Abstract
Background
Gestational diabetes mellitus (GDM) is usually diagnosed between 24th and 28th gestational week using the 75-g oral glucose tolerance test (OGTT). It is difficult to predict GDM before 24th gestational week because fast plasma glucose (FPG) decreases as the gestational age increases. It is controversial that if FPG ≥5.1 mmol/L before 24th gestational week should be intervened or not. The aim of this study was to evaluate the value of FPG to screen GDM before 24th gestational week in women with different pre-pregnancy body mass index (BMI).
Methods
This was a multi-region retrospective cohort study in China. Women who had a singleton live birth between June 20, 2013 and November 30, 2014, resided in Beijing, Guangzhou and Chengdu, and received prenatal care in 21 selected hospitals, were included in this study. Pre-pregnancy BMI, FPG before the 24th gestational week, and one-step GDM screening with 75 g-OGTT at the 24th to 28th gestational weeks were extracted from medical charts and analyzed. The pregnant women were classified into four groups based on pre-pregnancy BMI: Group A (underweight, BMI < 18.5 kg/m2), Group B (normal, BMI 18.5–23.9 kg/m2), Group C (overweight, BMI 24.0–27.9 kg/m2) and Group D (obesity, BMI ≥28.0 kg/m2). The trend of FPG before 24th week of gestation was described, and the sensitivity and specificity of using FPG before the 24th gestational week to diagnose GDM among different pre-pregnancy BMI groups were reported. Differences in the means between groups were evaluated using independent sample t-test and analysis of variance. Pearson Chi-square test was used for categorical variables.
Results
The prevalence of GDM was 20.0% (6806/34,087) in the study population. FPG decreased gradually as the gestational age increased in all pre-pregnancy BMI groups until the 19th gestational week. FPG was higher in women with higher pre-pregnancy BMI. FPG before the 24th gestational week and pre-pregnancy BMI could be used to predict GDM. The incidence of GDM in women with FPG ≥5.10 mmol/L in the 19th to 24th gestational weeks and pre-pregnancy overweight or obesity was significantly higher than that in women with FPG ≥5.10 mmol/L and pre-pregnancy BMI <24.0 kg/m2 (78.5% [62/79] vs. 52.9% [64/121], χ2 = 13.425, P < 0.001).
Conclusions
FPG decreased gradually as the gestational age increased in all pre-pregnancy BMI groups until the 19th gestational week. Pre-pregnancy overweight or obesity was associated with an increased FPG value before the 24th gestational week. FPG ≥5.10 mmol/L between 19 and 24 gestational weeks should be treated as GDM in women with pre-pregnancy overweight and obesity.
Keywords: Body mass index, Fasting plasma glucose, Gestational diabetes mellitus
Introduction
China has a large burden of diabetes in recent years.[1] Developmental origins of health and disease (DOHaD) hypothesis indicated that environmental exposures during pregnancy and postnatal development can affect health years or even decades later.[2] Gestational diabetes mellitus (GDM) is one of the most common pregnancy complications and is associated with a higher risk of maternal morbidity and perinatal/neonatal morbidity.[3] Intrauterine hyperglycemia has long-term consequences for the offspring, including increased risk for obesity and type 2 diabetes in later childhood and adulthood. It has been reported that good blood glucose control in GDM could decrease the risk of maternal/fetal complications, such as macrosomia, cesarean section and the risk of diabetes in adults.
The diagnosis of hyperglycemia in pregnancy (pre-gestational diabetes mellitus and GDM) has been a hot area of research for several years.[4–6] GDM is usually diagnosed between the 24th and 28th gestational weeks using the 75-g oral glucose tolerance test (OGTT); therefore, women with GDM only have about 14 to 16 weeks to control blood glucose. Earlier diagnosis of GDM may trigger earlier blood glucose management and benefit the patients, but it is difficult to predict GDM before the 24th gestational week because fast plasma glucose (FPG) decreases as the gestational age increases.[7,8] It is controversial that if FPG ≥5.10 mmol/L before the 24th gestational week should be intervened or not. The aim of this study was to evaluate if FPG before the 24th gestational week could be used to predict GDM, which is often diagnosed at the 24th to 28th gestational weeks, and examine if considering pre-pregnancy body mass index (BMI) in addition to FPG can increase the accuracy of prediction.
Methods
Ethical approval
Clinical Research Ethics Committee of Peking University First Hospital approved this study (No. 2013 [572]). The participants have given the written informed consent for their clinical records to be used in this study.
Study population
This study recruited 15 hospitals in Beijing, five hospitals in Guangzhou, and one hospital in Chengdu. Women who had a singleton live birth between June 20, 2013 to November 30, 2014, performed an FPG test at the first prenatal visit before the 24th gestational week, and had 75-g OGTT during the 24th to 28th gestational week at study hospitals were included.
Women with pre-pregnancy diabetes mellitus, without an FPG test before the 24th gestational week or 75-g OGTT during pregnancy, without pre-pregnancy BMI, or with other fetal factors (fetal malformations and fetal death) were excluded.
Data collection
Pre-pregnancy weight and height, the dates and values of FPG test before the 24th gestational week, the results of 75-g OGTT during pregnancy, and the diagnosis of GDM were extracted from medical records by trained residents and nurses at study hospitals.
Definitions
The diagnosis of GDM was made when any one of the following values was met or exceeded in 75-g OGTT at the 24th to 28th gestational week: 0 h (fasting), 5.1 mmol/L; 1 h, 10.0 mmol/L; and 2 h, 8.5 mmol/L. The cutoff points were from the guideline established by the Ministry of Health (MOH) in China according to International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria.[7]
Pre-pregnancy BMI was calculated based on self-reported pre-pregnancy weight and measured height obtained at the first prenatal visit. Then the women were classified into four groups based on World Health Organization (WHO) recommendations for Asian population[9]: Group A (underweight): BMI <18.5 kg/m2; Group B (normal): 18.5 to 23.9 kg/m2; Group C (overweight): 24.0 to 27.9 kg/m2; and Group D (obesity): ≥28.0 kg/m2.
Statistical analysis
As FPG values decreased with gestational age, this study compared FPG values across pre-pregnancy BMI groups, after controlling for gestational age, using linear regression models with and without pre-pregnancy BMI-gestational age interaction [Equations. (1) and (2)]. As the decrease of FPG with gestational age might not be linear, this study tried adding square terms of gestational age in the model to improve fit [Equation (3)]:
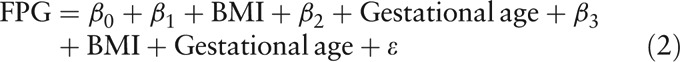 |
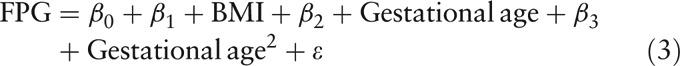 |
Then the logistic regression was used to build the predictive model, in which GDM was the dependent variable, while FPG and pre-pregnancy BMI were independent variables. This study tested the predictive power of the models with and without pre-pregnancy BMI [Equations (5) and (6)], and checked if adding pre-pregnancy BMI can significantly increase the predictive power of the model by checking the change of C-statistic. This study also tested adding FPG gestational age and interaction terms to test if they could elevate the predictive power.
Lastly, this study tested if the models have better predictive power in patients with 19th to 24th gestational weeks FPG to predict GDM in patients with overweight/obesity.
Data were analyzed using SPSS version 17.0 statistical software (SPSS Inc., Chicago, IL, USA). Continuous variables are presented as the mean ± standard deviation, and categorical variables are presented as numbers and percentages. Differences in the means between groups were evaluated using independent sample t-test and analysis of variance (ANOVA). Pearson Chi-square test was used for categorical variables. The level of statistical significance was set at 0.05.
Results
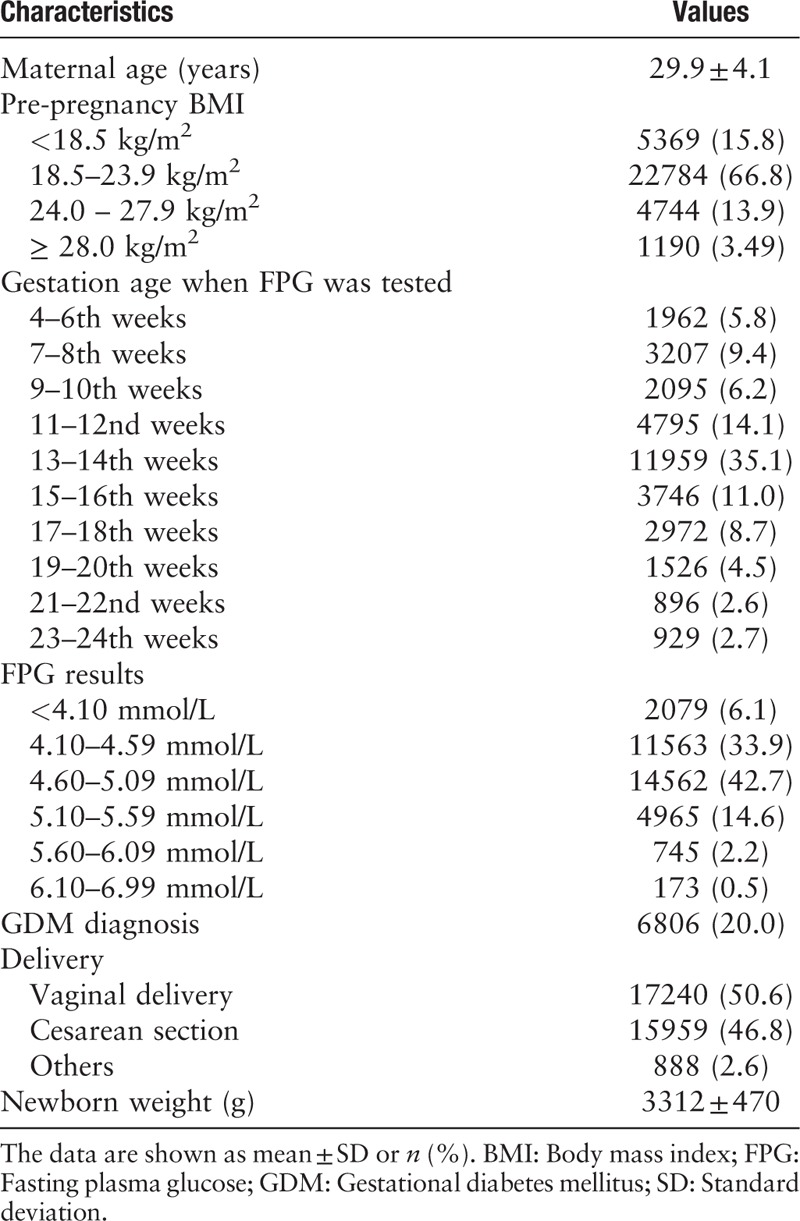
The demographic and clinical characteristics of the study population are shown in Table 1. The prevalence of gestational diabetes mellitus (GDM) was 20.0% (6806/34,087). The pregnancy week and value for FPG test before the 24th weeks of gestation were 12.7 ± 4.0 weeks of gestation and 4.70 ± 0.44 mmol/L, respectively.
Table 1.
Demographic and clinical characteristics of the study population (N = 34,087).
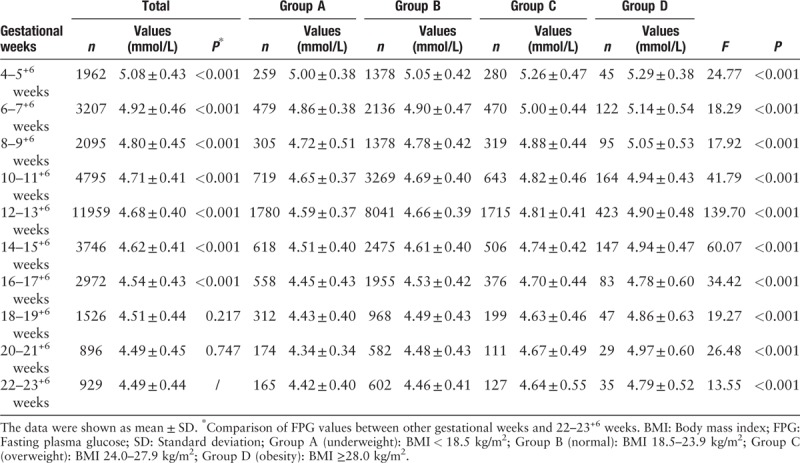
The variation of FPG in women with different pre-pregnancy BMI as the gestational age increasing is shown in Table 2. FPG decreased with increasing gestational age in different pre-pregnancy BMI groups from 4 to 5+6 gestational weeks. In fact, in study population, FPG declined gradually until the 19th week to reach a steady state. After controlling for pre-pregnancy BMI, compared to FPG of 22 to 23+6 gestational weeks, the FPG of 4 to 22 gestational weeks were 0.59 mmol/L (4–5+6 weeks), 0.43 mmol/L (6–7+6 weeks), 0.30 mmol/L (8–9+6 weeks), 0.22 mmol/L (10–11+6 weeks), 0.19 mmol/L (12–13+6 weeks), 0.13 mmol/L (14–15+6 weeks), 0.06 mmol/L (16–17+6 weeks), 0.02 mmol/L (18–19+6 weeks) and 0.01 mmol/L (20–21+6 weeks) higher (all P < 0.001), except 18 to 19+6 and 20 to 21+6 weeks (P = 0.217 and 0.747, respectively).
Table 2.
Variation of FPG in different pre-pregnancy BMI women as the gestational age increasing.
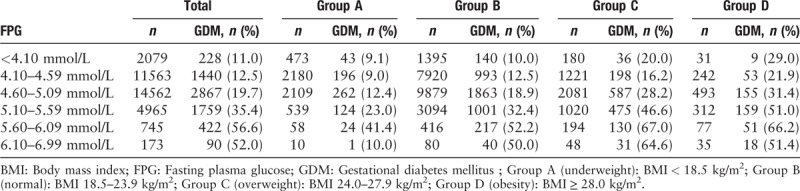
The proportion of patients diagnosed with GDM in four groups of FPG and pre-pregnancy BMI combinations is shown in Table 3. With every 0.50 mmol/L increase in FPG level >4.10 mmol/L at the first prenatal visit, the incidence of GDM diagnosis later in pregnancy increased in pre-pregnancy BMI groups. Logistic regression indicated patients with higher FPG (OR: 3.1, 95% CI: 2.90–3.30) and higher pre-pregnancy BMI (OR: 1.1, 95% CI: 1.06–1.13) were more likely to be diagnosed with GDM.
Table 3.
Proportion of patients diagnosed with GDM in four groups of FPG and pre-pregnancy BMI combinations.
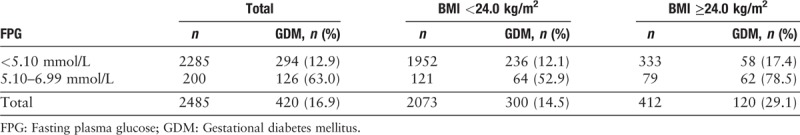
The prevalence of GDM was associated with FPG in 19 to 24 weeks of gestation and pre-pregnancy BMI [Table 4]. The incidence of GDM in women with FPG ≥5.10 mmol/L and pre-pregnancy overweight or obesity was higher than that in women with FPG ≥5.10 mmol/L and pre-pregnancy BMI <24.0 kg/m2 (78.5% [62/79] vs. 52.9% [64/121], χ2 = 13.425, P < 0.001).
Table 4.
FPG ≥5.10 mmol/L in 19th to 24th gestational weeks to screen GDM among different pre-pregnancy BMI women.
The receiver operating characteristic curve of using FPG in 19th to 24th gestational week to screen GDM is shown in Figure 1A–1C. The area under the curve (AUC) in women with pre-pregnancy BMI ≥24.0 kg/m2, <24.0 kg/m2 and total were 0.803, 0.679, and 0.718, respectively.
Figure 1.
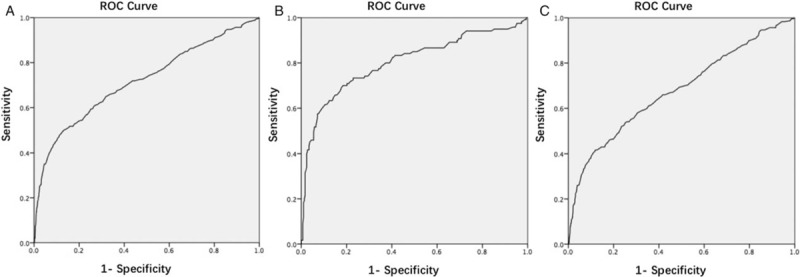
ROC curve of using FPG in the 19th to 24th gestational weeks to screen GDM in total (A), pre-pregnancy BMI ≥ 24.0 kg/m2 (B), and pre-pregnancy BMI < 24.0 kg/m2 (C) (P < 0.001). BMI: Body mass index; FPG: Fasting plasma glucose; GDM: Gestational diabetes mellitus; ROC: Receiver operating characteristic.
Discussion
The results of the multi-region retrospective cohort study showed the predictive value of FPG before the 24th gestational week for GDM in China. As we all know, type 2 diabetes has become a global epidemic. It has been reported that the prevalence of diabetes was 9.7% for the entire population (8.8% for women) and the prevalence of pre-diabetes was 15.5% (14.9% for women) in China.[10] Both GDM mothers and babies have the high risk of DM in their further lives, and the risk would decrease significantly after good glycemic control during pregnancy.
It is recommended that all pregnant women should have FPG test to exclude pre-pregnancy diabetes mellitus by the International Association of Diabetes and Pregnancy Study Groups (IADPSG).[11,12] One issue with GDM screening test is that the test is usually performed between the 24th and 28th gestational week using 75-g OGTT,[13–15] resulting in only about 14 to 16 weeks for GDM management. In recent years, studying the predictive value of FPG to GDM has become a hot topic.[16–19]
FPG is a well predictive index for GDM diagnosis, but it has been reported that it is inappropriate to use FPG as the diagnostic basis of GDM at the early stage of pregnancy as FPG decreases with increasing gestational age.[8] Pre-pregnancy obesity or overweight is an independent risk factor for GDM. Therefore, we evaluated if combining pre-pregnancy BMI and FPG before the 24th gestational week can better predict GDM.
Our study showed that the downtrend of FPG level was not significant after the 19th gestational week, suggesting FPG at the 19th to 24th gestational weeks would be a good predictor for GDM. In women pre-pregnancy with overweight and obesity, the predictive power of FPG was even higher. For example, the incidence of GDM was up to 78.5% when FPG ≥5.10 mmol/L in the 19th to 24th gestational week in women with pre-pregnancy overweight or obesity, and the AUC of FPG ≥5.10 mmol/L was as high as 0.803; these women should be treated as GDM earlier. We also recommended the screening test of PFG at the 19th to 24th gestational week for GDM in women who were overweight or obese before pregnancy.
However, several limitations existed in this study. First, the study targeted only pregnancy women in Beijing, Chengdu and Guangzhou in China. Second, the pre-pregnancy weight was based on self-report of the participants, which was at risk of self-report bias.
In conclusion, FPG decreased as gestational age increased in different pre-pregnancy BMI groups, and the downward trend became insignificant after 19 weeks of gestation. Pre-pregnancy overweight or obesity was associated with an increased FPG value before the 24th gestational week. FPG ≥5.10 mmol/L between the 19th and 24th gestational week was a good predictor for GDM in women with pre-pregnancy overweight and obesity.
Funding
This study was supported by grants from National Key Research and Development Program of Reproductive Health & Major Birth Defects Control and Prevention (No. 2016YFC1000402) and World Diabetes Foundation (No. WDF14–908).
Conflicts of interest
None.
Footnotes
How to cite this article: Wei YM, Liu XY, Shou C, Liu XH, Meng WY, Wang ZL, Wang YF, Wang YQ, Cai ZY, Shang LX, Sun Y, Yang HX. Value of fasting plasma glucose to screen gestational diabetes mellitus before the 24th gestational week in women with different pre-pregnancy body mass index. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000158
References
- 1.Chan JC, Zhang Y, Ning G. Diabetes in China: a societal solution for a personal challenge. Lancet Diabetes Endocrinol 2014; 2:969–979. doi: 10.1016/S2213-8587(14)70144-5. [DOI] [PubMed] [Google Scholar]
- 2.Yajnik CS, Deshmukh US. Maternal nutrition intrauterine programming and consequential risks in the offspring. Rev Endocr Med Disord 2008; 9:203–211. doi 10.1007/s11154-008-9087-z. [DOI] [PubMed] [Google Scholar]
- 3.Waters TP, Dyer AR, Scholtens DM, Dooley SL, Herer E, Lowe LP, et al. Maternal and neonatal morbidity for women who would be added to the diagnosis of GDM using IADPSG criteria: a secondary analysis of the hyperglycemia and adverse pregnancy outcome study. Diabetes Care 2016; 39:2204–2210. doi: 10.2337/dc16-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei Y, Yang H, Zhu W, Yang H, Li H, Yan J, et al. International Association of Diabetes and Pregnancy Study Group criteria is suitable for gestational diabetes mellitus diagnosis: further evidence from China. Chin Med J 2014; 127:3553–3556. doi: 0.3760/cma.j.issn.0366-6999.20140898. [PubMed] [Google Scholar]
- 5.Zhu W, Yang H, Wei Y, Wang Z, Li X, Wu H, et al. Comparing the diagnostic criteria for gestational diabetes mellitus of World Health Organization 2013 with 1999 in Chinese population. Chin Med J 2015; 128:125–127. doi: 10.4103/0366-6999.147858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song G, Wang C, Yang HX. Diabetes management beyond pregnancy. Chin Med J 2017; 130:1009–1011. doi: 10.4103/0366-6999.204938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang HX. Diagnostic criteria for gestational diabetes mellitus (WS 331-2011). Chin Med J 2012; 125:1212–1213. doi: 10.3760/cma.j.issn.0366-6999.2012.07.004. [PubMed] [Google Scholar]
- 8.Zhu WW, Yang HX, Wei YM, Yan J, Wang ZL, Li XL, et al. Evaluation of the value of fasting plasma glucose in the first prenatal visit to diagnose gestational diabetes mellitus in china. Diabetes Care 2013; 36:586–590. doi: 10.2337//dc12-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004; 363:157–163. doi: 10.1016/S0140-6736 (03)15268-3. [DOI] [PubMed] [Google Scholar]
- 10.Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, et al. China National Diabetes and Metabolic Disorders Study Group. Prevalence of diabetes among men and women in China. N Engl J Med 2010; 362:1090–1101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 11.Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, et al. HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008; 358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 12.Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, et al. International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010; 33:676–682. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Diabetes Association. Standards of medical care in diabetes-2017: summary of revisions. Diabetes Care 2017; 40:S4–S5. doi: 10.2337/dc17-S003. [DOI] [PubMed] [Google Scholar]
- 14.Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: a World Health Organization Guideline. Diabetes Res Clin Pract 2014;103:341–363. doi: 10.1016/j.diabres.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Hod M, Kapur A, Sacks DA, Hadar E, Agarwal M, Di Renzo GC, et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on gestational diabetes mellitus: a pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet 2015; 131:S173–211. doi: 10.1016/S0020-7292(15)30007-2. [DOI] [PubMed] [Google Scholar]
- 16.Li P, Yin Y, Lin S, Cui J, Zhou S, Li L, et al. Utility of pregestational body mass index and initial fasting plasma glucose in predicting gestational diabetes mellitus. Am J Med Sci 2016; 351:420–425. doi: 10.1016/j.amjms.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Ozgu-Erdinc AS, Yilmaz S, Yeral MI, Seckin KD, Erkaya S, Danisman AN. Prediction of gestational diabetes mellitus in the first trimester: comparison of C-reactive protein, fasting plasma glucose, insulin and insulin sensitivity indices. J Matern Fetal Neonatal Med 2015; 28:1957–1962. doi: 10.3109/14767058.2014.973397. [DOI] [PubMed] [Google Scholar]
- 18.Jesmin S, Akter S, Akashi H, Al-Mamun A, Rahman MA, Islam MM, et al. Screening for gestational diabetes mellitus and its prevalence in Bangladesh. Diabetes Res Clin Pract 2014; 103:57–62. doi: 10.1016/j.diabres.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 19.Yeral MI, Ozgu-Erdinc AS, Uygur D, Seckin KD, Karsli MF, Danisman AN. Prediction of gestational diabetes mellitus in the first trimester, comparison of fasting plasma glucose, two-step and one-step methods: a prospective randomized controlled trial. Endocrine 2014; 46:512–518. doi: 10.1007/s12020-013-0111-z. [DOI] [PubMed] [Google Scholar]


