/chemotherapy group (HCT), which included 48 patients, where 16 patients were with malignant pleural effusion. All these patients accepted gemcitabine/cisplatin chemotherapy with radio-frequency deep hyperthermia. In addition, patients with malignant pleural effusion underwent thoracentesis and intrapleural injection chemotherapy. (B) Chemotherapy group (CT) included 45 patients, and among them there were 10 patients with malignant pleural effusion. These patients accepted gemcitabine/cisplatin chemotherapy. None of them underwent hyperthermia alone.
Treatment methods
Chemotherapy
Standard intravenous chemotherapy treatment with gemcitabine and cisplatin was given every 3 weeks. The dose levels of 1000 mg/m2 of gemcitabine on days 1 and 8, 75 mg/m2 of cisplatin were divided into 2 to 4 days, and were administered in each cycle. Tropisetron and other similar medications were routinely administered to stop vomiting during chemotherapy.
Hyperthermia
Hyperthermia was applied by HY7000-I radiofrequency deep hyperthermia system (Nanjing GREATHOPE Corporation, Nanjing, China), which heats up the locoregional lesions that are located deep inside the body. Hyperthermia was applied before or after chemotherapy twice a week or after intrapleural injection chemotherapy. The heat was applied using a 40.68 ± 1.00 MHz RF-capacitive regional HT. Heating duration was adjusted from 40 to 60 min based on patient's tolerance (median, 50 min). Both the upper/lower or left/right electrodes were 30 cm in diameter and placed on opposite sides of the whole thoracic region, and the electrodes were alternatively used to reduce the degree of pain caused by heating. The objective of hyperthermia treatment was to achieve a skin temperature of 40°C. The patient lied in the supine position and the body temperature was recovered naturally. Patients were carefully instructed to mention any unpleasant sensation, suggesting a hot spot.
Intrapleural injection chemotherapy
Thoracocentesis and central venous catheter were performed under B-type ultrasonic location, and drained using pleural effusion as much as possible. Interleukin-2 was dissolved in 50 mL of normal saline for intrapleural injection. In order to improve the drug bioavailability, patients were required to turnover once every 15 min to ensure full access to drugs within the chest wall. Doses were administered twice a week for an average of 4 weeks. Detailed response for the treatment was recorded, and B-type ultrasonic was reviewed weekly.
Assessment of efficacy
Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 was utilized to evaluate the response. A complete response (CR) was defined as the disappearance of all target lesions for at least 4 weeks. For a partial response (PR), at least a 30% decrease in the sum of the greatest unidimensional diameters of the targeted lesions was required, taking the baseline sum of the diameters of target lesions as reference. Progressive disease (PD) required an increase of at least 20% in the sum of the diameters of target lesions, taking the smallest sum of the diameters of target lesions recorded since treatment started as reference. Any cases that do not qualify for either PR or PD are classified as stable disease (SD). WHO criteria for evaluating therapeutic effects in patients with solid tumors were modified to assess the outcomes of treatment for malignant pleural effusion include CR: effusion and disappearance of symptoms, and staying stable for more than 4 weeks; PR: effusion was reduced by 50%, symptoms were improved, and residual effusion showed no growth through 4 weeks of observation; SD: pleural effusion was decreased by <50% or showed no change; and PD: pleural effusion was increased than before. CR and PR were summed up to be objective response rate (ORR). Disease control rate (DCR) contained CR, PR, and SD. PFS was calculated from the day of treatment until the documentation of disease progression or death. Overall survival (OS) was defined from the day of treatment till death or last day of follow-up. Toxicities were classified as grade 0 to 4 according to the WHO standard, except for the HT-related toxicity of skin burn.
Statistical analysis
The SPSS 19.0 software (IBM Corp., Armonk, NY, USA) was used for statistical data processing. Proportions were compared by Chi-squared and Fisher exact probability tests. The overall survival time was estimated by Kaplan-Meier method. Survival curves were compared using the log-rank test. P values less than 0.05 were accepted to be statistically significant.
Results
Patient characteristics
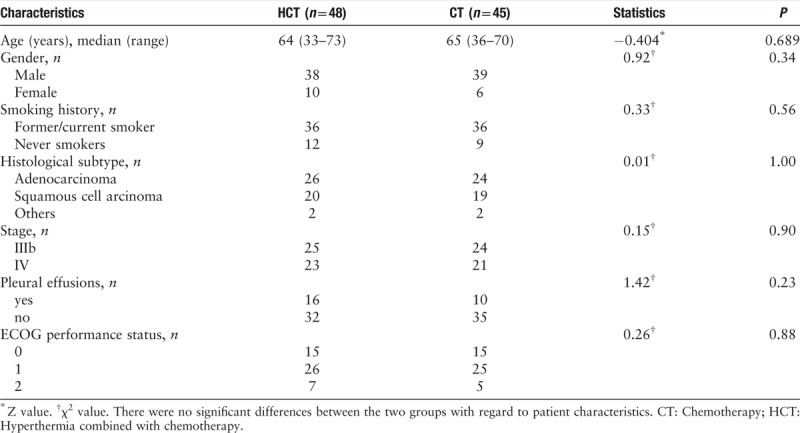
The study included 93 patients, where 48 patients received hyperthermia and chemotherapy, and 45 patients received chemotherapy only. There was no significant difference between the two groups in age, gender, smoking habit, performance status, disease stage, and tumor histology. The median age of the patients was 64 years old in HCT group and 65 years old in CT group, respectively. About 80% of the patients were male, and approximately 80% had a smoking habit. Almost half of the patients had a performance-status score of 1, and about 20% to 30% had malignant pleural effusion [Table 1].
Table 1.
Patient characteristics of the two study groups of non-small cell lung cancer patients.
Objective tumor response and survival
In the HCT group, the mean number of chemotherapy cycles was 3.5 ± 0.3, and radiofrequency hyperthermia was eight times for a case. No patient achieved a CR, 18 (37.5%) patients showed a PR, 16 (33.3%) had SD, and 14 (29.2%) had PD. ORR was 37.5%, and disease control rate (DCR) was 70.8%. In the CT group, the mean number of treatment cycles was 2.8 ± 0.4, no CR was observed, 15 (33.3%) patients had PR, 15 (33.3%) had SD, and 15 (33.3%) had PD. The ORR was 33.3%, and DCR was 66.7%.
Follow-up ranged from 5 to 35 months (median, 11.0 months). In the HCT and CT groups, the survival rate at 1 year was 54% and 40%, and the rate at 2 years was 14.6% and 13.3%, respectively. The median PFS was 5.65 and 5.5 months, and the median OS was 13.2 and 10.9 months, respectively. There were no significant differences in the objective tumor response and survival [Table 2, Figure 1].
Table 2.
Comparisons of curative effect in the two study groups of non-small cell lung cancer patients.
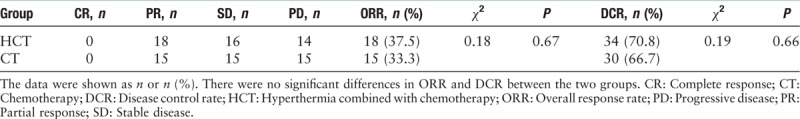
Figure 1.
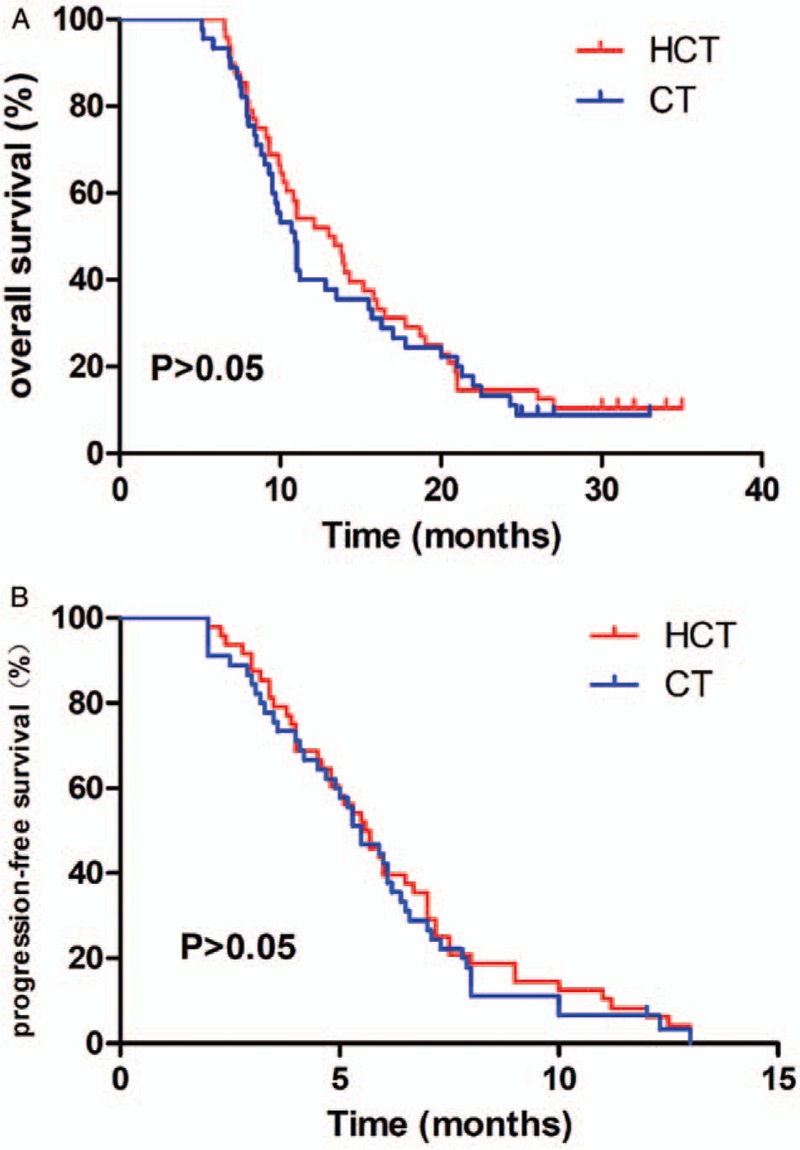
Kaplan-Meier estimates of overall survival (A) and Kaplan-Meier estimates of progression-free survival (B) in the patients of HCT (n = 48) and CT groups (n = 45). There were no significant differences in survival. CT: Chemotherapy; HCT: Hyperthermia combined with chemotherapy.
Toxic effects
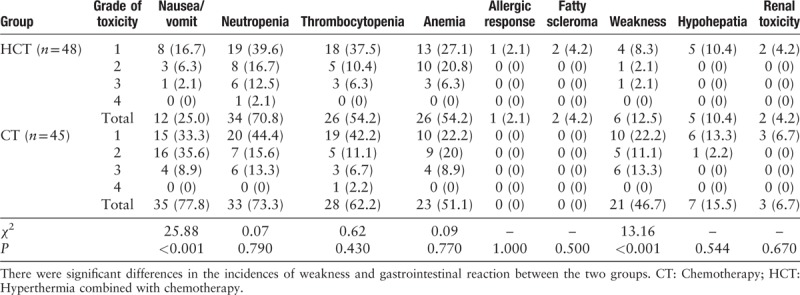
The toxic effects are presented in Table 3. There was no difference between the two groups in hematological toxicity, hepatic dysfunction, renal toxicity as well as allergic response. In two patients, a fatty scleroma was observed after hyperthermia and was disappeared spontaneously. A significant difference was observed in the incidences of weakness (12.5% and 46.7%, χ2 = 13.16, P = 0.0003) and gastrointestinal reaction (25.0% and 77.8%, χ2 = 25.88, P < 0.0001) between HCT and CT groups.
Table 3.
Comparisons of toxicity and adverse reaction of patients in the two groups of non-small cell lung cancer patients, n (%).
Response evaluation of pleural effusions
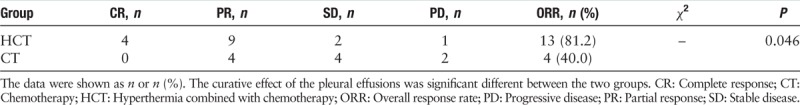
We analyzed the response evaluation of pleural effusions between the two groups after a median of 3.5 cycles (range 2–6) treatment. In the HCT group, four patients (25.0%) had CR status, nine patients (56.3%) had PR status, and two patients (12.5%) had SD status, and one patient (6.3%) experienced a PD. The ORR was 81.2%. In the CT group, no patients achieved CR status, four patients (40.0%) achieved PR status, and four patients (40.0%) achieved SD status, and two patients (20.0%) had PD status. The ORR was 40.0%. There was a significant difference between the two groups (P = 0.046) [Table 4].
Table 4.
Comparisons of curative effect of the pleural effusions in the two groups.
Discussion
Hyperthermia is an important treatment strategy for advanced malignant tumors, and has been reported to improve the therapeutic efficacy in some tumors such as prostate cancer, ovarian cancer, rectal cancer, soft tissue sarcoma through acting synergistically with radiotherapy and chemotherapy.[7,13–16] Although the exact molecular mechanisms are still elusive, some researches demonstrated that hyperthermia could lead to cellular DNA, protein and membrane damage, and interfere with cell cycle as well as DNA and protein synthesis. All these may cause cell death, either directly or by triggering the apoptotic pathways.[3] Interestingly, hyperthermia could hinder DNA repair, and can sensitize the cancer cells to DNA damaging agents, such as gemcitabine,[17,18] pyrimidine analogs,[19] cisplatin,[20–22] cyclophosphamide,[23,24] melphalan,[25,26] camptothecin,[27] PARP inhibitors-olaparib, PJ-34[28,29] and so on. Additional studies suggested that hyperthermia could elevate the activity of quinoneoxido reductase (NQO1) in tumors to improve the cytotoxicity of β-lapachone against cancer cells both in vitro and in vivo.[30] Moreover, hyperthermia could enhance radio-sensitivity by depressing DNA-PK kinase activity and lacking nonhomologous end joining and/or homologous recombination pathways during the double strand break repair.[31] In clinical perspective, Ohguri et al have reported that radiotherapy combined with regional hyperthermia demonstrated better clinical outcomes in patients with stage III NSCLC.[32] For patients with recurrent NSCLC, re-irradiation plus regional hyperthermia might be a promising treatment that contributes for better local control and acceptable toxicity.[33] In a randomized phase 3 multi-center study, the neo-adjuvant chemotherapy with regional hyperthermia resulted in significantly better local progression-free survival (LPFS) and DFS for patients with localized high-risk soft-tissue sarcoma than neo-adjuvant chemotherapy alone.[34] Docetaxel combined with intraperitoneal hyperthermic perfusion chemotherapy could effectively improve the total effective rate in controlling the ascites rate, remission rate of tumor and descent rate of CA125 in patients with advanced ovarian cancer.[13]
To our knowledge, this is one of the few retrospective studies that included chemotherapy and hyperthermia combined with chemotherapy in patients with advanced NSCLC. Our results suggested that hyperthermia combined with chemotherapy for the treatment of advanced NSCLC showed better trends in objective tumor response and clinical outcomes, but showed no significant differences. Hence, it is necessary to get more cases in the following studies for chemotherapy and HCT in patients with advanced NSCLC. However, we discovered some interesting results, as a significant difference was found in the incidences of weakness and gastrointestinal reactions between HCT and CT groups. Hyperthermia as a physical therapy could relax patients and promote metabolism. According to a study, hyperthermic effect of non-selective 5-HT agonists was dose-dependently antagonized by 5-HT receptor antagonists and dopamine receptor antagonist spiperone,[35] and so hyperthermia might be correlated with something relevant to emotion and nausea. We speculated that hyperthermia could also improve the therapeutic efficacy of antiemetics, such as metoclopramide, tropisetron, and ondansetron, or it could inhibit the secretion of 5-hydroxytryptamine and neurokinin-1. Overall, this supported the patients to have full dose and full course chemotherapy.
The malignant pleural effusion often occurs in the advanced NSCLC, weakening the pulmonary function, and diminishing the quality of life due to symptoms like dyspnea, pain, cough, etc.[36] In this study, we discovered that the regional hyperthermia combined with interleukin-2 intrapleural injections was one of the effective means for managing pleural effusion, with an ORR of 81.2% in HCT group compared to 40% in CT group. No major complications, such as respiratory events and fever, were encountered. There were no minor complications, except the pain at the chest tube insertion sites. It might be due to that the alignment of these two could strongly induce expression of heat-shock protein 70, CD8-positive and CD4-positive T cells, promoting tumor cells apoptosis and necrosis and then reducing the formation of malignant pleural effusion.[37] Hyperthermia could also promote permeation of chemotherapeutics into the pleural cavity.
In conclusion, hyperthermia combined with chemotherapy might lead to the development of a better therapeutic strategy in advanced NSCLC patients with malignant pleural effusion. Also, it could greatly reduce the chemotherapeutic toxic effects in the incidences of weakness and gastrointestinal adverse reactions in advanced NSCLC patients. However, this study was a retrospective study including single-institution information, and has small sample size. Additional investigations are warranted to confirm our study findings.
Funding
This work was supported by the grants from the Scientific Research Foundation of Shanxi Province Healthy Commission (No. 2017068), and Shanxi Province Science Foundation for Youths (No. 201801D221259).
Conflicts of interest
None.
Footnotes
How to cite this article: Yang WH, Xie J, Lai ZY, Yang MD, Zhang GH, Li Y, Mu JB, Xu J. Radiofrequency deep hyperthermia combined with chemotherapy in the treatment of advanced non-small cell lung cancer. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000156
References
- 1.Chen W, Zheng R, Zhang S, Zeng H, Xia C, Zuo T, et al. Cancer incidence and mortality in China, 2013. Cancer Lett 2017; 401:63–71. doi: 10.1016/j.canlet.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin 2012; 62:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 3.Oei AL, Vriend LE, Crezee J, Franken NA, Krawczyk PM. Effects of hyperthermia on DNA repair pathways: one treatment to inhibit them all. Radiat Oncol (London, England) 2015; 10:165.doi: 10.1186/s13014-015-0462-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Z. Clinical effects of high frequency hyperthermia-assisted irinotecan chemotherapy on patients with middle and advanced colorectal cancer and its safety assessment. Oncol Lett 2019; 17:215–220. doi: 10.3892/ol.2018.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao S, Zheng M, Ren X, Tang Y, Liang X. Local hyperthermia in head and neck cancer: mechanism, application and advance. Oncotarget 2016; 7:57367–57378. doi: 10.18632/oncotarget.10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verduijn GM, de Wee EM, Rijnen Z, Togni P, Hardillo JAU, Ten Hove I, et al. Deep hyperthermia with the HYPERcollar system combined with irradiation for advanced head and neck carcinoma - a feasibility study. Int J Hyperthermia 2018; 34:994–1001. doi: 10.1080/02656736.2018.1454610. [DOI] [PubMed] [Google Scholar]
- 7.Kalapurakal JA, Mittal BB, Sathiaseelan V. Re-irradiation and external hyperthermia in locally advanced, radiation recurrent, hormone refractory prostate cancer: a preliminary report. British J Radiol 2001; 74:745–751. doi: 10.1259/bjr.74.884.740745. [DOI] [PubMed] [Google Scholar]
- 8.Cohen J, Anvari A, Samanta S, Poirier Y, Soman S, Alexander A, et al. Mild hyperthermia as a localized radiosensitizer for deep-seated tumors: Investigation in an orthotopic prostate cancer model in mice. Br J Radiol 2019; 92:20180759.doi: 10.1259/bjr.20180759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z, Sun Q, Huang X, Zhang J, Hao J, Li Y, et al. The efficacy of radiofrequency hyperthermia combined with chemotherapy in the treatment of advanced ovarian cancer. Open Med (Warsaw, Poland) 2018; 13:83–89. doi: 10.1515/med-2018-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugimachi K, Kitamura K, Baba K, Ikebe M, Morita M, Matsuda H, et al. Hyperthermia combined with chemotherapy and irradiation for patients with carcinoma of the oesophagus--a prospective randomized trial. Int J Hyperthermia 1992; 8:289–295. doi: 10.3109/02656739209021783. [DOI] [PubMed] [Google Scholar]
- 11.Ohguri T, Imada H, Yahara K, Moon SD, Yamaguchi S, Yatera K, et al. Re-irradiation plus regional hyperthermia for recurrent non-small cell lung cancer: a potential modality for inducing long-term survival in selected patients. Lung Cancer(Amsterdam, Netherlands) 2012; 77:140–145. doi: 10.1016/j.lungcan.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 12.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. New Engl J Med 2002; 346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 13.Zhang T, Pan Q, Xiao S, Li L, Xue M. Docetaxel combined with intraperitoneal hyperthermic perfusion chemotherapy and hyperthermia in the treatment of advanced ovarian cancer. Oncol Lett 2016; 11:3287–3292. doi: 10.3892/ol.2016.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujimoto S, Takahashi M, Endoh F, Shrestha RD, Kokubun M, Takai M, et al. A clinical pilot study combining surgery with intraoperative pelvic hyperthermochemotherapy to prevent the local recurrence of rectal cancer. Ann Surg 1991; 213:43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlemmer M, Wendtner CM, Lindner L, Abdel-Rahman S, Hiddemann W, Issels RD. Thermochemotherapy in patients with extremity high-risk soft tissue sarcomas (HR-STS). Int J Hyperthermia 2010; 26:127–135. doi: 10.3109/02656730903335995. [DOI] [PubMed] [Google Scholar]
- 16.Issels RD, Lindner LH, Verweij J, Wessalowski R, Reichardt P, Wust P, et al. Effect of neoadjuvant chemotherapy plus regional hyperthermia on long-term outcomes among patients with localized high-risk soft tissue sarcoma: the EORTC 62961-ESHO 95 randomized clinical trial. JAMA Oncol 2018; 4:483–492. doi: 10.1001/jamaoncol.2017.4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raoof M, Zhu C, Cisneros BT, Liu H, Corr SJ, Wilson LJ, et al. Hyperthermia inhibits recombination repair of gemcitabine-stalled replication forks. JNCI: J Natl Cancer Inst 2014; 106:dju183.doi: 10.1093/jnci/dju183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohamed F, Marchettini P, Stuart OA, Urano M, Sugarbaker PH. Thermal enhancement of new chemotherapeutic agents at moderate hyperthermia. Ann Surg Oncol 2003; 10:463–468. doi: 10.1245/ASO.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Maehara Y, Sakaguchi Y, Takahashi I, Yoshida M, Kusumoto H, Masuda H, et al. 5-Fluorouracil's cytotoxicity is enhanced both in vitro and in vivo by concomitant treatment with hyperthermia and dipyridamole. Cancer Chemother Pharmacol 1992; 29:257–260. doi: 10.1007/BF00685941. [DOI] [PubMed] [Google Scholar]
- 20.Raaphorst GP, Yang DP. The evaluation of thermal cisplatin sensitization in normal and XP human cells using mild hyperthermia at 40 and 41 degrees C. Anticancer Res 2005; 25:2649–2653. [PubMed] [Google Scholar]
- 21.Takemoto M, Kuroda M, Urano M, Nishimura Y, Kawasaki S, Kato H, et al. The effect of various chemotherapeutic agents given with mild hyperthermia on different types of tumours. Int J Hyperthermia 2003; 19:193–203. doi: 10.1080/0265673021000035235. [DOI] [PubMed] [Google Scholar]
- 22.Raaphorst GP, Li LF, Yang DP, LeBlanc JM. Cisplatin sensitization by concurrent mild hyperthermia in parental and mutant cell lines deficient in homologous recombination and non-homologous endjoining repair. Oncol Rep 2005; 14:281–285. [PubMed] [Google Scholar]
- 23.Wiedemann G, Roszinski S, Biersack A, Weiss C, Wagner T. Local hyperthermia enhances cyclophosphamide, ifosfamide and cis-diamminedichloroplatinum cytotoxicity on human-derived breast carcinoma and sarcoma xenografts in nude mice. J Cancer Res Clin Oncol 1992; 118:129–135. doi: 10.1007/BF01187501. [DOI] [PubMed] [Google Scholar]
- 24.Gerad H, van Echo DA, Whitacre M, Ashman M, Helrich M, Foy J, et al. Doxorubicin, cyclophosphamide, and whole body hyperthermia for treatment of advanced soft tissue sarcoma. Cancer 1984; 53:2585–2591. doi: 10.1002/1097-0142(19840615)53:12<2585::AID-CNCR2820531203>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 25.Urano M, Ling CC. Thermal enhancement of melphalan and oxaliplatin cytotoxicity in vitro. Int J Hyperthermia 2002; 18:307–315. doi: 10.1080/02656730210123534. [DOI] [PubMed] [Google Scholar]
- 26.Orlandi L, Zaffaroni N, Bearzatto A, Costa A, Supino R, Vaglini M, et al. Effect of melphalan and hyperthermia on cell cycle progression and cyclin B1 expression in human melanoma cells. Cell Prolif 1995; 28:617–630. doi: 10.1111/j.2040-1124.2010.00046. [DOI] [PubMed] [Google Scholar]
- 27.Ng CE, Bussey AM, Raaphorst GP. Sequence of treatment is important in the modification of camptothecin induced cell killing by hyperthermia. Int J Hyperthermia 1996; 12:663–678. discussion 679-680. doi: 10.3109/02656739609027674. [DOI] [PubMed] [Google Scholar]
- 28.Krawczyk PM, Eppink B, Essers J, Stap J, Rodermond H, Odijk H, et al. Mild hyperthermia inhibits homologous recombination, induces BRCA2 degradation, and sensitizes cancer cells to poly (ADP-ribose) polymerase-1 inhibition. Proc Natl Acad Sci U S A 2011; 108:9851–9856. doi: 10.1073/pnas.1101053108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eppink B, Krawczyk PM, Stap J, Kanaar R. Hyperthermia-induced DNA repair deficiency suggests novel therapeutic anti-cancer strategies. Int J Hyperthermia 2012; 28:509–517. doi: 10.3109/02656736.2012.695427. [DOI] [PubMed] [Google Scholar]
- 30.Park HJ, Choi EK, Choi J, Ahn KJ, Kim EJ, Ji IM, et al. Heat-induced up-regulation of NAD(P)H:quinone oxidoreductase potentiates anticancer effects of beta-lapachone. Clin Cancer Res 2005; 11:8866–8871. doi: 10.1158/1078-0432.ccr-05-0818. [DOI] [PubMed] [Google Scholar]
- 31.Ihara M, Takeshita S, Okaichi K, Okumura Y, Ohnishi T. Heat exposure enhances radiosensitivity by depressing DNA-PK kinase activity during double strand break repair. Int J Hyperthermia 2014; 30:102–109. doi: 10.3109/02656736.2014.887793. [DOI] [PubMed] [Google Scholar]
- 32.Ohguri T, Imada H, Yahara K, Morioka T, Nakano K, Terashima H, et al. Radiotherapy with 8-MHz radiofrequency-capacitive regional hyperthermia for stage III non-small-cell lung cancer: the radiofrequency-output power correlates with the intraesophageal temperature and clinical outcomes. Int J Radiat Oncol Biol Phys 2009; 73:128–135. doi: 10.1016/j.ijrobp.2008.03.059. [DOI] [PubMed] [Google Scholar]
- 33.Augusto AC, Miguel F, Mendonca S, Pedrazzoli J, Jr, Gurgueira SA. Oxidative stress expression status associated to Helicobacter pylori virulence in gastric diseases. Clin Biochem 2007; 40:615–622. doi: 10.1016/j.clinbiochem.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 34.Issels RD, Lindner LH, Verweij J, Wust P, Reichardt P, Schem BC, et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomised phase 3 multicentre study. Lancet Oncol 2010; 11:561–570. doi: 10.1016/s1470-2045(10)70071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klodzinska A, Chojnacka-Wojcik E. Hyperthermia induced by m-trifluoromethylphenylpiperazine (TFMPP) or m-chlorophenylpiperazine (m-CPP) in heat-adapted rats. Psychopharmacology 1992; 109:466–472. doi: 10.1007/BF02247725. [DOI] [PubMed] [Google Scholar]
- 36.Thomas JM, Musani AI. Malignant pleural effusions: a review. Clin Chest Med 2013; 34:459–471. doi: 10.1016/j.ccm.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Hu R, Ma S, Ke X, Jiang H, Wei D, Wang W. Effect of interleukin-2 treatment combined with magnetic fluid hyperthermia on Lewis lung cancer-bearing mice. Biomed Rep 2016; 4:59–62. doi: 10.3892/br.2015.540. [DOI] [PMC free article] [PubMed] [Google Scholar]


